Submitted:
10 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
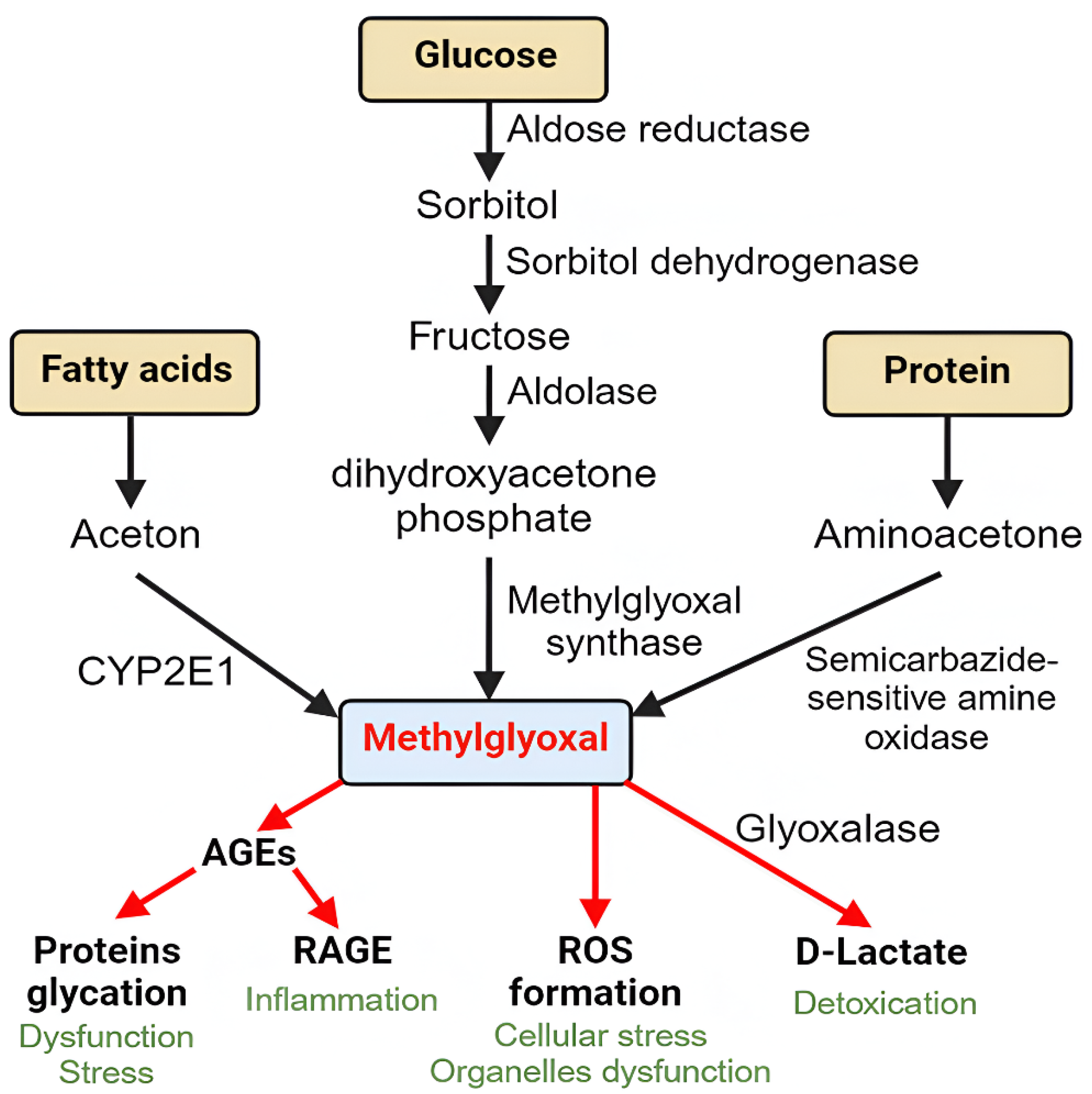
1. Sources of Methylglyoxal and Its Association with Diabetic Retinopathy
2. Modes of Action of MGO in DR
2.1. MGO-Induced Retina Proteins Glycation and Oxidative Stress
2.2. MGO-Induced RAGE Activation
3. MGO Alters Various Cellular Pathways
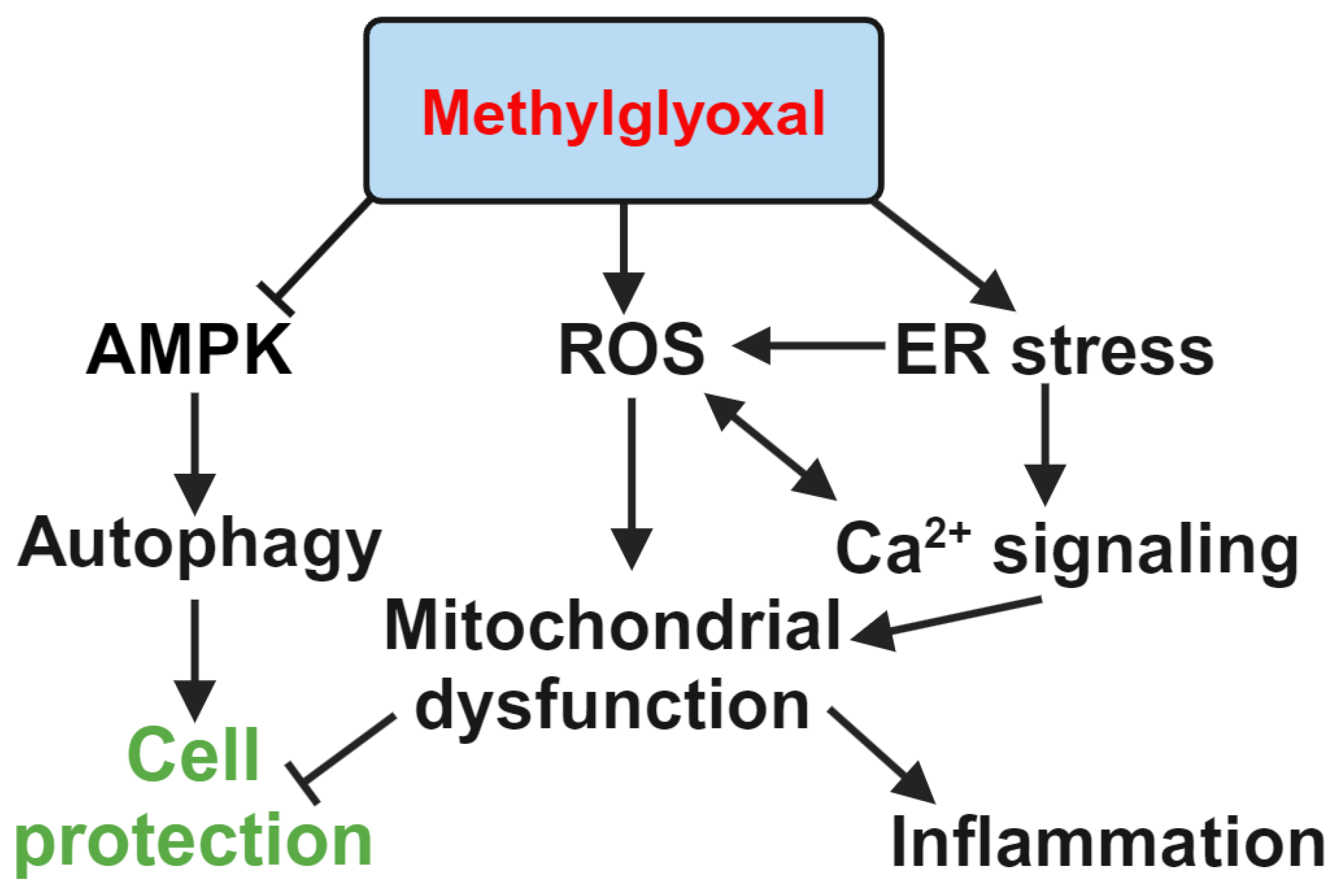
3.1. Inhibition of Autophagy in Retinal Cells
3.2. Induction of Oxidative Stress-Associated Inflammation in Retinal Cells
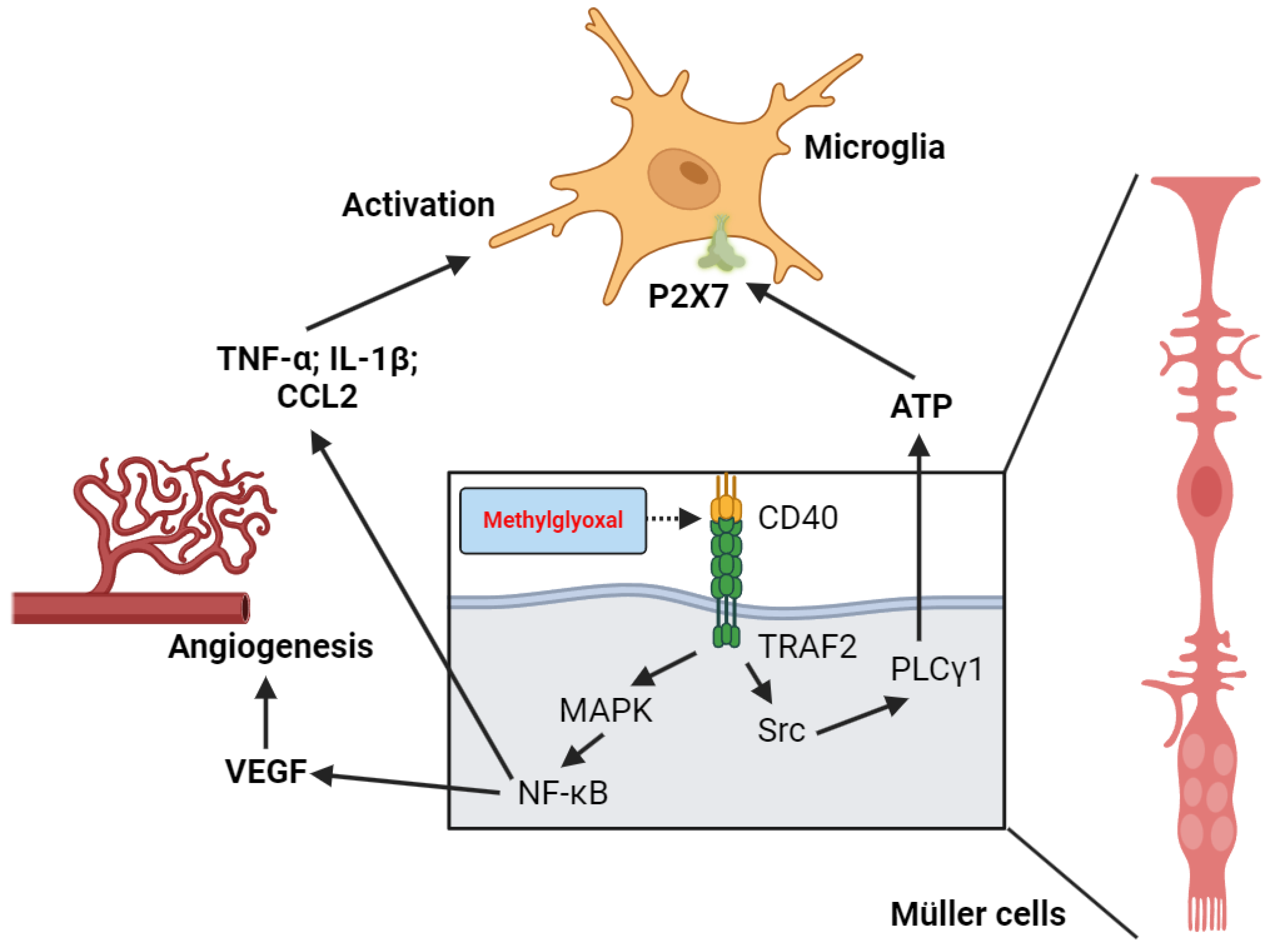
3.3. Inflammation and Microglia Activation
3.4. Endoplasmic Reticulum (ER)-Stress And Calcium Signaling
3.5. AMP-Activated Protein Kinase and Mitochondrial Stability
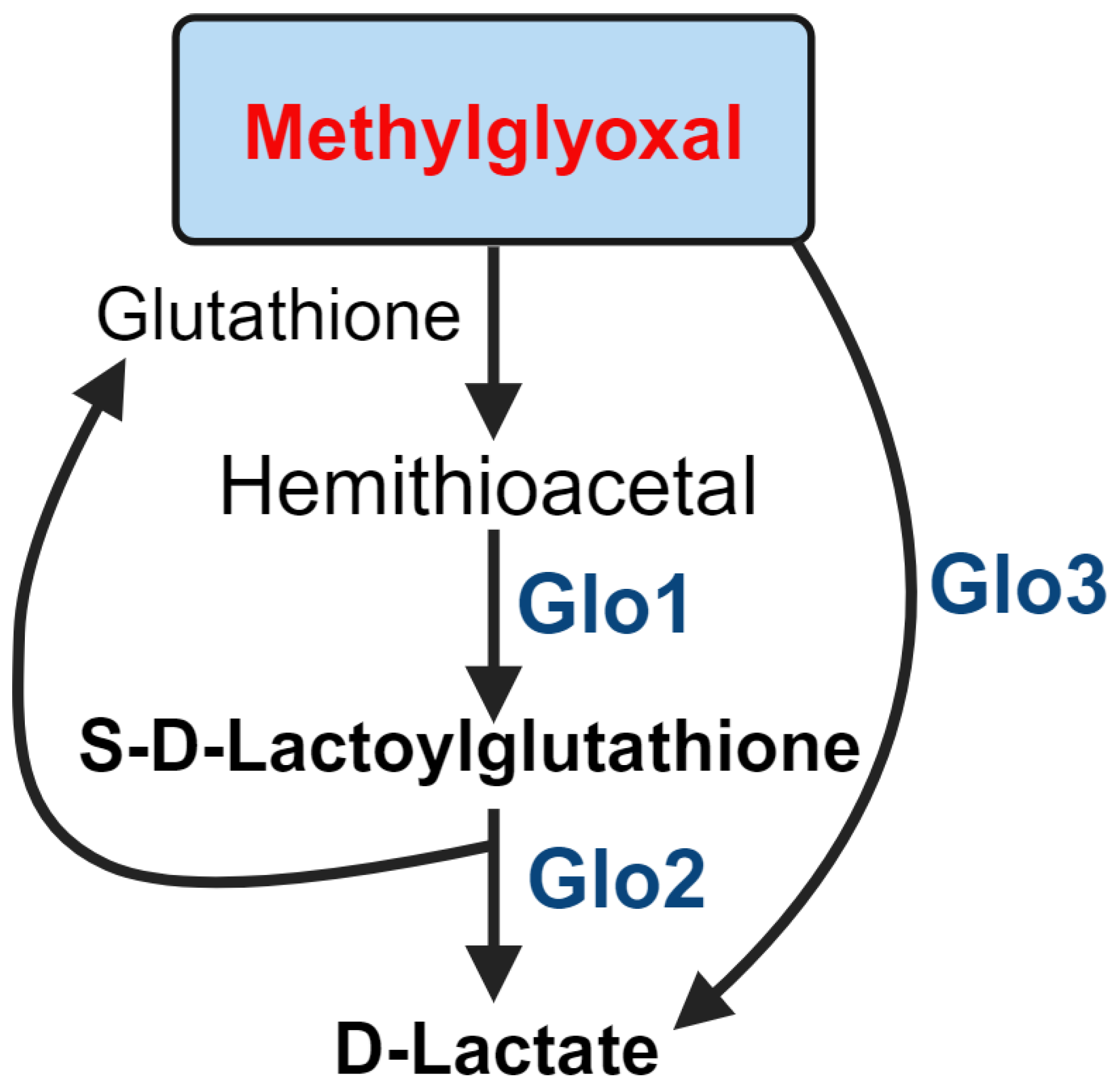
4. Role of Glyoxlases in MGO Detoxification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: International Diabetes Federation, 2021.
- Milne, R.; Brownstein, S. Advanced glycation end products and diabetic retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Karachalias, N.; Babaei-Jadidi, R.; Ahmed, N.; Thornalley, P.J. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem. Soc. Trans. 2003, 31 Pt 6, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diab. Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.H.; Patel, B.; Wilmot, E.G.; Amoaku, W.M. Diabetic retinopathy for the non-ophthalmologist. Clin. Med. (Lond.) 2022, 22, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, D.P.; Bebu, I.; Aiello, L.P.; Sivitz, W.; Gubitosi-Klug, R.; Malone, J.; White, N.H.; et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care 2019, 42, 875–882. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 93. [Google Scholar] [CrossRef]
- Sadikan, M.Z.; Abdul Nasir, N.A.; Lambuk, L.; Mohamud, R.; Reshidan, N.H.; Low, E.; Singar, S.A.; et al. Diabetic retinopathy: a comprehensive update on in vivo, in vitro and ex vivo experimental models. BMC Ophthalmol. 2023, 23, 421. [Google Scholar] [CrossRef]
- Schlotterer, A.; Kolibabka, M.; Lin, J.; Acunman, K.; Dietrich, N.; Sticht, C.; Fleming, T.; et al. Methylglyoxal induces retinopathy-type lesions in the absence of hyperglycemia: studies in a rat model. FASEB J. 2019, 33, 4141–4153. [Google Scholar] [CrossRef]
- Bellier, J.; Nokin, M.J.; Larde, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcene, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Mukohda, M.; Okada, M.; Hara, Yukio. ; Yamawaki, H. Exploring Mechanisms of Diabetes-Related Macrovascular Complications: Role of Methylglyoxal, a Metabolite of Glucose on Regulation of Vascular Contractility. J. of Pharmacol. Sci. 2012, 118, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.H.; Humpert, P.M.; Nawroth, P.P.; Bierhaus, A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: a mini-review. Gerontology 2011, 57, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Ashfaq, F.; Alsayegh, A.A.; Hamouda, A.; Khatoon, F.; Altamimi, T.N.; Alhodieb, F.S.; Beg, M.M.A. Advanced glycation end product signaling and metabolic complications: Dietary approach. World J. Diabetes 2023, 14, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; de Oliveira, M.G.; Mónica, F.Z.; Antunes, E. Methylglyoxal and Advanced Glycation End Products (AGEs): Targets for the Prevention and Treatment of Diabetes-Associated Bladder Dysfunction? Biomedicines 2024, 12, 939. [Google Scholar] [CrossRef]
- Yan, L.J. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model Exp. Med. 2018, 1, 7–13. [Google Scholar] [CrossRef]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Kalapos, M.P. Methylglyoxal and glucose metabolism: a historical perspective and future avenues for research. Drug Metabol. Drug. Interact. 2008, 23(1-2), 69-91. [CrossRef]
- Yu, P. H.; Wright, S.; Fan, E. H.; Lun, Z. R.; Gubisne-Harberle, D. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim. Biophys. Acta 2003, 1647, 193–199. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (2024). PubChem Pathway Summary for Pathway PWY-5451, acetone degradation I (to methylglyoxal), Source: BioCyc. Retrieved October 3, 2024 from https://pubchem.ncbi.nlm.nih. 3 October 5451.
- Yoon, K.D.; Yamamoto, K.; Ueda, K.; Zhou, J.; Sparrow, J.R. A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration. PLoS One 2012, 7, e41309. [Google Scholar] [CrossRef]
- Ueda, K.; Zhao, J.; Kim, H.J.; Sparrow, J.R. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 6904–6909. [Google Scholar] [CrossRef]
- Wu, Y.; Yanase, E.; Feng, X.; Siegel, M.M.; Sparrow, J.R. Structural characterization of bisretinoid A2E photocleavage pro Alouffi ducts and implications for age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 7275–7280. [Google Scholar] [CrossRef]
- Zhou, J.; Ueda, K.; Zhao, J.; Sparrow, J.R. Correlations between Photodegradation of Bisretinoid Constituents of Retina and Dicarbonyl Adduct Deposition. J. Biol. Chem. 2015, 290, 27215–27227. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Dutta, D.; Sen, A.; Chowdhury, I.H.; Mitra, B.; Mondal, L.K.; Saha, A.; et al. Role of N-ε- carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol. Vis. 2013, 19, 100–113. [Google Scholar] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Hexokinase-2-Linked Glycolytic Overload and Unscheduled Glycolysis-Driver of Insulin Resistance and Development of Vascular Complications of Diabetes. Int. J. Mol. Sci. 2022, 23, 2165. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Perez, R.; Cano-Martinez, A.; Gutierrez-Velazquez, E.; Martinez-Rosas, M.; Perez-Gutierrez, R.M.; Jimenez-Gomez, F.; Flores-Estrada, J. Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats. Antioxidants (Basel) 2021, 10, 717. [Google Scholar] [CrossRef]
- Alouffi, S.; Khan, M.W.A. Dicarbonyls Generation, Toxicities, Detoxifications and Potential Roles in Diabetes Complications. Curr. Protein Pept. Sci. 2020, 21, 890–898. [Google Scholar] [CrossRef]
- Muronetz, V.I. , Melnikova, A.K.; Seferbekova, Z.N.; Barinova, K.V.; Schmalhausen, E.V. Glycation, Glycolysis, and neurodegenerative diseases: Is There Any Connection? Biochemistry (Mosc.) 2017, 82, 874–886. [Google Scholar] [CrossRef]
- Oshitari, T. Advanced Glycation End-Products and Diabetic Neuropathy of the Retina. Int. J. Mol. Sci. 2023, 24, 2927. [Google Scholar] [CrossRef]
- Hammes, H.P.; Alt, A.; Niwa, T.; Clausen, J.T.; Bretzel, R.G.; Brownlee, M.; Schleicher, E.D. Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia 1999, 42, 728–736. [Google Scholar] [CrossRef]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Li, K.M.; Razmovski-Naumovski, V.; Kam, A.; Alam, P.; Li, G.Q. Attenuation of methylglyoxal-induced glycation and cellular dysfunction in wound healing by Centella cordifolia. Saudi. J. Biol. Sci. 2021, 28, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.S.; Machado, U.F.; Passarelli, M. Advanced glycation end products as biomarkers for cardiovascular disease: browning clarifying atherogenesis. Biomark. Med. 2020, 14, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Delligatti, C.E.; Kirk, J.A. Glycation in the cardiomyocyte. Vitam. Horm. 2024, 125, 47–88. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.Y.; Justino, A.B.; Caixeta, D.C.; Queiroz, J.S.; Sabino-Silva, R.; Salmen Espindola, F. Fructose and methylglyoxal-induced glycation alters structural and functional properties of salivary proteins, albumin and lysozyme. PLoS One 2022, 17, e0262369. [Google Scholar] [CrossRef]
- Bejarano, E.; Taylor, A. Too sweet: Problems of protein glycation in the eye. Exp. Eye Res. 2019, 178, 255–262. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Bhat, S.P. Crystallins, genes and cataract. Progress in Drug Res. 2003, 60. [Google Scholar] [CrossRef]
- Abdullah, E.M.; Haq, S.H.; Ahmed, M.A.; Khan, J.M.; Alamery, S.F.; Malik, A. Structural stability and solubility of glycated camel lens ζ-crystallin. Int. J. Biol. Macromol. 2020, 158, 384–393. [Google Scholar] [CrossRef]
- Jeong, W.J.; Rho, J.H.; Yoon, Y.G.; Yoo, S.H.; Jeong, N.Y.; Ryu, W.Y.; Ahn, H.B.; et al. Cytoplasmic and nuclear anti-apoptotic roles of αB-crystallin in retinal pigment epithelial cells. PLoS One 2012, 7, e45754. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.S.; Shinn, J.W.; Oh, Y.S.; Kim, H.T.; Jo, I.; Shinn, S.H. The effect of antioxidants on glycated albumin-induced cytotoxicity in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 2002, 292, 1010–1016. [Google Scholar] [CrossRef]
- Dahrouj, M.; Desjardins, D.M.; Liu, Y.; Crosson, C.E.; Ablonczy, Z. Receptor mediated disruption of retinal pigment epithelium function in acute glycated-albumin exposure. Exp. Eye Res. 2015, 137, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kim, J.; Jo, K.; Lee, Y.M.; Sohn, E.; Yoo, N.H.; Kim, J.S. OSSC1E-K19, a novel phytochemical component of Osteomeles schwerinae, prevents glycated albumin-induced retinal vascular injury in rats. Mol. Med. Rep. 2015, 12, 7279–7284. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, Y.; Masuda, K.; Hatano, E.; Inoue, T.; Matsuyama, T.; Iizuka, M.; Ono, Y.; et al. Novel Nrf2 activators from microbial transformation products inhibit blood-retinal barrier permeability in rabbits. Br. J. Pharmacol. 2015, 172, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Xu, B.; Shi, H. Long noncoding RNA MALAT1 acts as a competing endogenous RNA to regulate Amadori-glycated albumin-induced MCP-1 expression in retinal microglia by a microRNA-124-dependent mechanism. Inflamm. Res. 2018, 67, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Wang, J.J.; Dashti, A.; Wilson, K.; Zou, M.H.; Szweda, L.; Ma, J.X.; Lyons, T.J. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J. Mol. Endocrinol. 2008, 41, 135–143. [Google Scholar] [CrossRef]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef]
- Portillo, J.C.; Pfaff, A.; Vos, S.; Weng, M.; Nagaraj, R.H.; Subauste, C.S. Advanced Glycation End Products Upregulate CD40 in Human Retinal Endothelial and Müller Cells: Relevance to Diabetic Retinopathy. Cells 2024, 13, 429. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Augustine, J.; Troendle, E.P.; Barabas, P.; McAleese, C.A.; Friedel, T.; Stitt, A.W.; Curtis, T.M. The Role of Lipoxidation in the Pathogenesis of Diabetic Retinopathy. Front. Endocrinol. (Lausanne) 2021, 11, 621938. [Google Scholar] [CrossRef]
- Yatoh, S.; Mizutani, M.; Yokoo, T.; Kozawa, T.; Sone, H.; Toyoshima, H.; Suzuki, S.; et al. Antioxidants and an inhibitor of advanced glycation ameliorate death of retinal microvascular cells in diabetic retinopathy. Diabetes Metab. Res. Rev. 2006, 22, 38–45. [Google Scholar] [CrossRef]
- Sheikpranbabu, S.; Haribalaganesh, R.; Gurunathan, S. Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes Metab. 2011, 37, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef] [PubMed]
- Barile, G.R.; Pachydaki, S.I.; Tari, S.R.; Lee, S.E.; Donmoyer, C.M.; Ma, W.; Rong, L.L.; et al. The RAGE axis in early diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2005, 46, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Fukami, K. RAGE signaling regulates the progression of diabetic complications. Front. Pharmacol. 2023, 14, 1128872. [Google Scholar] [CrossRef]
- Wang, X.L.; Yu, T.; Yan, Q.C.; Wang, W.; Meng, N. : Li, X.J.; Luo, Y.H. AGEs Promote Oxidative Stress and Induce Apoptosis in Retinal Pigmented Epithelium Cells RAGE-dependently. J. Mol. Neurosci. [CrossRef]
- Bhattacharya, R.; Alam, M.R.; Kamal, M.A.; Seo, K.J.; Singh, L.R. AGE-RAGE axis culminates into multiple pathogenic processes: a central road to neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1155175. [Google Scholar] [CrossRef]
- McVicar, C.M.; Ward, M.; Colhoun, L.M.; Guduric-Fuchs, J.; Bierhaus, A.; Fleming, T.; Schlotterer, A.; et al. Role of the receptor for advanced glycation endproducts (RAGE) in retinal vasodegenerative pathology during diabetes in mice. Diabetologia 2015, 58, 1129–1137. [Google Scholar] [CrossRef]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, W.; Liu, Y.; Liang, H.; Chen, W.; Wang, H. Acute glucose fluctuation promotes RAGE expression via reactive oxygen species-mediated NF-κB activation in rat podocytes. Mol. Med. Rep. 2021, 23, 330. [Google Scholar] [CrossRef]
- Ramya, R.; Coral, K.; Bharathidevi, S.R. RAGE silencing deters CML-AGE induced inflammation and TLR4 expression in endothelial cells. Exp. Eye Res. 2021, 206, 108519. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, J.; Zhang, X.; Liu, Z.; Yang, Y.; Gong, Q.; Ren, B. The Role of HMGB1 in the Pathogenesis of Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2543268. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Sharma, E.; Sehgal, A.; Kaur, I.; Kumar, A.; Arora, R.; Pal, G.; et al. Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs. Mol. Biol. Rep. 2021, 48, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Hsiao, C.C.; Yang, I.H.; Chou, M.H.; Wu, C.L.; Wei, Y.C.; Chen, C.H.; Chuang, J.H. High-mobility group box 1 protein is implicated in advanced glycation end products-induced vascular endothelial growth factor A production in the rat retinal ganglion cell line RGC-5. Mol. Vis. 2012, 18, 838–850. [Google Scholar] [PubMed]
- Maeda, S.; Matsui, T.; Ojima, A.; Takeuchi, M.; Yamagishi, S. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr. Res. 2014, 34, 807–813. [Google Scholar] [CrossRef]
- Li, G.; Tang, J.; Du, Y.; Lee, C.A.; Kern, T.S. Beneficial effects of a novel RAGE inhibitor on early diabetic retinopathy and tactile allodynia. Mol. Vis. 2011, 17, 3156–3165. [Google Scholar]
- Wu, Q.; Liu, H.; Zhou, M. Fangchinoline Ameliorates Diabetic Retinopathy by Inhibiting Receptor for Advanced Glycation End-Products (RAGE)-Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB) Pathway in Streptozotocin (STZ)-Induced Diabetic Rats. Med. Sci. Monit. 2019, 25, 1113–1121. [Google Scholar] [CrossRef]
- Simon, M.V. , Vera, M.S.; Tenconi, P.E.; Soto, T.; Prado Spalm, F.H.; Torlaschi, C.; Mateos, M.V., Rotstein, N.P. Sphingosine-1-phosphate and ceramide-1-phosphate promote migration, pro-inflammatory and pro-fibrotic responses in retinal pigment epithelium cells. Exp. Eye Res. 2022, 224, 109222. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration. EXCLI J. 2020, 19, 1353–1371. [Google Scholar] [CrossRef]
- Zhang, S.M.; Fan, B.; Li, Y.L.; Zuo, Z.Y.; Li, G.Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Hashikawa, K.I.; Tsuruta, A.; Yamakawa, W.; Yasukochi, S.; Koyanagi, S.; Ohdo, S. Senescence-induced alteration of circadian phagocytic activity of retinal pigment epithelium cell line ARPE-19. Biochem. Biophys. Res. Commun. 2023, 658, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cai, B.; Li, Y.; Su, W.; Zhao, X.; Gong, B.; Li, Z.; Zhang, X.; Wu, Y.; et al. HMGB1 and Caveolin-1 related to RPE cell senescence in age-related macular degeneration. Aging (Albany NY) 2019, 11(13), 4323- 4337. [CrossRef]
- Qi, H.; Liu, T.; Liu, J.; Teng, Q.; Ma, Z.; Wang, S.; Wen, S.; et al. Thioredoxin1 is a target to attenuate diabetes-induced RPE cell dysfunction in human ARPE19 cells by alleviating oxidative stress. Mol. Med. Rep. 2023, 28, 134. [Google Scholar] [CrossRef] [PubMed]
- Clarkson-Townsend, D.A.; Douglass, A.J.; Singh, A.; Allen, R.S.; Uwaifo, I.N.; Pardue, M.T. Impacts of high fat diet on ocular outcomes in rodent models of visual disease. Exp. Eye Res. 2021, 204, 108440. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Hsiao, G.; Hsu, S.H.; Huang, D.Y.; Lin, W.W.; Chan, C.M. Metformin inhibits methylglyoxal-induced retinal pigment epithelial cell death and retinopathy via AMPK-dependent mechanisms: Reversing mitochondrial dysfunction and upregulating glyoxalase 1. Redox Biol. 2023, 64, 102786. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Huang, Y.P.; Hsu, S.H.; Kang, L.Y.; Shen, C.M.; Lin, W.W. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J. Cell. Mol. Med. 2016, 20, 1749–1760. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Intartaglia, D.; Giamundo, G.; Conte, I. Autophagy in the retinal pigment epithelium: a new vision and future challenges. FEBS J. 2022, 289, 7199–7212. [Google Scholar] [CrossRef]
- Si, Z.; Zheng, Y.; Zhao, J. The Role of Retinal Pigment Epithelial Cells in Age-Related Macular Degeneration: Phagocytosis and Autophagy. Biomolecules 2023, 13, 901. [Google Scholar] [CrossRef]
- Wu, A.Y.; Sekar, P.; Huang, D.Y.; Hsu, S.H.; Chan, C.M.; Lin, W.W. Spatiotemporal roles of AMPK in PARP-1- and autophagy-dependent retinal pigment epithelial cell death caused by UVA. J. Biomed. Sci. 2023, 30, 91. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 100846. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Peng, J.; He, F.; Tursun, X.; Li, S.P.; Xin, X.L.; Aisa, H.A. Shabyar Ameliorates High Glucose Induced Retinal Pigment Epithelium Injury Through Suppressing Aldose Reductase and AMPK/mTOR/ULK1 Autophagy Pathway. Front. Pharmacol. 2022, 13, 852945. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Tan, W.; Liu, K.; Chen, B.; Duan, T.; Xu, H. Wnt inhibitory factor 1 ameliorated diabetic retinopathy through the AMPK/mTOR pathway-mediated mitochondrial function. FASEB J. 2022, 36, e22531. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liang, L.; Zhang, S.; Yang, J.; Yue, Y.; Zhang, X. HMGB1 downregulation in retinal pigment epithelial cells protects against diabetic retinopathy through the autophagy-lysosome pathway. Autophagy 2022, 18, 320–339. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, L.; Xie, H.; Liu, Y.; Huang, P.; Zhang, J.; Luo, D.; Zhang, J. Glucose transport, transporters and metabolism in diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166995. [Google Scholar] [CrossRef]
- Singh, L.P. Thioredoxin Interacting Protein (TXNIP) and Pathogenesis of Diabetic Retinopathy. J Clin. Exp. Ophthalmol. 2013, 4. [Google Scholar] [CrossRef]
- Bang, E.; Park, C.; Hwangbo, H.; Shim, J.H.; Leem, S.H.; Hyun, J.W.; Kim, G.Y.; Choi, Y.H. Spermidine Attenuates High Glucose-Induced Oxidative Damage in Retinal Pigment Epithelial Cells by Inhibiting Production of ROS and NF-κB/NLRP3 Inflammasome Pathway. Int. J. Mol. Sci. 2023, 24, 10550. [Google Scholar] [CrossRef]
- Li, J.Q.; Shi, Y.H. ; Min-Xu; Shi, C.X., Teng-Wang, Wang, T.H., Zuo, Z.F., Eds.; Liu, X.Z. Discovery of astragaloside IV against high glucose-induced apoptosis in retinal ganglion cells: Bioinformatics and in vitro studies. Gene 2024, 905, 148219. [Google Scholar] [CrossRef]
- Gao, S.; Gao, S.; Wang, Y.; Li, N.; Yang, Z.; Yao, H.; Chen, Y.; et al. Inhibition of Ferroptosis Ameliorates Photoreceptor Degeneration in Experimental Diabetic Mice. Int. J. Mol. Sci. 2023, 24, 16946. [Google Scholar] [CrossRef]
- Liu, K.; Gao, X.; Hu, C.; Gui, Y.; Gui, S.; Ni, Q.; Tao, L.; Jiang, Z. Capsaicin ameliorates diabetic retinopathy by inhibiting poldip2-induced oxidative stress. Redox Biol. 2022, 56, 102460. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hsieh, M.C.; Wu, H.J.; Wu, W.C.; Kao, Y.H. Methylglyoxal, a reactive glucose metabolite, enhances autophagy flux and suppresses proliferation of human retinal pigment epithelial ARPE-19 cells. Toxicol. in Vitro 2015, 29, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Kim, J.; Kim, C.S.; Kim, N.H.; Kim, J.S. KIOM-79 prevents methyglyoxal-induced retinal pericyte apoptosis in vitro and in vivo. J. Ethnopharmacol. 2010, 129, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Zheng, Y.; Jiang, J.; Wang, L.; Wu, J.; Zhang, C.; Luo, M. Glucose metabolite methylglyoxal induces vascular endothelial cell pyroptosis via NLRP3 inflammasome activation and oxidative stress in vitro and in vivo. Cell Mol. Life Sci. 2024, 81, 401. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.L.; Oliveira, A.L.; Mello, G.C.; Antunes, E. Metformin Counteracts the Deleterious Effects of Methylglyoxal on Ovalbumin-Induced Airway Eosinophilic Inflammation and Remodeling. Int. J. Mol. Sci. 2023, 24, 9549. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Chemical Composition of Hazelnut Skin Food Waste and Protective Role against Advanced Glycation End-Products (AGEs) Damage in THP-1-Derived Macrophages. Molecules 2023, 28, 2680. [Google Scholar] [CrossRef]
- Kang, J.; Jeong, Y.J.; Ha, S.K.; Lee, H.H.; Lee, K.W. Glyoxal-derived advanced glycation end-products, Nε-carboxymethyl-lysine, and glyoxal-derived lysine dimer induce apoptosis-related gene expression in hepatocytes. Mol. Biol. Rep. 2023, 50, 2511–2520. [Google Scholar] [CrossRef]
- Ma, J.H.; Wang, J.J.; Zhang, S.X. The unfolded protein response and diabetic retinopathy. J. Diabetes Res. 2014, 2014, 160140. [Google Scholar] [CrossRef]
- Chandrakumar, S.; Santiago Tierno, I.; Agarwal, M.; Matisioudis, N.; Kern, T.S.; Ghosh, K. Subendothelial Matrix Stiffening by Lysyl Oxidase Enhances RAGE-Mediated Retinal Endothelial Activation in Diabetes. Diabetes 2023, 72, 973–985. [Google Scholar] [CrossRef]
- Tu, Z.; Li, Y.; Smith, D.S.; Sheibani, N.; Huang, S.; Kern, T.; Lin, F. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol. Vis. Sci. 2011, 52, 9005–9010. [Google Scholar] [CrossRef]
- Pickel, L.; Kim, S.J.; Hacibekiroglu, S.; Nagy, A.; Lee, J.; Sung, H.K. The Circadian Clock of Müller Glia is Necessary for Retinal Homeostasis and Neuronal Survival. Am. J. Pathol. 0002. [Google Scholar] [CrossRef]
- Rattner, A.; Williams, J.; Nathans, J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Invest. 2019, 129, 3807–3820. [Google Scholar] [CrossRef]
- Eastlake, K.; Luis, J.; Limb, G.A. Potential of Müller Glia for Retina Neuroprotection. Curr. Eye Res. 2020, 45, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, G.T.; Zhang, J.F. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural. Regen. Res. 2023, 18, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.S.; Allkabes, M.; Salsini, G.; Bonifazzi, C.; Perri, P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016, 162, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Reber, F.; Kasper, M.; Siegner, A.; Kniep, E.; Seigel, G.; Funk, R.H. Alteration of the intracellular pH and apoptosis induction in a retinal cell line by the AGE-inducing agent glyoxal. Graefes. Arch. Clin. Exp. Ophthalmol. 2002, 240, 1022–1032. [Google Scholar] [CrossRef]
- Portillo, J.C.; Yu, J.S.; Vos, S.; Bapputty, R.; Lopez Corcino, Y.; Hubal, A.; Daw, J.; et al. Disruption of retinal inflammation and the development of diabetic retinopathy in mice by a CD40-derived peptide or mutation of CD40 in Müller cells. Diabetologia 2022, 65, 2157–2171. [Google Scholar] [CrossRef]
- Portillo, J.C.; Lopez Corcino, Y.; Miao, Y.; Tang, J.; Sheibani, N.; Kern, T.S.; Dubyak, G.R.; Subauste, C.S. CD40 in Retinal Müller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes 2017, 66, 483–493. [Google Scholar] [CrossRef]
- Portillo, J.C.; Corcino, Y.L.; Dubyak, G.R.; Kern, T.S.; Matsuyama, S.; Subauste, C.S. Ligation of CD40 in Human Müller Cells Induces P2X7 Receptor–Dependent Death of Retinal Endothelial Cells. Invest. Ophthalmol. Vis. Sci. 2016, 57, 6278–6286. [Google Scholar] [CrossRef]
- Samuels, I.S.; Portillo, J.C.; Miao, Y.M.; Kern, T.S.; Subaste, C.S. Loss of CD40 attenuates experimental diabetes-induced retinal inflammation but does not protect mice from electroretinogram defects. Vis. Neurosci. 2017, 34, E009. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, J.X.; Guo, J.; Wang, J.; Zhu, M.; Chen, Y.; Le, Y.Z. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 2009, 219, 446–454. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Sang, S.; Leung, T. Methylglyoxal-Induced Retinal Angiogenesis in Zebrafish Embryo: A Potential Animal Model of Neovascular Retinopathy. J. Ophthalmol. 2019, 2019, 2746735. [Google Scholar] [CrossRef]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef]
- Karbasforooshan, H.; Karimi, G. The role of SIRT1 in diabetic retinopathy. Biomed. Pharmacother. 2018, 97, 190–194. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, X.; Bowers, M.; Jessberger, S.; Golczak, M.; Semenkovich, C.F.; Rajagopal, R. Increasing Energetic Demands on Photoreceptors in Diabetes Corrects Retinal Lipid Dysmetabolism and Reduces Subsequent Microvascular Damage. Am. J. Pathol. 2023, 193, 2144–2155. [Google Scholar] [CrossRef]
- Zou, J.; Tan, W.; Liu, K.; Chen, B.; Duan, T.; Xu, H. Wnt inhibitory factor 1 ameliorated diabetic retinopathy through the AMPK/mTOR pathway-mediated mitochondrial function. FASEB J. 2022, 36, e22531. [Google Scholar] [CrossRef]
- Song, S.; Bao, S.; Zhang, C.; Zhang, J.; Li, J.; Li, X.; Chudhary, M.; Ren, X.; Kong, L. Stimulation of AMPK Prevents Diabetes-Induced Photoreceptor Cell Degeneration. Oxid. Med. Cell. Longev. 2021, 2021, 5587340. [Google Scholar] [CrossRef]
- Qian, S.; Qian, Y.; Huo, D.; Wang, S.; Qian, Q. Tanshinone IIa protects retinal endothelial cells against mitochondrial fission induced by methylglyoxal through glyoxalase 1. Europ. J. Pharmacol. 2019, 857, 172419. [Google Scholar] [CrossRef]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef]
- Van Herreweghe, F.; Mao, J.; Chaplen, F.W.R.; Grooten, J.; Gevaert, K.; Vandekerckhove, J.; Vancompernolle, K. Tumor necrosis factor-induced modulation of glyoxalase I activities through phosphorylation by PKA results in cell death and is accompanied by the formation of a specific methylglyoxal-derived AGE. Proc. Natl. Acad. Sci. USA 2002, 99, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Alhujaily, M. Molecular Assessment of Methylglyoxal-Induced Toxicity and Therapeutic Approaches in Various Diseases: Exploring the Interplay with the Glyoxalase System. Life (Basel) 2024, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Activity, regulation, copy number and function in the glyoxalase system. Biochem. Soc. Trans. 2014, 42, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Maessen, D.E.; Stehouwer, C.D.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. (Lond) 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell. Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef]
- Rasul, A.; Rashid, A.; Waheed, P.; Khan, S.A. Expression analysis of glyoxalase I gene among patients of diabetic retinopathy. Pak. J. Med. Sci. 2018, 34, 139–143. [Google Scholar] [CrossRef]
- Sachdeva, R.; Schlotterer, A.; Schumacher, D.; Matka, C.; Mathar, I.; Dietrich, N.; Medert, R.; et al. TRPC proteins contribute to development of diabetic retinopathy and regulate glyoxalase 1 activity and methylglyoxal accumulation. Mol. Metab. 2018, 9, 156–167. [Google Scholar] [CrossRef]
- Lodd, E.; Wiggenhauser, L.M.; Morgenstern, J.; Fleming, T.H.; Poschet, G.; Büttner, M.; Tabler, C.T.; et al. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight 2019, 4, e126154. [Google Scholar] [CrossRef]
- Berner, A.K.; Brouwers, O.; Pringle, R.; Klaassen, I.; Colhoun, L.; McVicar, C.; Brockbank, S.; et al. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia 2012, 55, 845–854. [Google Scholar] [CrossRef]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated Levels of the Reactive Metabolite Methylglyoxal Recapitulate Progression of Type 2 Diabetes. Cell. Metab, 27. [CrossRef]
- Irshad, Z.; Xue, M.; Ashour, A.; Larkin, J.R.; Thornalley, P.J.; Rabbani, N. Activation of the unfolded protein response in high glucose treated endothelial cells is mediated by methylglyoxal. Sci. Rep. 2019, 2019 9, 7889. [Google Scholar] [CrossRef]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.B.; Anwar, A.; Waldron, M.; Shafie, A.; et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes 2016, 2016 65, 2282–2294. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




