1. Introduction
Marburg virus disease (MVD) is a zoonotic viral hemorrhagic fever that is commonly transmitted between humans and other animal hosts mainly fruit bats known as natural reservoirs, and it caused by the Marburg virus (MARV), a close relative of Ebola virus. The disease is characterized by a remarkably high fatality rate up to 90% [
1]. The virus was identified for the first during it is involvement two simultaneous outbreaks in Marburg in Germany and Belgrade in Serbia, in 1967 [
2]. According to the World Health Organization (WHO), the disease can be acquired from direct contact with infected animal and/or human or contaminated products and material [
3]. It is classified within the order
Mononegavirales, the family
Filoviridae, and the genus
Marburgvirus. This pathogen poses a significant public health and security threat to human, animal, and environmental health as well as wildlife biodiversity and socioeconomic stability of human populations due to its severity, rapid spread, lack of licensed vaccine or treatment [
4]. Therefore, the disease is on the WHO and The Global Alliance for Vaccines and Immunizations (GAVI) lists for pathogens that will very likely causes the next pandemic, and the WHO list of high priority diseases for research and development [
5,
6,
7].
2. Historical Epidemiology
The ever first recorded cases of MVD were among laboratory workers involved in medical research activities using African green monkeys, which were imported for the production of primary cell cultures [
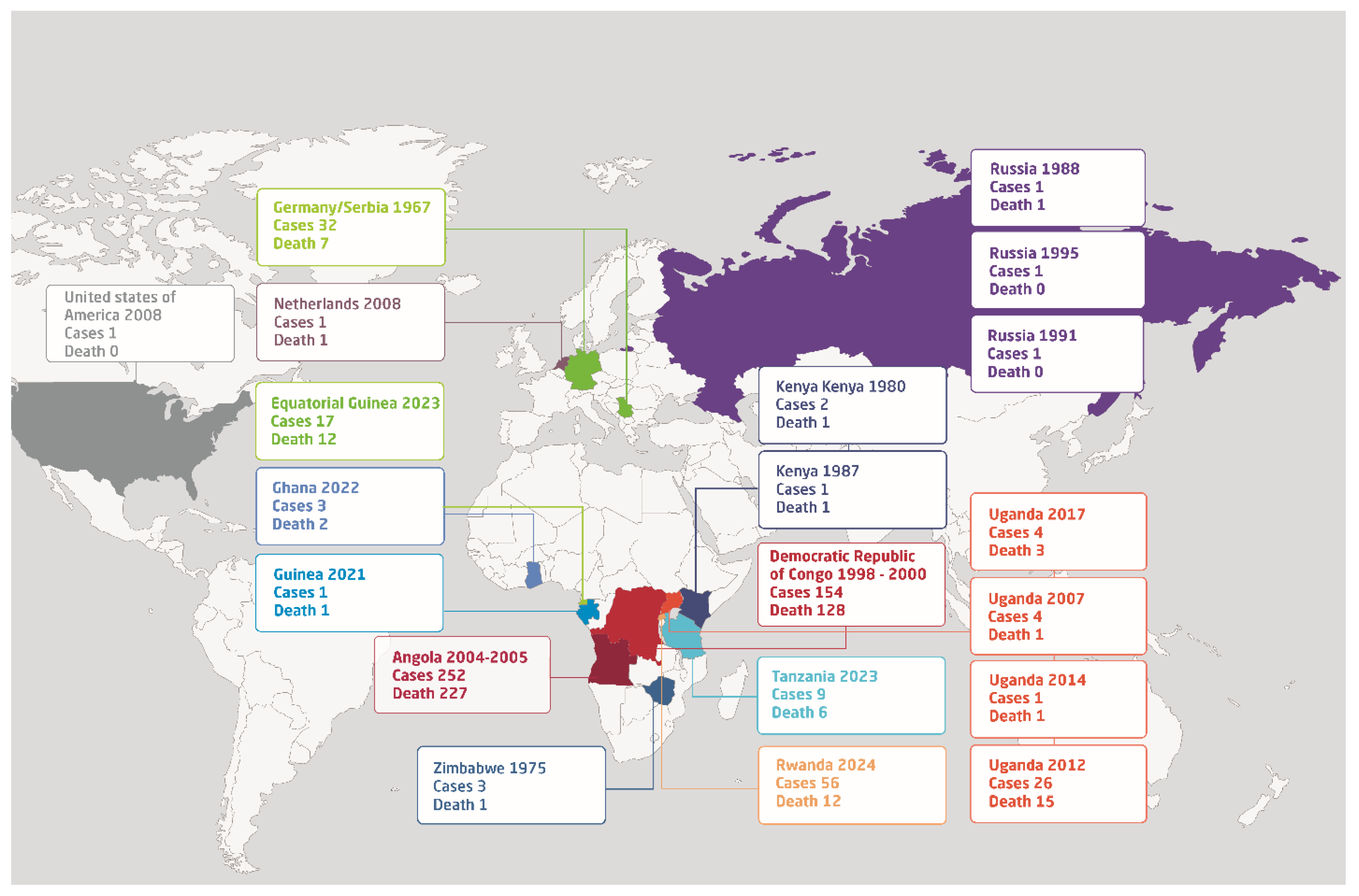
8]. Since its initial discovery, MARV has raised ongoing concerns regarding its potential for high mortality and the challenges associated with its management in outbreak situations. Due to it is natural circulation among animal reservoir that mainly existed in Africa, the disease predominantly affects individuals in Sub-Saharan Africa. To date, there have been a total of 19 reported outbreaks of MARV, primarily occurring in African nations such as Uganda, Ghana, Guinea, Tanzania, Angola, the Democratic Republic of the Congo, Zimbabwe, and most recently, Rwanda (
Figure 1). However, outbreaks of MARV have also occurred in Germany, Netherlands, Russia, and Serbia in Europe and in the USA (
Figure 1).
3. Natural Reservoirs and Routes of Transmission
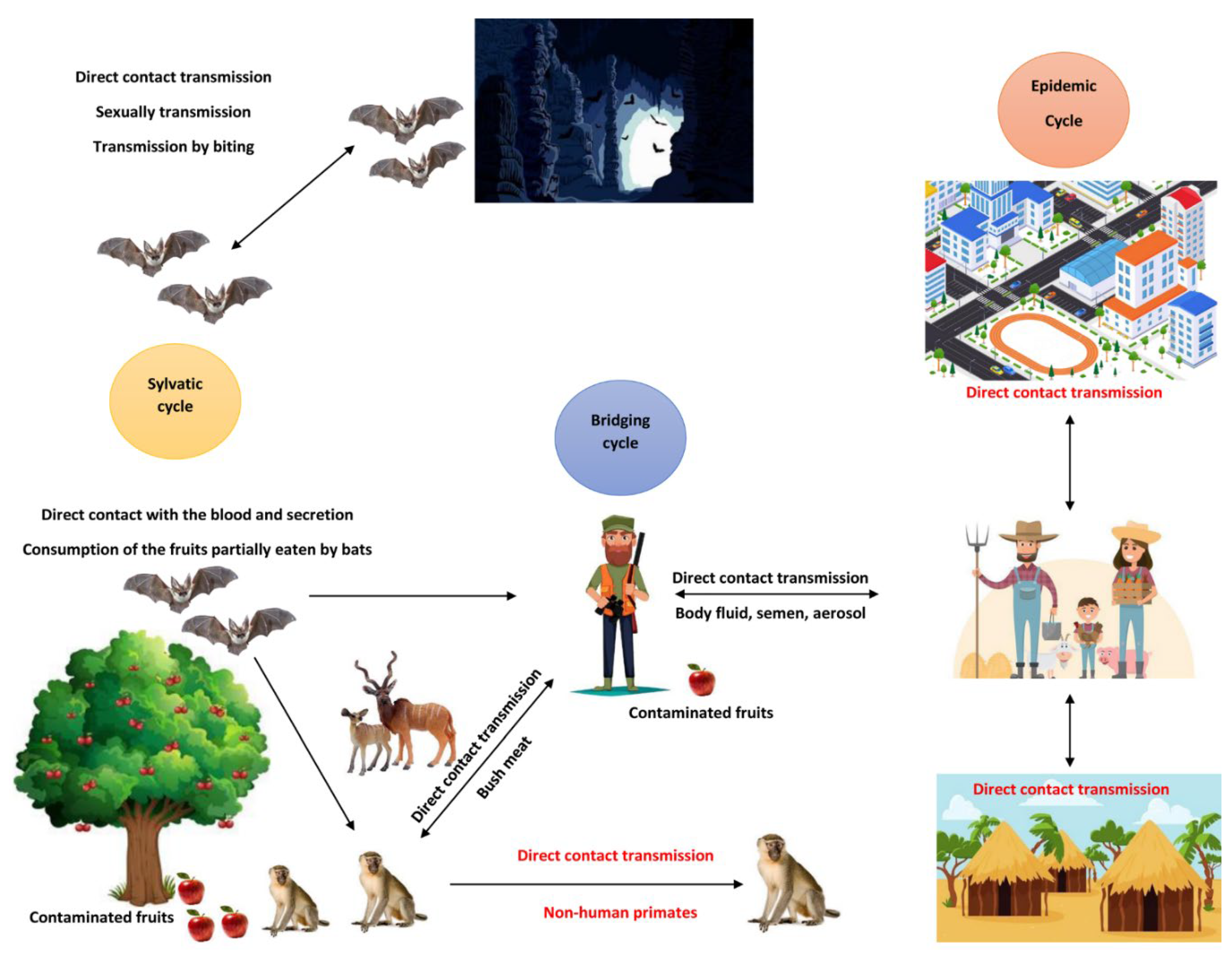
Interestingly, existed evidence shows that all identified and reported outbreak of MARV are involving contact with animal hosts, whether in nature or lab settings. This highlights the zoonotic nature of the virus and indicates a sustainable epizootic transmission that maintains the virus circulation in the environment. Various species of bats have been incriminated to be involved in the virus transmission and some species were identified as natural reservoirs for filoviruses including MARV. There is a compelling evidence about the critical role of
Rousettus aegyptiacus; commonly known as the Egyptian fruit bat, in the ecology and dynamics of MARV [
9,
10].
In order to investigate the role of the bats as a reservoirs host for MARV, researchers conducted a numerous studies and they succeeded in the isolations of live MARV from
R. aegyptiacus bats in Uganda, where miners diagnosed with MVD [
11,
12]. Furthermore, studies involving the direct infection of
R. aegyptiacus bats with MARV have revealed that infection with MARV in these bats are asymptomatic, therefore, they live normally including traveling between endemic and disease-free areas contributing to the spread of the virus [
13,
14]. Nevertheless, a mild immune response was noted, along with the detection of the virus in various organs of bat including liver, spleen, blood kidney, salivary gland, intestines, and in the inoculated site of the skin. Viral shedding was identified through oral and rectal swabs [
15]. Moreover, despite the presence of viremia and viral shedding, there was no observed transmission to other susceptible
R. aegyptiacus bats for up to 42 days. Additionally, they demonstrated the shedding of the virus from saliva and excreta of the bats [
16]. Therefore, the inhalation of aerosols or contaminated excreta from these bats might be a significant route for introducing the virus into the human population.
4. Transmission Dynamics
MARV transmission occurs through several pathways, primarily involving mucosal surfaces, broken skin, or parenteral routes. In outbreak scenarios, direct contact with infected humans or animals represents the most common source of infection. Notably, parenteral exposure -often occurring in healthcare settings- has proven to be one of the most lethal routes of infection (
Figure 2) [
17].
During the 1967 outbreak, a significant number of cases resulted from direct contact with the blood and organs of infected monkeys or involvement in post-mortem examinations [
18]. However, this was shortly followed by a secondary transmission among individuals with no contact with originally infected animals or their materials, providing the initial evidence of human-to-human transmission of MARV [
19].
Human-to-human transmission of MARV typically occurs via direct contact with bodily fluids, including blood, saliva, sweat, stool, urine, tears, semen and breast milk, particularly during the care of infected patients [
19,
20,
21]. Additionally, in the 1998-2000 outbreak in the Democratic Republic of the Congo (DRC), handling corpses during traditional burial practices was identified as a leading factor for infection with MARV [
22]. Furthermore, evidence from the 1967 outbreak suggested possible sexual transmission during the convalescence phase, as virus antigens were detected in the semen of an infected patient [
19].
Experimental study among group of non-human primate;
Cynomolgus macaques also known as Crab-eating macaque, has established a compiling evidence of aerosol transmission of MARV in confined space [
23]. This piece of evidence is of high concern, because it indicate the risk of nosocomial outbreaks of MARV in healthcare facilities, which threats the live and health of healthcare providers, community health workers, patients, co-patients, visitors, and supportive staff in these facilities. Particularly that, aerosolized particles can remain suspended in the air for extended period of time leading to a rapidly growing transmission rate of the disease.
5. Clinical Presentations
Marburg virus disease is characterized with an extended incubation period that ranging between two and 21 days [
24]. This highly depends on factors such as the infectious dose and route of infection. More importantly, during this incubation period individuals are not infectious and the transmission occurs after the onset of the disease [
4,
25]. The clinical course of MVD infection is generally divided into three phases (
Table 1).
The first phase, known as the generalized phase, lasts from days one to four and is characterized by an abrupt onset with nonspecific, flu-like symptoms, including high fever (typically between 39–40°C), severe headache, chills, myalgia, prostration, and malaise [
17].
The second phase, the early phase, spans from 5 to 13 days, during which 50–75% of patients experience gastrointestinal symptoms, such as anorexia, abdominal discomfort, severe nausea, vomiting, and diarrhea, within the first two to five days. The intensity of the disease often escalates between day five and seven, presenting with a maculopapular rash and symptoms of hemorrhagic fever, including petechiae, mucosal and gastrointestinal bleeding, as well as hemorrhage from venipuncture sites [
17].
The final phase, convalescence phase, begins after day 13, during which survivors may skip the most severe symptoms and may not reach the late organ phase altogether. Neurological symptoms like disorientation, agitation, seizures, and coma may manifest in the later stages of the disease [
26]. Moreover, in some cases infection with the virus can lead to complications even during the recovery period that can include joint pain, uveitis, orchitis, and pericarditis, with recovery often being slow. Furthermore, throughout the course of MVD, disseminated intravascular coagulation (DIC), lymphopenia, and thrombocytopenia are typically emerging within a week from the onset of symptoms. Patients ultimately, either recover with appropriate supportive care or end up with fatal outcomes, such as dehydration, internal bleeding, and multi-organ failure, usually occurring eight to sixteen days after symptoms begin.
6. Diagnosis
MVD diagnosis employs various laboratory-based techniques that adapt to the disease's stages for effective management. In the early stage, detection of viral antigens in the bloodstream is crucial, utilizing methods such as virus isolation via cell culture, antigen capture enzyme-linked immunosorbent assay (ELISA) with monoclonal antibodies produced to the recombinant nucleoprotein including MAb2A7 and MAb2H6, and immunohistochemical analysis of tissue samples to identify MARV antigens [
27,
28]. As the disease progresses, serological assays become essential for detecting IgM and IgG antibodies, employing indirect immunofluorescence assays and IgM/IgG capture ELISA to assess infection history and immune response.
Molecular diagnostics, including reverse transcription polymerase chain reaction (RT-PCR), nested RT-PCR, and real-time quantitative RT-PCR (qRT-PCR), which can be done from blood samples as well as buccal swab, offer sensitive and specific detection of viral RNA, aiding diagnosis in both early and late stages [
29].
In addition to above mentioned specific tests, complementary diagnostic evaluations are essential for physicians to suspect arboviral infections, including Marburg Virus Disease (MVD). A Complete Blood Count (CBC) often reveals hallmark laboratory findings such as thrombocytopenia (reduced platelet count) and leukopenia (decreased white blood cell count), both of which are critical indicators of viral infection and the body’s immune response. Moreover, MVD patients frequently exhibit elevated liver enzyme levels, particularly aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which signal hepatic involvement and can provide valuable insight into liver function, aiding in clinical management decisions. Additionally, the presence of proteins in urine samples can indicate renal dysfunction associated with MVD, making urinalysis an important component of a comprehensive patient evaluation. Collectively, these diagnostic tools enhance the clinician's ability to assess disease severity and tailor interventions effectively.
7. Case Management
Currently, there are no specific antiviral treatments that is approved and licensed for MVD, so medical management primarily involves supportive care. This includes rehydration, managing symptoms, and maintaining electrolyte balance.
Experimental treatments, such as monoclonal antibodies (e.g., REGN3470-3471-3479) [
30]. In addition to monoclonal antibodies, researchers tried some antiviral in animal models including Galidesivir (BCX4430) which is an antiviral agent that functions by terminating RNA chains and inhibiting the activity of viral RNA polymerase. Study conducted on six
cynomolgus macaques infected with MARV demonstrated its efficacy, resulting in increased survival rates, reduced levels of viremia [
31,
32]. However, it is important to note that data from human trials regarding Galidesivir's effectiveness have not yet been published.
Another antiviral therapy included, Favipiravir (T-705), a broad-spectrum antiviral, was previously utilized during the Ebola virus disease outbreak in West Africa. In the context MVD, promising outcomes were observed when intravenous Favipiravir was administered to six
cynomolgus macaques infected with MARV, with five of the animals surviving the disease [
33]. Furthermore, remdesivir (GS-5734) has shown effectiveness against both Ebola and MVD in non-human primate studies [
34].
8. Integrated Multisectoral One Health Strategy for Prevention and Control of MVD
Due the zoonotic nature of MDV, cost-effective prevention and control measures should target the main hosts and reservoirs. Particularly that MVD is a pandemic-prone disease with high mortality and socioeconomic impacts that imposes serious threat on the Global Health Security, therefore, affected countries and countries at risk should invest on strengthening pandemic preparedness, prevention, and response (PPPR) [
35,
36]. To enhance the cost-effectiveness of such PPPR framework, it needs to be implemented through an integrated multisectoral transdisciplinary One Health strategy that includes integrated collaborative surveillance supported with genomics analysis [
37,
38,
39,
40]. This should take account of strengthening the diagnostic capacity for the early detection among humans, animals, and the environment, isolating confirmed cases, and tracking the contact of confirmed infections to identify all the suspected cases and monitor their health for three weeks. Additional public health interventions include community engagement by health education to raising awareness as well as improving hygiene and sanitation [
41]. Other major interventions are strengthening the implementation of the International Health Regulations (IHRs 2005) [
41].
Infection prevention and control measures are vital. This should be strictly implemented in public facilities including healthcare units, restaurants, Airports, and public toilets. It also essential to enforce the use of proper personal protective equipment (PPE) for healthcare provider and community health workers, strict adherence to hygiene protocols, safe burial practices to prevent post-mortem transmission, and firm waste management for medical and personal waste that came in direct and/or indirect contact with confirmed and suspected cases [
42]. Furthermore, collaboration with international health organizations can enhance response capabilities, ensuring resources and expertise are mobilized to address and contain outbreaks effectively. Therefore, live communication and immediate public sharing of information is a key for effective community and stakeholders engagement [
43]. During outbreak, health authorities should considering administrating the experimental vaccine of MARV for at high risk populations mainly including frontline responders such as healthcare providers and community health workers [
44,
45]. However, this should be done under close monitoring and observation in the immediate availability and accessibility to a supportive care whenever it is needed.
Moreover, additional One Health interventions include reducing the contact between humans and bats, pigs, and non-human primates particularly those show signs of illness. Careful attention should be given to endangered species such as the mountain gorilla as they are susceptible for fatal infection with MARV. Therefore, it important to improve the living environment including the housing for humans and domestic animals to avoid the manifestation with bats [
46]. Also, avoid the consumption of food contaminated came in contact with bats or their feces, animal products must be cocked thoroughly, and consider vaccinating the mountain gorilla [
46]. Recovered males should avoid non-protected sex until their semen was tested and confirmed to be virus-free [
47].
9. Conclusions
Marburg virus remains a major threat to the Global Health Security due to its potential for high mortality, devastating health, socioeconomic, and political impacts, and it challenges the health systems to manage it is outbreaks. Evident by historical outbreaks and recent emergences, mainly in Africa, countries at risk need to invest in strengthening the Global Health Security and the pandemic preparedness, prevention, and response (PPPR) framework through the implementation of an integrated multisectoral and transdisciplinary One Health strategy. Such investment should include improving the diagnostic capacity for the early detection, integrated collaborative surveillance system that monitor the health metrics and dynamics of zoonotic diseases in humans, animals, and the environment. Cost-effective healthcare and public health interventions including response protocols to health emergencies, risk communication, health education, and community and stakeholders engagement are essential for health emergencies response and containment. Strict implementation of the International Health Regulations (IHRs 2005), effective infection prevention and control, and adequate hygiene and sanitation are cost-effective measures for prevention and control of MARV outbreaks. Considering the global lack of safe, effective, and affordable licensed vaccine and treatment for the disease, per the WHO recommendation, stakeholders of human and animal health should invest and collaborate on research and development of vaccines and treatment to cost-effectively prevent and response to future pandemics.
Author Contributions
Conceptualization, C.M.M., E.E.S. and A.A.; methodology, C.M.M., J.C.S.N., N.B., E.E.S., and A.A.; investigation, C.M.M., J.C.S.N., N.B., E.E.S., and A.A.; resources, C.M.M.; data curation, E.E.S.; writing—original draft preparation, C.M.M., J.C.S.N., N.B., E.E.S., and A.A.; writing—review and editing, C.M.M., J.C.S.N., N.B., Y.B., S.N., J.K., E.E.S., and A.A.; visualization, J.C.S.N., N.B., and E.E.S.; supervision, C.M.M.; project administration, C.M.M.; funding acquisition, J.C.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
The APCs for this were paid from grant provided by the Belgian Directorate General for Development Cooperation to institutional collaboration, Rwanda Biomedical Centre and the Institute of Tropical Medicine.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data collected during the development of this review is presented in the published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shifflett, K.; Marzi, A. Marburg Virus Pathogenesis – Differences and Similarities in Humans and Animal Models. Virol J 2019, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Brauburger, K.; Hume, A.J.; Mühlberger, E.; Olejnik, J. Forty-Five Years of Marburg Virus Research. Viruses 2012, 4, 1878–1927. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO) Marburg Virus Disease. Available online: https://www.who.int/health-topics/marburg-virus-disease (accessed on 9 October 2024).
- Srivastava, S.; Sharma, D.; Kumar, S.; Sharma, A.; Rijal, R.; Asija, A.; Adhikari, S.; Rustagi, S.; Sah, S.; Al-qaim, Z.H.; et al. Emergence of Marburg Virus: A Global Perspective on Fatal Outbreaks and Clinical Challenges. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- The Global Alliance for Vaccines and Immunizations (GAVI) The next Pandemic: Marburg? Available online: https://www.gavi.org/vaccineswork/next-pandemic/marburg (accessed on 10 October 2024).
- The World Health Organization (WHO) Prioritizing Diseases for Research and Development in Emergency Contexts Available online:. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 10 October 2024).
- The World Health Organization (WHO) Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness. 2024.
- Ristanović, E.S.; Kokoškov, N.S.; Crozier, I.; Kuhn, J.H.; Gligić, A.S. A Forgotten Episode of Marburg Virus Disease: Belgrade, Yugoslavia, 1967. Microbiology and Molecular Biology Reviews 2020, 84, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Schuh, A.J.; Amman, B.R.; Guito, J.C.; Graziano, J.C.; Sealy, T.K.; Kirejczyk, S.G.M.; Towner, J.S. Natural Reservoir Rousettus Aegyptiacus Bat Host Model of Orthonairovirus Infection Identifies Potential Zoonotic Spillover Mechanisms. Sci Rep 2022, 12, 20936. [Google Scholar] [CrossRef]
- Guito, J.C.; Prescott, J.B.; Arnold, C.E.; Amman, B.R.; Schuh, A.J.; Spengler, J.R.; Sealy, T.K.; Harmon, J.R.; Coleman-McCray, J.D.; Kulcsar, K.A.; et al. Asymptomatic Infection of Marburg Virus Reservoir Bats Is Explained by a Strategy of Immunoprotective Disease Tolerance. Current Biology 2021, 31, 257–270.e5. [Google Scholar] [CrossRef]
- Timen, A.; Koopmans, M.P.G.; Vossen, A.C.T.M.; van Doornum, G.J.J.; Günther, S.; van den Berkmortel, F.; Verduin, K.M.; Dittrich, S.; Emmerich, P.; Osterhaus, A.D.M.E.; et al. Response to Imported Case of Marburg Hemorrhagic Fever, the Netherlands. Emerg Infect Dis 2009, 15, 1171–1175. [Google Scholar] [CrossRef]
- CDC, C. Transmission of Colorado Tick Fever Virus by Blood Transfusion - Montana. Morbidity and Mortality Weekly Report 1975. [Google Scholar]
- Amman, B.R.; Jones, M.E.B.; Sealy, T.K.; Uebelhoer, L.S.; Schuh, A.J.; Bird, B.H.; Coleman-McCray, J.D.; Martin, B.E.; Nichol, S.T.; Towner, J.S. ORAL SHEDDING OF MARBURG VIRUS IN EXPERIMENTALLY INFECTED EGYPTIAN FRUIT BATS (ROUSETTUS AEGYPTIACUS). Journal of Wildlife Diseases 2015, 51, 113–124. [Google Scholar] [CrossRef]
- Paweska, J.T.; Vuren, P.J. van; Masumu, J.; Leman, P.A.; Grobbelaar, A.A.; Birkhead, M.; Clift, S.; Swanepoel, R.; Kemp, A. Virological and Serological Findings in Rousettus Aegyptiacus Experimentally Inoculated with Vero Cells-Adapted Hogan Strain of Marburg Virus. PLOS ONE 2012, 7, e45479. [Google Scholar] [CrossRef]
- Paweska, J.T.; Jansen van Vuren, P.; Fenton, K.A.; Graves, K.; Grobbelaar, A.A.; Moolla, N.; Leman, P.; Weyer, J.; Storm, N.; McCulloch, S.D.; et al. Lack of Marburg Virus Transmission From Experimentally Infected to Susceptible In-Contact Egyptian Fruit Bats. The Journal of Infectious Diseases 2015, 212, S109–S118. [Google Scholar] [CrossRef] [PubMed]
- Amman, B.R.; Carroll, S.A.; Reed, Z.D.; Sealy, T.K.; Balinandi, S.; Swanepoel, R.; Kemp, A.; Erickson, B.R.; Comer, J.A.; Campbell, S.; et al. Seasonal Pulses of Marburg Virus Circulation in Juvenile Rousettus Aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLOS Pathogens 2012, 8, e1002877. [Google Scholar] [CrossRef] [PubMed]
- Mehedi, M.; Groseth, A.; Feldmann, H.; Ebihara, H. Clinical Aspects of Marburg Hemorrhagic Fever. Future Virology 2011, 6, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Hennessen, W. Epidemiology of “Marburg Virus” Disease. In Marburg Virus Disease; Martini, G.A., Siegert, R., Eds.; Springer: Berlin, Heidelberg, 1971; pp. 161–165. ISBN 978-3-662-01593-3. [Google Scholar]
- Martini, G.A.; Schmidt, H.A. [Spermatogenic transmission of the “Marburg virus”. (Causes of “Marburg simian disease”)]. Klin Wochenschr 1968, 46, 398–400. [Google Scholar] [CrossRef]
- Gear, J.S.; Cassel, G.A.; Gear, A.J.; Trappler, B.; Clausen, L.; Meyers, A.M.; Kew, M.C.; Bothwell, T.H.; Sher, R.; Miller, G.B.; et al. Outbreake of Marburg Virus Disease in Johannesburg. Br Med J 1975, 4, 489–493. [Google Scholar] [CrossRef]
- Borchert, M.; Muyembe-Tamfum, J.J.; Colebunders, R.; Libande, M.; Sabue, M.; Van der Stuyft, P. Short Communication: A Cluster of Marburg Virus Disease Involving an Infant. Tropical Medicine & International Health 2002, 7, 902–906. [Google Scholar] [CrossRef]
- Bausch, D.G.; Borchert, M.; Grein, T.; Roth, C.; Swanepoel, R.; Libande, M.L.; Talarmin, A.; Bertherat, E.; Muyembe-Tamfum, J.-J.; Tugume, B.; et al. Risk Factors for Marburg Hemorrhagic Fever, Democratic Republic of the Congo. Emerg Infect Dis 2003, 9, 1531–1537. [Google Scholar] [CrossRef]
- Alves, D.A.; Glynn, A.R.; Steele, K.E.; Lackemeyer, M.G.; Garza, N.L.; Buck, J.G.; Mech, C.; Reed, D.S. Aerosol Exposure to the Angola Strain of Marburg Virus Causes Lethal Viral Hemorrhagic Fever in Cynomolgus Macaques. Vet Pathol 2010, 47, 831–851. [Google Scholar] [CrossRef]
- The World Health Organization (WHO) Marburg Virus Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease (accessed on 10 October 2024).
- Mitu, R.A.; Islam, Md.R. The Current Pathogenicity and Potential Risk Evaluation of Marburg Virus to Cause Mysterious “Disease X”—An Update on Recent Evidences. Environ. Health. Insights 2024, 18, 11786302241235809. [Google Scholar] [CrossRef]
- Chauhan, L.; Matthews, E.; Piquet, A.L.; Henao-Martinez, A.; Franco-Paredes, C.; Tyler, K.L.; Beckham, D.; Pastula, D.M. Nervous System Manifestations of Arboviral Infections. Curr Trop Med Rep 2022, 9, 107–118. [Google Scholar] [CrossRef]
- Grolla, A.; Lucht, A.; Dick, D.; Strong, J.E.; Feldmann, H. Laboratory Diagnosis of Ebola and Marburg Hemorrhagic Fever. Bull Soc Pathol Exot 2005, 98, 205–209. [Google Scholar] [PubMed]
- Saijo, M.; Niikura, M.; Ikegami, T.; Kurane, I.; Kurata, T.; Morikawa, S. Laboratory Diagnostic Systems for Ebola and Marburg Hemorrhagic Fevers Developed with Recombinant Proteins. Clin Vaccine Immunol 2006, 13, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, Y.; Grolla, A.; Fukuma, A.; Feldmann, H.; Yasuda, J. Development and Evaluation of a Simple Assay for Marburg Virus Detection Using a Reverse Transcription-Loop-Mediated Isothermal Amplification Method. J Clin Microbiol 2010, 48, 2330–2336. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Coates, E.E.; Novik, L.; Widge, A.; Houser, K.V.; Burch, E.; Holman, L.A.; Gordon, I.J.; Chen, G.L.; Carter, C.; et al. Safety, Tolerability, Pharmacokinetics, and Immunogenicity of the Therapeutic Monoclonal Antibody mAb114 Targeting Ebola Virus Glycoprotein (VRC 608): An Open-Label Phase 1 Study. The Lancet 2019, 393, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Kortepeter, M.G.; Dierberg, K.; Shenoy, E.S.; Cieslak, T.J. Marburg Virus Disease: A Summary for Clinicians. International Journal of Infectious Diseases 2020, 99, 233–242. [Google Scholar] [CrossRef]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G. Protection against Filovirus Diseases by a Novel Broad-Spectrum Nucleoside Analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef]
- Bixler, S.L.; Bocan, T.M.; Wells, J.; Wetzel, K.S.; Van Tongeren, S.A.; Dong, L.; Garza, N.L.; Donnelly, G.; Cazares, L.H.; Nuss, J. Efficacy of Favipiravir (T-705) in Nonhuman Primates Infected with Ebola Virus or Marburg Virus. Antiviral Research 2018, 151, 97–104. [Google Scholar] [CrossRef]
- Porter, D.P.; Weidner, J.M.; Gomba, L.; Bannister, R.; Blair, C.; Jordan, R.; Wells, J.; Wetzel, K.; Garza, N.; Van Tongeren, S.; et al. Remdesivir (GS-5734) Is Efficacious in Cynomolgus Macaques Infected With Marburg Virus. The Journal of Infectious Diseases 2020, 222, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Muvunyi, C.M.; Bigirimana, N.; Tuyishime, A.; Mukagatare, I.; Ngabonziza, J.C.; Ahmed, A. Initiatives and Strategies to Strengthen the National, Regional, and International Global Health Security: A Case Study of Rwanda Biomedical Centre. 2024. Available online: https://ssrn.com/abstract=4957490. [CrossRef]
- Gashema, P.; Musafiri, T.; Ndahimana, F.; Iradukunda, H.; Saramba, E.; Nyakatswau, S.T.; Gahamanyi, N.; Iradukunda, P.G.; Ahmed, A.; Dzinamarira, T.; et al. Mpox in East Africa: Learning from COVID-19 and Ebola to Strengthen Public Health Responses. Viruses 2024, 16, 1578. [Google Scholar] [CrossRef]
- Remera, E.; Rwagasore, E.; Muvunyi, C.M.; Ahmed, A. Emergence of the First Molecularly Confirmed Outbreak of Rift Valley Fever among Humans in Rwanda, Calls for Institutionalizing the One Health Strategy. IJID One Health 2024, 4. [Google Scholar] [CrossRef]
- Remera, E.; Rwagasore, E.; Nsekuye, O.; Semakula, M.; Gashegu, M.; Rutayisire, R.; Ishema, L.; Musanabaganwa, C.; Butera, Y.; Nsanzimana, S.; et al. Rift Valley Fever Epizootic, Rwanda, 2022. Emerg Infect Dis 2024, 30, 2191–2193. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, Y.; Ibrahim, N.A.; Mohamed, S.I.; Zinsstag, J.; Siddig, E.E.; Mohamed, N.S.; Muvunyi, C.M. One Health Response for Rift Valley Fever Outbreak in Sudan. 2024.
- Nsengimana, I.; Juma, J.; Roesel, K.; Gasana, M.N.; Ndayisenga, F.; Muvunyi, C.M.; Hakizimana, E.; Hakizimana, J.N.; Eastwood, G.; Chengula, A.A.; et al. Genomic Epidemiology of Rift Valley Fever Virus Involved in the 2018 and 2022 Outbreaks in Livestock in Rwanda. Viruses 2024, 16, 1148. [Google Scholar] [CrossRef] [PubMed]
- Wirsiy, F.S.; Nkfusai, C.N.; Bain, L.E. The SPIN Framework to Control and Prevent the Marburg Virus Disease Outbreak in Equatorial Guinea. Pan Afr Med J 2023, 44, 110. [Google Scholar] [PubMed]
- Brainard, J.; Hooper, L.; Pond, K.; Edmunds, K.; Hunter, P.R. Risk Factors for Transmission of Ebola or Marburg Virus Disease: A Systematic Review and Meta-Analysis. International Journal of Epidemiology 2016, 45, 102–116. [Google Scholar] [CrossRef]
- Ahmed, A. Urgent Call for a Global Enforcement of the Public Sharing of Health Emergencies Data: Lesson Learned from Serious Arboviral Disease Epidemics in Sudan. Int Health 2020, 12, 238–240. [Google Scholar] [CrossRef]
- Manno, D. Developing a Vaccine against Marburg Virus Disease. The Lancet 2023, 401, 251–253. [Google Scholar] [CrossRef]
- O’Donnell, K.L.; Feldmann, F.; Kaza, B.; Clancy, C.S.; Hanley, P.W.; Fletcher, P.; Marzi, A. Rapid Protection of Nonhuman Primates against Marburg Virus Disease Using a Single Low-Dose VSV-Based Vaccine. eBioMedicine 2023, 89. [Google Scholar] [CrossRef]
- The World Health Organization (WHO) Marburg Virus Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease (accessed on 10 October 2024).
- CDC About Marburg. Available online: https://www.cdc.gov/marburg/about/index.html (accessed on 10 October 2024).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).