Submitted:
11 October 2024
Posted:
13 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Size and Zeta Potential Measurement
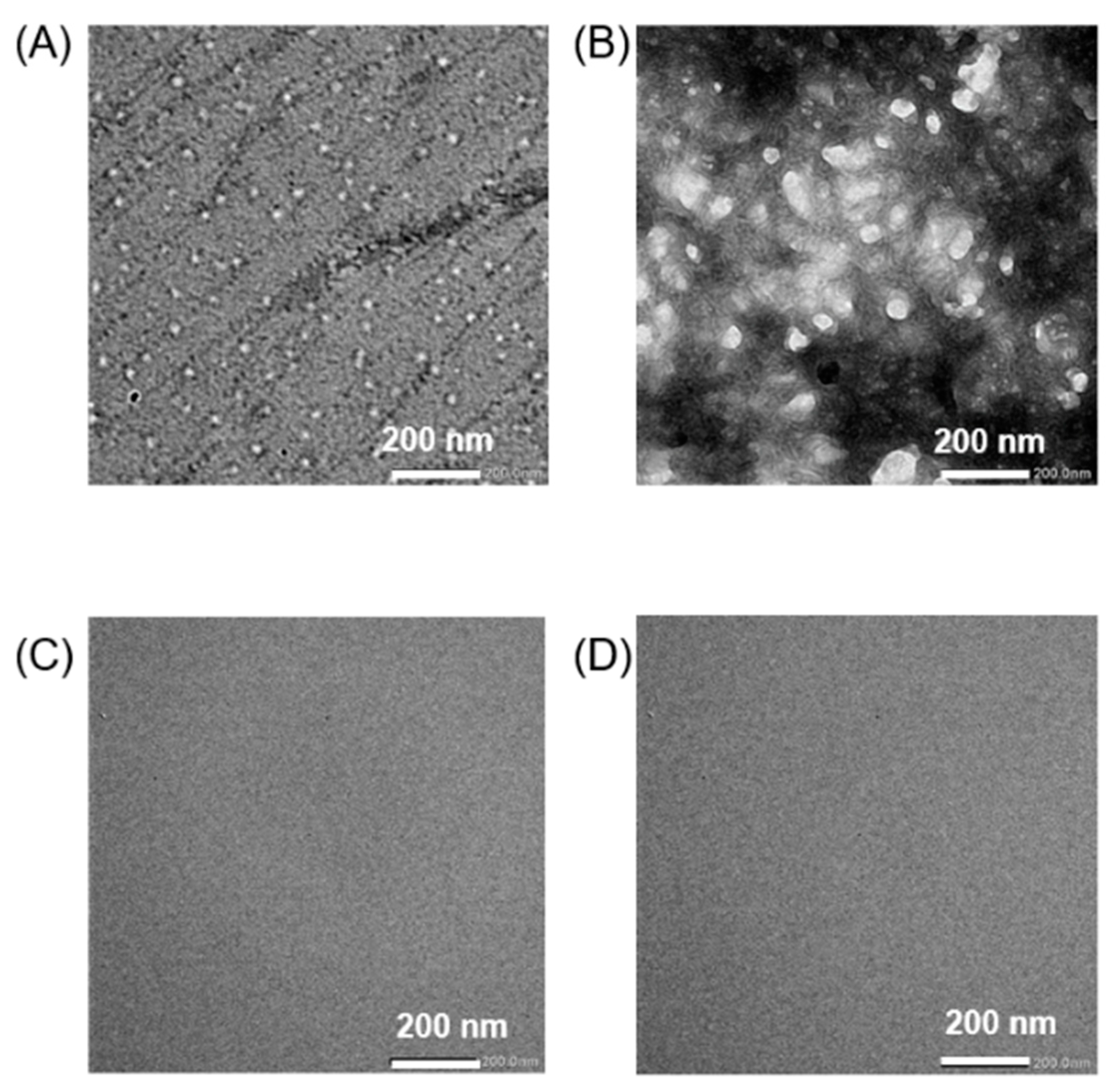
2.3. Transmission Electron Microscopy (TEM) Observations
2.4. ThT Assay
2.5. Naive Polyacrylamide Gel Electrophoresis (Native-PAGE)
3. Results
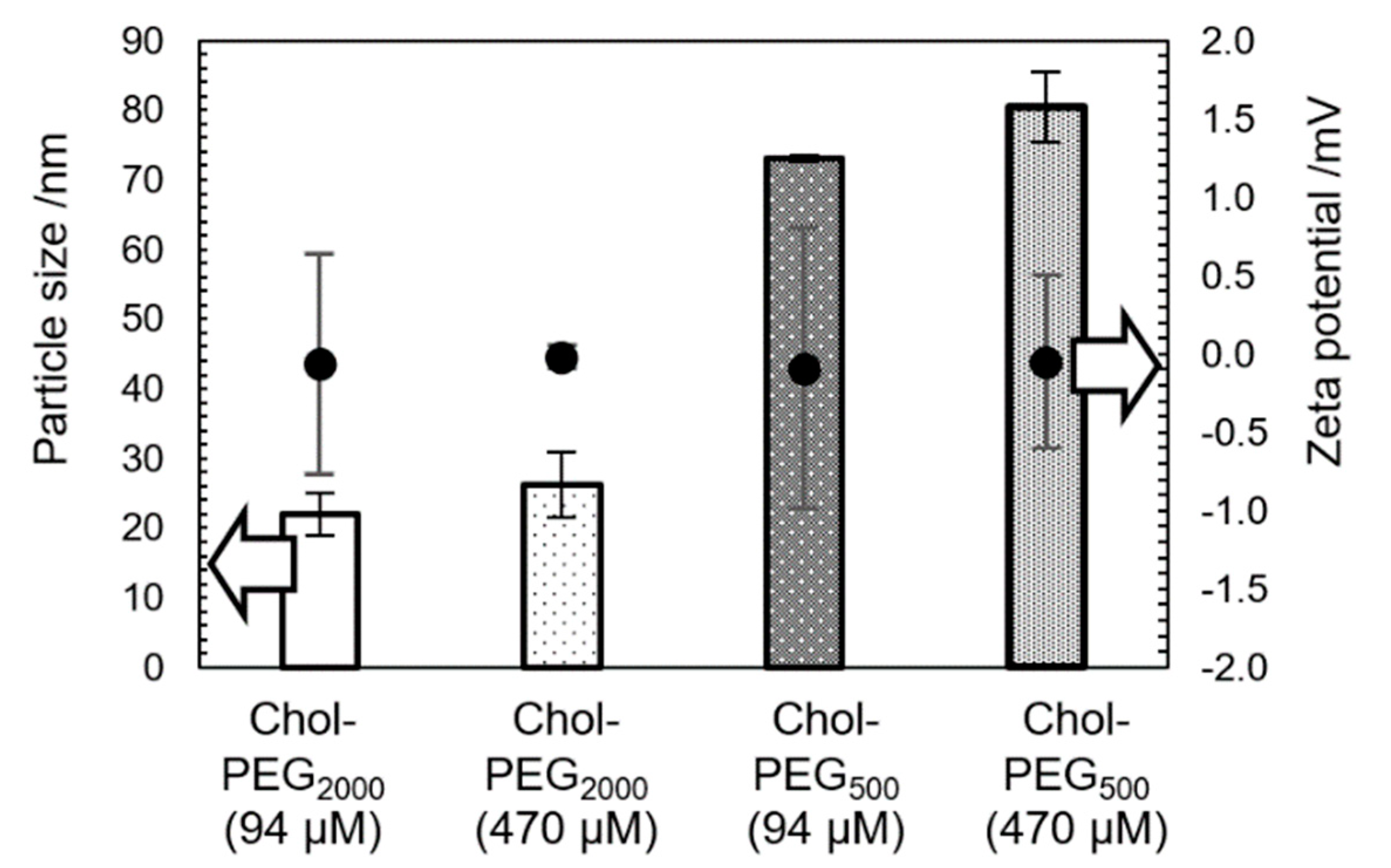
3.1. Physical Properties of Chol-PEG Assemblies
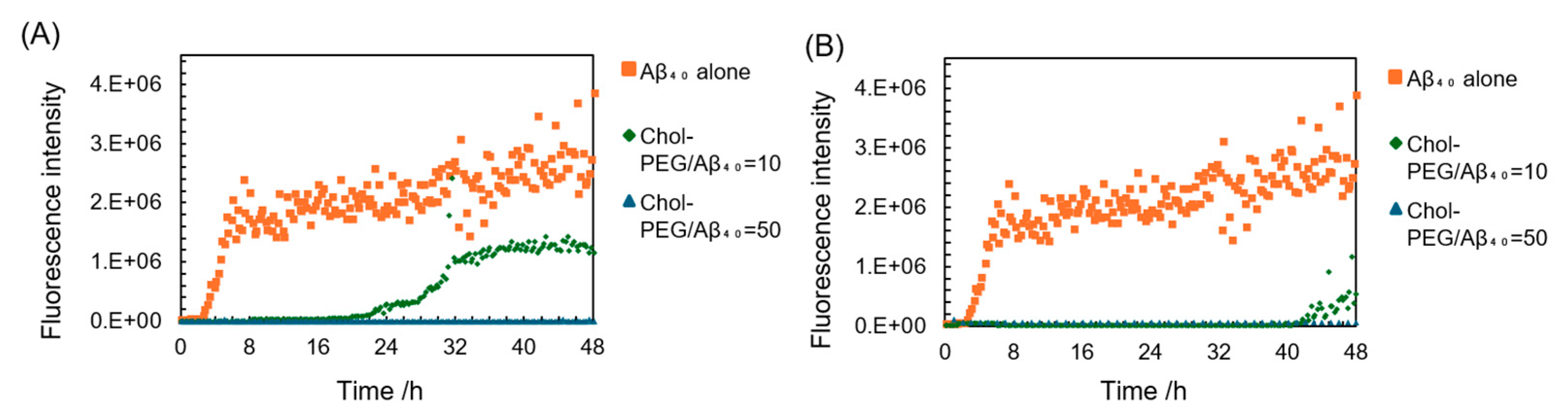
3.2. ThT Assay of Aβ40/Chol-PEG Assemblies
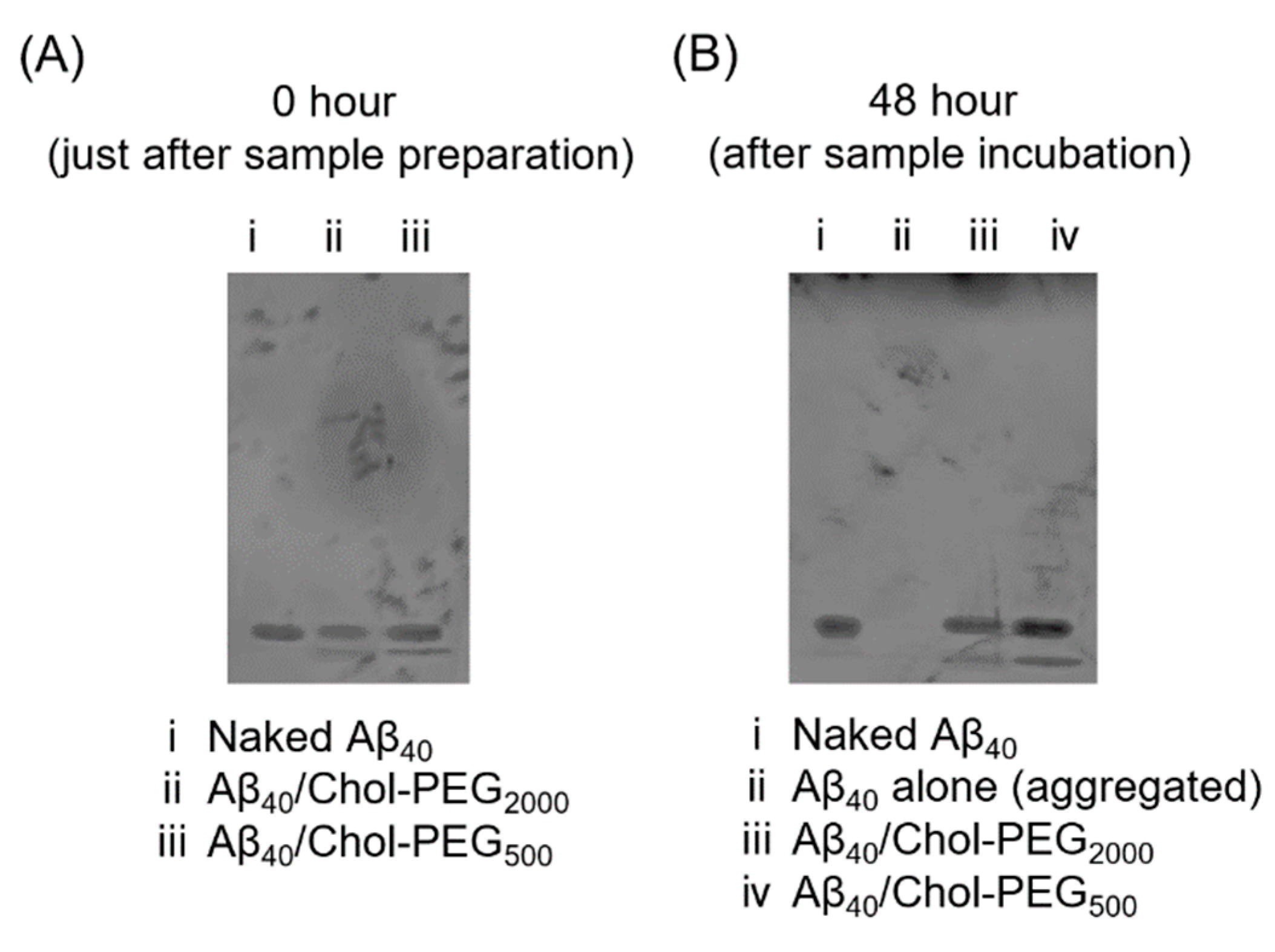
3.3. Aggregation Evaluation Using Polyacrylamide Gel Electrophoresis (Native-PAGE)
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beshir SA, Hussain N, Menon VB, Al Haddad AHI, Al Zeer RAK, Elnour AA, Int J Alzheimers Dis. 2024, 2052142. [CrossRef]
- Hur JY, Exp Mol Med. 2022, 433. [CrossRef]
- Grøntvedt GR, Schröder TN, Sando SB, White L, Bråthen G, Doeller CF, Curr Biol. 2018, R645. [CrossRef]
- Aleksis R, Oleskovs F, Jaudzems K, Pahnke J, Biverstål H, Biochimie. 2017, 176. [CrossRef]
- Leong YQ, Ng KY, Chye SM, Ling APK, Koh RY, Metab Brain Dis. 2020, 11. [CrossRef]
- Choi H, Kim C, Song H, Cha MY, Cho HJ, Son SM, Kim HJ, Mook-Jung I, Aging Cell. 2019, e12872. [CrossRef]
- Carrell RW, Lomas DA, Lancet. 1997, 134. [CrossRef]
- Yamin G, Ono K, Inayathullah M, Teplow DB, Curr Pharm Des. 2008, 3231. [CrossRef]
- Kapadia A, Sharma KK, Maurya IK, Singh V, Khullar M, Jain R, Eur J Med Chem. 2021, 212:113126. [CrossRef]
- Loeffler DA, J Alzheimers Dis Rep. 2023, 7(1):873. [CrossRef]
- Amano A, Sanjo N, Araki W, Anraku Y, Nakakido M, Matsubara E, Tomiyama T, Nagata T, Tsumoto K, Kataoka K, Yokota T, J Nanobiotechnology. 2023, 21(1):36. [CrossRef]
- Xiong H, Callaghan D, Jones A, Walker DG, Lue LF, Beach TG, Sue LI, Woulfe J, Xu H, Stanimirovic DB, Zhang W, Neurobiol Dis. 2008, 29(3):422. [CrossRef]
- Lazar AN, Bich C, Panchal M, Desbenoit N, Petit VW, Touboul D, Dauphinot L, Marquer C, Laprévote O, Brunelle A, Duyckaerts C, Acta Neuropathol. 2013, 125, 1, 133. [CrossRef]
- Ledesma MD, Abad-Rodriguez J, Galvan C, Biondi E, Navarro P, Delacourte A, Dingwall C, Dotti CG, EMBO Rep. 2003, 4, 12, 1190. [CrossRef]
- Egawa J, Pearn ML, Lemkuil BP, Patel PM, Head BP, J Physiol. 2016, 594, 16, 4565. [CrossRef]
- Rushworth JV, Hooper NM, Int J Alzheimers Dis. 2010, 2011. [CrossRef]
- Avdulov NA, Chochina SV, Igbavboa U, Warden CS, Vassiliev AV, Wood WG, J Neurochem. 1997, 69, 4, 746. [CrossRef]
- Panchal M, Loeper J, Cossec JC, Perruchini C, Lazar A, Pompon D, Duyckaerts C, J Lipid Res. 2010, 51, 3, 598. [CrossRef]
- Di Scala C, Chahinian H, Yahi N, Garmy N, Fantini J, Biochemistry. 2014, 53, 28, 4489. [CrossRef]
- Harris JR, Micron. 2008, 8, 1192. [CrossRef]
- Del Mar Martínez-Senac M, Villalaín J, Gómez-Fernández JC, Eur J Biochem. 1999, 265, 2, 744. [CrossRef]
- Ahyayauch H, Masserini ME, Alonso A, Goñi FM, Int J Mol Sci. 2024, 25, 12, 6401. [CrossRef]
- Asayama S, Nagashima K, Negishi Y, Kawakami H, Bioconjug Chem. 2018, 1, 67. [CrossRef]
- Watanabe S, Asayama S, Chemistry Letters. 2024, upae166. [CrossRef]
- Groenning M, J Chem Biol. 2010, 1, 1. [CrossRef]
- LeVine H 3rd, Methods Enzymol. 1999, 309, 274. [CrossRef]
- Batzli KM, Love BJ, Mater Sci Eng C Mater Biol Appl. 2015, 48, 359. [CrossRef]
- Arosio P, Knowles TP, Linse S, Phys Chem Chem Phys. 2015, 17, 12, 7606. [CrossRef]
- Biancalana M, Koide S, Biochim Biophys Acta. 2010, 1804, 7, 1405. [CrossRef]
- Tsuchie Y, Kusuda S, Kawabe H, Mori W, Lindgren M, Watanabe Y, Zako T, Int J Mol Sci. 2024, 25, 6, 3112. [CrossRef]
- Cheung DL, Molecules. 2024, 29, 15, 3634. [CrossRef]
- Qu L, Fudo S, Matsuzaki K, Hoshino T, Chem Pharm Bull. 2019, 67, 9, 959. [CrossRef]
- Ahyayauch H, García-Arribas AB, Masserini ME, Pantano S, Goñi FM, Alonso A, Int J Biol Macromol. 2020, 164, 2651. [CrossRef]
- Lockhart C, Klimov DK, Biochim Biophys Acta. 2016, 1858, 6, 1118. [CrossRef]
- Dey C, Roy M, Ghosh R, Pal P, Roy D, Ghosh Dey S, Chemistry. 2024, e202401531. [CrossRef]
- Salahuddin P, Fatima MT, Abdelhameed AS, Nusrat S, Khan RH, Eur J Med Chem. 2016, 114, 41. [CrossRef]
- Mueller C, Capelle MA, Seyrek E, Martel S, Carrupt PA, Arvinte T, Borchard G, J Pharm Sci. 2012, 101, 6, 1995. [CrossRef]
- Pozzi D, Colapicchioni V, Caracciolo G, Piovesana S, Capriotti AL, Palchetti S, De Grossi S, Riccioli A, Amenitsch H, Laganà A, Nanoscale. 2014, 6, 5, 2782. [CrossRef]
- Yu X, Zheng J, J Mol Biol. 2012, 421, 4, 561. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




