Submitted:
10 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
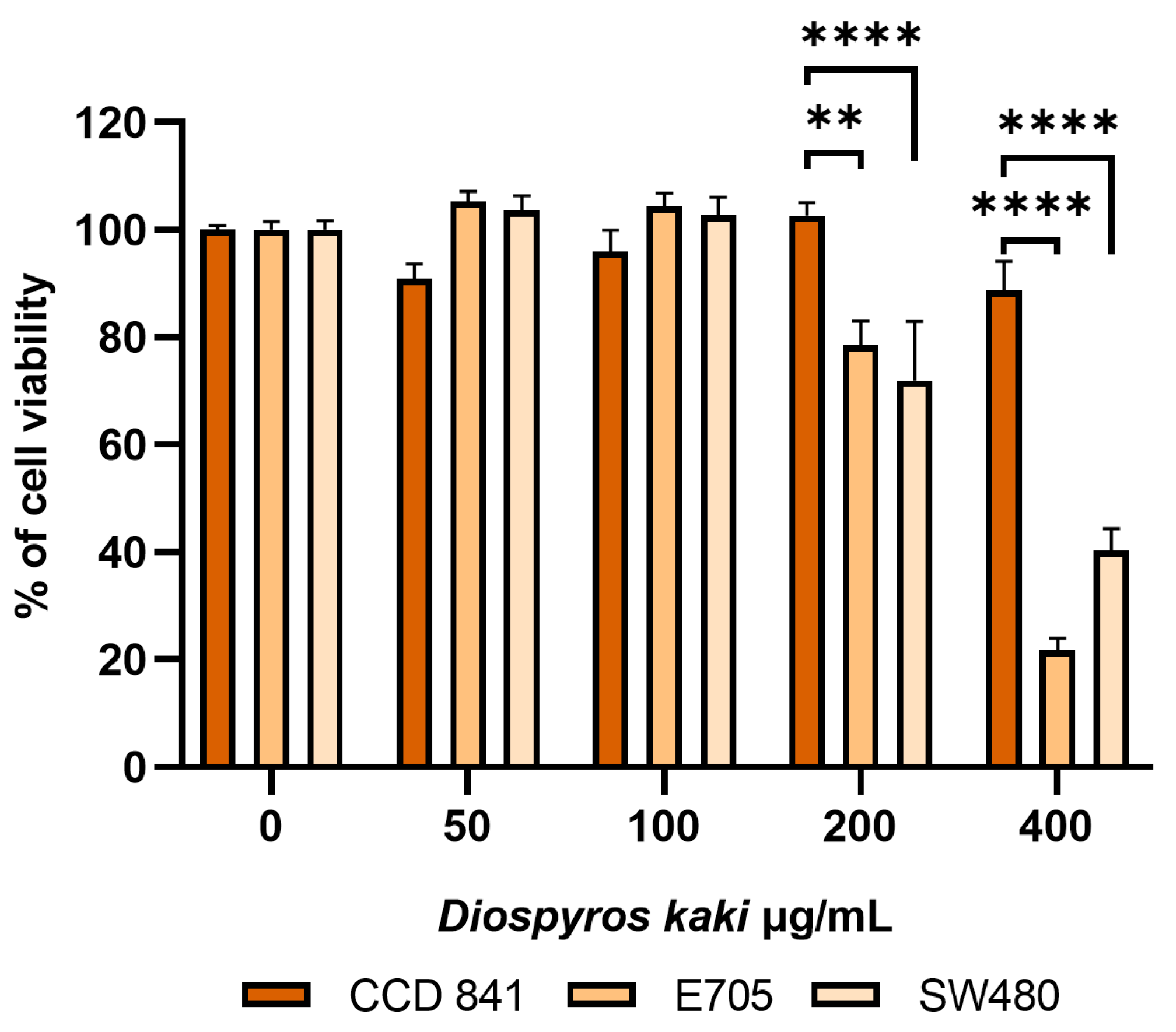
Diospyros kaki, the most widely cultivated species of persimmon, has been long used in traditional medicine since its leaves extracts contain high amounts of flavonoids and terpenoids, endowed with potential antioxidant, anti-inflammatory, anticancer, antidiabetic and antimicrobial effects. Re-cently, many in vitro and in vivo studies have assessed different potential health benefits of per-simmon leaves; however, its anticancer activity towards colorectal cancer (CRC), the second deadliest cancer in Western countries, has not been investigated in depth. Since CRC current therapies are associated with serious side effects and show no efficacy towards patients carrying RAS/BRAF mutations, the search for new and more effective therapies has turned to plant extracts, which could help reduce conventional drugs dosages and toxicity. The effect of Diospyros kaki al-coholic extract has been investigated on E705 CRC cell line, representative of most CRC patients, and on SW480 cells, carrying a KRAS activating mutation. This extract is effective in reducing tumor cells viability, without affecting the healthy mucosa cell line CCD 841, and it triggers apoptosis in CRC cells, by disrupting mitochondrial functionality and increasing oxidative stress.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Plant Material and Preparation of D. kaki Extract
2.3. UHPLC-DAD-HRMS/MS Analysis
2.4. Viability Assay
2.5. SDS-PAGE and Western Blotting
2.6. Intracellular Reactive Oxygen Species (ROS) Measurement
2.7. Glutathione Detection
2.8. Mitochondrial Transmembrane Potential (MTP) Evaluation
2.9. Seahorse Mito Stress Test and ATP Rate Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Profiling of Bioactive Compounds of D. kaki Leaf Extract
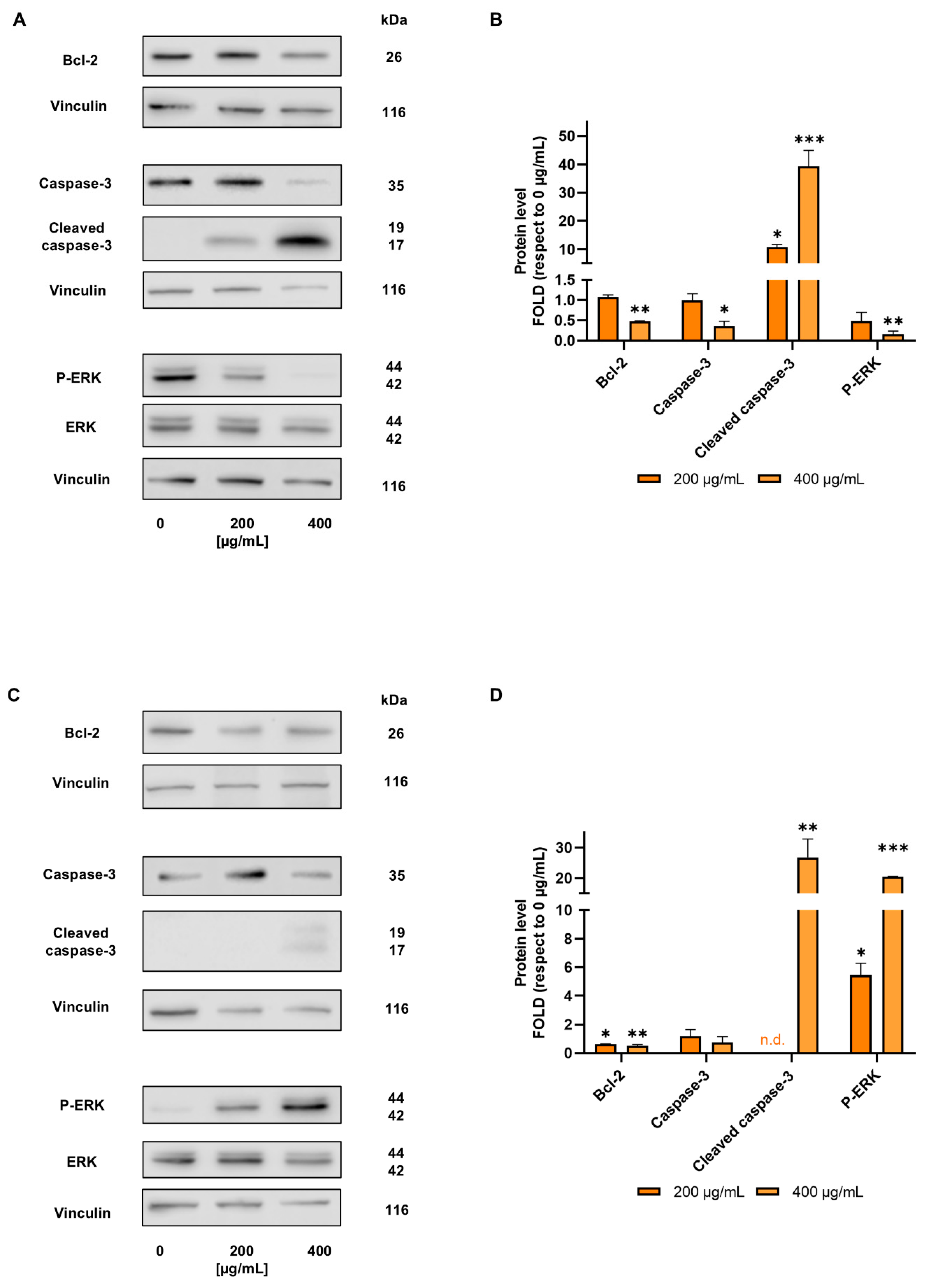
3.2. D. kaki Extract Reduces CRC Cells Viability, Triggering Apoptosis [26]
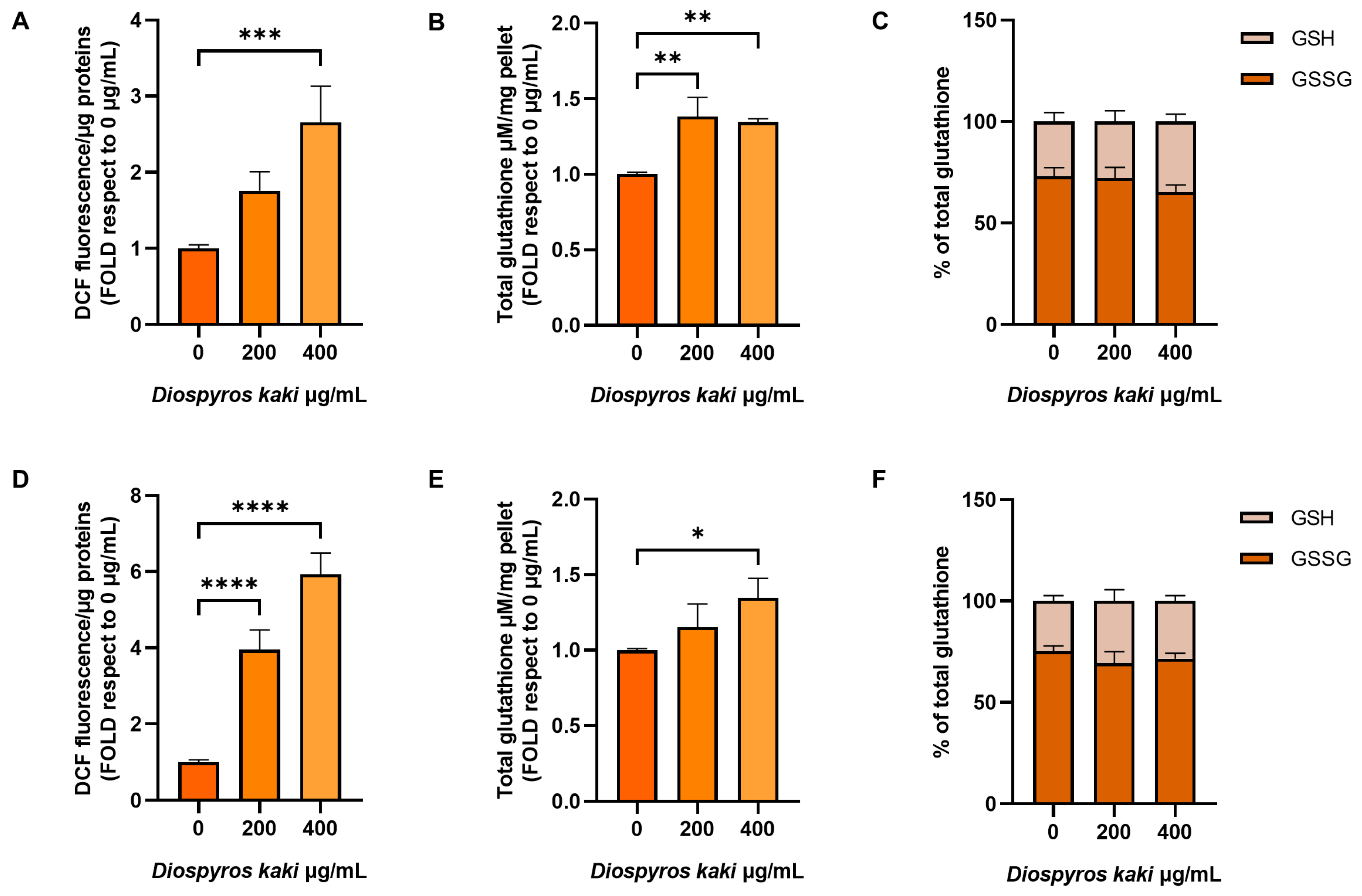
3.3. D. kaki Extract Increases Oxidative Stress in CRC Cells
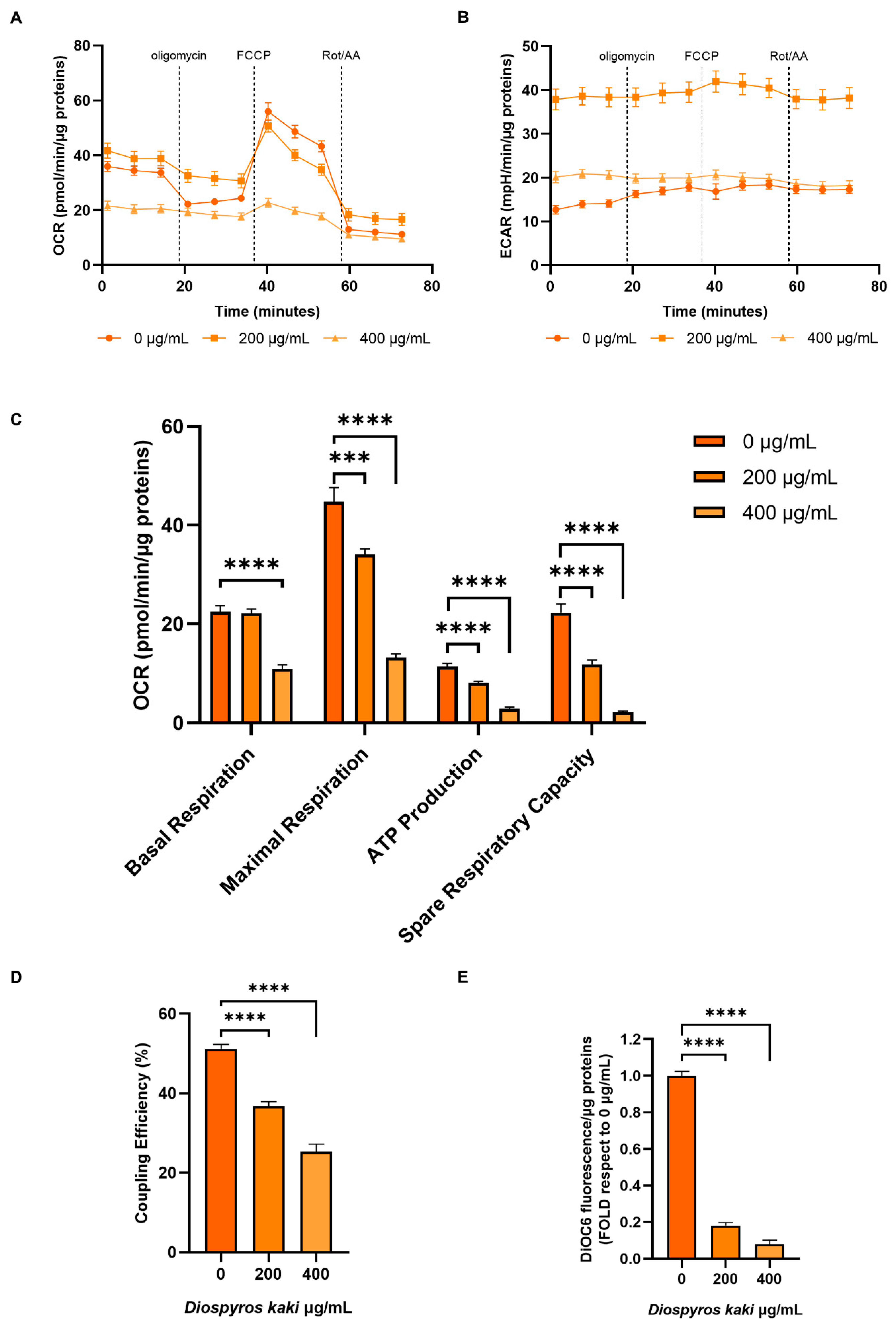
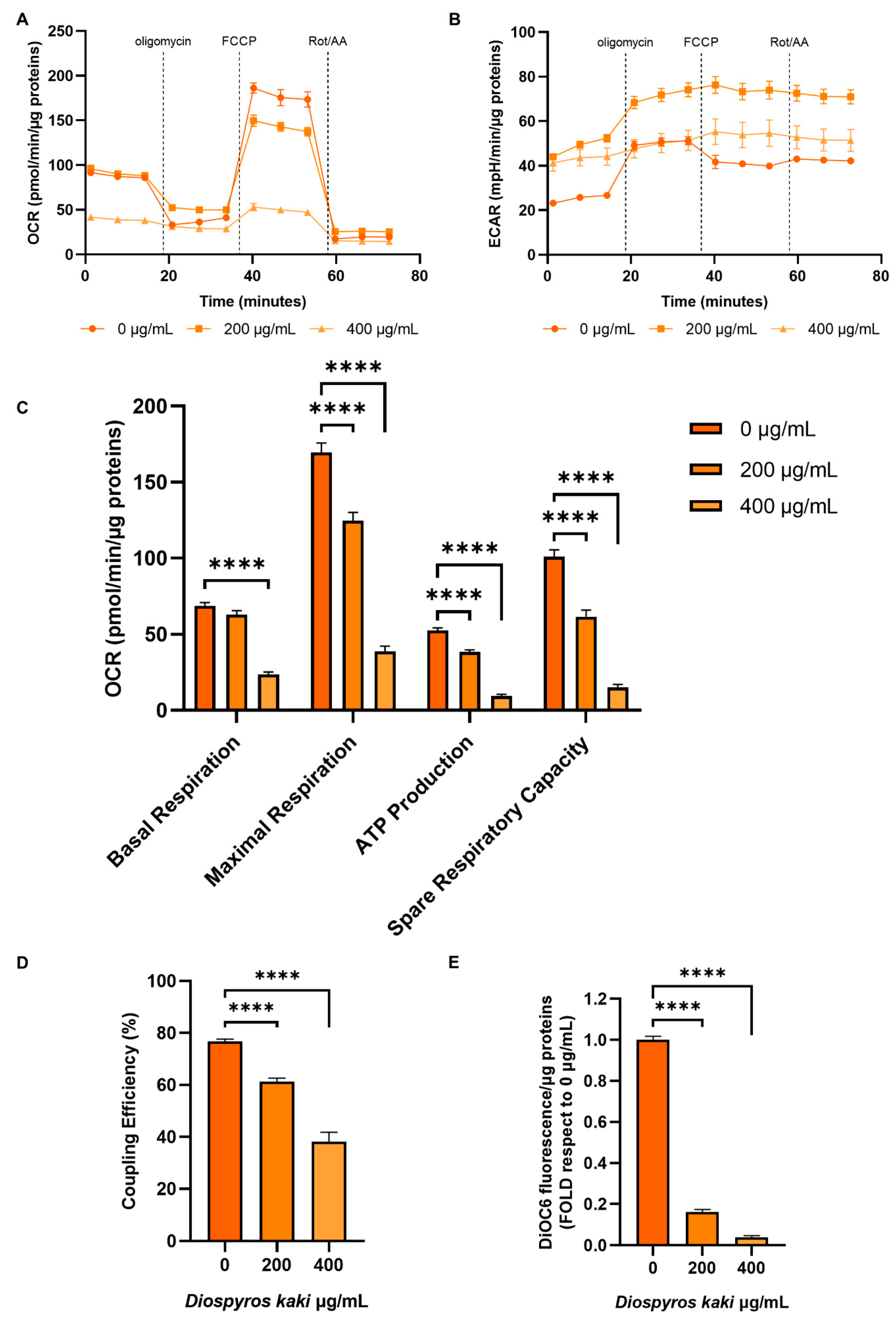
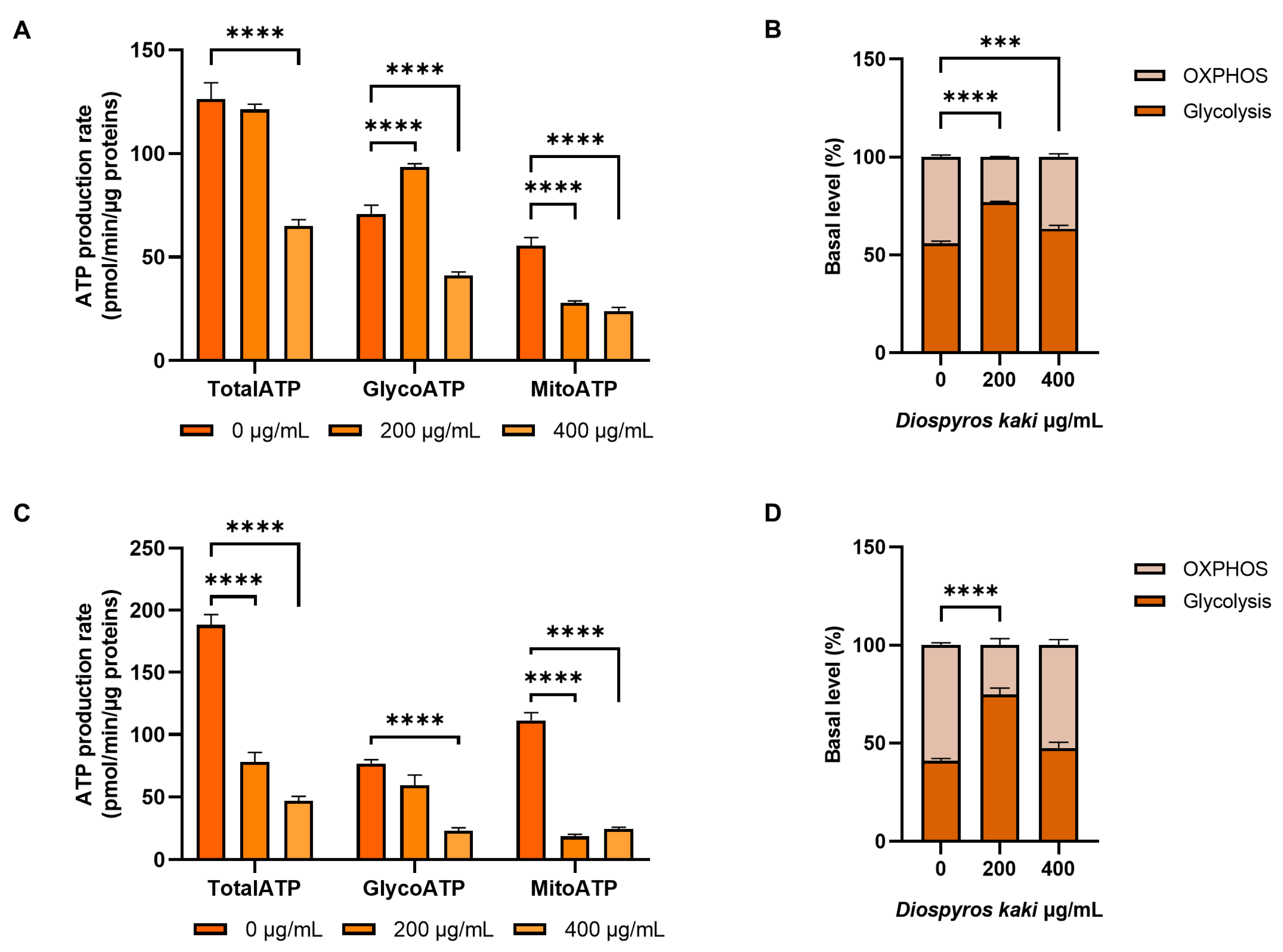
3.4. Mitochondria Dysfunction Induced by D. kaki Extract is Not Rescued by Glycolysis Upregulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hossain, A.; Shahidi, F. Persimmon Leaves: Nutritional, Pharmaceutical, and Industrial Potential—A Review. Plants 2023, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ren, K.; Dong, H.; Song, F.; Chen, J.; Guo, Y.; Liu, Y.; Tao, W.; Zhang, Y. Flavonoids from Persimmon (Diospyros Kaki L.) Leaves Inhibit Proliferation and Induce Apoptosis in PC-3 Cells by Activation of Oxidative Stress and Mitochondrial Apoptosis. Chem Biol Interact 2017, 275, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Suh, J.-S.; Jang, Y.-K.; Ahn, S.-H.; Raja, G.; Kim, J.-C.; Jung, Y.; Jung, S.H.; Kim, T.-J. Anti-Cancer Potential of Persimmon (Diospyros Kaki) Leaves via the PDGFR-Rac-JNK Pathway. Sci Rep 2020, 10, 18119. [Google Scholar] [CrossRef]
- Lim, W.C.; Choi, J.W.; Song, N.E.; Cho, C.W.; Rhee, Y.K.; Hong, H. Do Polysaccharide Isolated from Persimmon Leaves (Diospyros Kaki Thunb.) Suppresses TGF-Β1-Induced Epithelial-to-Mesenchymal Transition in A549 Cells. Int J Biol Macromol 2020, 164, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Wang, X.; Xu, J.; Guo, J.; Peng, H.; Zhang, Y. A Review: Pharmacological Effect of Natural Compounds in Diospyros Kaki Leaves from the Perspective of Oxidative Stress. Molecules 2023, 29. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Huh, G.; Jung, S.H.; Kwon, H.; Jeon, Y.; Park, Y.N.; Kim, Y.J. Diospyros Kaki Leaves Inhibit HGF/Met Signaling-Mediated EMT and Stemness Features in Hepatocellular Carcinoma. Food and Chemical Toxicology 2020, 142, 111475. [Google Scholar] [CrossRef]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, R.B.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.; Alves, P.; Bronze, R.; et al. Dyospiros Kaki Phenolics Inhibit Colitis and Colon Cancer Cell Proliferation, but Not Gelatinase Activities. J Nutr Biochem 2017, 46, 100–108. [Google Scholar] [CrossRef]
- CHEN, L.I.; GUO, Y.; ALSAIF, G.; GAO, Y. Total Flavonoids Isolated from <Em>Diospyros Kaki</Em> L. f. Leaves Induced Apoptosis and Oxidative Stress in Human Cancer Cells. Anticancer Res 2020, 40, 5201. [Google Scholar] [CrossRef]
- Keskin, C.; Ölçekçi, A.; Baran, A.; Baran, M.F.; Eftekhari, A.; Omarova, S.; Khalilov, R.; Aliyev, E.; Sufianov, A.; Beilerli, A.; et al. Green Synthesis of Silver Nanoparticles Mediated Diospyros Kaki L. (Persimmon): Determination of Chemical Composition and Evaluation of Their Antimicrobials and Anticancer Activities. Front Chem 2023, 11. [Google Scholar] [CrossRef]
- Park, S. Bin; Park, G.H.; Song, H.M.; Son, H.-J.; Um, Y.; Kim, H.-S.; Jeong, J.B. Anticancer Activity of Calyx of Diospyros Kaki Thunb. through Downregulation of Cyclin D1 via Inducing Proteasomal Degradation and Transcriptional Inhibition in Human Colorectal Cancer Cells. BMC Complement Altern Med 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.; Zulli, A. A Synopsis of Modern - Day Colorectal Cancer: Where We Stand. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2022, 1877, 188699. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Hoyo, A.; Monzonís, X.; Vidal, J.; Linares, J.; Montagut, C. Unveiling Acquired Resistance to Anti-EGFR Therapies in Colorectal Cancer: A Long and Winding Road. Front Pharmacol 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. The Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst 2019, 111, 158–169. [Google Scholar] [CrossRef]
- Masci, D.; Puxeddu, M.; Silvestri, R.; La Regina, G. Metabolic Rewiring in Cancer: Small Molecule Inhibitors in Colorectal Cancer Therapy. Molecules 2024, 29. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal Biochem 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bovio, F.; Perciballi, E.; Melchioretto, P.; Ferrari, D.; Forcella, M.; Fusi, P.; Urani, C. Morphological and Metabolic Changes in Microglia Exposed to Cadmium: Cues on Neurotoxic Mechanisms. Environ Res 2024, 240, 117470. [Google Scholar] [CrossRef] [PubMed]
- Korchak, H.M.; Rich, A.M.; Wilkenfeld, C.; Rutherford, L.E.; Weissmann, G. A Carbocyanine Dye, DiOC6(3), Acts as a Mitochondrial Probe in Human Neutrophils. Biochem Biophys Res Commun 1982, 108, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, B.E.; Gallin, J.I. Use of Lipophilic Probes of Membrane Potential to Assess Human Neutrophil Activation. Abnormality in Chronic Granulomatous Disease. J Clin Invest 1980, 66, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Cannazza, G.; Capriotti, A.L.; Citti, C.; La Barbera, G.; Laganà, A.; Montone, C.M.; Piovesana, S.; Cavaliere, C. A New Software-Assisted Analytical Workflow Based on High-Resolution Mass Spectrometry for the Systematic Study of Phenolic Compounds in Complex Matrices. Talanta 2020, 209, 120573. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Pagano, I.; Esposito, T.; Mencherini, T.; Porta, A.; Petrone, A.M.; Gazzerro, P.; Picerno, P.; Sansone, F.; Rastrelli, L.; et al. HRMS Profile of a Hazelnut Skin Proanthocyanidin-Rich Fraction with Antioxidant and Anti- Candida Albicans Activities. J Agric Food Chem 2016, 64, 585–595. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Li, L.; Sun, B. UHPLC-MS/MS Analysis and Protective Effects on Neurodegenerative Diseases of Phenolic Compounds in Different Parts of Diospyros Kaki L. Cv. Mopan. Food Research International 2024, 184, 114251. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Palmioli, A.; Forcella, M.; Oldani, M.; Angotti, I.; Sacco, G.; Fusi, P.; Airoldi, C. Adjuvant Effect of Cinnamon Polyphenolic Components in Colorectal Cancer Cell Lines. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
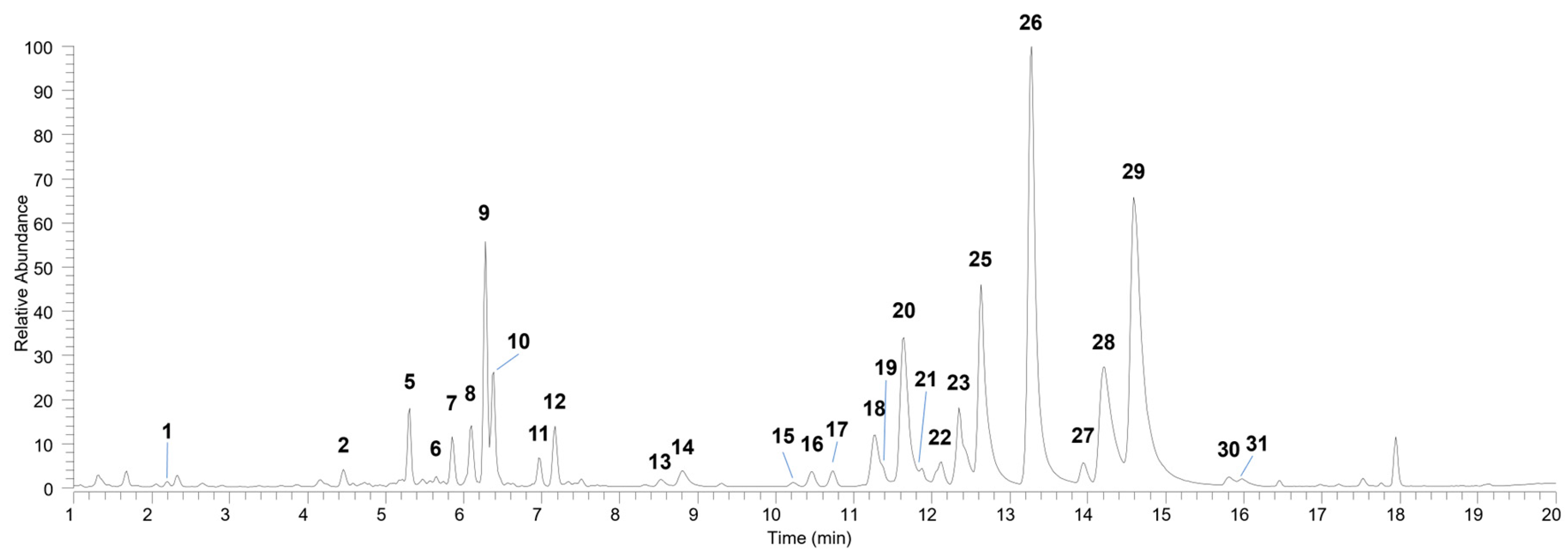
| N | Compound | Molecular Formula | Rt (min) |
[M-H]- (m/z) |
Error (ppm) |
Diagnostic product ions (m/z) |
MSI levela |
|---|---|---|---|---|---|---|---|
| 1 | (epi)Gallocatechin-(epi)gallocatechin | C30H26O14 | 2.2 | 609.1248 | -0.2 | 483.0952, 441.0816, 423.0715, 305.0663, 177.0183, 125.0231 | 2 |
| 2 | Gallocatechin | C15H14O7 | 4.5 | 305.0663 | -1.0 | 167.0339, 165.0182, 137.0232, 125.0231 | 1 |
| 3 | (epi)Gallocatechin-(epi)catechin | C30H26O13 | 4.7 | 593.1296 | -0.7 | 467.0996, 441.0825, 425.0873, 407.0765, 303.0512, 289.0715 | 2 |
| 4 | (epi)Catechin-(epi)gallocatechin | C30H26O13 | 5.0 | 593.1294 | -1.2 | 467.0985, 441.0829, 423.0714, 305.0663, 289.0714, 287.0559 | 2 |
| 5 | Hydroxyroseoside | C19H30O9 | 5.3 | 447.1867 b | -1.3 | 371.1716, 239.1287 | 3 |
| 6 | Procyanidin B1 | C30H26O12 | 5.6 | 577.1342 | -1.2 | 451.1023, 425.0874, 407.0766, 299.0557, 289.0715, 287.0557 | 1 |
| 7 | Catechin | C15H14O6 | 5.9 | 289.0713 | -1.6 | 151.0389, 149.0233, 137.0232, 125.0231 | 1 |
| 8 | Coumaroyl-hexoside-pentoside | C20H26O12 | 6.1 | 457.1347 | -1.0 | 325.0926, 1630.390, 119.0490 | 2 |
| 9 | Roseoside | C19H30O8 | 6.3 | 431.1914 b | -2.0 | 223.1321, 205.0498 | 2 |
| 10 | Roseoside pentoside | C24H38O12 | 6.4 | 563.2335 b | -2.1 | 3 | |
| 11 | Iridoid glycoside | C19H32O8 | 7.0 | 433.2077 b | -0.3 | 4 | |
| 12 | Iridoid glycoside | C19H28O10 | 7.2 | 415.1606 | -0.9 | 4 | |
| 13 | Myricetin 3-O-hexoside | C21H20O13 | 8.5 | 479.0829 | -0.3 | 317.0283, 316.0220, 287.0195, 271.0246, 178.9976, 151.0026 | 2 |
| 14 | Myricetin 3-O-hexoside | C21H20O13 | 8.8 | 479.0829 | -0.3 | 317.0280, 316.0220, 287.0196, 271.0246, 178.9975, 151.0026 | 2 |
| 15 | Quercetin-3-O-hexoside-deoxyhexoside | C27H30O16 | 10.2 | 609.1464 | 0.7 | 301.0318, 300.0271, 271.0246, 255.0296, 178.9969, 151.0022 | 2 |
| 16 | Iridoid glycoside | C24H42O11 | 10.5 | 551.2708 b | -0.2 | 4 | |
| 17 | Iridoid glycoside | C24H42O11 | 10.7 | 551.2705 b | -0.6 | 4 | |
| 18 | Quercetin-3-O-hexoside | C21H20O12 | 11.3 | 463.0875 | -1.3 | 301.0343, 300.0272, 271.0245, 255.0295, 178.9974, 151.0025 | 2 |
| 19 | Iridoid glycoside | C24H42O11 | 11.4 | 551.2700 b | -1.8 | 2 | |
| 20 | Quercetin-3-O-glucoside (isoquercitrin) | C21H20O12 | 11.6 | 463.0874 | -1.7 | 301.0342, 300.0272, 271.0245, 255.0294, 178.9973, 151.0026 | 1 |
| 21 | Kaempferol-3-O-hexoside-deoxyhexoside | C27H30O15 | 11.9 | 593.1506 | -0.8 | 285.0378, 284.0322, 255.0294, 227.0343, 151.0022 | 2 |
| 22 | Laricitrin 3-O-hexoside | C22H22O13 | 12.1 | 493.0985 | -0.5 | 331.0461, 330.0375, 316.01930, 315.0144, 287.0195, 178.9975, 151.0022 | 2 |
| 23 | Quercetin-7(4’)-O-galloylhexoside | C28H24O16 | 12.4 | 615.0981 | -1.6 | 313.0559, 301.0349, 178.9975, 169.0129, 151.0025 | 2 |
| 24 | Quercetin-7(4’)-O-galloylhexoside | C28H24O16 | 12.7 | 615.0983 | -1.6 | 463.0864, 313.0566, 301.0351, 178.9977, 169.0127, 151.0026 | 2 |
| 25 | Kaempferol-3-O-hexoside | C21H20O11 | 12.6 | 447.0924 | -2.1 | 285.0392, 284.0324, 255.0296, 227.0344, 151.0025 | 2 |
| 26 | Kaempferol-3-O-glucoside (Astragalin) | C21H20O11 | 13.3 | 447.0923 | -2.2 | 285.0394, 284.0323, 255.0295, 227.0343, 151.0026 | 1 |
| 27 | Kaempferol-3-O-pentose | C20H18O10 | 13.9 | 417.0825 | -0.4 | 285.0387, 284.0323, 255.0295, 227.0343, 151.0023 | 2 |
| 28 | Kaempferol 7(4’)-O-galloylhexoside | C28H24O15 | 14.2 | 599.1032 | -1.9 | 313.0563, 285.0402, 257.0455, 229.0498, 169.0132, 151.0025 | 2 |
| 29 | Kaempferol 7(4’)- O-galloylhexoside | C28H24O15 | 14.6 | 599.1033 | -1.7 | 313.0562, 285.0402, 257.0452, 229.0502, 169.0133, 151.0025 | 2 |
| 30 | Kaempferol 7(4’)-O-galloylpentoside | C27H22O14 | 15.8 | 569.0937 | 0.2 | 285.0402, 283.0457, 257.0452, 229.0500, 169.0130, 151.0025 | 2 |
| 31 | Kaempferol 7(4’)-O-galloylpentoside | C27H22O14 | 16.0 | 569.0939 | 0.5 | 285.0402, 283.0458, 257.0453, 229.0500, 169.0129, 151.0026 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





