Submitted:
10 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
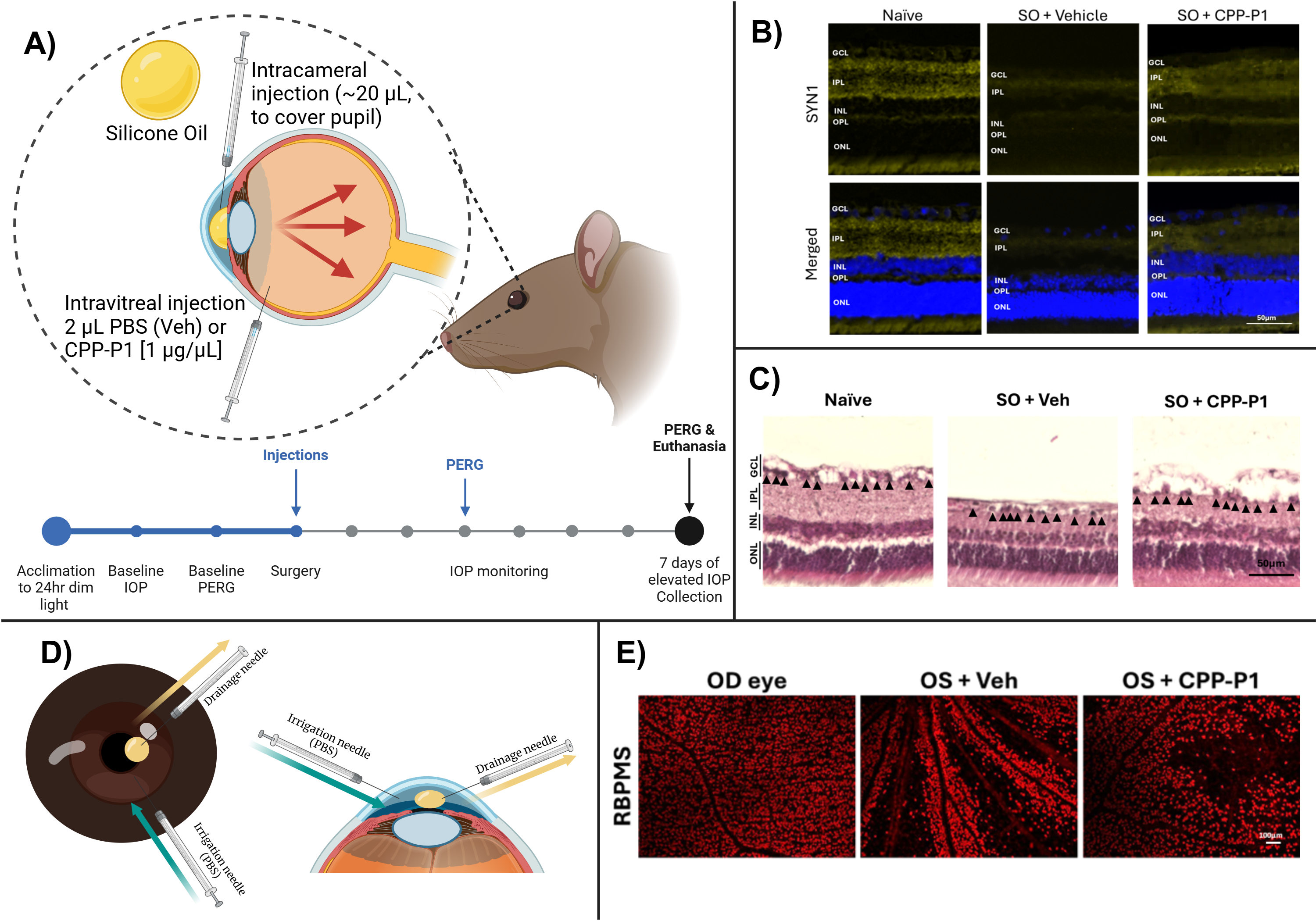
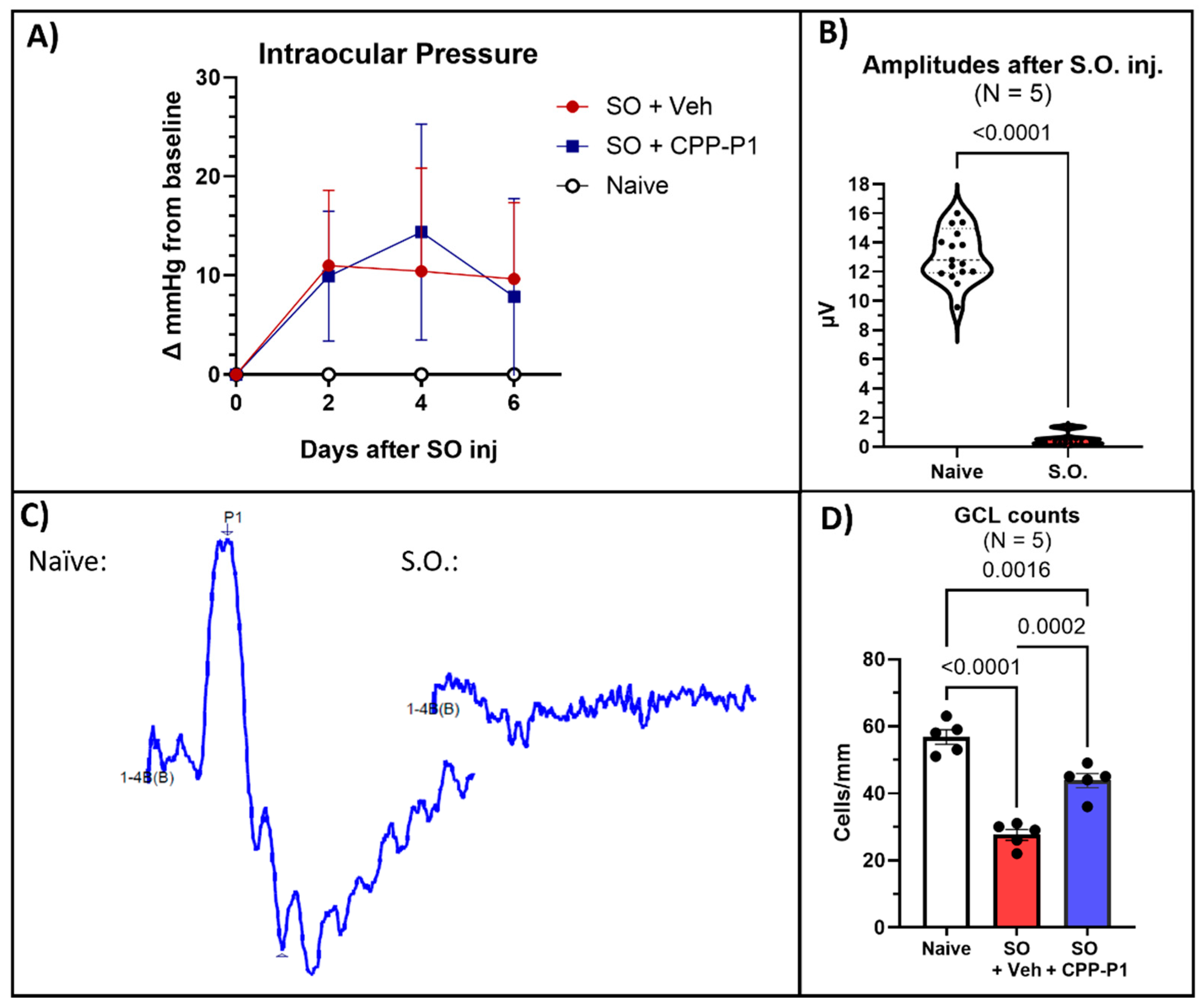
3.1. Intraocular Pressure (IOP)
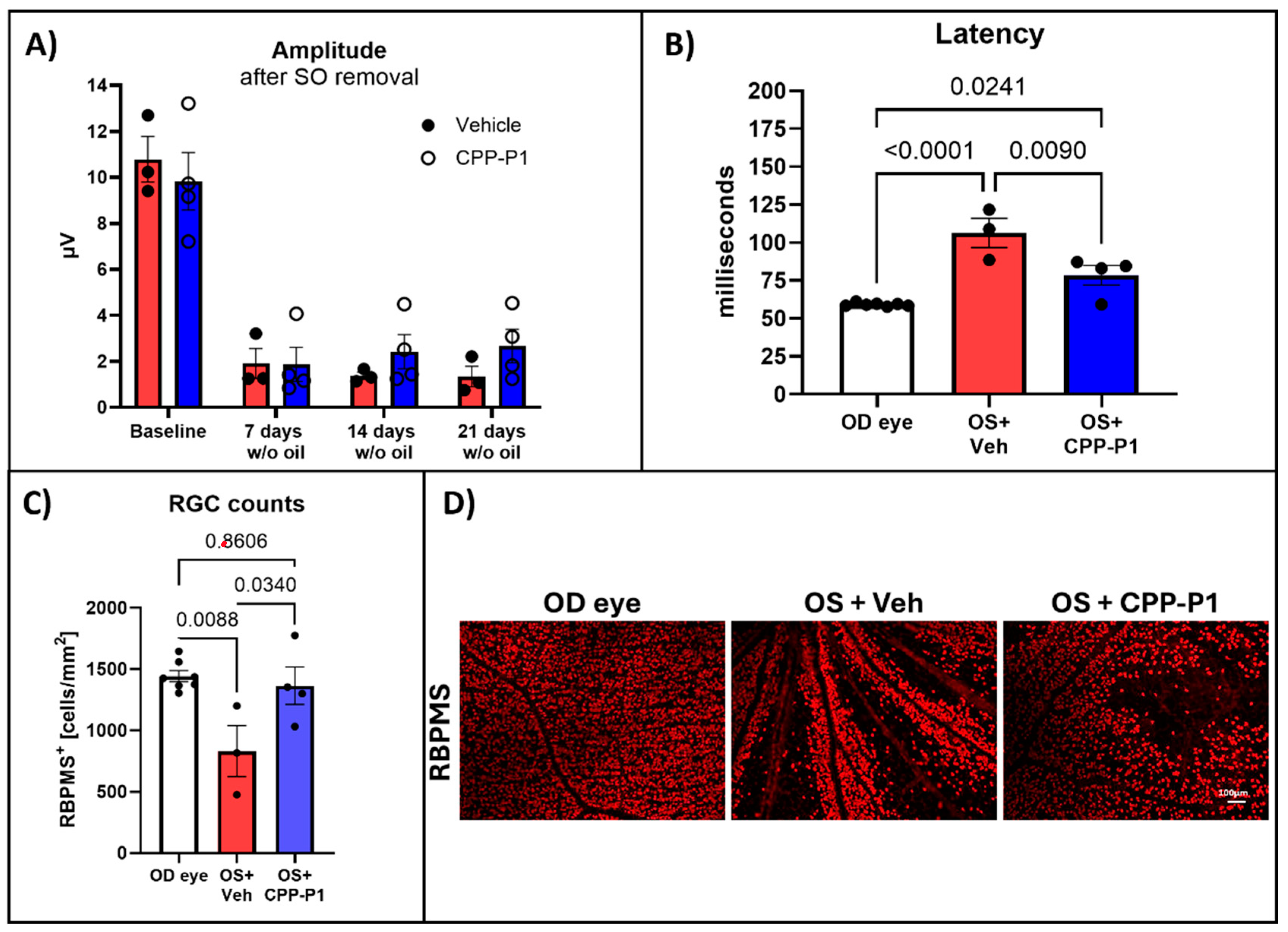
3.2. Pattern Electroretinography (PERG)
3.3. CPP-P1 Prevented the Loss of Cells in the GCL
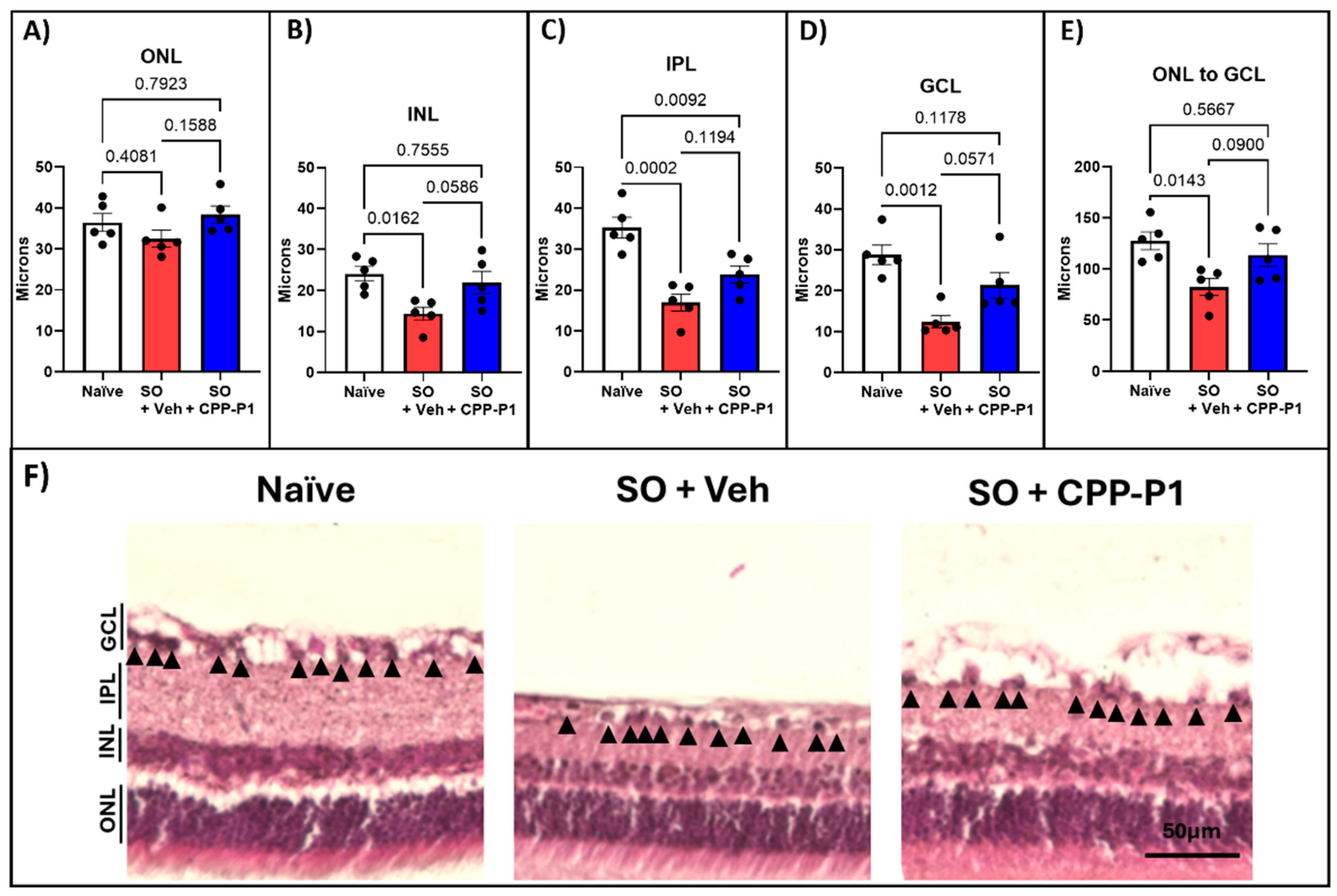
3.4. Retinal Layers Thickness
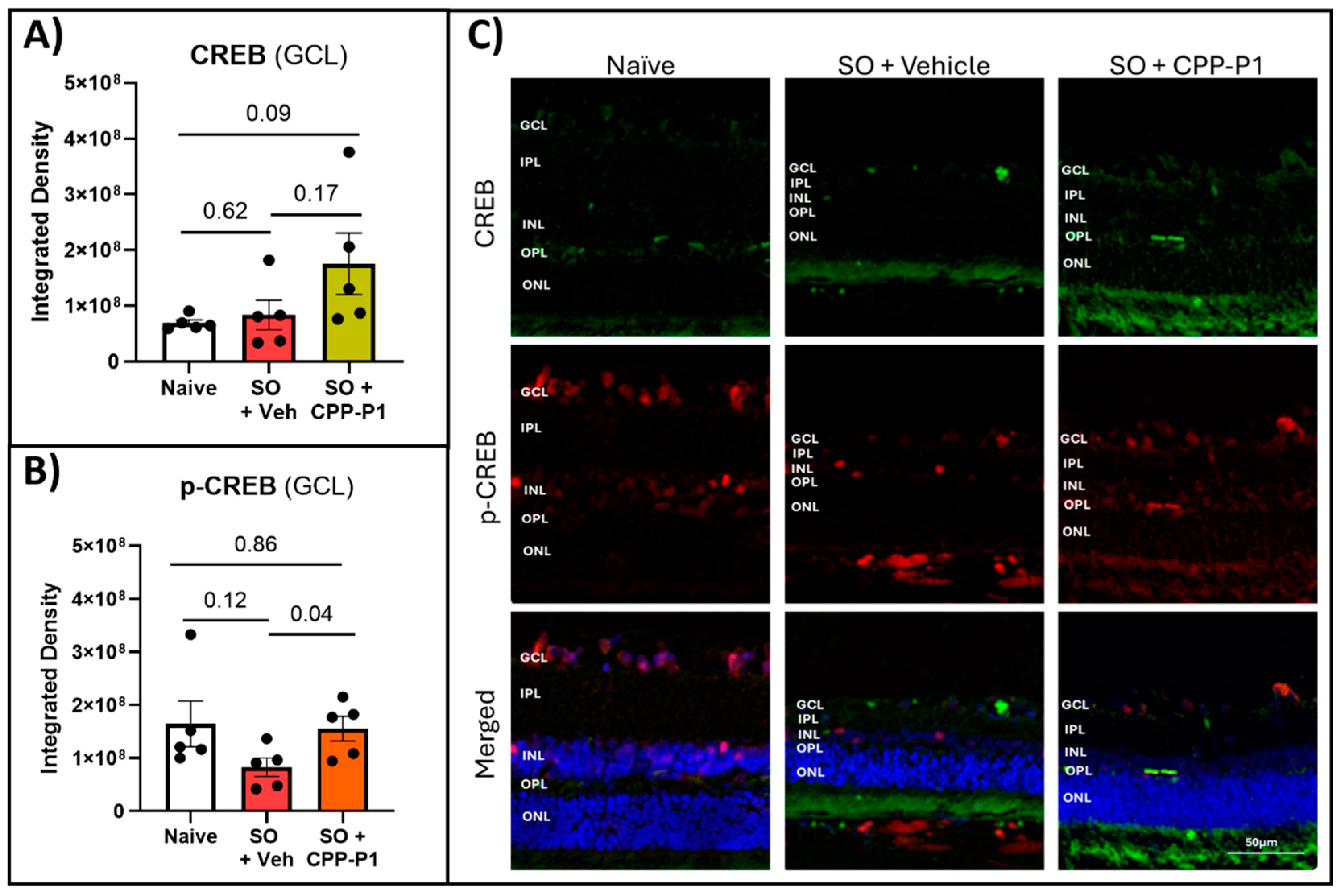
3.5. CREB and Phospho-CREB Analysis
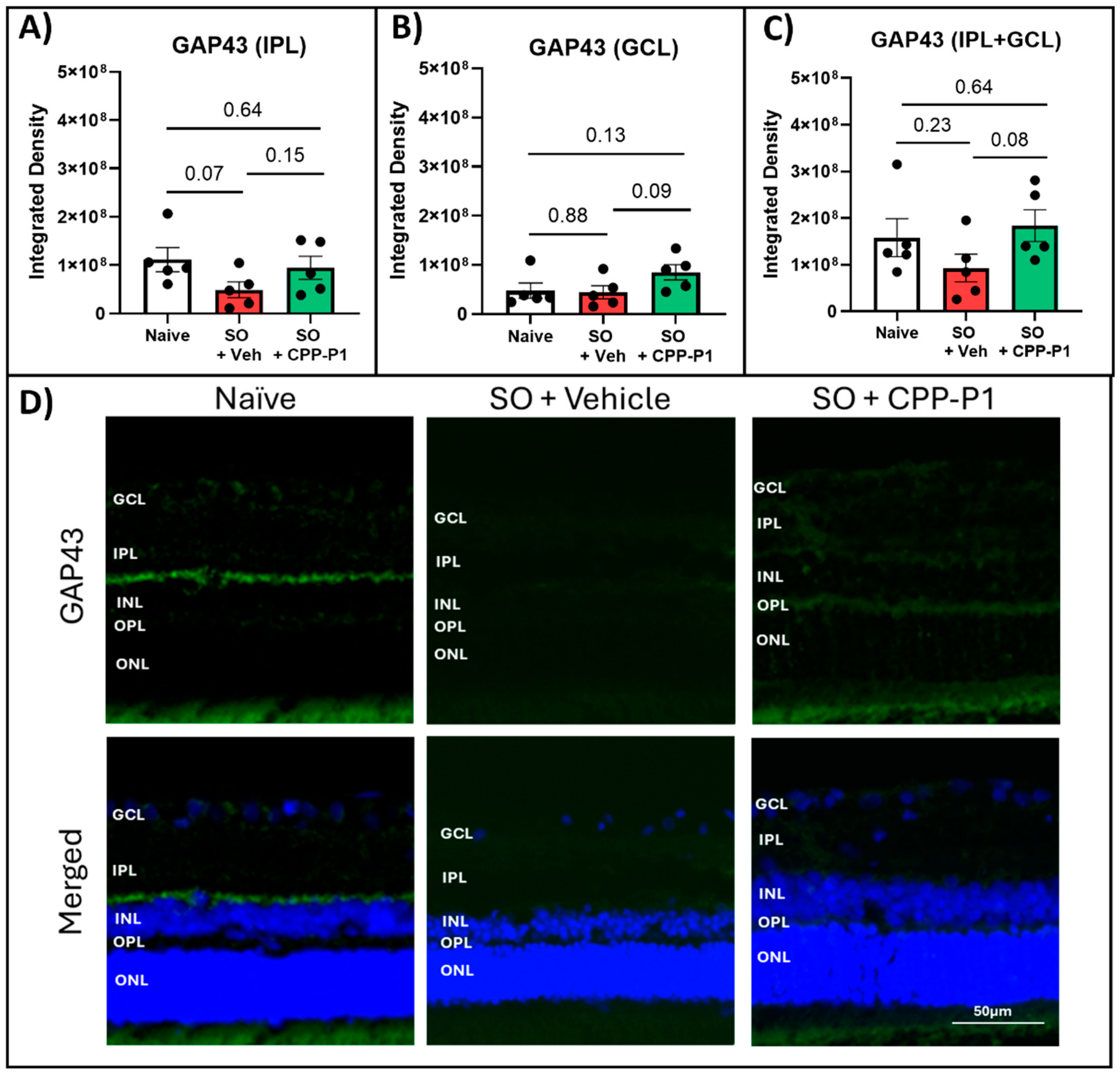
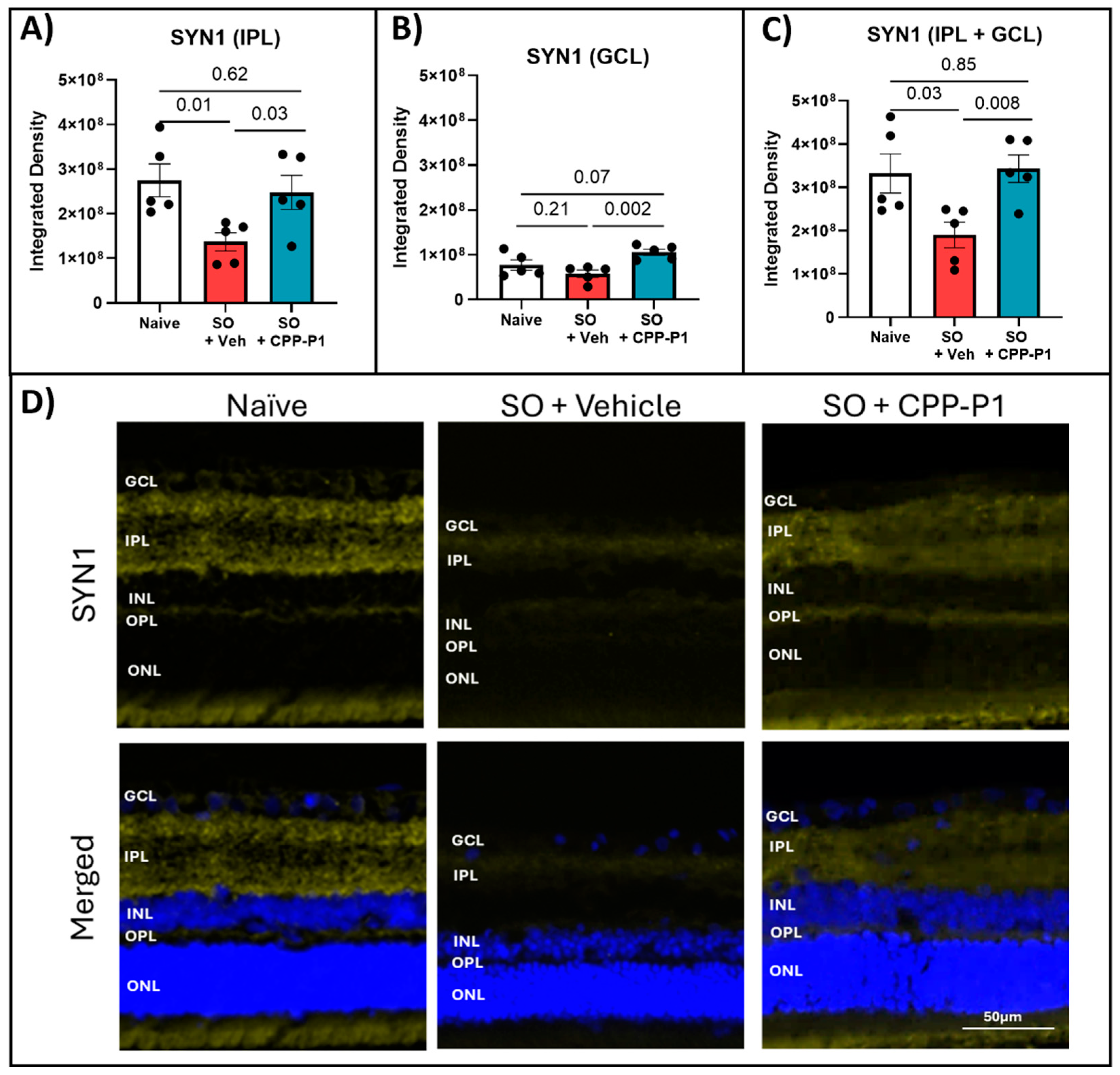
3.6. Expression of GAP43 and SYN1s following IOP Elevation and Treatment with CPP-P1
3.7. Mitochondrial SOD2 Levels Evaluation
3.8. Assessment of RGC Survival via RBPMS-Positive Cell Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allison, K., Patel, D., & Alabi, O., Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus, 2020. 12. [CrossRef]
- De Moraes, C.G., J.M. Liebmann, and L.A. Levin, Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog Retin Eye Res, 2017. 56: p. 107-147. [CrossRef]
- Erb, C., et al., Electrical neurostimulation in glaucoma with progressive vision loss. Bioelectron Med, 2022. 8(1): p. 6. [CrossRef]
- Quigley, H.A., Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol, 2012. 23(2): p. 144-54. [CrossRef]
- Akerfelt, M., R.I. Morimoto, and L. Sistonen, Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol, 2010. 11(8): p. 545-55. [CrossRef]
- Andley, U.P., The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones. Int J Biochem Cell Biol, 2008. 40(3): p. 317-23. [CrossRef]
- Fischer, D., et al., Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci, 2008. 37(3): p. 471-9.
- Fort, P.E. and K.J. Lampi, New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp Eye Res, 2011. 92(2): p. 98-103. [CrossRef]
- Piri, N., J.M. Kwong, and J. Caprioli, Crystallins in retinal ganglion cell survival and regeneration. Mol Neurobiol, 2013. 48(3): p. 819-28. [CrossRef]
- Rajeswaren, V., et al., Small Heat Shock Proteins in Retinal Diseases. Front Mol Biosci, 2022. 9: p. 860375. [CrossRef]
- Sreekumar, P.G., et al., Antiapoptotic properties of α-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci, 2013. 54(4): p. 2787-98. [CrossRef]
- Vendredy, L., E. Adriaenssens, and V. Timmerman, Small heat shock proteins in neurodegenerative diseases. Cell Stress & Chaperones, 2020. 25(4): p. 679-699. [CrossRef]
- Anders, F., et al., The Small Heat Shock Protein α-Crystallin B Shows Neuroprotective Properties in a Glaucoma Animal Model. Int J Mol Sci, 2017. 18(11). [CrossRef]
- Kannan, R., P.G. Sreekumar, and D.R. Hinton, Novel roles for α-crystallins in retinal function and disease. Prog Retin Eye Res, 2012. 31(6): p. 576-604. [CrossRef]
- Kannan, R., et al., Alpha Crystallin Derived Peptide Chaperone Protects Human RPE Cells From Oxidative Injury. Investigative Ophthalmology & Visual Science, 2010. 51(13): p. 1441-1441.
- Kourtis, N., V. Nikoletopoulou, and N. Tavernarakis, Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature, 2012. 490(7419): p. 213-218. [CrossRef]
- Liu, H., et al., Crystallins Play a Crucial Role in Glaucoma and Promote Neuronal Cell Survival in an In Vitro Model Through Modulating Muller Cell Secretion. Invest Ophthalmol Vis Sci, 2022. 63(8): p. 3. [CrossRef]
- Liu, J.P., et al., Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res, 2004. 79(6): p. 393-403.
- McGreal, R.S., et al., αB-crystallin/sHSP protects cytochrome c and mitochondrial function against oxidative stress in lens and retinal cells. Biochim Biophys Acta, 2012. 1820(7): p. 921-30.
- Munemasa, Y., et al., The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci, 2009. 50(8): p. 3869-75. [CrossRef]
- Nahomi, R.B., et al., Chaperone peptides of α-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem, 2013. 288(18): p. 13022-35.
- Nam, M.-H., et al., Peptains block retinal ganglion cell death in animal models of ocular hypertension: implications for neuroprotection in glaucoma. Cell Death & Disease, 2022. 13(11): p. 958. [CrossRef]
- Reddy, V.S. and G.B. Reddy, Emerging therapeutic roles of small heat shock protein-derived mini-chaperones and their delivery strategies. Biochimie, 2022. [CrossRef]
- Sreekumar, P.G., et al., Intra-vitreal αB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J Control Release, 2018. 283: p. 94-104. [CrossRef]
- Sreekumar, P.G., et al., Mechanisms of RPE senescence and potential role of αB crystallin peptide as a senolytic agent in experimental AMD. Exp Eye Res, 2022. 215: p. 108918. [CrossRef]
- Stankowska, D.L., Nam, M. H., Nahomi, R. B., Chaphalkar, R. M., Nandi, S. K., Fudala, R., Krishnamoorthy, R. R., & Nagaraj, R. H., Systemically administered peptain-1 inhibits retinal ganglion cell death in animal models: Implications for neuroprotection in glaucoma. Cell Death Discovery, 2019. 5. [CrossRef]
- Webster, J.M., et al., Small Heat Shock Proteins, Big Impact on Protein Aggregation in Neurodegenerative Disease. Front Pharmacol, 2019. 10: p. 1047. [CrossRef]
- Wu, N., et al., α-Crystallin protects RGC survival and inhibits microglial activation after optic nerve crush. Life Sciences, 2014. 94(1): p. 17-23. [CrossRef]
- Yan, H., et al., The Protective Effects of αB-Crystallin on Ischemia-Reperfusion Injury in the Rat Retina. J Ophthalmol, 2017. 2017: p. 7205408.
- Johnson, G.A., et al., Mechanisms contributing to inhibition of retinal ganglion cell death by cell permeable peptain-1 under glaucomatous stress. Cell Death Discovery, 2024. 10(1): p. 305. [CrossRef]
- Arthur, J.S., et al., Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci, 2004. 24(18): p. 4324-32.
- Caracciolo, L., et al., CREB controls cortical circuit plasticity and functional recovery after stroke. Nat Commun, 2018. 9(1): p. 2250. [CrossRef]
- Kandel, E.R., The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain, 2012. 5: p. 14.
- Walton, M.R. and I. Dragunow, Is CREB a key to neuronal survival? Trends Neurosci, 2000. 23(2): p. 48-53.
- Wang, L., et al., The transcription factor CREB acts as an important regulator mediating oxidative stress-induced apoptosis by suppressing αB-crystallin expression. Aging (Albany NY), 2020. 12(13): p. 13594-13617.
- Guo, X.J., et al., Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp Eye Res, 2014. 125: p. 156-63. [CrossRef]
- Guo, X., et al., Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell, 2021. 184(16): p. 4299-4314.e12.
- Zhu, S., et al., Schwann cell-derived extracellular vesicles as a potential therapy for retinal ganglion cell degeneration. J Control Release, 2023. 363: p. 641-656. [CrossRef]
- Eastwood, S.L. and P.J. Harrison, Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull, 2001. 55(5): p. 569-78. [CrossRef]
- Jin, Y., Synaptogenesis. WormBook, 2005: p. 1-11.
- Kaneda, M., et al., Growth-associated protein43 (GAP43) is a biochemical marker for the whole period of fish optic nerve regeneration. Adv Exp Med Biol, 2010. 664: p. 97-104.
- Cho, K.S., et al., Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J Cell Sci, 2005. 118(Pt 5): p. 863-72. [CrossRef]
- Leon, S., et al., Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci, 2000. 20(12): p. 4615-26. [CrossRef]
- John, A., et al., The neurodevelopmental spectrum of synaptic vesicle cycling disorders. J Neurochem, 2021. 157(2): p. 208-228. [CrossRef]
- Parenti, I., et al., The different clinical facets of SYN1-related neurodevelopmental disorders. Front Cell Dev Biol, 2022. 10: p. 1019715. [CrossRef]
- Ferreira, S.M., et al., Oxidative stress markers in aqueous humor of glaucoma patients. American Journal of Ophthalmology, 2004. 137(1): p. 62-69. [CrossRef]
- Ghanem, A.A., L.F. Arafa, and A. El-Baz, Oxidative Stress Markers in Patients with Primary Open-Angle Glaucoma. Current Eye Research, 2010. 35(4): p. 295-301. [CrossRef]
- Zhang, J., et al., Silicone oil-induced ocular hypertension and glaucomatous neurodegeneration in mouse. Elife, 2019. 8. [CrossRef]
- Fang, F., et al., Chronic mild and acute severe glaucomatous neurodegeneration derived from silicone oil-induced ocular hypertension. Scientific Reports, 2021. 11(1): p. 9052. [CrossRef]
- Zhang, J., et al., A Reversible Silicon Oil-Induced Ocular Hypertension Model in Mice. J Vis Exp, 2019(153).
- Kodati, B., et al., Oral administration of a dual ET(A)/ET(B) receptor antagonist promotes neuroprotection in a rodent model of glaucoma. Mol Vis, 2022. 28: p. 165-177.
- Dattilo, M., N.J. Newman, and V. Biousse, Acute retinal arterial ischemia. Annals of Eye Science, 2018. 3: p. 28. [CrossRef]
- Khakha, N., et al., Therapeutic implications of phosphorylation- and dephosphorylation-dependent factors of cAMP-response element-binding protein (CREB) in neurodegeneration. Pharmacol Rep, 2023. 75(5): p. 1152-1165.
- Fontanella, R.A., et al., Tirzepatide prevents neurodegeneration through multiple molecular pathways. J Transl Med, 2024. 22(1): p. 114. [CrossRef]
- Jiang, Y.Y., et al., Ginsenoside Rg1 promotes neurite growth of retinal ganglion cells through cAMP/PKA/CREB pathways. J Ginseng Res, 2024. 48(2): p. 163-170. [CrossRef]
- Yan, D., et al., Subchronic Acrylamide Exposure Activates PERK-eIF2α Signaling Pathway and Induces Synaptic Impairment in Rat Hippocampus. ACS Chem Neurosci, 2022. 13(9): p. 1370-1381.
- Naguib, S., et al., Retinal oxidative stress activates the NRF2/ARE pathway: An early endogenous protective response to ocular hypertension. Redox Biol, 2021. 42: p. 101883.
- Jiang, W., et al., Adeno-associated virus mediated SOD gene therapy protects the retinal ganglion cells from chronic intraocular pressure elevation induced injury via attenuating oxidative stress and improving mitochondrial dysfunction in a rat model. Am J Transl Res, 2016. 8(2): p. 799-810.
- Chae, J.J., M.R. Prausnitz, and C.R. Ethier, Effects of General Anesthesia on Intraocular Pressure in Rabbits. J Am Assoc Lab Anim Sci, 2021. 60(1): p. 91-95. [CrossRef]
- Tsuchiya, S., et al., Effect of inhalation anesthesia with isoflurane on circadian rhythm of murine intraocular pressure. Exp Eye Res, 2021. 203: p. 108420. [CrossRef]
| Group |
% loss in Vehicle |
p-value Vehicle: Naïve |
% loss in CPP-P1 |
p-value CPP-P1: Vehicle |
p-value CPP-P1: Naïve |
| ONL | 11% | 0.408 | 0% | 0.159 | 0.792 |
| INL | 41% | *0.016 | 9% | 0.059 | 0.756 |
| IPL | 52% | ***0.0002 | 33% | 0.119 | **0.009 |
| GCL | 57% | ***0.001 | 26% | 0.057 | 0.118 |
| Total | 65% | *0.014 | 11% | 0.090 | 0.567 |
| Naïve | p-value Vehicle: Naïve |
Vehicle | p-value CPP-P1: Vehicle |
CPP-P1 | p-value CPP-P1: Naive |
|
|---|---|---|---|---|---|---|
| CREB | 6.9E7 | 0.62 | 8.3E7 | 0.17 | 1.8E8 | 0.09 |
| p-CREB | 1.6E8 | 0.12 | 8.3E7 | *0.04 | 1.6E8 | 0.86 |
| p-CREB/ CREB | 2.3 | 1 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





