1. Introduction
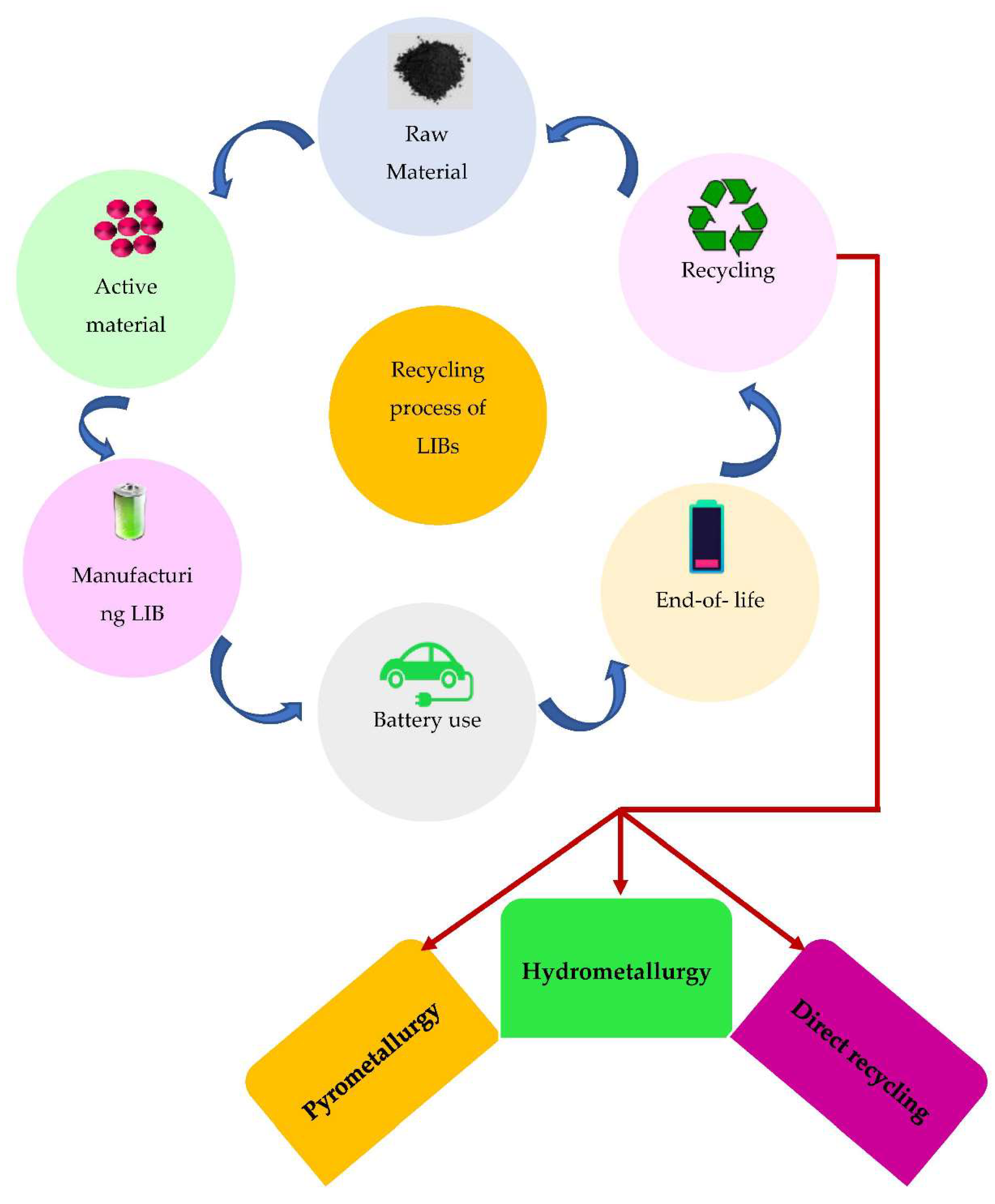
The widespread adoption of lithium-ion batteries (LIBs) in various applications, including electric vehicles, portable electronics, and grid storage systems, has led to an increased demand for efficient and sustainable battery recycling technologies [1, 2]. Among the valuable components of LIBs, lithium metal oxide holds particular significance due to its crucial role in battery performance and its limited availability as a finite resource [
3]. This introduction provides an overview of the importance of lithium metal oxide recovery from spent LIBs, discusses the application of microwave technology in LIB recycling, and outlines the scope of this review, which focuses on the utilization of microwave technology in conjunction with froth flotation and magnetic processes for lithium metal oxide recovery.
Lithium metal oxide, primarily found in the cathode of LIBs, is a key component responsible for the storage and release of lithium ions during charge and discharge cycles [
4]. Common lithium metal oxides used in LIB cathodes include lithium cobalt oxide (LiCoO2), lithium nickel manganese cobalt oxide (NMC), and lithium iron phosphate (LiFePO4). These materials play a crucial role in determining the energy density, power output, and cycle life of LIBs. Lithium is a finite resource, primarily extracted from mineral deposits such as spodumene and brine [
5]. The increasing demand for LIBs necessitates the efficient recovery and recycling of lithium metal oxide to conserve this valuable resource and reduce reliance on virgin materials. The extraction and processing of lithium metal oxide have environmental implications, including energy consumption, greenhouse gas emissions, and water usage [
6]. By recycling lithium metal oxide from spent LIBs, we can mitigate these environmental impacts and contribute to a more sustainable battery supply chain [
7]. Recovering lithium metal oxide from spent LIBs supports the principles of a circular economy by closing the loop in battery production. Instead of disposing of spent batteries in landfills or incinerating them, recycling allows us to recover valuable materials and reintroduce them into the manufacturing process, reducing waste and conserving resources.
Microwave technology has emerged as a promising tool in LIB recycling due to its ability to selectively heat materials and accelerate chemical reactions [
8]. In microwave-assisted processes, electromagnetic waves in the microwave frequency range (300 MHz to 300 GHz) interact with materials, causing polar molecules and ions to rotate and generate heat. This phenomenon enables rapid and uniform heating of materials, making microwave technology well-suited for various stages of LIB recycling. Microwaves can selectively heat specific components within LIBs, such as lithium metal oxide, while leaving other materials relatively unaffected [
9]. This selective heating allows for targeted processing and enhances the efficiency of recovery processes. It heating is characterized by its rapid heating rates and uniform temperature distribution. Compared to conventional heating methods, microwave-assisted processes can significantly reduce processing times, leading to higher throughput and energy savings [
10]. The application of microwave radiation can accelerate chemical reactions, including dissolution, leaching, and phase transformations. This acceleration of reaction kinetics improves the efficiency of LIB recycling processes and allows for more thorough extraction of valuable materials.
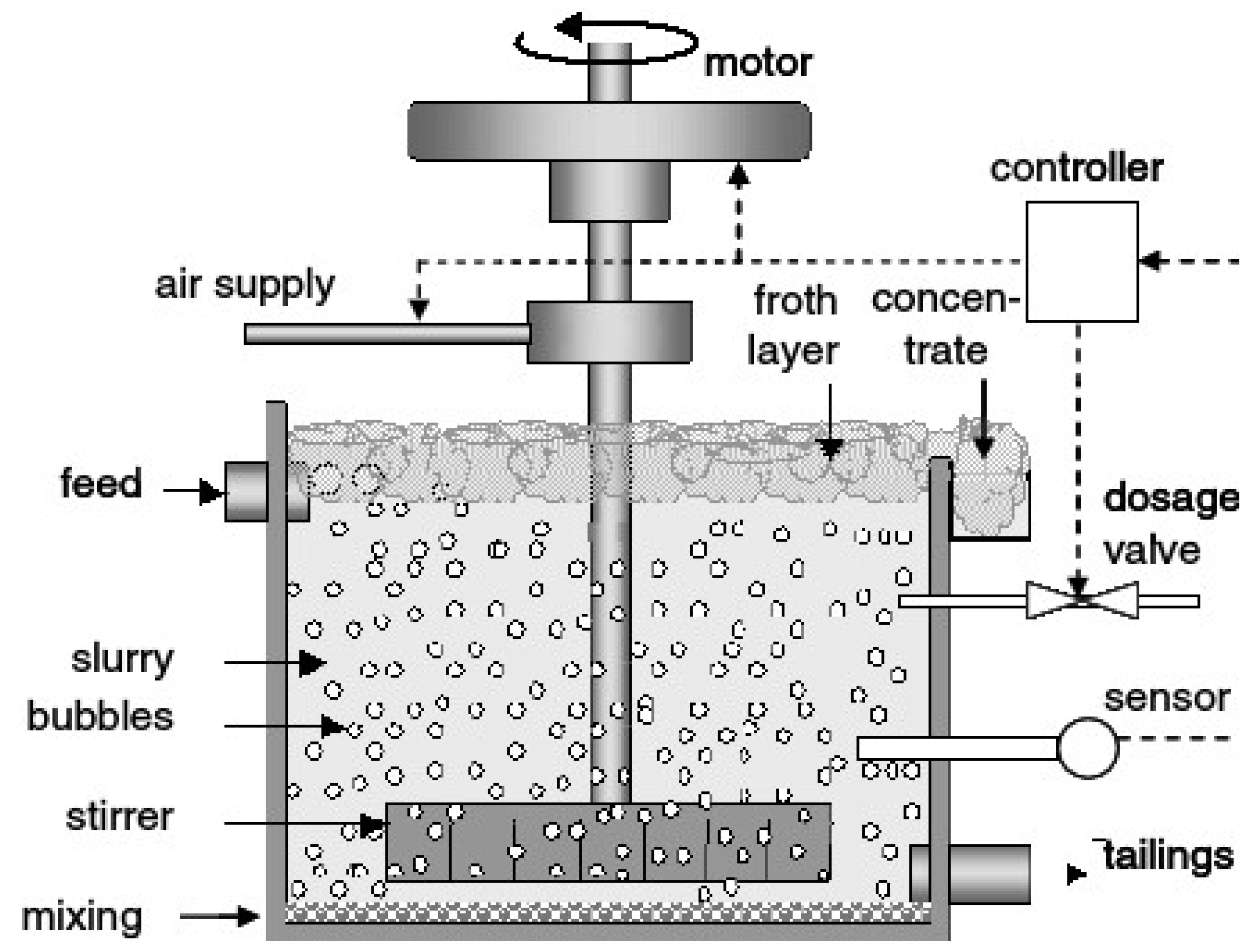
Froth flotation and magnetic separation are well-established separation techniques commonly used in mineral processing and LIB recycling, as illustrated in
Figure 1 [11, 12]. By integrating microwave technology with these processes, we aim to explore the synergistic effects and potential benefits of combined approaches for lithium metal oxide recovery. Froth flotation is a separation technique that exploits differences in surface wettability to separate minerals [
13]. In LIB recycling, froth flotation can be used to separate lithium metal oxide from electrode materials. The integration of microwave technology with froth flotation processes offers opportunities for enhanced recovery efficiency, reduced energy consumption, and improved selectivity [
14]. Magnetic separation relies on the magnetic properties of materials to separate them from non-magnetic components. In LIB recycling, magnetic separation is used to recover magnetic materials such as cobalt and nickel from electrode materials. By incorporating microwave technology into magnetic separation processes, we can enhance the liberation of magnetic materials and improve separation efficiency [
15]. This provides insights into the application of microwave technology in conjunction with froth flotation and magnetic processes for lithium metal oxide recovery from spent LIBs. By examining the principles, advantages, challenges, and potential synergies of these combined approaches, we can contribute to the development of more efficient and sustainable LIB recycling technologies.
2. Microwave Technology in LIB Recycling
Lithium-ion batteries (LIBs) have revolutionized modern technology by providing efficient energy storage solutions for various applications [
16]. However, as the demand for LIBs continues to grow, so does the need for effective recycling methods to recover valuable materials and reduce environmental impact. Microwave technology has emerged as a promising tool in LIB recycling due to its ability to selectively heat materials and accelerate chemical reactions [17, 18]. This explores the principles of microwave heating, the advantages of microwave technology in LIB recycling, and microwave-assisted processes for lithium metal oxide recovery as illustrated in
Figure 2.
Microwave heating relies on the interaction between electromagnetic waves and polar molecules within materials [
19]. When exposed to microwave radiation, polar molecules within materials attempt to align themselves with the alternating electric field. This continuous realignment of molecules results in the conversion of electromagnetic energy into heat, a process known as dielectric heating. The penetration depth of microwave radiation depends on the frequency of the microwaves and the dielectric properties of the material. Materials with higher dielectric constants absorb microwaves more effectively and heat up more rapidly. For most materials, microwave radiation penetrates to a depth of a few centimeters. Unlike conventional heating methods, which rely on heat conduction from the surface, microwave heating generates heat uniformly throughout the material volume [
20]. This uniform heating minimizes temperature gradients and allows for rapid heating of bulk materials. In addition to thermal effects, microwave radiation can induce non-thermal effects such as molecular vibration, chemical reactions, and phase transformations. These non-thermal effects can accelerate chemical processes and modify material properties.
Microwave technology offers several advantages for LIB recycling, making it an attractive option for recovering valuable materials from spent batteries. Microwave heating enables rapid and uniform heating of materials, reducing processing times and increasing throughput [
21]. Unlike conventional heating methods, which heat materials from the outside in, microwave heating heats materials volumetrically, ensuring uniform temperature distribution. It can selectively heat specific components within LIBs, such as lithium metal oxide, while leaving other materials relatively unaffected. This selective heating allows for targeted processing and enhances the efficiency of recovery processes by minimizing energy consumption and reducing the risk of thermal degradation. The application of microwave radiation can accelerate chemical reactions, including dissolution, leaching, and phase transformations. The rapid heating and high temperatures achieved with microwaves promote faster reaction kinetics, leading to shorter reaction times and improved process efficiency. Microwave-assisted processes generally require lower energy input compared to conventional heating methods, resulting in energy savings and reduced operating costs [
22]. The rapid heating and efficient energy transfer of microwave technology contribute to lower energy consumption and environmental impact.
Microwave technology has been applied to various stages of LIB recycling, including electrode material pretreatment, leaching, and metal recovery. In the context of lithium metal oxide recovery, microwave-assisted processes offer several advantages. Pretreatment of spent electrode materials with microwaves can enhance the liberation of lithium metal oxide and other valuable components [
23]. Microwave irradiation weakens the bonds between active materials and electrode matrices, facilitating their separation and recovery. Microwave-assisted leaching processes involve the dissolution of lithium metal oxide and other metals from spent electrode materials using acidic or alkaline solutions. The rapid heating and enhanced mass transfer kinetics of microwaves accelerate the dissolution of target compounds, leading to higher metal recovery rates. Microwave-assisted pyrolysis can be used to decompose electrode materials at high temperatures, producing a mixture of metals and carbonaceous residues. The selective heating of microwaves promotes the decomposition of organic binders and coatings, allowing for more efficient recovery of lithium metal oxide and other metals. Microwaves can be used to enhance separation processes such as froth flotation and magnetic separation. Selective heating of target compounds improves their liberation and separation from gangue materials, leading to higher purity and recovery rates. Microwave technology offers significant potential for improving the efficiency, selectivity, and sustainability of LIB recycling processes [
24]. By leveraging the advantages of microwave heating, researchers and industry stakeholders can develop innovative solutions for recovering lithium metal oxide and other valuable materials from spent LIBs, contributing to the advancement of a circular economy and the transition towards a more sustainable energy future.
2.1. Froth Flotation for Lithium Metal Oxide Recovery
Froth flotation is a widely used separation technique in mineral processing that exploits differences in surface hydrophobicity to separate minerals from gangue [
25]. In the context of lithium-ion battery (LIB) recycling, froth flotation has been adapted to recover lithium metal oxide from spent electrode materials. This examines the principles of froth flotation, its application in LIB recycling, challenges associated with froth flotation of LIBs, and the integration of microwave technology with froth flotation for lithium metal oxide recovery. Additionally, case studies and experimental results are presented to illustrate the effectiveness of this approach.
Froth flotation relies on the ability of certain minerals to attach to air bubbles and float to the surface of a flotation cell, while others remain in the bulk solution [
11]. The principle of froth flotation can be summarized as follows. Spent electrode materials are first crushed and ground to liberate the lithium metal oxide particles. The ground material is then mixed with water and flotation reagents, including collectors, frothers, and modifiers. Air is introduced into the flotation cell, creating a froth layer on the surface of the pul The air bubbles attach to hydrophobic particles, lifting them to the surface and forming a froth layer. Hydrophobic particles, including lithium metal oxide, attach to the air bubbles and are carried to the surface of the flotation cell, where they form a froth layer. Meanwhile, hydrophilic particles remain in the bulk solution. The froth containing the hydrophobic particles is skimmed off the surface of the flotation cell, while the hydrophilic particles are discharged as tailings. The froth containing the desired minerals, including lithium metal oxide, is collected and further processed to concentrate the valuable components [
26].
Froth flotation has been successfully applied to LIB recycling for the recovery of lithium metal oxide from spent electrode materials. The application of froth flotation in LIB recycling involves several steps. Spent LIBs are disassembled, and the electrode materials are crushed and ground to a suitable particle size [
27]. The ground material is then subjected to pretreatment to remove surface contaminants and enhance the hydrophobicity of lithium metal oxide particles. The pretreated electrode material is mixed with water and flotation reagents in a flotation cell. Collectors, such as fatty acids or alkyl sulfates, are added to promote the attachment of air bubbles to hydrophobic particles, while frothers are added to stabilize the froth. Air is injected into the flotation cell, creating a froth layer on the surface of the pulp. Lithium metal oxide particles, which are hydrophobic, attach to the air bubbles and rise to the surface, forming a froth layer. The froth containing lithium metal oxide is skimmed off the surface of the flotation cell and collected for further processing [
28]. Meanwhile, hydrophilic particles, such as graphite and electrolyte residues, are discharged as tailings. The froth containing lithium metal oxide is further processed to concentrate the valuable components, typically through additional flotation stages or other separation techniques.
Despite its effectiveness, froth flotation of LIBs presents several challenges that can affect the efficiency and selectivity of the process. Spent LIBs contain a complex mixture of materials, including lithium metal oxide, graphite, electrolyte residues, and binder materials [
29]. Achieving efficient separation of lithium metal oxide from other components can be challenging due to their similar physical and chemical properties. Surface contamination of electrode materials, such as organic coatings and oxidation layers, can interfere with the attachment of flotation reagents and reduce the hydrophobicity of lithium metal oxide particles. The particle size distribution of electrode materials can vary widely, affecting the efficiency of froth flotation. Fine particles may not attach to air bubbles efficiently, while coarse particles may sink rapidly and be lost in the tailings. The selection of flotation reagents, including collectors and frothers, is critical for achieving efficient separation [
30]. However, finding suitable reagents that selectively target lithium metal oxide while minimizing interference from other components can be challenging.
The integration of microwave technology with froth flotation offers opportunities to enhance the efficiency, selectivity, and sustainability of lithium metal oxide recovery from spent LIBs [
31]. Microwave irradiation can selectively heat lithium metal oxide particles, promoting their detachment from electrode surfaces and enhancing their recovery. The selective heating of microwaves can target lithium metal oxide particles while leaving other materials relatively unaffected, improving selectivity and reducing processing costs. Microwave-assisted pretreatment of electrode materials can promote the liberation of lithium metal oxide and other valuable components. By weakening the bonds between active materials and electrode matrices, microwave irradiation facilitates their separation and enhances recovery rates. Microwave-assisted froth flotation processes require lower energy input compared to conventional methods, resulting in energy savings and reduced environmental impact [
32]. The rapid and efficient heating of microwaves contributes to shorter processing times, leading to increased throughput and lower operating costs.
Several studies have investigated the integration of microwave technology with froth flotation for lithium metal oxide recovery from spent LIBs, yielding promising results. In a study by Liu
et al., 2020 [
33], microwave-assisted flotation was employed to recover lithium cobalt oxide (LiCoO2) from spent LIBs. Microwave pretreatment of electrode materials enhanced the liberation of LiCoO2 particles, resulting in higher recovery rates and purity compared to conventional flotation. Microwave irradiation of flotation pulp improved the hydrophobicity of lithium metal oxide particles, leading to increased flotation efficiency and reduced reagent consumption. Jiang
et al., 2023 [
34] studied the integration of microwave heating with froth flotation for the recovery of lithium nickel cobalt manganese oxide (NCM) from spent LIBs. Microwave-assisted flotation achieved higher recovery rates and selectivity compared to conventional flotation, with reduced energy consumption and processing times. These case studies demonstrate the effectiveness of integrating microwave technology with froth flotation for lithium metal oxide recovery from spent LIBs. The selective heating and enhanced liberation of active materials contribute to improved recovery rates, higher purity, and reduced environmental impact, highlighting the potential of this approach for advancing LIB recycling technologies. Froth flotation is a valuable separation technique for recovering lithium metal oxide from spent LIBs, offering high efficiency and selectivity. However, challenges such as complex feed composition, surface contamination, and particle size distribution can impact the performance of froth flotation in LIB recycling. The integration of microwave technology with froth flotation provides opportunities to address these challenges and enhance the efficiency and sustainability of lithium metal oxide recovery [
35]. By selectively heating lithium metal oxide particles, promoting their liberation, and reducing energy consumption, microwave-assisted froth flotation offers a promising approach for advancing LIB recycling practices. Further research and development in this area are needed to optimize process parameters, scale up operations, and address challenges associated with commercial implementation. With continued innovation and collaboration, microwave-assisted froth flotation has the potential to play a significant role in meeting the growing demand for sustainable battery recycling solutions [
36].
2.2. Magnetic Separation for Lithium Metal Oxide Recovery
The ever-increasing demand for lithium-ion batteries (LIBs) due to the growing popularity of electric vehicles and portable electronics has led to concerns regarding the sustainability of lithium resources and the environmental impact of LIBs disposal [
37]. Recycling of LIBs has emerged as a crucial strategy to address these issues. Magnetic separation, as a promising technique, has gained significant attention in the recycling of LIBs, particularly in the recovery of lithium metal oxides. This paper explores the principles of magnetic separation, its application in LIBs recycling, challenges, and limitations, as well as the potential of microwave technology in enhancing magnetic separation processes.
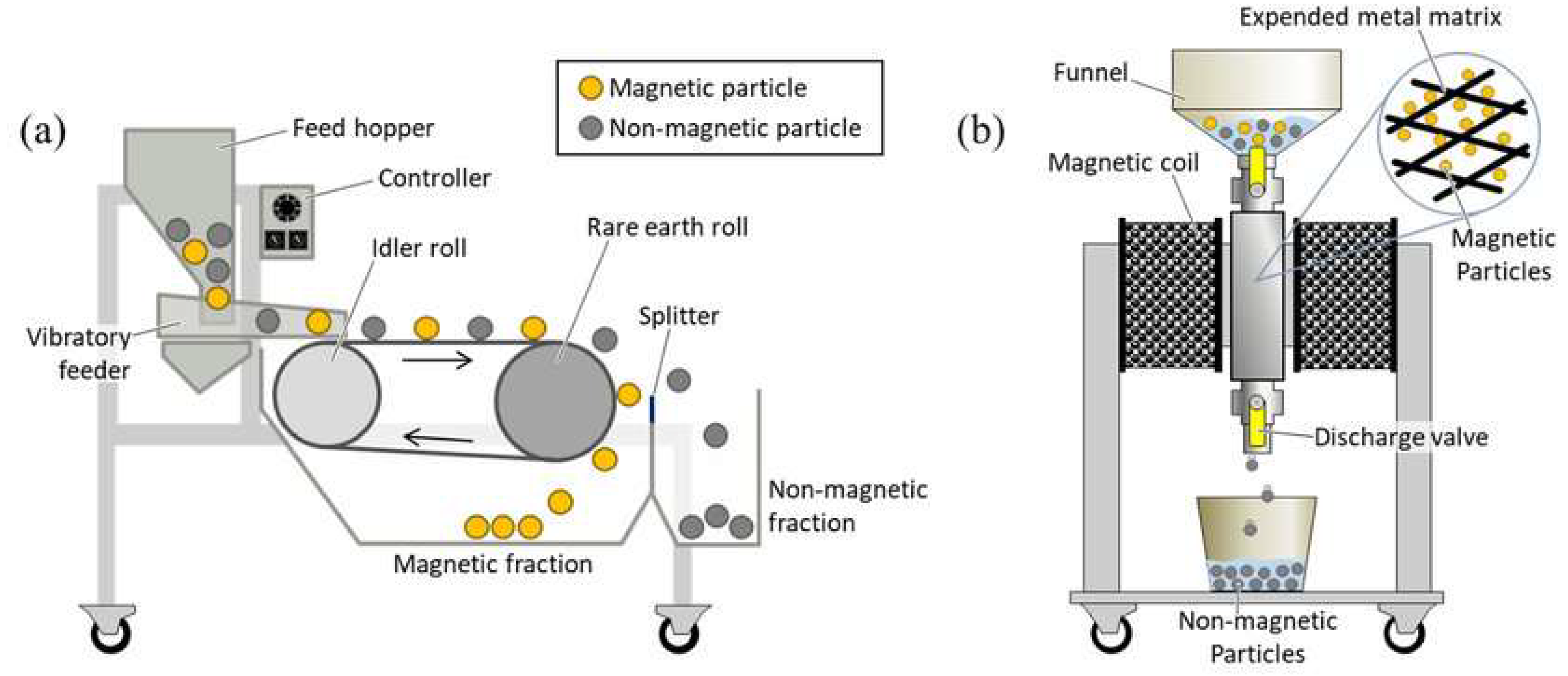
Magnetic separation is a process that utilizes the magnetic properties of materials to separate them from a mixture as illustrated in figure 3 [38, 39]. It is based on the principle that materials with different magnetic properties can be separated by applying a magnetic field. In the context of LIBs recycling, magnetic separation is employed to recover valuable materials such as lithium metal oxides from spent battery materials. The principle of magnetic separation relies on the magnetic susceptibility of materials, which determines their response to a magnetic field. Materials with high magnetic susceptibility are attracted to magnets, while those with low magnetic susceptibility are not. This difference in response allows for the separation of materials based on their magnetic properties. Magnetic separators typically consist of a magnetized material and a non-magnetic material. When a mixture containing magnetic and non-magnetic materials is passed through the magnetic field generated by the separator, the magnetic materials are attracted to the magnetized material and separated from the non-magnetic materials [
40].
In LIBs recycling, magnetic processes play a crucial role in recovering valuable materials, particularly lithium metal oxides, from spent battery materials [
41]. The key steps involved in magnetic processes for LIBs recycling include; Spent battery materials are first crushed and ground to liberate the active materials, including lithium metal oxides, from the electrode materials. The crushed and ground materials are then subjected to magnetic separation to recover the lithium metal oxides. This process involves passing the mixture through a magnetic separator, where the magnetic materials are attracted to the magnetized material and separated from the non-magnetic materials. The recovered lithium metal oxides may undergo further purification steps to remove impurities and improve their quality for reuse in battery manufacturing [
42].
Despite its effectiveness, magnetic separation in LIBs recycling faces several challenges and limitations. Spent battery materials contain a complex mixture of components, including lithium metal oxides, graphite, electrolytes, and other metals [
43]. Separating these components efficiently requires advanced separation techniques. Achieving effective separation depends on the particle size and magnetic properties of the materials. Fine particles or materials with low magnetic susceptibility may be challenging to separate. The efficiency of magnetic separation processes and the yield of recovered materials can vary depending on the operating conditions and the quality of the spent battery materials. Magnetic separation processes may involve the use of chemicals or generate waste, raising concerns about their environmental impact [
44]. The cost of implementing magnetic separation technology in LIBs recycling facilities, including equipment and operating expenses, may affect its economic viability.
Microwave technology has shown promise in enhancing magnetic separation processes in LIBs recycling. The application of microwaves can lead to increased reaction rates, selective heating of target materials, scalability, and cost-effectiveness. Microwave irradiation can accelerate chemical reactions, including the separation of lithium metal oxides from spent battery materials [
45]. The rapid heating induced by microwaves promotes the decomposition of organic binders and facilitates the release of lithium metal oxides, improving the efficiency of magnetic separation. Microwave heating is selective, meaning it heats only the materials with high dielectric loss properties. In LIBs recycling, this selective heating can be utilized to target specific components, such as lithium metal oxides, while minimizing the heating of other materials. This selective heating enhances the efficiency and selectivity of magnetic separation processes. Microwave technology offers scalability and cost-effectiveness for large-scale implementation in LIBs recycling facilities. Compared to conventional heating methods, microwave heating requires less energy and shorter processing times, resulting in reduced operating costs and improved overall efficiency. Magnetic processes, including magnetic separation, play a vital role in the recycling of lithium-ion batteries, particularly in the recovery of valuable materials like lithium metal oxides [
46]. While these processes face challenges and limitations, the integration of microwave technology holds promise for enhancing efficiency, selectivity, and cost-effectiveness in LIBs recycling.
2.3. Combined Microwave-Froth Flotation-Magnetic Processes
The increasing demand for sustainable and efficient processes for material recovery has driven the exploration of combined microwave-assisted techniques [
47]. This focuses on the integration of microwave technology with froth flotation and magnetic processes, highlighting their synergistic effects, benefits, and providing case studies and experimental results to support their efficacy.
Froth flotation is a widely used technique for separating valuable minerals from gangue minerals based on differences in their surface properties. Integration of microwave technology in froth flotation involves the application of microwaves to enhance the separation process by improving the liberation of valuable minerals and increasing the efficiency of bubble-particle collision. Microwaves can heat the mineral particles, weakening their bonds with gangue minerals and facilitating their detachment, leading to improved flotation recovery [
48]. Microwave-assisted froth flotation has been applied in various mineral processing operations, including the recovery of copper, lead, and zinc ores. Magnetic separation utilizes magnetic properties of materials to separate them from a mixture. Integrating microwave technology with magnetic separation involves the application of microwaves to enhance the magnetic properties of target materials or to induce selective heating, improving the efficiency of separation. Microwaves can increase the temperature of magnetic materials, promoting their magnetic susceptibility and facilitating separation. Microwave-assisted magnetic separation has been employed in various fields, including the recovery of magnetic nanoparticles, rare earth elements, and metals from waste materials [
49].
The integration of microwave technology with froth flotation and magnetic processes enhances the selectivity and recovery of valuable materials. Microwaves can selectively heat target minerals or increase their liberation, leading to improved separation efficiency. Combined microwave-assisted processes result in higher recovery rates and purity of recovered materials compared to conventional methods. Microwave-assisted processes require less energy and shorter processing times compared to conventional methods. The rapid and selective heating induced by microwaves reduces the energy consumption and overall processing time, resulting in cost savings and increased productivity. Microwave-assisted processes offer environmental benefits by reducing the consumption of chemicals and minimizing waste generation [
50]. The use of microwaves eliminates the need for some harsh chemicals used in conventional processes, leading to reduced environmental impact. Combined microwave-assisted processes, integrating microwave technology with froth flotation and magnetic separation, offer significant advantages in material recovery. These processes result in enhanced selectivity, reduced energy consumption, and environmental benefits.
2.4. Environmental and Economic Implications of Microwave-Assisted Recycling
Microwave-assisted recycling has emerged as a promising approach for sustainable materials recovery, offering both environmental benefits and economic feasibility [
51]. Microwave-assisted recycling typically requires less energy compared to conventional methods. Microwaves directly heat the target materials, reducing the need for energy-intensive processes such as melting or chemical treatments. This results in lower energy consumption and reduced greenhouse gas emissions. Microwave-assisted recycling often requires fewer chemicals compared to conventional methods [
52]. The rapid and selective heating provided by microwaves can eliminate the need for certain chemical treatments. This reduces the use of hazardous chemicals and minimizes the generation of toxic waste. It improves resource efficiency by enhancing the recovery of valuable materials. The selective heating and rapid reaction rates achieved with microwaves allow for higher recovery rates and purity of recovered materials. This reduces the need for virgin resources and lowers the environmental impact associated with resource extraction. Overall, microwave-assisted recycling offers a reduced environmental footprint compared to conventional methods. By minimizing energy consumption, chemical usage, and waste generation, microwave-assisted recycling contributes to sustainable resource management and environmental conservation [
53].
The initial capital investment for microwave-assisted recycling facilities can be higher compared to conventional recycling facilities. Microwave equipment and specialized reactors may require significant investment [
54]. However, advancements in microwave technology and increased production volumes can lower equipment costs over time. Microwave-assisted recycling often has lower operating costs compared to conventional methods. Reduced energy consumption and fewer chemical treatments result in lower utility and material costs. Additionally, shorter processing times and higher throughput contribute to cost savings. Despite the higher initial investment, microwave-assisted recycling can achieve a lower cost per unit of material processed compared to conventional methods. The increased efficiency and resource recovery lead to higher yields and reduced processing costs. Cost analysis studies have shown that microwave-assisted recycling can be economically feasible, particularly for high-value materials such as precious metals or rare earth elements [
55].
Microwave-assisted recycling is more energy-efficient compared to conventional methods. Conventional methods often involve high-energy processes such as melting or prolonged heating. Microwaves provide rapid and selective heating, minimizing energy losses and reducing overall energy consumption [
56]. Microwave-assisted recycling reduces the need for chemicals compared to conventional methods. Conventional methods may require extensive chemical treatments for leaching, separation, or purification. Microwaves can achieve similar or better results without the use of hazardous chemicals, leading to reduced environmental impact and cost savings. Microwave-assisted recycling enhances resource recovery compared to conventional methods [
57]. Conventional methods may result in lower recovery rates or lower purity of recovered materials. Microwaves enable higher recovery rates and improved purity due to their selective heating and rapid reaction kinetics. Overall, microwave-assisted recycling has a lower environmental impact compared to conventional methods. Reduced energy consumption, minimized chemical usage, and enhanced resource recovery contribute to environmental benefits [
58]. Conventional methods may generate more waste or emissions, leading to greater environmental harm. Microwave-assisted recycling offers significant environmental benefits and economic feasibility compared to conventional recycling methods. By reducing energy consumption, minimizing chemical usage, and enhancing resource recovery, microwave-assisted recycling contributes to sustainable materials management and environmental conservation. Despite the higher initial investment, the lower operating costs and improved efficiency make microwave-assisted recycling economically viable, particularly for high-value materials. Overall, microwave-assisted recycling represents a promising approach for meeting the challenges of resource scarcity and environmental sustainability in materials recycling [
59].
2.5. Future Directions and Challenges
Emerging trends in microwave-assisted recycling of lithium-ion batteries (LIBs) focus on selective recovery of materials, particularly lithium, cobalt, and nickel [
60]. Researchers are exploring innovative approaches to selectively recover these valuable materials from spent battery materials using microwave-assisted techniques [61, 62]. There is a growing emphasis on optimizing microwave-assisted recycling processes to improve efficiency and reduce energy consumption. Researchers are investigating the use of advanced microwave reactors, optimized heating profiles, and novel reactor configurations to enhance process efficiency. Another emerging trend is the integration of microwave-assisted recycling with other techniques such as froth flotation, magnetic separation, and hydrometallurgical processes. Combining microwave technology with complementary techniques can enhance the overall efficiency and selectivity of LIBs recycling [
63]. As microwave-assisted recycling technologies mature, efforts are being made to scale up these processes for commercial implementation. Research and development focus on scaling up microwave-assisted recycling processes while ensuring cost-effectiveness and environmental sustainability.
One of the main challenges in microwave-assisted recycling is achieving uniform heating and temperature control throughout the material. Variations in material composition, particle size, and microwave absorption properties can lead to non-uniform heating, affecting process efficiency and product quality [
64]. Understanding the reaction kinetics and mechanisms involved in microwave-assisted recycling processes is crucial for process optimization. Research gaps exist in elucidating the specific mechanisms by which microwaves interact with materials and promote desired reactions. Scaling up microwave-assisted recycling processes for industrial application presents significant challenges. Ensuring consistent and reliable performance at larger scales while minimizing energy consumption and operating costs requires further research and development. Efficient material handling and reactor design are critical for successful implementation of microwave-assisted recycling. Research is needed to develop optimized reactor designs that can accommodate different types of spent battery materials and ensure uniform heating.
Developing advanced reactor designs tailored for microwave-assisted recycling can address challenges related to uniform heating and scalability [
65]. Novel reactor configurations, such as multi-mode reactors or continuous flow systems, can improve process efficiency and enable scale-up. Computational modeling and simulation techniques can provide insights into the underlying mechanisms of microwave-assisted recycling processes [
66]. Modeling efforts should focus on predicting temperature distribution, reaction kinetics, and material behavior under microwave irradiation. Comprehensive characterization of spent battery materials is essential for optimizing microwave-assisted recycling processes. Research should focus on characterizing material properties such as particle size distribution, composition, and microwave absorption properties. Integration of AI and machine learning algorithms can facilitate process optimization and control in microwave-assisted recycling. AI-based approaches can analyze complex data sets, optimize process parameters, and predict optimal operating conditions. Conducting life cycle assessments of microwave-assisted recycling processes can evaluate their environmental impact and identify areas for improvement. LCA studies should consider the entire life cycle of the recycling process, including energy consumption, emissions, and waste generation. Microwave-assisted recycling of lithium-ion batteries holds great promise for addressing the challenges of resource scarcity and environmental sustainability. Emerging trends focus on selective recovery of materials, process optimization, integration with other techniques, and scale-up for commercialization. However, technological challenges such as uniform heating, reaction kinetics, scalability, and reactor design need to be addressed. Potential solutions include advanced reactor designs, modeling and simulation, materials characterization, integration of AI, and life cycle assessment [
67]. Further research in these areas is crucial for realizing the full potential of microwave-assisted recycling in the future.
3. Conclusion
Microwave-assisted processes offer a promising approach for the recovery of lithium metal oxides from spent LIBs. These processes utilize microwave energy to efficiently and selectively recover valuable materials from battery waste. Its offer several advantages over traditional methods, including shorter processing times, higher yields, lower energy consumption, and reduced environmental impact. Despite the potential benefits, microwave-assisted processes for LIB recycling still face challenges such as scalability, process optimization, and cost-effectiveness. However, ongoing research and development efforts are addressing these challenges and unlocking new opportunities for sustainable battery recycling. Sustainable recycling of lithium-ion batteries is crucial for mitigating environmental impacts, conserving valuable resources, and reducing reliance on virgin materials. Microwave-assisted processes play a significant role in achieving these objectives by enabling efficient recovery of lithium metal oxides. Its processes offer high efficiency and selectivity in recovering lithium metal oxides from battery waste. The selective heating provided by microwaves enables the targeted recovery of valuable materials while minimizing energy consumption and waste generation. By reducing the need for harsh chemicals and long processing times, microwave-assisted processes contribute to lower environmental impacts compared to conventional recycling methods. This aligns with the principles of sustainable development and environmental stewardship. Recovering lithium metal oxides from spent LIBs through microwave-assisted processes helps conserve valuable resources and reduce the reliance on virgin materials. This is crucial considering the increasing demand for lithium-ion batteries in various applications, including electric vehicles and renewable energy storage. Microwave-assisted processes offer economic benefits through reduced processing times, lower energy consumption, and higher yields of valuable materials. This enhances the economic viability of battery recycling operations and encourages the development of a circular economy for lithium-ion batteries.
While microwave-assisted processes show great promise for LIB recycling, there is a need for further research and development to address existing challenges and optimize the technology. Research efforts should focus on scaling up microwave-assisted processes to handle large volumes of battery waste efficiently. This involves optimizing process parameters, such as microwave power, temperature, and reaction time, to maximize yields and minimize energy consumption. Improving the cost-effectiveness of microwave-assisted processes is essential for their widespread adoption in the battery recycling industry. This requires innovative approaches to reduce operating costs, such as the development of cost-effective microwave reactors and the use of alternative microwave-absorbing materials. Ensuring the recovery of high-purity lithium metal oxides is critical for their reuse in battery manufacturing. Research should focus on developing methods to enhance the purity and quality of recovered materials, including the removal of impurities and contaminants. Conducting comprehensive life cycle assessments (LCAs) of microwave-assisted LIB recycling processes is essential to evaluate their environmental impacts and identify opportunities for improvement. LCAs can help identify hotspots in the recycling process and guide efforts to enhance sustainability. Microwave-assisted processes hold great promise for the recovery of lithium metal oxides from spent LIBs, offering efficiency, selectivity, and environmental benefits. However, further research and development are needed to overcome existing challenges and optimize the technology for large-scale implementation. By investing in research and innovation, we can advance sustainable LIB recycling practices and contribute to a cleaner and more sustainable future.
Acknowlegdment: The author wish to appreciate the German Academic Exchange Service, (DAAD) for funding this research.
References
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Del Campo, F.J.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Bashir, T.; Ismail, S.A.; Song, Y.; Irfan, R.M.; Yang, S.; Zhou, S.; Zhao, J.; Gao, L. A review of the energy storage aspects of chemical elements for lithium-ion based batteries. Energy Mater 2021, 1, 100019. [Google Scholar] [CrossRef]
- Akhilash, M.; Salini, S.; John, B.; Mercy, T.D. A journey through layered cathode materials for lithium ion cells–from lithium cobalt oxide to lithium-rich transition metal oxides. J. Alloys Compd. 2021, 869, 159239. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of lithium production and recovery from minerals, brines, and lithium-ion batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Chordia, M.; Nordelöf, A.; Ellingsen, L.A.W. Environmental life cycle implications of upscaling lithium-ion battery production. Int. J. Life Cycle Assess. 2021, 26, 2024–2039. [Google Scholar] [CrossRef]
- Kelly, J.C.; Wang, M.; Dai, Q.; Winjobi, O. Energy, greenhouse gas, and water life cycle analysis of lithium carbonate and lithium hydroxide monohydrate from brine and ore resources and their use in lithium ion battery cathodes and lithium ion batteries. Resour. Conserv. Recycl. 2021, 174, 105762. [Google Scholar] [CrossRef]
- Song, D.; Yu, J.; Wang, M.; Tan, Q.; Liu, K.; Li, J. Advancing recycling of spent lithium-ion batteries: From green chemistry to circular economy. Energy Storage Mater. 2023, 102870. [Google Scholar] [CrossRef]
- Ibrahim, K.B.; Shifa, T.A.; Zorzi, S.; Sendeku, M.G.; Moretti, E.; Vomiero, A. Emerging 2D materials beyond mxenes and TMDs: Transition metal carbo-chalcogenides. Prog. Mater. Sci. 2024, 101287. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: a review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Mondal, S.; Acharjee, A.; Mandal, U.; Saha, B. Froth flotation process and its application. Vietnam J. Chem. 2021, 59, 417–425. [Google Scholar] [CrossRef]
- Nazari, S.; Vakylabad, A.B.; Asgari, K.; Li, J.; Khoshdast, H.; He, Y.; Hassanzadeh, A. Bubbles to batteries: A review of froth flotation for sustainably recycling spent lithium-ion batteries. J. Energy Storage 2024, 84, 110702. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.; Liu, Q. Entrainment of gangue minerals in froth flotation: mechanisms, models, controlling factors, and abatement techniques—a review. Min. Metall. Explor. 2021, 38, 673–692. [Google Scholar] [CrossRef]
- Milian, Y.E.; Jamett, N.; Cruz, C.; Herrera-León, S.; Chacana-Olivares, J. A comprehensive review of emerging technologies for recycling spent lithium-ion batteries. Sci. Total Environ. 2023, 168543. [Google Scholar] [CrossRef]
- You, J.; Qin, Z.; Wang, G.; Rao, M.; Luo, J.; Peng, Z.; Zou, S.; Li, G. Recycling valuable metals from spent lithium-ion battery cathode materials based on microwave-assisted hydrogen reduction followed by grind-leaching and magnetic separation. J. Clean. Prod. 2023, 428, 139488. [Google Scholar] [CrossRef]
- Ahmed, M.D.; Maraz, K.M. Revolutionizing energy storage: Overcoming challenges and unleashing the potential of next generation Lithium-ion battery technology. Mater. Eng. Res. 2023, 5, 265–278. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of lithium-ion batteries—current state of the art, circular economy, and next generation recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Kuterbekov, K.; Kabyshev, A.; Kubenova, M.M.; Bekmyrza, K.Z.; Azad, A.K. Emerging and Recycling of Li-Ion Batteries to Aid in Energy Storage, A Review. Recycling 2023, 8, 48. [Google Scholar] [CrossRef]
- Yadav, A.R.; Mohite, S.K. A brief review: Microwave chemistry and its applications. Res. J. Pharm. Dos. Forms Technol. 2020, 12, 191–197. [Google Scholar] [CrossRef]
- Singh, C.; Khanna, V.; Singh, S. Sustainability of microwave heating in materials processing technologies. Mater. Today: Proc. 2023, 73, 241–248. [Google Scholar] [CrossRef]
- Naik, T.; Singh, I.; Sharma, A.K. Processing of polymer matrix composites using microwave energy: A review. Compos. Part A: Appl. Sci. Manuf. 2022, 156, 106870. [Google Scholar] [CrossRef]
- Radoiu, M.; Mello, A. Technical advances, barriers, and solutions in microwave—assisted technology for industrial processing. Chem. Eng. Res. Des. 2022, 181, 331–342. [Google Scholar] [CrossRef]
- Yan, S.X.; Jiang, Y.Z.; Chen, X.; Yuan, L.; Min, T.T.; Cao, Y.; Peng, W.L.; Zhou, T. Engineering classification recycling of spent lithium-ion batteries through pretreatment: a comprehensive review from laboratory to scale-up application. Rare Met. 2024, 43, 915–941. [Google Scholar] [CrossRef]
- Roy, J.J.; Rarotra, S.; Krikstolaityte, V.; Zhuoran, K.W.; Cindy, Y.D.I.; Tan, X.Y.; Carboni, M.; Meyer, D.; Yan, Q.; Srinivasan, M. Green recycling methods to treat lithium-ion batteries E-waste: a circular approach to sustainability. Adv. Mater. 2022, 34, 2103346. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, S.; Acuña, C.; Vera, M. New technology to improve the recovery of fine particles in froth flotation based on using hydrophobized glass bubbles. Miner. Eng. 2020, 156, 106364. [Google Scholar] [CrossRef]
- Salces, A.M.; Bremerstein, I.; Rudolph, M.; Vanderbruggen, A. Joint recovery of graphite and lithium metal oxides from spent lithium-ion batteries using froth flotation and investigation on process water re-use. Miner. Eng. 2022, 184, 107670. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Xu, C.; Li, X.G.; Deng, C.Z.; Yan, S.; Zhu, X.N. Mixed crushing and competitive leaching of all electrode material components and metal collector fluid in the spent lithium battery. J. Environ. Manag. 2024, 358, 120818. [Google Scholar] [CrossRef]
- Verdugo, L.; Zhang, L.; Saito, K.; Bruckard, W.; Menacho, J.; Hoadley, A. Flotation behavior of the most common electrode materials in lithium ion batteries. Sep. Purif. Technol. 2022, 301, 121885. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. An urgent call to spent LIB recycling: whys and wherefores for graphite recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Kadagala, M.R.; Nikkam, S.; Tripathy, S.K. A review on flotation of coal using mixed reagent systems. Miner. Eng. 2021, 173, 107217. [Google Scholar] [CrossRef]
- Traore, N.; Kelebek, S. Characteristics of spent lithium ion batteries and their recycling potential using flotation separation: A review. Miner. Process. Extr. Metall. Rev. 2023, 44, 231–259. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hafez, I.; Tajvidi, M.; Amirbahman, A. Fundamentals of Hybrid Cellulose Nanofibril Foam Production by Microwave-Assisted Thawing/Drying Mechanism. ACS Sustain. Chem. Eng. 2023, 11, 13240–13250. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Nie, C.C.; Li, X.G.; Shi, S.X.; Gao, Q.; Wang, Y.S.; Zhu, X.N.; Wang, Z. Review on comprehensive recycling of spent lithium-ion batteries: A full component utilization process for green and sustainable production. Sep. Purif. Technol. 2023, 123684. [Google Scholar] [CrossRef]
- Zante, G.; Elgar, C.E.; Hartley, J.; Mukherjee, R.; Kettle, J.; Horsfall, L.; Walton, A.; Harper, G.; Abbott, A. A toolbox for improved recycling of critical metals and materials in low-carbon technologies. RSC Sustainability 2024. [CrossRef]
- Akhmetov, N.; Manakhov, A.; Al-Qasim, A.S. Li-Ion Battery Cathode Recycling: An Emerging Response to Growing Metal Demand and Accumulating Battery Waste. Electronics 2023, 12, 1152. [Google Scholar] [CrossRef]
- Mrozik, W. , Rajaeifar, M.A., Heidrich, O. and Christensen, Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Nithya, R.; Thirunavukkarasu, A.; Sathya, A.B.; Sivashankar, R. Magnetic materials and magnetic separation of dyes from aqueous solutions: a review. Environ. Chem. Lett. 2021, 19, 1275–1294. [Google Scholar] [CrossRef]
- Park, I. , Kanazawa, Y., Sato, N., Galtchandmani,, Jha, M.K., Tabelin, C.B., Jeon, S., Ito, M. and Hiroyoshi, N. Beneficiation of low-grade rare earth ore from Khalzan Buregtei deposit (Mongolia) by magnetic separation. Minerals 2021, 11, 1432. [Google Scholar] [CrossRef]
- Ajibola, O.O. , Adebayo, A.O., Borisade, S.G., Oladimeji, H.T., Oluwalana, E., Daramola, M., Adeleke, A.E. and Omoyeni, D.O. DESIGN AND FABRICATION OF COMBINED ELECTROMAGNETIC AND MAGNETIC DRUM-BELT CONVEYOR SEPARATOR. LAUTECH J. Eng. Technol. 2021, 15, 67–77. [Google Scholar]
- Du, K.; Ang, E.H.; Wu, X.; Liu, Y. Progresses in sustainable recycling technology of spent lithium-ion batteries. Energy Environ. Mater. 2022, 5, 1012–1036. [Google Scholar] [CrossRef]
- Cheng, Q.; Marchetti, B.; Chen, X.; Xu, S.; Zhou, X.D. Separation, purification, regeneration and utilization of graphite recovered from spent lithium-ion batteries-A review. J. Environ. Chem. Eng. 2022, 10, 107312. [Google Scholar] [CrossRef]
- Leal, V.M.; Ribeiro, J.S.; Coelho, E.L.D.; Freitas, M.B.J.G. Recycling of spent lithium-ion batteries as a sustainable solution to obtain raw materials for different applications. J. Energy Chem. 2023, 79, 118–134. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Fahimi, A. , Alessandri, I., Cornelio, A., Frontera,, Malara, A., Mousa, E., Ye, G., Valentim, B. and Bontempi, E. A microwave-enhanced method able to substitute traditional pyrometallurgy for the future of metals supply from spent lithium-ion batteries. Resour. Conserv. Recycl. 2023, 194, 106989. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; Part, F. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef]
- Arpia, A.A.; Chen, W.H.; Lam, S.S.; Rousset; De Luna, M. D.G. Sustainable biofuel and bioenergy production from biomass waste residues using microwave-assisted heating: A comprehensive review. Chem. Eng. J. 2021, 403, 126233. [Google Scholar] [CrossRef]
- Niu, C.; Xia, W.; Li, Y.; Bu, X.; Wang, Y.; Xie, G. Insight into the low-rank coal flotation using amino acid surfactant as a promoter. Fuel 2022, 307, 121810. [Google Scholar] [CrossRef]
- Stein, R.T.; Kasper, A.C.; Veit, H.M. Recovery of rare earth elements present in mobile phone magnets with the use of organic acids. Minerals 2022, 12, 668. [Google Scholar] [CrossRef]
- Luo, Y.; Selvam, E.; Vlachos, D.G.; Ierapetritou, M. Economic and environmental benefits of modular microwave-assisted polyethylene terephthalate depolymerization. ACS Sustain. Chem. Eng. 2023, 11, 4209–4218. [Google Scholar] [CrossRef]
- Alam, S.S.; Khan, A.H. Microwave-assisted pyrolysis for waste plastic recycling: a review on critical parameters, benefits, challenges, and scalability perspectives. Int. J. Environ. Sci. Technol. 2024, 21, 5311–5330. [Google Scholar] [CrossRef]
- Risco, A.; Sucunza, D.; Gonzalez-Egido, S. Chemical recovery of waste electrical and electronic equipment by microwave-assisted pyrolysis: A review. J. Anal. Appl. Pyrolysis 2021, 159, 105323. [Google Scholar] [CrossRef]
- Tuli, V.; Luo, C.; Robinson, B.; Hu, J.; Wang, Y. Microwave-assisted catalytic technology for sustainable production of valuable chemicals from plastic waste with enhanced catalyst reusability. Chemical Engineering Journal 2024, 151551. [Google Scholar] [CrossRef]
- Muley, D. , Wang, Y., Hu, J. and Shekhawat, D., 2021. Microwave-assisted heterogeneous catalysis.
- Dhawan, N.; Tanvar, H. A critical review of end-of-life fluorescent lamps recycling for recovery of rare earth values. Sustain. Mater. Technol. 2022, 32, e00401. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Liu, C.; Mahmoodi, R.; Li, Q.; Naebe, M. Development of a low cost and green microwave assisted approach towards the circular carbon fibre composites. Compos. Part B: Eng. 2020, 184, 107750. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Sanaeepur, H.; Amooghin, A.E.; Shirazi, M.M.A. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.V.N.; Van Le, Q.; Aghbashlo, M. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Siyu, G.; Enhua, D.; Bingguo, L.; Chao, Y.; Yifan, N.; Guangxiong, J.; Wang, C.; Keren, H.; Shenghui, G.; Libo, Z. Eco-friendly closed-loop recycling of nickel, cobalt, manganese, and lithium from spent lithium-ion battery cathodes. Separation and Purification Technology 2024, 127771. [Google Scholar] [CrossRef]
- Zhu, A.; Bian, X.; Han, W.; Wen, Y.; Ye, K.; Wang, G.; Yan, J.; Cao, D.; Zhu, K.; Wang, S. Microwave-ultra-fast recovery of valuable metals from spent lithium-ion batteries by deep eutectic solvents. Waste Manag. 2023, 156, 139–147. [Google Scholar] [CrossRef]
- Raj, B.; Sahoo, M.K.; Sourav, S.; Basu, S.; Mohapatra, M. Recycling and regeneration of energy storage materials from spent lithium-ion batteries (LIBs) through microwave leaching. Ionics 2023, 29, 4365–4377. [Google Scholar] [CrossRef]
- Andrade, D.F.; Castro, J.; Garcia, J.A.; Machado, R.C.; Pereira-Filho, E.R.; Amarasiriwardena, D. Analytical and reclamation technologies for identification and recycling of precious materials from waste computer and mobile phones. Chemosphere 2022, 286, 131739. [Google Scholar] [CrossRef] [PubMed]
- Gamit, D.N.; Chudasama, M.K. Size-effect in microwave processing of engineering materials-A review. J. Mech. Eng. Sci. 2020, 14, 6770–6788. [Google Scholar] [CrossRef]
- Saleem, Q.; Torabfam, M.; Fidan, T.; Kurt, H.; Yuce, M.; Clarke, N.; Bayazit, M.K. Microwave-Promoted Continuous Flow Systems in Nanoparticle Synthesis—A Perspective. ACS Sustain. Chem. Eng. 2021, 9, 9988–10015. [Google Scholar] [CrossRef]
- Bandi, F.; Sulttan, S.; Rohani, S. Modeling and simulation of microwave assisted catalytic pyrolysis system of waste plastics polymer for fuel production. Finite Elem. Anal. Des. 2024, 229, 104073. [Google Scholar] [CrossRef]
- Balbaud, F.; Cabet, C.; Cornet, S.; Dai, Y.; Gan, J.; Mayoral, M.H.; Hernández, R.; Jianu, A.; Malerba, L.; Maloy, S.A.; Marrow, J. A NEA review on innovative structural materials solutions, including advanced manufacturing processes for nuclear applications based on technology readiness assessment. Nucl. Mater. Energy 2021, 27, 101006. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).