1. Anatomy of the Retina and Light Transduction
The retina is an essential part of the eye that holds various components responsible for the interpretation of light stimulus into electrical signals for the brain to develop visual images1. The three essential cells that comprise the retina are the photoreceptors that connect to bipolar neural cells, and ganglion cells connecting after. This large-scale connection of the retina can further be divided into ten specific cellular sublayers for maintaining cells and interpreting light. The innermost layer, named the inner limiting membrane, is an exception to the composition of retinal layers as it is made up of basement membranes that separate the retina from the vitreous cavity of the eye2. Hereafter, all ganglia axons begin to converge to create the next layer called the nerve fiber layer (NFL). These fibers eventually thicken as they enter the optic disc, the gateway from the retina to the optic nerve3. The following ganglion cell layer is where ganglion cell bodies reside and where they project their axons to form the optic nerve1. The inner plexiform layer follows and is named for the connection between the synapse of ganglion cells and bipolar cells, moreover, bipolar cells are seen to form various types of connections between amacrine cells4. Next is the inner nuclear layer which includes bipolar, horizontal, and amacrine cells1. The next three layers are counterparts to the inner layers: the outer plexiform layer (OPL), the outer nuclear layer (ONL), and the external limiting membrane (ELM). The OPL describes the synaptic connection between the bipolar cells and photoreceptors, the ONL describes the layer of photoreceptor cell bodies, and the ELM is filled with gap junctions that separate photoreceptor cell bodies from their inner and outer segments1. After the ELM is the photoreceptor layer consisting of photoreceptors’ inner and outer segments responsible for housing mitochondria for metabolism demands and light-sensitive rhodopsin for light transduction, respectively1. The final layer is the retinal pigment epithelial (RPE) which is below choroid vascularization and has essential roles in absorbing unnecessary light stimulus, providing physiological support, and secreting growth factors5.
When light enters the eye, it is first processed by the two types of photoreceptors, rods and cones. The process of light transduction begins with the chemical breakdown of rhodopsin. A quick overlook of the process shows that rhodopsin, composed of protein Opsin and 11-cis Retinal, isomerizes into all-trans retinal and detaches the protein Opsin through photobleaching6. A more detailed process shows that when rhodopsin is photobleached, the inactivated protein scotopsin or photopsin, respective to rods and cones, will isomerize through varying opsin intermediates before settling into metarhodopsin, the product in both photoreceptors that activates transducin7. Specifically, metarhodopsin exists in equilibrium between metarhodopsin I and metarhodopsin II, where metarhodopsin II is favored in high temperatures and pH is the active form essential for transducin activation8. Transducin, the G-protein bounded with GDP, then becomes activated through the photolysis of rhodopsin to exchange GDP for GTP9. The protein phosphodiesterase is then activated by GTP and regulated by ATP to turn cyclic GMP (cGMP) to GMP through hydrolysis10. This breakdown of cGMP is done through phosphodiesterase, a light-activating enzyme. Mediated by cGMP, the breakdown to GMP closes positive-ion channels and produces a hyperpolarized electrical signal of the photoreceptors6. In dark settings, photoreceptors possess sodium and calcium ion channels located in synapse terminals that bring in a constant current of positive ions within the outer segment that trigger the release of the neurotransmitter glutamate11. In the presence of light, cation levels consequently drop alongside glutamate release. This change in the rate of neurotransmitter release is then decoded by bipolar cells. Bipolar cells have been seen to differ between rods and cones as each photoreceptor has its respective bipolar cell it interacts with12. Regardless of the photoreceptor, all variations of bipolar cells are still mediated by glutamate expression within synapse terminals. Communicating through synaptic potential instead of action potentials, bipolar cells send inputs toward retinal ganglion cells that eventually form a bundle of axons termed the optic nerve that sends information toward the brain.
1.1. Immunological and Homeostatic Role of Retinal Cells
As seen in the immune system, microglia play pivotal roles in the retinal microenvironment. They are tasked with constantly observing the body for immunoregulation, tissue repairment, and defending the body against foreign stimuli13. Alongside microglia, the blood-retinal barrier (BRB) and the complement system play roles in retinal immunity. The BRB supports the retina layers through an inner layer comprised of tight vascular endothelial cell junctions and an outer tight junction layer between RPE cells14. This defense protects the retina from potential foreign pathogens and any harmful responses from the systemic immune system. In the event of a breach within the BRB, the retina suppresses immune response to reduce inflammation. Lowering immunopathology is achieved through suppressing immune activation mediating by neural-immune regulatory proteins that influence microglia, macrophages, and the activation of the complement system14. As part of the innate immune system, the complement system is involved in retinal immunity through retinal microglia. Initially synthesized from hepatocytes, complement proteins are circulated throughout the body awaiting stimuli from BRB injury. Depending on the protein, three pathways may be activated that trigger membrane ruptures through osmotic pressure15 of impacted cells. Furthermore, past studies show that protein synthesis involved in activating complement pathways is also locally synthesized in retinal cells, demonstrating a higher involvement of retinal cells within immunity16.
In the context of homeostasis, retinal Müller cells are glial cells that are heavily involved in maintaining the retinal environment. Located in all nuclear and plexiform layers, Müller cells are seen to regulate the excitatory and inhibitory neurotransmitters glutamate and GABA17.
Additionally, neurotrophic and growth therapeutic agents are synthesized for neuronal and other glial cells that facilitate retinal development and reduce reactive oxygen species17. Like microglia, Müller cells can phagocytose external cell factors and debris for microenvironment maintenance and may trigger pro-inflammatory responses when pathogens appear 17. Moreover, Müller cells behave as a secondary defense when misplaced serum or proteins flow into the cell18. Horizontal and amacrine cells are seen to play roles in homeostasis. Horizontal cells mediate glutamate transmission with photoreceptors while amacrine cells interpret and form signals within the inner retina toward ganglion cells19.
1.2. Pathology Regarding the Retina
Findings within the year 2020 report that within patients 50 years or older, diseases such as Glaucoma, Age-related Macular Degeneration, and Diabetic Retinopathy were among the highest causes of eye blindness20. With over 40 million blind cases, the alternative for addressing irreversible blindness is the transplantation of retinal progenitor, pigment epithelium, and ganglion cells21. In this section, we highlight the workings of retinal degeneration diseases that result in blindness.
Age-related Macular Degeneration, or AMD, is the progressive loss of photoreceptors and RPE through the abnormal buildup of drusen, or ECM components seen on RPE and Bruch’s membrane BrM23. At first, manifesting in moderate drusen sizes termed “early AMD,” the progression into larger aggregates is termed Geographic Atrophy (GA) or neovascular AMD20.
Eventually leading to blindness, AMD causation can originate from aging, genetics, and even biochemical pathways such as the complement system. It has been studied that the complement system heavily influences the retina, and dysregulation of this pathway becomes a main driver for AMD pathology23. Specifically, it is through the combination of local and system complement proteins that affects tissues and has been validated through higher complement protein concentration in patients with AMD compared to controls21. Moreover, RPE and immune cells influenced by the complement system to activate and secrete pro-inflammatory substances to further cause pathology 21.
Diabetic retinopathy (DR) is one of the implications of both variations of diabetes mellitus, where hyperglycemic blood flow leads to retinal endothelial cell damage. This change in retinal blood flow also leads to basement membrane thickening and blood vessel ischemia which trigger further complications24. Through ischemia, the frequent occurrence of endothelial lesions and microaneurysms can result in retinal detachment, ultimately causing vision loss22.
Regarding inflammation, microglia have been confirmed to play roles in retina pathology- induced animal models, produce factors that induce retinal neuronal death in cell culture, and have been identified in post-mortem DR patients at different pathology progression states25.
Glaucoma is a retinal disease that damages the foundation of the optic nerve head through the loss of retinal ganglion cell axons26. The risk factors that facilitate the development of glaucoma include aging, genetic heritage, and intraocular pressure that ultimately leads to optic nerve damage and vision loss. This degeneration of retinal ganglion cells is caused by elevated intraocular pressure attributed to the unmediated outflow of clear fluid produced in the eye, known as aqueous humor27. Though the focus for therapies addresses intraocular pressure, studies have reported that glaucoma pathophysiology changes with disease progression, incorporating aspects of the innate immune response28. Thus, demonstrating that mechanical and biochemical origins contribute to disease progression.
Of the retinal diseases mentioned, Retinitis Pigmentosa (RP) arises from genetic predispositions that lead to vision and color impairment through photoreceptor degeneration. RP displays a range of genetic origins such as being inherited through dominant and recessive autosomal chromosomes and X-linked genes and additional genes have been implicated in critical retinal roles such as phototransduction29. The presence of these genes leads to the execution of detrimental necrotic and apoptotic signaling which affects rods first, followed by the degeneration of cones30. Specifically, apoptosis pathways are characterized as three-caspase- dependent mechanisms that, when altered through gene mutations, become activated. This activation causes many downstream proteins to be cleaved and released which results in irreversible damage to DNA31. Additionally, unregulated necrotic pathways are activated through the presence of reactive oxygen species and cell death attributed to ischemia29.
2. Commercial Alternatives to Eye Therapeutics
Biobanks provide an efficient way for patients to find alternatives for their best match in tissue replacements. It has been through biobanking that most patients impacted by eye diseases turn to for their therapeutic needs. Moreover, biobanks provide useful information for researchers to study pathologies such as Diabetic Retinopathy or Glaucoma within retinas through gaining thousands of measurements in visual acuity, fundus photography, and refraction32. Over the past couple of years, biobanks have been heavily involved in the contribution of organ transplantations for patients through compatible biomarker identification for reducing risk of organ rejection33. Termed population-based, these biobanks focus on common to complex diseases, and biobanks defined as disease-oriented possess tissue samples and clinical data34, both of which have made accessibility to retinal or other eye tissues easier. Although biobanks have made it easier to access compatible tissue sources, these therapies rely on patients or institutions that ultimately do not address the shortage of donors. Additionally, the many databases available for doctors to decide on what may or may not improve patient compatibility are set to have a margin of error as it is not truly personalized to the patient. To address such concerns, stem cells are a viable option for their ability to revert to a pluripotent state from any patient cell, making it truly personalized.
3. Induced Pluripotent Stem Cells to Retinal Cells
Stem cells are the foundational cells of the body that differentiate into different cell types and can also self-renew. These exist in both embryos and adults. Embryonic stem cells are present in the inner cell mass of blastocysts i.e., from the 4th to the 7th day after fertilization.
These stem cells carry the potential to differentiate into the three germinal layers35. Adult stem cells (ASCs) are also known as somatic stem cells and are undifferentiated cells that can be isolated from the bone marrow, central nervous system, skin, intestine, etc. These can only be induced to form epithelial cells as they are composed of mature cells that brings higher genetic stability for extended-duration cultures35.
These cells can also be classified into totipotent, pluripotent, multipotent, oligopotent and unipotent stem cells based off their differentiation potential.
Totipotent stem cells can divide into cells of the entire organism, while pluripotent stem cells are usually specific to a certain germ layer. Multipotent and oligopotent stem cells have narrower differentiation spectrums while unipotent stem cells have the narrowest differentiation capability but with a rapid dividing ability36.
Yamanaka, Takahashi and their group reprogrammed multipotent adult stem cells to pluripotent stem cells. They successfully induced fibroblasts to become pluripotent, naming them pluripotent stem cells37. IPSCs show similar characteristics to embryonic stem cells within their genetic markers and differentiation potential. They can form the three germ layers, hence why they are preferred for developmental studies38.
Retinal cells are highly specialized cells and hence do not divide. Damage to these cells gives rise to irreversible blindness and other retinal diseases. Stem cell therapy is a treatment option that has recently been explored for retinal disorders. Stem cells originating from blastocyst-stage embryos or adult fibroblasts can be reprogrammed into pluripotent stem cells and utilized for retinal regeneration39.
The retina is made up of neuroretina and retinal pigmented epithelium. The undifferentiated induced pluripotent stem cells must be programmed toward neuronal lineage. The pivotal stages from iPSCs to retinal cells include the formation of the anterior neural plate, the optic vesicle emerging from the diencephalon and an invagination to form a bilayered optic cup. These iPSCs are introduced to proneural N2 media which involves SMAD inhibition and the addition of IGF1 to promote retinogenesis40. SMAD inhibition allows for neuroectodermal development while IGF-1 plays a role in neural/eye field development and Notch inhibition is required for photoreceptor development41. Retinoic acid and FGF-2 in B27 and N2 media promote the neural retinal cell types while Activin-A moves the cells from progenitor to maturing neurons42. Barnea-Cramer and colleagues introduced retinal induction media and neural differentiation media to pluripotent stem cells which became eye field progenitors. Neural differentiation media was then removed to give retinal neuronal progenitor cells and photoreceptor progenitors. Photoreceptor-like cells were formed when BDNF (brain-derived neurotrophic factor), retinoic acid, DAPT, and ciliary neurotrophic factor (CNTF)43.
3.1. Clinical Trials for Retinal iPSCs
Clinical trials using iPSC replacing RPE cells have been conducted as early as 2013. In the trial and study by Mandai et al, the group utilized a patient's cell line to develop RPE cells that exhibited a consistent genetic profile of the RPE tissue. Moreover, this transplantation aimed to restore conditions from AMD pathology without implementing immunosuppressants44.
Though this trial only had one patient, it is important not to disregard the impact of the immune system in the aspect of cell rejection. As recent as 2023, various trials have been aimed at minimizing stem cell rejection by immunosuppressing the body through medication and steroids
45. Through rare trials mentioned in Gowrishankar et al. that showed severe side effects such as pneumonia or urinary tract infections, most trials and patients were successful in their immunosuppression. Though the longevity and efficacy of transplanted retinal stem cells are yet to be elucidated, mediating the immune response is appealing when considering past animal model studies reporting no identification or rejection of hESC RPE cells when no immunosuppression was administered
45.
Table 1 provides up-to-date information on the ongoing clinical trials utilizing iPSCs for addressing retina-associated diseases.
4. Miniature Organs: Organoids
Cells can grow into three-dimensional structures where they accumulate to form small masses of cells that can self-organize and differentiate into various cell types. These originate from stem cells to form three-dimensional structures called organoids which resemble an organ’s functional and structural characteristics. These mimic the organ biochemically better than 2D cultures.
Scientists Smith and Cochrea were the first to use the term organoids for cystic teratomas, in 194651. In 1987, Li et al. used mouse mammary epithelial cells to form ducts, ductules, lumina, and secretory alveoli-like structures that secreted milk proteins52.
Ronnovjessen et al, in 1990, co-cultured tumor cells along with fibroblasts to recreate breast cancer organoids53. 2009 brought a milestone in organoids. Sato et al, used adult intestinal stem cells, that expressed a single leucine-rich repeat containing G protein-coupled receptor 5, to form a 3D intestinal organoid with villus-like structures54,55. Following this, we have made organoids of multiple kinds such as the heart, liver, kidney, brain, pancreas, and retina56.
Stem cells can be induced to take up a certain cell type lineage by intrinsic cellular components, introducing growth factors or environmental cues such as through media or extracellular matrix57. Cell-cell interactions and external forces from the extracellular matrix can also provide active and passive biochemical cues56. The most used extracellular matrix is matrigel which is composed of collagen, entactin, laminin, and heparin sulfate proteoglycans58. However, matrigel is derived from Engelbreth-Holm-Swarm mouse sarcomas and hence contains tumor growth factors that could affect the maturation process of organoids. Other alternatives to matrigel include hydrogels like collagen, alginate, or functionalizing the hydrogel with RGD (Arg-Gly-Asp) peptides, and synthetic polymer hydrogels of poly-ethylene glycol (PEG) or poly lactic-co-glycolic acid (PLGA)59.
Soluble factors such as biologics, growth factors, or small molecule drugs help activate or inhibit pathways to direct cells to a particular lineage resulting in the differentiation of stem cells to form organoids60. Furthermore, the use of microfluidics aids in creating concentration gradients of growth factors or morphogens that allow tissue patterning.
Organoids are formed from human-derived cells with high efficiency and stability, making them advantageous to research human physiological systems. The added benefit of individualization contributes to precision and personalized medicine. The three major organoid applications are drug discovery, transplantation alternatives, and disease modeling.
5. Retinal Organoids
Stem cell-derived 2D cultures are unable to replicate all aspects of the retina and hence an efficient model that offers stability and allows the study of cell-cell interactions and signaling pathways to interpret the development of retinogenesis as well as retinal disorders.
The first successfully developed retinal organoid was done by the Sasai lab in 2011. They used mouse ESCs which spontaneously formed a hollow vesicle of the neuroepithelium and further differentiated into the rostral forebrain tissue to give a retina-forming field. These ESCs self-organized to take up an optic cup structure with six different cell types that included the photoreceptors, Muller cells, bipolar cells, ganglion cells, horizontal cells, and amacrine cells61. The same research group subsequently demonstrated using human ESCs to form self-organizing retinal organoids with an optic cup structure and layers of distinct retinal cells using a serum-free floating culture-like aggregates method62.
Earlier work indicated using Noggin and DKK1 for early differentiation in neural induction63. The use of antagonists of Wnt, IGF1, and BMP464,65,66 is an alternative guided approach for the differentiation of development of the neural retina. The inactivation of motor proteins, myosin, is said to facilitate the invagination of the optic cup62. Neural induction media is usually B27 media with antagonists of Wnt/Notch/BMP4 signaling. This influences the cells to take up the neural retinal fate. For the further differentiation and maturation of these organoids, lipid supplements, taurine67, and retinoic acid68 are used. All of which help in developing retinal ganglion cells and photoreceptors69,70.
5.1. Retinal Organoids for Medicine
5.1.1. Age-Related Macular Degeneration (AMD)
IPSC-derived retinal pigmental epithelium from patients has been used to create successful disease models. These are known to have the appropriate biomarkers, the required immune complement systems, and pro-inflammatory factors71,72. Studies done using retinal organoids indicated extracellular vesicles in the RPE of AMD as disease messengers and hence could be considered therapeutic targets73. The disease model for AMD is in the works along with research being done on potential therapeutics.
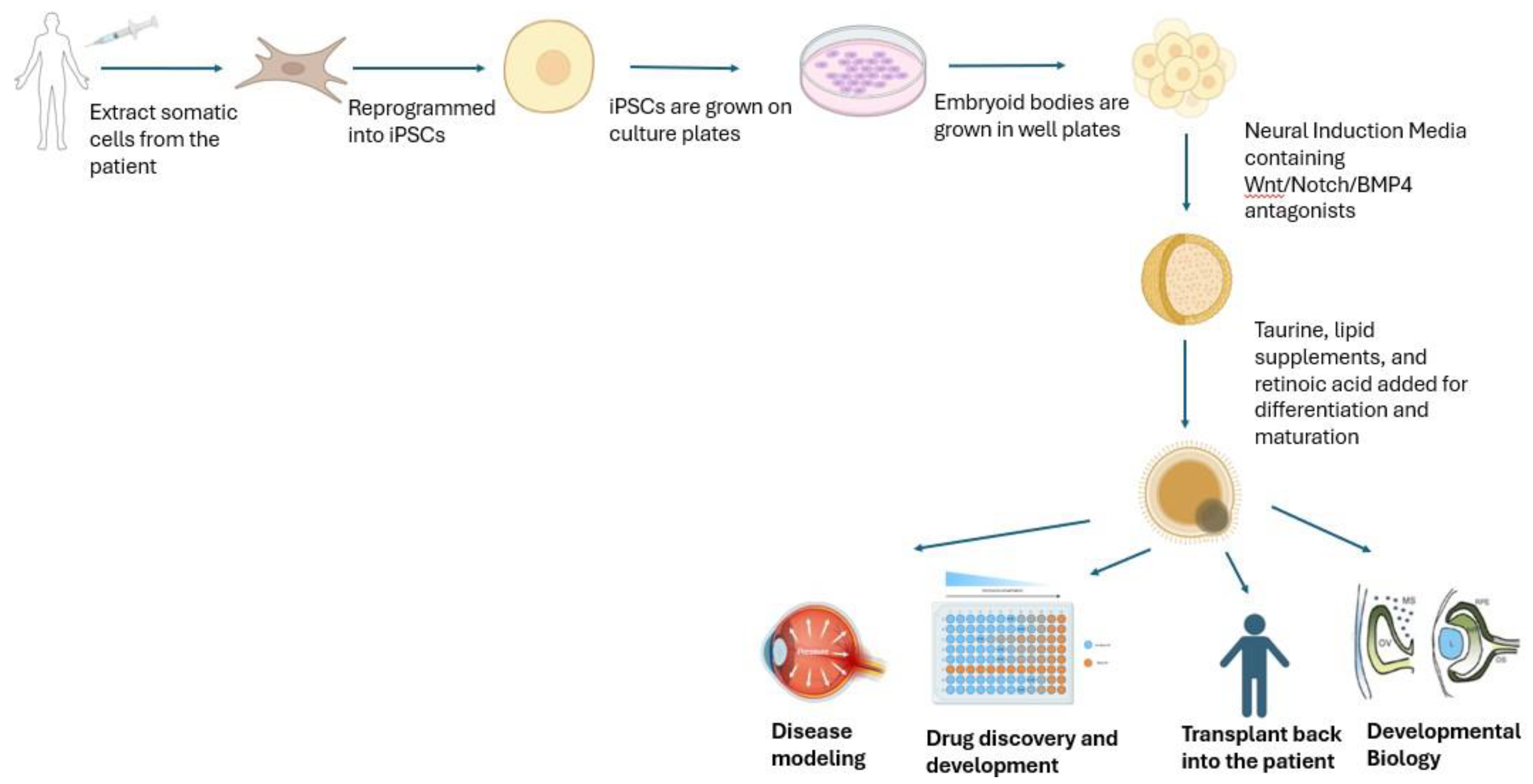
Figure 1.
Somatic cells can be extracted from a patient to generate induced pluripotent stem cells. These are grown on culture dishes and well plates to get embryoid bodies (clusters). Neural Induction Media along with supplements allow these to grow into retinal organoids. Drug discovery and development, transplant alternatives, disease modeling, and developmental biology97 are examples of their applications. (Created in Biorender).
Figure 1.
Somatic cells can be extracted from a patient to generate induced pluripotent stem cells. These are grown on culture dishes and well plates to get embryoid bodies (clusters). Neural Induction Media along with supplements allow these to grow into retinal organoids. Drug discovery and development, transplant alternatives, disease modeling, and developmental biology97 are examples of their applications. (Created in Biorender).
5.1.2. Retinitis Pigmentosa
RP is a hereditary retinal degenerative disorder in which an irreversible loss of photoreceptor cells is observed. Cyclic GMP concentrations are elevated along with an influx of Ca ions through the CNG channels. The use of retinal organoids as a disease model for early- onset and late-onset retinal degeneration has been established using patient-derived iPSCs74,75,76. A mutation in RP2 accounts for 15% of X-linked retinitis pigmentosa, Cheetham and his scientists investigated this mutation and a rescue mechanism using iPSC-derived retinal organoids77. These organoids are also now emerging as transplantation alternatives. One such study was done in patients of retinitis pigmentosa, and the grafts led to no adverse effects and helped in improving full-field light stimulus78.
5.1.3. Glaucoma
Photoreceptor cells, retinal pigment epithelium cells, and retinal ganglion cells (RGC) have been widely studied using retinal organoids. The genetically inherited form of glaucoma leads to the loss of RGCs. These were rescued using neuroprotective factors introduced to patient-derived retinal organoids79. A recent study showed the use of retinal ganglion-like cells from retinal organoids by adding surface markers like CD184 and CD171. These cells survived, integrated with the receptor retina, and even helped with optic nerve damage80.
5.1.4. Neurodegenerative Disorders
A 3D disease model of the retina was created to study the early-onset phenotypes of Alzheimer’s disease81. Neurodegeneration and inflammation studies of early diabetic retinopathy have also been modeled in retinal organoids82.
5.1.5. Bacterial Conjunctivitis
Pharmacological and toxicological studies have also been conducted on these retinal organoids. The drug Moxifloxacin, which is a wide-spectrum antibiotic, when introduced to these organoids had toxic effects, especially on the amacrine cells and photoreceptors83.
5.1.6. X-Linked Juvenile Retinoschisis
A retinal organoid model for X-linked juvenile retinoschisis was developed and a CRISPR/Cas9 base modification was made to show that the condition could be repaired in that manner84.
6. Organ – On – A – Chip
Organ-on-a-chips are microfluidic devices in which living single or multiple cell cultures are grown to research organ-level pathophysiology. The first ever device created was a lung alveolus, that had hollow channels like the lung airway. This chip had endothelium-lined channels to mimic vasculature, and a cyclin suction recreated the breathing motions85,86. Multi- organ systems can also be developed by creating separate chambers for each organ87.
Retinal organoids have emerged as a useful tool in developing new therapeutic strategies.
However, these lack vascularization and hence the physiological interactions between matured photoreceptors and retinal pigment epithelium have not been achieved. This led to the advent of the retina-on-a-chip. This enables vasculature and interactions, providing a closer model of the human retina due to the development of outer segment-like structures as well88.
The lack of the blood-retinal barrier (BRB) leads to a lack of neuronal signaling, resulting in vision loss. Organs on chips facilitate the organization of multi-layer complexes with the different transporters to investigate the entirety of the BRB. Another advantage to this is the assessment of immune infiltration in diseased conditions. Gradients can be created that allow the study of drug thresholds to predict drug dosages89,90.
The specificity and security of the eye lead to challenges in ocular drug delivery. Topical eye drops or intraocular injection administration seem the best and most widely adopted treatment option in ophthalmology. Organ-on-a-chip provides a platform to study the long-term effects of these treatment options due to their high stability91.
The platform provides a nutrient supply for the organoids using microfluidics. This allows for a stable and uniform nutrient supply facilitating the longer survival of these organoids88,92. This was done to help expand the retinal ganglion cells for drug screening, RGC- related disorders, and cell transplantation93.
7. Future Directions of Retinal Studies
Notable progress within retinal studies ranges from understanding disease pathology to learning more about retinal development. In a study published in 2024, retinal organoids were utilized to uncover the mechanisms behind long or medium cone fate, which dictates red or green cone reception, respectively94. Previously two models were proposed that attempted to explain how cone fate was dictated, and by referencing past animal studies95 and mechanisms observed in this study, researchers report retinoic acid playing a vital role in human cone fate.
Furthermore, a 2023 study uses human-derived retinal organoids to study how the subretinal space of animal models impacts the transplanted cells. The successful transplantation of human retinal organoids demonstrated that the in vivo environment supports efficient rod and cone development compared to in vitro counterparts and follow-up sequencing data exhibited a variety of cells seen in the retina96.
Scientists Busskamp and Sharma addressed the challenge of vasculature in the retinal organoids by mixing endothelial cells and iPSCs before cell differentiation. At the 30-week mark, the endothelial cells formed branching and even helped to improve the retinal organoid size97,98. The integration of vasculature and the immune system in these organoids is still a standing challenge. Another limitation is non-uniform differentiation patterns from iPSCs99.
8. Conclusion
Retinal disorders are highly prevalent in adults above the age of 60. These disorders do not have complete treatment options and hence the emergence of retinal organoids as a model system to help in the development of drugs and as a transplantation alternative aid in accomplishing that. Their use as disease models to assist in fully understanding the pathogenesis of these disorders is a step towards better treatment options. The role of these organoids in personalized medicine is promising for developing cures and therapy for retinal disorders in the coming years.
References
- Nguyen, K. H. , Patel, B. C., & Tadi, P. (2023). Anatomy, head and neck: Eye retina. In StatPearls [Internet]. StatPearls Publishing.
- Zhang, K. Y. , & Johnson, T. V. (2021). The internal limiting membrane: Roles in retinal development and implications for emerging ocular therapies. Experimental eye research, 206, 108545.
- Hildebrand, G. D., & Fielder, A. R. (2011). Anatomy and physiology of the retina. Pediatric retina, 39-65.
- Kolb, H. (2007). Inner plexiform layer. Webvision: The Organization of the Retina and Visual System [Internet].
- Spencer, C., Abend, S., McHugh, K. J., & Saint-Geniez, M. (2017). Identification of a synergistic interaction between endothelial cells and retinal pigment epithelium. Journal of cellular and molecular medicine, 21(10), 2542-2552.
- Pepe I., M. (1999). Rhodopsin and phototransduction. Journal of photochemistry and photobiology. B, Biology, 48(1), 1–10. [CrossRef]
- Imamoto, Y. , & Shichida, Y. (2014). Cone visual pigments. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1837(5), 664-673.
- Filipek, S. , Stenkamp, R. E., Teller, D. C., & Palczewski, K. (2003). G protein-coupled receptor rhodopsin: a prospectus. Annual review of physiology, 65(1), 851-879.
- Kwok-Keung Fung, B., & Stryer, L. (1980). Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proceedings of the National Academy of Sciences, 77(5), 2500-2504.
- Sitaramayya, A., & Liebman, P. A. (1983). Phosphorylation of rhodopsin and quenching of cyclic GMP phosphodiesterase activation by ATP at weak bleaches. Journal of Biological Chemistry, 258(20), 12106-12109.
- Haeseleer, F. , & Palczewski, K. (2002). Calmodulin and Ca 2+-binding proteins (CaBPs): variations on a theme. Photoreceptors and calcium, 303-317.
- Euler, T. , Haverkamp, S., Schubert, T., & Baden, T. (2014). Retinal bipolar cells: elementary building blocks of vision. Nature Reviews Neuroscience, 15(8), 507-519.
- Fan, W. , Huang, W., Chen, J., Li, N., Mao, L., & Hou, S. (2022). Retinal microglia: Functions and diseases. Immunology, 166(3), 268-286.
- Chen, M., Luo, C., Zhao, J., Devarajan, G., & Xu, H. (2019). Immune regulation in the aging retina. Progress in retinal and eye research, 69, 159-172.
- Xie, C. B. , Jane-Wit, D., & Pober, J. S. (2020). Complement membrane attack complex: new roles, mechanisms of action, and therapeutic targets. The American journal of pathology, 190(6), 1138-1150.
- Luo, C. , Chen, M., & Xu, H. (2011). Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Molecular vision, 17, 1588.
- Subirada, P. V. , Paz, M. C., Ridano, M. E., Lorenc, V. E., Vaglienti, M. V., Barcelona, P. F.,... & Sánchez, M. C. (2018). A journey into the retina: Müller glia commanding survival and death. European Journal of Neuroscience, 47(12), 1429-1443.
- Bringmann, A. , Iandiev, I., Pannicke, T., Wurm, A., Hollborn, M., Wiedemann, P.,... & Reichenbach, A. (2009). Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Progress in retinal and eye research, 28(6), 423-451.
- Masri, R. A. , Weltzien, F., Purushothuman, S., Lee, S. C., Martin, P. R., & Grünert, U. (2021). Composition of the inner nuclear layer in human retina. Investigative Ophthalmology & Visual Science, 62(9), 22-22.
- Steinmetz, J. D. , Bourne, R. R., Briant, P. S., Flaxman, S. R., Taylor, H. R., Jonas, J. B.,... & Morse, A. R. F. (2021). Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. The Lancet Global Health, 9(2), e144-e160.
- Van Gelder, R. N., Chiang, M. F., Dyer, M. A., Greenwell, T. N., Levin, L. A., Wong, R. O., & Svendsen, C. N. (2022). Regenerative and restorative medicine for eye disease. Nature medicine, 28(6), 1149-1156.
- Fleckenstein, M. , Keenan, T. D., Guymer, R. H., Chakravarthy, U., Schmitz-Valckenberg, S., Klaver, C. C.,... & Chew, E. Y. (2021). Age-related macular degeneration. Nature reviews Disease primers, 7(1), 31.
- Armento, A. , Ueffing, M., & Clark, S. J. (2021). The complement system in age-related macular degeneration. Cellular and Molecular Life Sciences, 78, 4487-4505.
- Antonetti, D. A. , Silva, P. S., & Stitt, A. W. (2021). Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nature Reviews Endocrinology, 17(4), 195- 206.
- Sachdeva, M. M. (2021). Retinal neurodegeneration in diabetes: an emerging concept in diabetic retinopathy. Current diabetes reports, 21(12), 65.
- Jayaram, H. , Kolko, M., Friedman, D. S., & Gazzard, G. (2023). Glaucoma: now and beyond. The Lancet, 402(10414), 1788-1801.
- Lee, D. A. , & Higginbotham, E. J. (2005). Glaucoma and its treatment: a review. American journal of health-system pharmacy, 62(7), 691-699.
- Shestopalov, V. I. , Spurlock, M., Gramlich, O. W., & Kuehn, M. H. (2021). Immune responses in the glaucomatous retina: regulation and dynamics. Cells, 10(8), 1973.
- Newton, F., & Megaw, R. (2020). Mechanisms of photoreceptor death in retinitis pigmentosa. Genes, 11(10), 1120.
- Liu, W. , Liu, S., Li, P., & Yao, K. (2022). Retinitis pigmentosa: progress in molecular pathology and biotherapeutical strategies. International Journal of Molecular Sciences, 23(9), 4883.
- Wu, K. Y. , Kulbay, M., Toameh, D., Xu, A. Q., Kalevar, A., & Tran, S. D. (2023). Retinitis pigmentosa: novel therapeutic targets and drug development. Pharmaceutics, 15(2), 685.
- Chua, S. Y. L. , Thomas, D., Allen, N., Lotery, A., Desai, P., Patel, P.,... & Foster, P. J. (2019). Cohort profile: design and methods in the eye and vision consortium of UK Biobank. BMJ open, 9(2), e025077.
- Hanif, Z., Sufiyan, N., Patel, M., & Akhtar, M. Z. (2018). Role of biobanks in transplantation. Annals of medicine and surgery, 28, 30-33.
- Kinkorová, J. (2016). Biobanks in the era of personalized medicine: objectives, challenges, and innovation: overview. EPMA Journal, 7, 1-12.
- National Research Council and Institute of Medicine. 2002. Stem Cells and the Future of Regenerative Medicine. Washington, DC: The National Academies Press. [CrossRef]
- Zakrzewski, W. , Dobrzyński, M., Szymonowicz, M. et al. Stem cells: past, present, and future. Stem Cell Res Ther 10, 68 (2019). [CrossRef]
- Takahashi, K., & Yamanaka, S. (2013). Induced pluripotent stem cells in medicine and biology. Development, 140(12), 2457-2461.
- Xu, Z. , Yang, J., Xin, X., Liu, C., Li, L., Mei, X., & Li, M. (2023). Merits and challenges of iPSC-derived organoids for clinical applications. Frontiers in cell and developmental biology, 11, 1188905. [CrossRef]
- Holmes, D. (2018). Reconstructing the retina. Nature, 561(7721), S2-S3.
- Rathod, R. , Surendran, H., Battu, R., Desai, J., & Pal, R. (2019). Induced pluripotent stem cells (iPSC)-derived retinal cells in disease modeling and regenerative medicine. Journal of Chemical Neuroanatomy, 95, 81-88.
- Wiley, L. A., Burnight, E. R., Songstad, A. E., Drack, A. V., Mullins, R. F., Stone, E. M., & Tucker, B. A. (2015). Patient-specific induced pluripotent stem cells (iPSCs) for the study and treatment of retinal degenerative diseases. Progress in retinal and eye research, 44, 15-35.
- Carla B. Mellough, Evelyne Sernagor, Inmaculada Moreno-Gimeno, David H.W. Steel, Majlinda Lako, Efficient Stage-Specific Differentiation of Human Pluripotent Stem Cells Toward Retinal Photoreceptor Cells, Stem Cells, Volume 30, Issue 4, April 2012, Pages 673– 686. [CrossRef]
- Barnea-Cramer, A. O. , Wang, W., Lu, S. J., Singh, M. S., Luo, C., Huo, H.,... & Lanza, R. (2016). Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Scientific reports, 6(1), 29784.
- Mandai, M. , Watanabe, A., Kurimoto, Y., Hirami, Y., Morinaga, C., Daimon, T.,... & Takahashi, M. (2017). Autologous induced stem-cell–derived retinal cells for macular degeneration. New England Journal of Medicine, 376(11), 1038-1046.
- Gowrishankar, S. , Smith, M. E., Creber, N., Muzaffar, J., & Borsetto, D. (2024). Immunosuppression in stem cell clinical trials of neural and retinal cell types: A systematic review. Plos one, 19(7), e0304073.
- Eyestem Research Pvt. Ltd. Safety & Efficacy of Eyecyte-RPE™ in Patients With Geographic Atrophy Secondary to Dry Age-related Macular Degeneration. ClinicalTrials.gov identifier: NCT06394232. Updated September 3, 2024. Accessed September 5, 2024.
- Zhongshan Ophthalmic Center, Sun Yat-sen University. Preparation of Patient Autologous Induced Pluripotent Stem Cell-derived Retinal Cells for AMD. ClinicalTrials.gov identifier: NCT05991986. Updated August 15, 2023. Accessed September 5, 2024.
- Beijing Tongren Hospital. Safety and Efficacy of Autologous Transplantation of iPSC-RPE in the Treatment of Macular Degeneration. ClinicalTrials.gov identifier: NCT05445063. Updated July 6, 2022. Accessed September 5, 2024.
- National Eye Institute (NEI). Autologous Transplantation of Induced Pluripotent Stem Cell- Derived Retinal Pigment Epithelium for Geographic Atrophy Associated With Age-Related Macular Degeneration. ClinicalTrials.gov identifier: NCT04339764. Updated June 18, 2024. Accessed September 5, 2024.
- University of Alabama at Birmingham. Human iPSC for Repair of Vasodegenerative Vessels in Diabetic Retinopathy (iPSC). ClinicalTrials.gov identifier: NCT03403699. Updated December 13, 2023. Accessed September 5, 2024.
- Rauth, S. , Karmakar, S., Batra, S. K., & Ponnusamy, M. P. (2021). Recent advances in organoid development and applications in disease modeling. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1875(2), 188527.
- Li, M. L., Aggeler, J., Farson, D. A., Hatier, C., Hassell, J., & Bissell, M. J. (1987). Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proceedings of the National Academy of Sciences, 84(1), 136-140.
- Rdnnov-Jessen, L., Petersen, O. W., Koteliansky, V., & Bissell, M. J. (1995). The origin of the myofibroblasts in breast cancer. J Clin Invest, 95, 859-873.
- Sato, T. , Vries, R. G., Snippert, H. J., Van De Wetering, M., Barker, N., Stange, D. E.,... & Clevers, H. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 459(7244), 262-265.
- Corrò, C. , Novellasdemunt, L., & Li, V. S. (2020). A brief history of organoids. American Journal of Physiology-Cell Physiology, 319(1), C151-C165.
- Qian, S., Mao, J., Liu, Z., Zhao, B., Zhao, Q., Lu, B., ... & Sun, X. (2022). Stem cells for organoids. Smart Medicine, 1(1), e20220007.
- Bartfeld, S. , & Clevers, H. (2017). Stem cell-derived organoids and their application for medical research and patient treatment. Journal of molecular medicine, 95, 729-738.
- Aisenbrey, E.A. , Murphy, W.L. Synthetic alternatives to Matrigel. Nat Rev Mater 5, 539– 551 (2020). [CrossRef]
- Heo, J. H., Kang, D., Seo, S. J., & Jin, Y. (2022). Engineering the extracellular matrix for organoid culture. International Journal of Stem Cells, 15(1), 60-69.
- Zhao, Z. , Chen, X., Dowbaj, A.M. et al. Organoids. Nat Rev Methods Primers 2, 94 (2022). [CrossRef]
- Eiraku, M. , Takata, N., Ishibashi, H. et al. Self-organizing optic-cup morphogenesis in three- dimensional culture. Nature 472, 51–56 (2011). [CrossRef]
- Nakano, T. , Ando, S., Takata, N., Kawada, M., Muguruma, K., Sekiguchi, K.,... & Sasai, Y. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell stem cell, 10(6), 771-785.
- Meyer, J. S. , Howden, S. E., Wallace, K. A., Verhoeven, A. D., Wright, L. S., Capowski, E. E.,... & Gamm, D. M. (2011). Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem cells, 29(8), 1206-1218.
- Kuwahara, A. , Ozone, C., Nakano, T., Saito, K., Eiraku, M., & Sasai, Y. (2015). Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nature communications, 6(1), 1-15.
- Mellough, C. B. , Collin, J., Khazim, M., White, K., Sernagor, E., Steel, D. H., & Lako, M. (2015). IGF-1 signaling plays an important role in the formation of three-dimensional laminated neural retina and other ocular structures from human embryonic stem cells. Stem cells, 33(8), 2416-2430.
- Singh, R. K. , Mallela, R. K., Cornuet, P. K., Reifler, A. N., Chervenak, A. P., West, M. D.,... & Nasonkin, I. O. (2015). Characterization of three-dimensional retinal tissue derived from human embryonic stem cells in adherent monolayer cultures. Stem cells and development, 24(23), 2778-2795.
- Cowan, C. S. , Renner, M., De Gennaro, M., Gross-Scherf, B., Goldblum, D., Hou, Y.,... & Roska, B. (2020). Cell types of the human retina and its organoids at single-cell resolution. Cell, 182(6), 1623-1640.
- Capowski, E. E., Samimi, K., Mayerl, S. J., Phillips, M. J., Pinilla, I., Howden, S. E., ... & Gamm, D. M. (2019). Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development, 146(1), dev171686.
- Móvio, M. I. , de Lima-Vasconcellos, T. H., Dos Santos, G. B., Echeverry, M. B., Colombo, E., Mattos, L. S., Resende, R. R., & Kihara, A. H. (2023). Retinal organoids from human- induced pluripotent stem cells: From studying retinal dystrophies to early diagnosis of Alzheimer's and Parkinson's disease. Seminars in cell & developmental biology, 144, 77–86. [CrossRef]
- O'Hara-Wright, M. , & Gonzalez-Cordero, A. (2020). Retinal organoids: a window into human retinal development. Development, 147(24), dev189746.
- Saini, J. S., Corneo, B., Miller, J. D., Kiehl, T. R., Wang, Q., Boles, N. C., ... & Temple, S. (2017). Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell stem cell, 20(5), 635-647.
- Kruczek, K., & Swaroop, A. (2020). Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells, 38(10), 1206-1215.
- Kurzawa-Akanbi, M., Whitfield, P., Burté, F., Bertelli, P. M., Pathak, V., Doherty, M., ... & Lako, M. (2022). Retinal pigment epithelium extracellular vesicles are potent inducers of age-related macular degeneration disease phenotype in the outer retina. Journal of Extracellular Vesicles, 11(12), 12295.
- Shimada, H., Lu, Q., Insinna-Kettenhofen, C., Nagashima, K., English, M. A., Semler, E. M., ... & Swaroop, A. (2017). In vitro modeling using ciliopathy-patient-derived cells reveals distinct cilia dysfunctions caused by CEP290 mutations. Cell reports, 20(2), 384-396.
- Deng, W. L., Gao, M. L., Lei, X. L., Lv, J. N., Zhao, H., He, K. W., ... & Jin, Z. B. (2018). Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem cell reports, 10(4), 1267-1281.
- Gao, M. L., Lei, X. L., Han, F., He, K. W., Jin, S. Q., Zhang, Y. Y., & Jin, Z. B. (2020). Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa. Frontiers in cell and developmental biology, 8, 128.
- Lane, A., Jovanovic, K., Shortall, C., Ottaviani, D., Panes, A. B., Schwarz, N., ... & Cheetham, M. E. (2020). Modeling and rescue of RP2 retinitis pigmentosa using iPSC- derived retinal organoids. Stem cell reports, 15(1), 67-79.
- Hirami, Y., Mandai, M., Sugita, S., Maeda, A., Maeda, T., Yamamoto, M., ... & Kurimoto, Y. (2023). Safety and stable survival of stem-cell-derived retinal organoid for 2 years in patients with retinitis pigmentosa. Cell Stem Cell, 30(12), 1585-1596.
- Ohlemacher, S. K., Sridhar, A., Xiao, Y., Hochstetler, A. E., Sarfarazi, M., Cummins, T. R., & Meyer, J. S. (2016). Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem cells, 34(6), 1553-1562.
- Li, G., & Luo, Y. (2024). Enriching new transplantable RGC-like cells from retinal organoids for RGC replacement therapy. Biochemical and Biophysical Research Communications, 700, 149509.
- Lavekar, S. S., Harkin, J., Hernandez, M., Gomes, C., Patil, S., Huang, K. C., ... & Meyer, J. S. (2023). Development of a three-dimensional organoid model to explore early retinal phenotypes associated with Alzheimer’s disease. Scientific Reports, 13(1), 13827.
- de Lemos, L., Antas, P., Ferreira, I. S., Santos, I. P., Felgueiras, B., Gomes, C. M., ... & Tenreiro, S. (2024). Modelling neurodegeneration and inflammation in early diabetic retinopathy using 3D human retinal organoids. In vitro models, 3(1), 33-48.
- Hallam, D., Hilgen, G., Dorgau, B., Zhu, L., Yu, M., Bojic, S., ... & Lako, M. (2018). Human-induced pluripotent stem cells generate light responsive retinal organoids with variable and nutrient-dependent efficiency. Stem cells, 36(10), 1535-1551.
- Huang, K. C., Wang, M. L., Chen, S. J., Kuo, J. C., Wang, W. J., Nguyen, P. N. N., ... & Chiou, S. H. (2019). Morphological and molecular defects in human three-dimensional retinal organoid model of X-linked juvenile retinoschisis. Stem Cell Reports, 13(5), 906-923.
- Huh, D., Fujioka, H., Tung, Y. C., Futai, N., Paine III, R., Grotberg, J. B., & Takayama, S. (2007). Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proceedings of the National Academy of Sciences, 104(48), 18886-18891.
- Varone, A., Nguyen, J. K., Leng, L., Barrile, R., Sliz, J., Lucchesi, C., ... & Hinojosa, C. D. (2021). A novel organ-chip system emulates three-dimensional architecture of the human epithelia and the mechanical forces acting on it. Biomaterials, 275, 120957.
- Ingber, D. E. (2022). Human organs-on-chips for disease modelling, drug development and personalized medicine. Nature Reviews Genetics, 23(8), 467-491.
- Achberger, K., Probst, C., Haderspeck, J., Bolz, S., Rogal, J., Chuchuy, J., ... & Loskill, P. (2019). Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. elife, 8, e46188.
- Kim, S., Kim, H. J., & Jeon, N. L. (2010). Biological applications of microfluidic gradient devices. Integrative Biology, 2(11-12), 584-603.
- Ragelle, H., Goncalves, A., Kustermann, S., Antonetti, D. A., & Jayagopal, A. (2020). Organ-on-a-chip technologies for advanced blood–retinal barrier models. Journal of ocular pharmacology and therapeutics, 36(1), 30-41.
- Yu, J., Yin, Y., Leng, Y., Zhang, J., Wang, C., Chen, Y., ... & Fan, Y. (2023). Emerging strategies of engineering retinal organoids and organoid-on-a-chip in modeling intraocular drug delivery: Current progress and future perspectives. Advanced Drug Delivery Reviews, 197, 114842.
- Zhao, H., & Yan, F. (2024). Retinal Organoids: A Next-Generation Platform for High- Throughput Drug Discovery. Stem Cell Reviews and Reports, 20(2), 495-508.
- Gong, J., Gong, Y., Zou, T., Zeng, Y., Yang, C., Mo, L., ... & Yang, J. (2023). A controllable perfusion microfluidic chip for facilitating the development of retinal ganglion cells in human retinal organoids. Lab on a Chip, 23(17), 3820-3836.
- Hadyniak, S. E., Hagen, J. F., Eldred, K. C., Brenerman, B., Hussey, K. A., McCoy, R. C., ... & Johnston Jr, R. J. (2024). Retinoic acid signaling regulates spatiotemporal specification of human green and red cones. PLoS biology, 22(1), e3002464.
- Mitchell, D. M. , Stevens, C. B., Frey, R. A., Hunter, S. S., Ashino, R., Kawamura, S., & Stenkamp, D. L. (2015). Retinoic acid signaling regulates differential expression of the tandemly-duplicated long wavelength-sensitive cone opsin genes in zebrafish. PLoS genetics, 11(8), e1005483.
- Liu, Y. V. , Santiago, C. P., Sogunro, A., Konar, G. J., Hu, M. W., McNally, M. M.,... & Singh, M. S. (2023). Single-cell transcriptome analysis of xenotransplanted human retinal organoids defines two migratory cell populations of nonretinal origin. Stem Cell Reports, 18(5), 1138-1154.
- Sharma, K., Krohne, T. U., & Busskamp, V. (2020). The rise of retinal organoids for vision research. International Journal of Molecular Sciences, 21(22), 8484.
- Busskamp, V.; Sharma, K. Advanced vascularized human stem cell-derived retinal organoids. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1894. [Google Scholar]
- Cooke, J. A., Voigt, A. P., Collingwood, M. A., Stone, N. E., Whitmore, S. S., DeLuca, A. P., ... & Tucker, B. A. (2023). Propensity of patient-derived iPSCs for retinal differentiation: implications for autologous cell replacement. Stem cells translational medicine, 12(6), 365- 378.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).