1. Introduction
Anxiety is a psychological state characterized by excessive fear and avoidance behaviors triggered by anticipated threats, which can lead to notable changes in brain structure and function [
1]. Multiple anxiety disorders, including social anxiety disorder, are common and debilitating, substantially affecting individuals' quality of life and social interactions. Social anxiety disorder alone affects approximately 13% of individuals and 4.05% of the global population, representing a significant threat to overall well-being. The treatment of anxiety disorders constitutes over 50% of global mental health expenditure, with a heavy reliance on pharmacotherapy, adding considerable social and economic burdens [
2,
3]. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are common initial treatments; however, these medications can face limitations, including treatment resistance, compliance challenges, and varying social, economic, and cultural factors [
3]. Drug therapy often suffers from issues like suboptimal effectiveness, adverse effects, delayed onset of therapeutic action, and a high risk of relapse. Cognitive Behavioral Therapy (CBT), a non-pharmacological approach that targets the root causes of anxiety, has shown effectiveness but generally requires continuous maintenance to sustain results [
3,
4].
An increasing number of patients are seeking complementary and alternative therapies, such as relaxation techniques, nutritional supplements, massage, and acupuncture, to mitigate medication-related side effects [
5]. Evidence suggests that acupuncture can be beneficial in managing chronic anxiety disorders, particularly when traditional treatments prove inadequate [
6]. Studies report that acupuncture, either as a standalone treatment or combined with medication, may alleviate anxiety symptoms; however, its mechanisms of action remain not fully elucidated [
7,
8].
Recent years have seen numerous randomized controlled trials (RCTs) and systematic reviews that underscore the effectiveness of electroacupuncture in treating anxiety disorders. For instance, a double-blind RCT by Amorim et al. found that electroacupuncture significantly alleviated anxiety symptoms, with the treatment group demonstrating notable reductions in anxiety scores compared to the control group [
9]. Moreover, a systematic review by Li et al. consolidated clinical studies on electroacupuncture, confirming its effectiveness not only in reducing anxiety symptoms but also in enhancing patients' quality of life [
10]. Research involving specific acupoints such as GV20 (Baihui) and GV29 (Yintang) has shown that stimulation of these sites can influence brain regions involved in emotional and stress responses, such as the amygdala, prefrontal cortex, and hypothalamus. This stimulation has been shown to reduce oxidative stress and regulate neural function [
11,
12]. These findings collectively support the clinical application of electroacupuncture, particularly targeting GV20 and GV29, highlighting its potential therapeutic benefits in anxiety management.
Social isolation, limited social connections, and loneliness are closely associated with social anxiety disorders, impacting emotional regulation, physical health, and overall well-being [
13]. Historically, social isolation was prevalent among elderly individuals, children left behind, people with disabilities, low-income earners, and immigrants, often due to limited resources, mobility restrictions, language barriers, and the loss of peer support [
14]. However, Generation Z (Gen Z), born between 1997 and 2012, has experienced heightened social media influence compared to prior generations [
15]. Although social media facilitates communication, studies indicate that it may also negatively impact mental health [
16,
17]. Social media has been shown to foster feelings of loneliness and social exclusion, contributing to social anxiety and isolation within Gen Z [
18,
19]. Consequently, we employed a socially isolated mouse model of anxiety to explore the effects of social isolation, drawing parallels to social anxiety disorder and providing insights for future research and treatment development [
20].
Basolateral amygdala (BLA) neurons are integral to emotional regulation, as they respond acutely to external stimuli and receive input from various brain regions [
21]. The BLA regulates emotional responses and stress by integrating sensory inputs from multiple areas, modulating anxiety-related behaviors and physiological processes [
22]. The amygdala collaborates with the hippocampus, which assists in recognizing and responding to stress, thereby helping the amygdala regulate emotional reactions [
23]. Studies with animal models indicate that activating the neural pathway between the BLA and vCA1 in mice can reduce anxiety during certain tasks [
24]. Furthermore, the medial prefrontal cortex (mPFC) plays a key role in modulating amygdala (AMY) activity, curtailing excessive emotional responses; disruptions in this regulation can lead to increased connectivity between the mPFC and AMY, potentially contributing to maladaptive responses and anxiety symptoms under sustained stress [
25]. Research highlights the BLA’s role in detecting threat signals from the environment and controlling the brain's endocannabinoid signaling, particularly 2-AG, which is essential for stress management and adaptation [
26]. Chronic stressors, such as social isolation, activate the BLA, which subsequently engages the HPA axis, triggering stress responses that may lead to inflammation, metabolic disturbances, and the progression of anxiety and other psychiatric disorders [
27,
28]. Optogenetic and chemogenetic studies of the BLA have demonstrated that modulating its activity can elicit anxiety-like behaviors [
29,
30]. Electrophysiological studies confirm heightened BLA activity in response to anxiety-provoking stimuli, corresponding with observable anxiety behaviors [
31]. Functional MRI studies have similarly shown elevated BLA activity in individuals with anxiety disorders, with activity levels positively correlated with symptom severity [
32]. Lesion studies have further illustrated that BLA damage leads to reduced anxiety-like behaviors, underscoring its pivotal role in anxiety regulation [
33].
The brain’s high oxygen demand renders it particularly susceptible to oxidative stress-related damage [
34]. Oxidative stress disrupts cellular integrity by damaging membranes, proteins, and DNA, leading to neuronal dysfunction and apoptosis [
35]. Regions crucial for emotion and memory, like the BLA, are especially vulnerable; oxidative stress can activate microglia, promoting the release of pro-inflammatory cytokines that impair neuronal function, synaptic plasticity, and signal transmission [
36]. Studies reveal that oxidative stress in the AMY/BLA region can induce anxiety symptoms, underscoring its significance in anxiety pathophysiology [
37].
NOX2, an enzyme in the nervous system, is a primary source of oxidative stress, producing superoxide anions and hydrogen peroxide that are pivotal in cellular signaling and immune response [
38]. Chronic stress, such as social isolation, upregulates NOX2 in microglia, leading to reactive oxygen species (ROS) production and potentially contributing to neurodegeneration [
36]. Excessive NOX2 activity is associated with ROS overproduction, triggering oxidative damage, neuronal dysfunction, synaptic toxicity, and cellular death, which ultimately disrupts the excitatory-inhibitory balance in neurons [
35,
37]. NOX2 upregulation under chronic stress has also been closely associated with changes in anxiety behaviors, highlighting its role in anxiety-related oxidative stress (
Figure 1) [
39].
Microglia, as primary immune cells in the central nervous system, maintain vigilance in their resting state, detecting ROS signals and participating in neuroinflammation [
38]. Upon recognizing pathogens or cellular damage, microglia release pro-inflammatory cytokines and oxidants like hydrogen peroxide to protect neurons. NOX2 expression in microglia underscores their role as regulators of oxidative stress within the brain [
36]. Under chronic stress, upregulated NOX2 activates microglia, leading to ROS overproduction and promoting neuroinflammation. This cycle of oxidative stress and microglial activation can exacerbate neuronal damage and inflammatory responses, contributing to anxiety and other psychiatric conditions under prolonged pathological conditions [
40].
This study aims to bridge the current knowledge gap by examining the effects of EA on microglial morphology and function in the BLA, a critical brain region for anxiety regulation. Using a social isolation-induced anxiety model in mice, we assessed NOX2 expression, microglial activation, and oxidative stress markers in the BLA to elucidate the role of microglia in EA’s anxiolytic effects. Our findings offer new insights into EA's cellular mechanisms and underscore its potential in modulating neuroinflammation and oxidative stress in the context of anxiety disorders.
2. Materials and Methods
2.1. Animals and Materials
Male C57BL/6 mice (8-10 weeks old, 20–25 g) were purchased from Guangzhou ZhiYuan Biopharmaceutical Technology Co., Ltd. Mice were randomly assigned to groups and housed in a standard laboratory environment with controlled temperature, humidity, bedding, food, water, and a 12-hour light/dark cycle. All animal procedures followed the approved protocol of the Animal Care and Use Committee of Guangzhou University of Traditional Chinese Medicine, adhering to ARRIVE guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A seven-day acclimatization period was provided before the experiments commenced.
2.2. Antibodies
The antibodies used in this study for Western Blot analysis included mouse anti-gp91 antibody (BDB611414) from BD Biosciences (USA) and mouse anti-β-ACTIN antibody (AF5003) and HRP-conjugated goat anti-mouse secondary antibody (AF0208) from Beyotime Biotech (China). For immunofluorescence, mouse anti-gp91 antibody (BDB611414) from BD Biosciences (USA), rabbit anti-IBA1 antibody (019-19741) from Wako (Japan), CoraLite488-labeled goat anti-mouse IgG(H+L) (SA00013-1), and CoraLite594-labeled goat anti-rabbit IgG(H+L) (SA00013-4) from Proteintech (China) were used.
2.3. Social Isolation
Social isolation is widely recognized for inducing anxiety- and depression-like behaviors in rodents [
40,
41]. In this study, a total of 48 male C57BL/6 mice (8-10 weeks, 20–25 g) were used, with 12 mice allocated to each group. After a seven-day acclimation, mice in the social isolation group were housed individually for four weeks, while those in the control group were housed in groups of 4-5 mice per cage. Weekly cage cleaning was the only human interaction. The groups were as follows:
Control Group: Mice housed in groups of four per cage with ad libitum access to food and water for four weeks, followed by anesthesia.
Control Group with EA Treatment: Group-housed mice with ad libitum access to food and water for four weeks, followed by anesthesia and EA treatment.
Social Isolation Group: Mice individually housed for four weeks with ad libitum access to food and water, followed by anesthesia.
Social Isolation Group with EA Treatment: Mice individually housed for four weeks with ad libitum access to food and water, followed by anesthesia and EA treatment.
2.4. Electroacupuncture (EA)
EA treatments were conducted daily at a set time, generally between 8 pm and 10 pm, corresponding to the nighttime activity period of the mice. To minimize stress, the EA treatment room was prepared 30 minutes before treatment. Mice were anesthetized with isoflurane, administered through a gas anesthesia machine (RWD Medical Co., Ltd., Shenzhen, China), with a 3% isoflurane induction at a flow rate of 1.0–1.5 L/min and maintenance at 1.5% isoflurane at a rate of 0.4–0.8 L/min. Anesthesia depth was monitored by observing hind limb muscle tone and body twitching, adjusting as necessary to ensure minimal discomfort.
Once anesthesia was stabilized, sterile needles (0.25 mm diameter, 13 mm length) were inserted horizontally into the GV20 and GV29 acupoints on the head, corresponding to traditional treatment sites for emotional disorders in humans. EA was applied with a 1 mA current at 2 Hz using a HANS stimulator (HANS-200A/100 B, Beijing, China) for 30 minutes on alternate days over four weeks. Control group mice were anesthetized without EA.
2.5. Anxiety Behavior Test
The Elevated Plus Maze (EPM) and Open Field Test (OFT) were used to assess anxiety behaviors 24 hours after the final treatment. Testing was conducted between 8 pm and midnight to align with mice's active period and minimize circadian influences. Open Field Test (OFT): Mice were habituated to the testing room for 1 hour every 3 days before testing. For the OFT, mice were placed in a 45 cm × 45 cm × 45 cm cubic testing box with a brightly illuminated 25 cm × 25 cm central zone. Mice were allowed 10 minutes of free exploration per trial, after which droppings were removed, and the box was cleaned with 75% ethanol to eliminate residual scents. Time spent and distance traveled in the central area were recorded and analyzed using LabState software.
2.6. Elevated Plus Maze (EPM)
The Elevated Plus Maze test was conducted following a standard protocol in an acoustically isolated room with consistent lighting. The maze was configured in a “+” shape, with two open arms and two closed arms, and was elevated 50 cm above the floor. In cases where a mouse fell, the trial was restarted after the previous round. Each mouse was initially placed in a 5 × 5 cm square facing an open arm and allowed to explore for 5 minutes. After each trial, excrement was removed, and the maze was sanitized with 75% ethanol to eliminate olfactory cues. The Any-maze software (Shanghai Xiansoft Information Technology Co., Ltd.) was used to analyze the time spent in the open arms.
2.7. DHE Experiment
Oxidative stress was assessed using two methods: quantification of reactive oxygen species (ROS) through dihydroethidium (DHE) staining and evaluation of lipid peroxidation and antioxidant status via malondialdehyde (MDA) and superoxide dismutase (SOD) measurements, respectively. These analyses provide insights into the extent of cellular damage caused by oxygen free radicals [
42]. For DHE detection, mice were maintained in a non-stressed state before intraperitoneal injection. DHE was dissolved in DMSO, diluted to 0.1 mg/ml with physiological saline, and administered at a dose of 2 mg/kg. After one hour, mice were anesthetized, perfused with PBS, and their brains were harvested. Brain tissue was embedded in OCT, sectioned into 10 μm slices, and mounted on glass slides. Following a drying phase, the sections were washed in PBS for 15 minutes, then immersed in a glycerol solution for coverslip application. Finally, the slides were sealed and subjected to fluorescence imaging.
2.8. Fluorescence Detection
Laser scanning confocal microscopy was used to detect fluorescence at 594 nm in the brain sections. Fluorescence images were acquired, with three to five brain slices from each region of interest selected for analysis. Average fluorescence intensity was calculated using ImageJ software based on three to four slices from each of three to five groups. Data standardization and processing were subsequently performed.
2.9. MDA/SOD Detection
The levels of MDA and SOD in the mouse amygdala were measured using an MDA detection kit (A003-1, Nanjing Jiancheng) according to the manufacturer’s protocol. Amygdala tissue was collected, homogenized in lysis buffer, and incubated with reagents at 95°C for 30 minutes. Following centrifugation, the absorbance was measured at 532 nm using a UV spectrophotometer to determine the MDA content.
2.10. Western Blot Analysis (WB)
Prior to Western Blot analysis, sterilized Eppendorf tubes were prepared and weighed to calculate tissue weight. Tissue samples were collected and placed in Eppendorf tubes kept on ice. RIPA lysis buffer and a protease-phosphatase inhibitor cocktail were added to the tubes. Samples were lysed for 30 minutes, homogenized using an ultrasonic processor, and centrifuged at 4°C for 15 minutes. Protein concentrations were determined with a BCA protein assay kit. SDS-PAGE loading buffer was added, and the mixture was heated before loading onto an 8% Gly SDS-PAGE gel for separation. Proteins were then transferred to PVDF membranes. The membranes were washed with TBST and blocked with a quick-blocking buffer. Primary antibodies targeting gp91 and ACTIN were applied for immunostaining. Following incubation with secondary antibodies, the membranes were rinsed and treated with ECL solution. Protein blotting was visualized using a chemiluminescence detection system.
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was employed to quantify NOX levels in tissue samples using a detection kit. Tissues were mixed with reagents per the manufacturer’s protocol and disrupted by ultrasound. Following disruption, samples were centrifuged at 4°C and 600 × g for 5 minutes to extract the supernatant, which was further centrifuged at 4°C and 11,000 × g for 10 minutes to obtain the cytosolic fraction. The precipitate was then mixed with reagents 2 and 3 from the kit, repeatedly agitated, and distributed into the measurement and control wells of a 96-well plate following the manufacturer’s instructions (Solarbio, China). Absorbance was initially recorded at 600 nm for 20 seconds using a microplate spectrophotometer (BioTek, USA).
2.12. Immunofluorescence Chemical Analysis (IF)
Brain tissue was embedded in Tissue-Tek OCT compound and sectioned using a freezing microtome. The sections were stored in freezing solution at -20°C. After staining, sections were washed with PBS and blocked with a solution containing bovine serum albumin (BSA) and Triton X-100. Primary antibodies (anti-NOX2 and anti-Iba1) were diluted in the blocking solution and incubated with the sections overnight. The following day, sections were washed with PBS containing Tween-20 and incubated with secondary antibodies. After washing with PBS-T, sections were mounted onto microscope slides with DAPI for nuclear staining. They were then air-dried, sealed with a glycerol/PBS solution, and stored at -20°C until imaging. Fluorescence imaging was performed using a laser confocal microscope.
2.13. Confocal Microscope Fluorescent Imaging
Confocal laser-scanning microscopy was used to capture Z-stack images at 20x magnification with a consistent setting (40 µm Z-stack, 2 µm intervals, Nikon A1). Four groups, each containing three animals, were included in the experimental design. Brain slices from specific BLA regions were obtained, and four random slices per animal were selected for imaging. Any slices containing impurities were excluded. This resulted in 48 images, showing the colocalization of gp91 and IBA1. Image analysis was conducted using Fiji (ImageJ) with suitable plugins. For additional analysis of microglial activation, 40x magnification images were captured from the AMY-BLA region. A total of 36 images were captured and analyzed using Fiji.
2.14. Skeletal Analysis of Microglia Cells
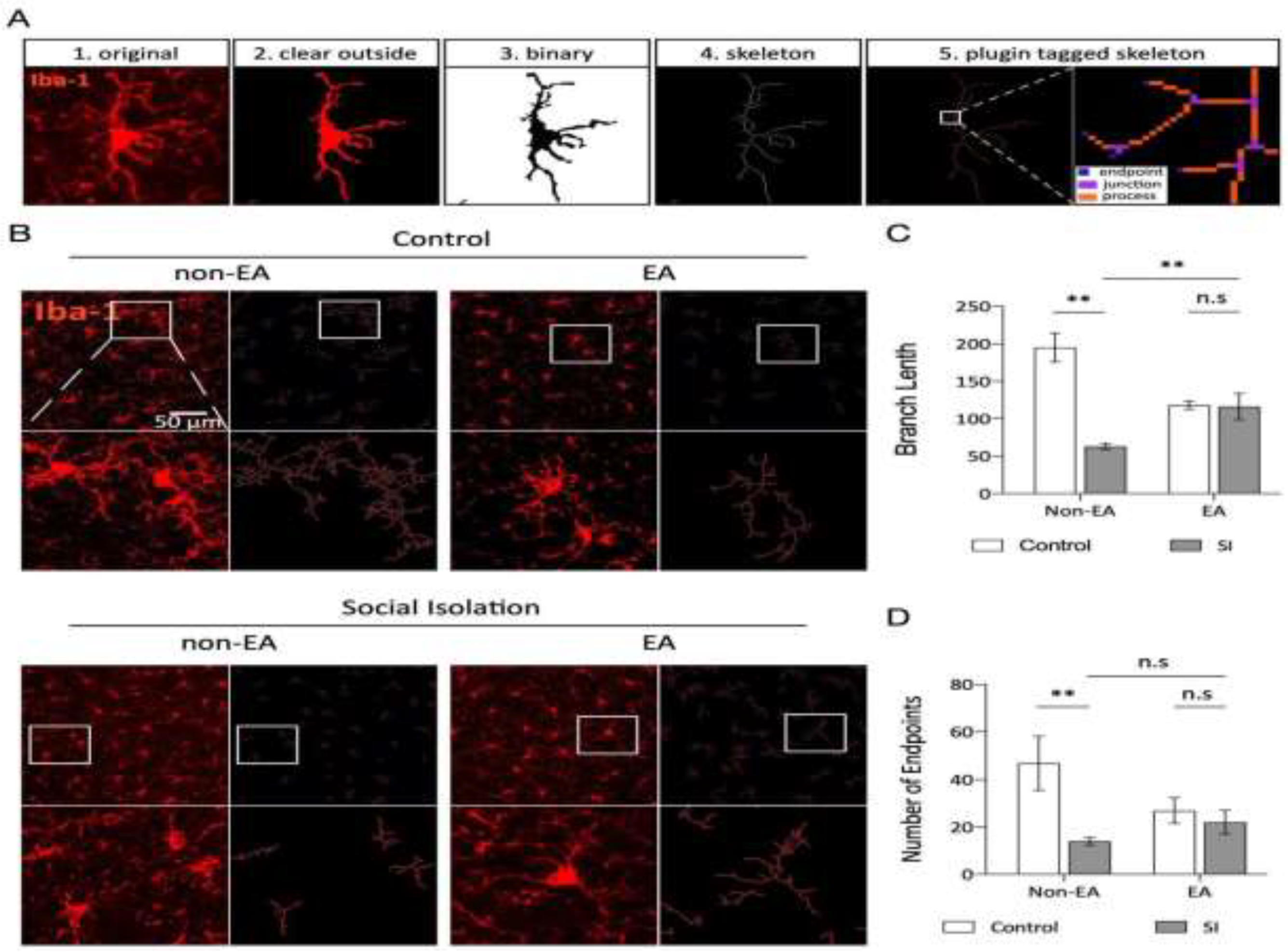
To analyze microglial morphology, appropriate parameters were set in the filter menu. The "despeckle" function removed salt-and-pepper noise from the image, followed by conversion to binary format with adjusted threshold settings. The “despeckle,” “close,” and “outlier” functions were applied to further reduce background noise. Functions were accessed via: Process > Noise > Despeckle, Process > Binary > Close, and Process > Noise > Remove Outliers. The final binary image isolated microglial cells, enabling skeletal analysis. Microglial cells were quantified by analyzing particles within a 40-infinity area size range. Using the ROImanager tool, all identified microglial cells were designated as “594.” After duplicating the backup image, ROI594 was marked, and Edit/Clear Outside was applied to remove the background and retain the microglial cells for analysis. Images were saved, binarized, and skeletonized. The “Skeleton” plugin was used to assess the number of branches and endpoints in microglial cells across the BLA region.
2.15. Data Analysis and Visualization
Data analysis was performed using SPSS version 29.0, and data visualization was achieved with GraphPad Prism version 9.0. Rigorous statistical procedures were followed, with quantitative data presented as mean ± SEM. Independent-sample t-tests were employed for pairwise comparisons. For multiple group comparisons under normal distribution and variance assumptions, the LSD test or Bonferroni correction was applied. When these assumptions were unmet, Tamhane’s test or the Kruskal-Wallis test was used. Statistical significance was set at p < 0.05 for pairwise comparisons and p < 0.01 for multiple group comparisons.
4. Discussion
Anxiety disorders are increasingly prevalent, particularly among Generation Z, due to rising social pressures and mental health challenges [
15]. While current pharmacological treatments can be effective, they often come with significant side effects, and their long-term efficacy remains uncertain. This has driven interest in non-drug therapies, such as electroacupuncture (EA), which has shown promise in treating mental health conditions, including anxiety [
7,
8]. However, the specific neurobiological mechanisms behind EA’s therapeutic effects remain underexplored.
Our study investigated the effects of EA in a social isolation-induced anxiety model in mice, providing new insights into the neurobiological pathways affected by EA. We found that EA significantly reduced anxiety-like behaviors, as evidenced by increased time spent in the open arms of the elevated plus maze and in the center of the open field test. These behavioral improvements were accompanied by reductions in oxidative stress markers, including NOX2 and malondialdehyde (MDA), and an increase in superoxide dismutase (SOD) activity. This suggests that EA may exert its anxiolytic effects, at least partially, through modulation of oxidative stress pathways within the basolateral amygdala (BLA), a region central to emotional regulation.
Recent research increasingly implicates oxidative stress in the pathophysiology of anxiety disorders [45]. Reactive oxygen species (ROS), such as those generated by NOX2, can impair neuronal function and plasticity by promoting lipid peroxidation and protein oxidation, leading to synaptic dysfunction and neurotransmitter dysregulation [46]. Our findings align with this body of work, showing that EA treatment reduced ROS production and restored oxidative balance, likely contributing to the observed reduction in anxiety-like behaviors. This result is consistent with studies that highlight the role of antioxidants like SOD in counteracting oxidative damage and alleviating anxiety symptoms [47,48].
The observation that EA treatment alleviated both oxidative stress and anxiety-like behaviors in this model suggests that oxidative stress modulation may be a key mechanism by which EA improves anxiety-related behaviors. Although multiple members of the NADPH oxidase family are associated with ROS generation, NOX2 is particularly relevant within the central nervous system, especially in the context of microglial activation and immune responses [
38]. Evidence suggests that NOX2 expression is strongly linked to anxiety progression [
39]. Microglia, the primary immune cells of the central nervous system, play a crucial role in neurological diseases, particularly through processes involving oxidative stress and inflammation [
38].
To further explore the relationship between NOX2 and microglia in anxiety, we conducted immunofluorescence colocalization experiments. In the social isolation (SI) group, compared to the control, we observed a significant increase in colocalization of NOX2 with microglia, an effect that was reduced following EA treatment (
Figure 4). This supports a strong association between anxiety and NOX2 overexpression in microglia. Additionally, we examined microglial cell counts across treatment and control groups. While the SI group exhibited an increase in microglial cells compared to the control, there was no significant difference in cell numbers between the SI + EA and SI groups or between the control + EA and control groups. Thus, EA treatment reduced NOX2 expression associated with microglial activation without affecting microglial proliferation.
Research indicates that chronic stress and molecular patterns associated with pathogens and damage (PAMPs and DAMPs, respectively) can accelerate microglial proliferation [
40,49]. Although EA has been shown to reduce neuroinflammation-related cognitive decline by inhibiting microglial proliferation in some studies [50,51], microglial function is closely associated with activation phenotype rather than cell count alone [
43,52]. For example, in an Alzheimer’s disease study, the treatment group showed reduced microglial activation with anti-inflammatory effects despite similar cell counts between groups [53]. In studies where the R47H gene, associated with microglial activation, was knocked out, gene mutation effects on microglial count were minimal [54]. Another study on sleep found that fragmented sleep affects microglial activation morphology through transcriptional regulation of specific genes, rather than by changing cell numbers [55].
These findings underscore the importance of understanding the specific activation states of microglia to elucidate their functional roles in anxiety and response to EA. This study contributes valuable insights into the potential mechanisms of EA, suggesting that it may reduce anxiety through modulation of oxidative stress and microglial activity in the BLA, providing a promising non-pharmacological approach for anxiety management.
Overall, these studies emphasize the complex relationship between microglial cell count, function, and activation status. While some research suggests that inhibiting microglial proliferation may alleviate neuroinflammation, other studies indicate that pathways regulating gene expression and activation states significantly impact microglial function, independent of changes in cell numbers. We considered the variability in microglial cell numbers across disease models and brain regions, along with reactive changes in microglia in response to various interventions, all of which may influence the extent of microglial activation induced by stressors.
Microglial number and morphological changes are linked to several neurological diseases, with morphology closely tied to activation status [
43]. Through colocalization experiments, we observed activated microglia, suggesting that chronic stress may increase microglial numbers while EA therapy potentially alleviates anxiety by modulating microglial morphology rather than inhibiting proliferation. To test this hypothesis, we conducted skeletal analysis of microglial morphology across groups. Our findings showed that EA increased branch length and endpoints in microglia without significantly altering cell count, indicating primarily adaptive morphological changes in response to EA rather than fluctuations in quantity (
Figure 4). This suggests that EA does not trigger an excessive immune response, as microglial stability is linked to controlled immune activation. The increase in branch length implies that EA may enhance microglial surveillance and coverage, supporting local neuroenvironmental regulation and neuronal function. These morphological adjustments likely improve immune monitoring and resource allocation, enhancing overall cell regulation, as supported by several studies [56,57].
Social and psychological stress activates microglia and is associated with inflammation and oxidative stress. However, the interactions among oxidative stress, inflammation, and neural cells during stress events remain unclear. Oxidative stress directly impacts nervous system function and can also activate inflammatory pathways within the nervous system, prompting immune cells, such as microglia, to release inflammatory cytokines. These cytokines affect neuronal function and may contribute to anxiety-like behaviors [
36,
37].
Prolonged or severe stress, such as social isolation, activates the HPA axis and stress response, leading to increased oxidative stress. This response releases stress hormones like cortisol, resulting in excess free radicals that damage neuronal structures, causing synaptic dysfunction and disrupting neurotransmitter balance and neural network integrity [
35,
36]. Whether oxidative stress acts as a cause or consequence of anxiety-like psychological stress, chronic stress compounds oxidative stress and inflammation [58]. The resulting vicious cycle among oxidative stress, inflammation, and anxiety exacerbates symptoms and may lead to additional health issues.
4.1. Study Limitations
While our study provides valuable insights into the mechanisms by which EA may reduce anxiety, there are certain limitations to consider. First, translating findings from animal models to human anxiety disorders presents challenges due to differences in neuroanatomy and behavioral responses. Second, although we observed changes in oxidative stress levels and microglial morphology, the precise molecular pathways through which EA exerts these effects remain unclear. Future research should aim to elucidate these pathways, potentially utilizing advanced techniques such as RNA sequencing or proteomics to reveal specific signaling mechanisms.
4.2. Practical Application Value and Future Directions
Our findings suggest that EA holds promise as a therapeutic approach for anxiety disorders, particularly for individuals who are resistant to traditional pharmacological treatments. With increasing interest in non-drug therapies for mental health conditions, EA could serve as a complementary or alternative treatment option, offering fewer side effects than conventional treatments. However, further research, including human clinical trials, is necessary to confirm these findings and to investigate the long-term effects of EA on anxiety and other stress-related disorders.
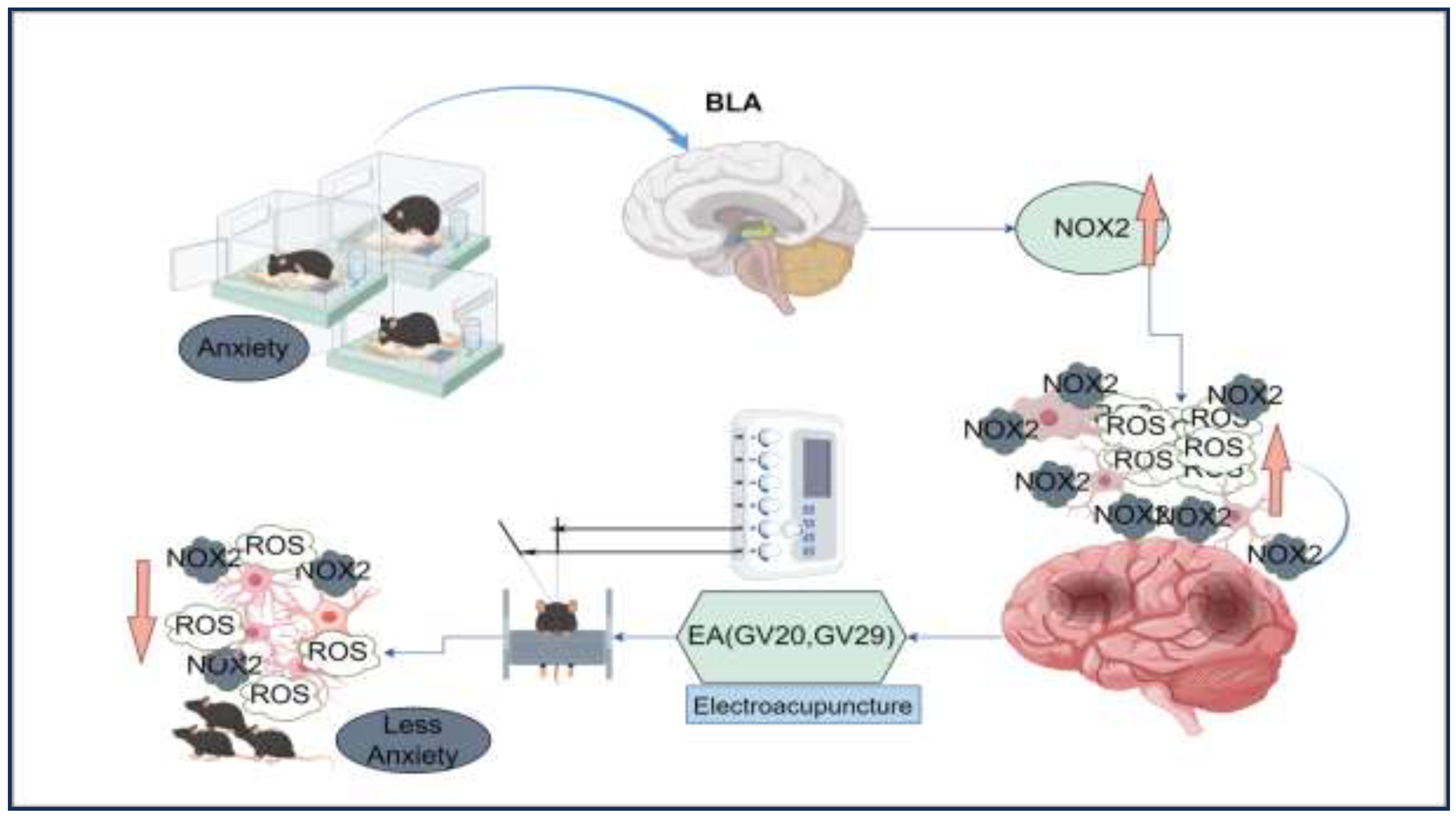
Figure 1.
Proposed
Mechanism of Electroacupuncture in Modulating Oxidative Stress and Microglial
Activity Induced by Social Isolation.
Figure 1.
Proposed
Mechanism of Electroacupuncture in Modulating Oxidative Stress and Microglial
Activity Induced by Social Isolation.
Figure 2.
EA treatment improved social isolation-induced anxiety-like behavior. A. Graphical representation of activity traces in each group of mice during the open field test (OFT). B. Tracking of activity patterns in each group of mice in the elevated plus maze (EPM). C. The total distance traveled in the open field test (OFT) and the total time spent by grouped mice in the central area. The results of the two-way ANOVA indicated no significant main effect of EA (F (1, 44) = 0.0074, p = 0.9318) or SI (F (1, 44) = 0.5093, p = 0.4792) on the total distance traveled in the OFT. Additionally, there was no significant interaction between EA and SI (F (1, 44) = 1.942, p = 0.1705). In contrast, the analysis of time spent in the center revealed a significant main effect of EA (F = 24.92, p < 0.001) and SI (F = 5.338, p = 0.0256) and a significant interaction between the two factors (F = 4.124, p = 0.0483) in a sample size of n=12. The results suggest that EA had a significant impact on the time spent in the center, whereas social isolation did not have a significant effect. Total time spent on the EPM open-arm activity. EA×SI two-way ANOVA: main effect of EA, F (1,44) =17.89, p<0.001; main effect of SI, F (1,44)=3.696, p=0.061. Interaction: F (1,44) =5.051, p=0.0297; n=12; ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001. D. Total time spent on EPM open-arm activity. EA×SI two-way ANOVA:main effect of EA, F (1,44) =17.89,P<0.001;main effect of SI, F(1,44)=3.696,P=0.061.Interaction: F (1,44) =5.051, P=0.0297; n=12; ns, not significant.
Figure 2.
EA treatment improved social isolation-induced anxiety-like behavior. A. Graphical representation of activity traces in each group of mice during the open field test (OFT). B. Tracking of activity patterns in each group of mice in the elevated plus maze (EPM). C. The total distance traveled in the open field test (OFT) and the total time spent by grouped mice in the central area. The results of the two-way ANOVA indicated no significant main effect of EA (F (1, 44) = 0.0074, p = 0.9318) or SI (F (1, 44) = 0.5093, p = 0.4792) on the total distance traveled in the OFT. Additionally, there was no significant interaction between EA and SI (F (1, 44) = 1.942, p = 0.1705). In contrast, the analysis of time spent in the center revealed a significant main effect of EA (F = 24.92, p < 0.001) and SI (F = 5.338, p = 0.0256) and a significant interaction between the two factors (F = 4.124, p = 0.0483) in a sample size of n=12. The results suggest that EA had a significant impact on the time spent in the center, whereas social isolation did not have a significant effect. Total time spent on the EPM open-arm activity. EA×SI two-way ANOVA: main effect of EA, F (1,44) =17.89, p<0.001; main effect of SI, F (1,44)=3.696, p=0.061. Interaction: F (1,44) =5.051, p=0.0297; n=12; ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001. D. Total time spent on EPM open-arm activity. EA×SI two-way ANOVA:main effect of EA, F (1,44) =17.89,P<0.001;main effect of SI, F(1,44)=3.696,P=0.061.Interaction: F (1,44) =5.051, P=0.0297; n=12; ns, not significant.
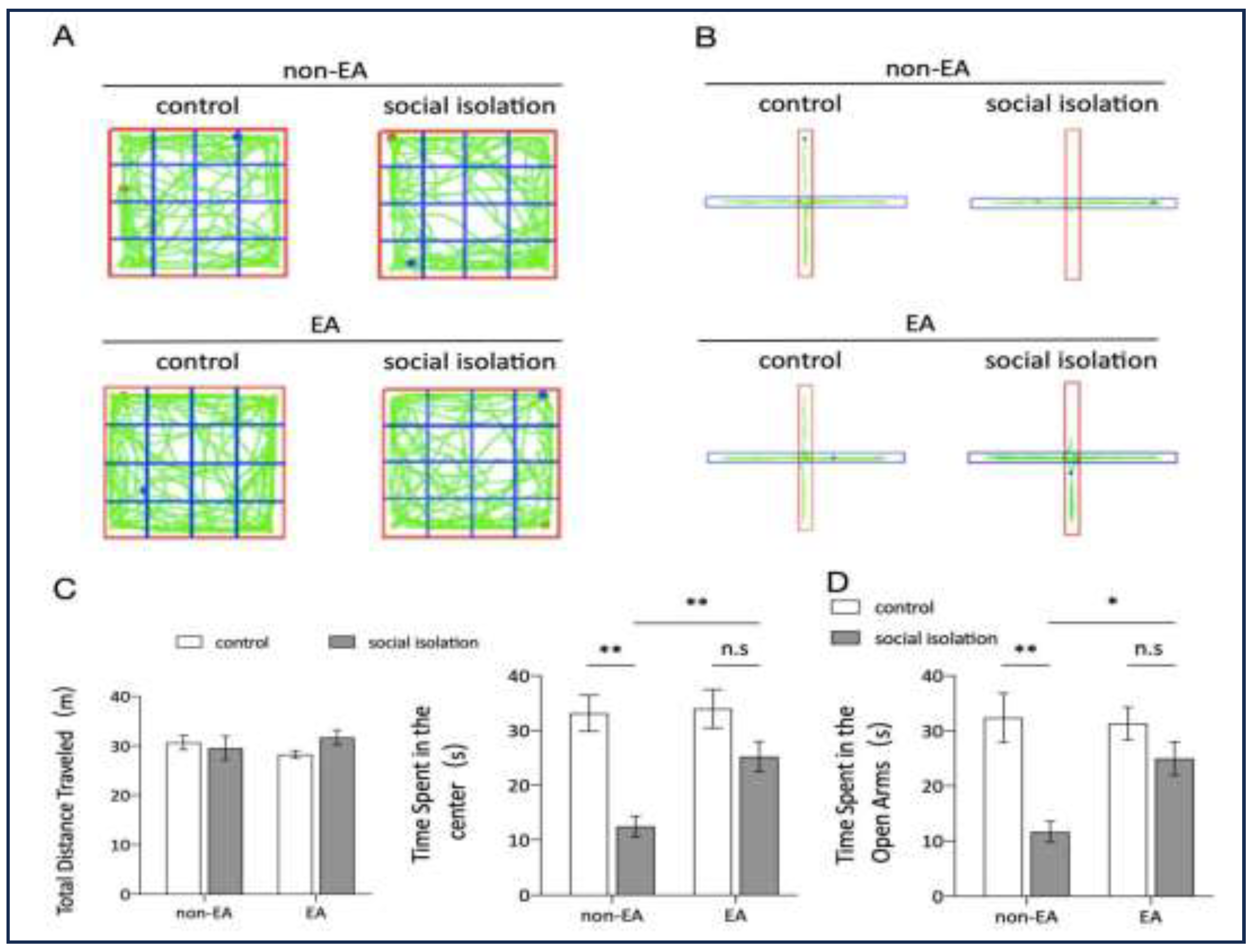
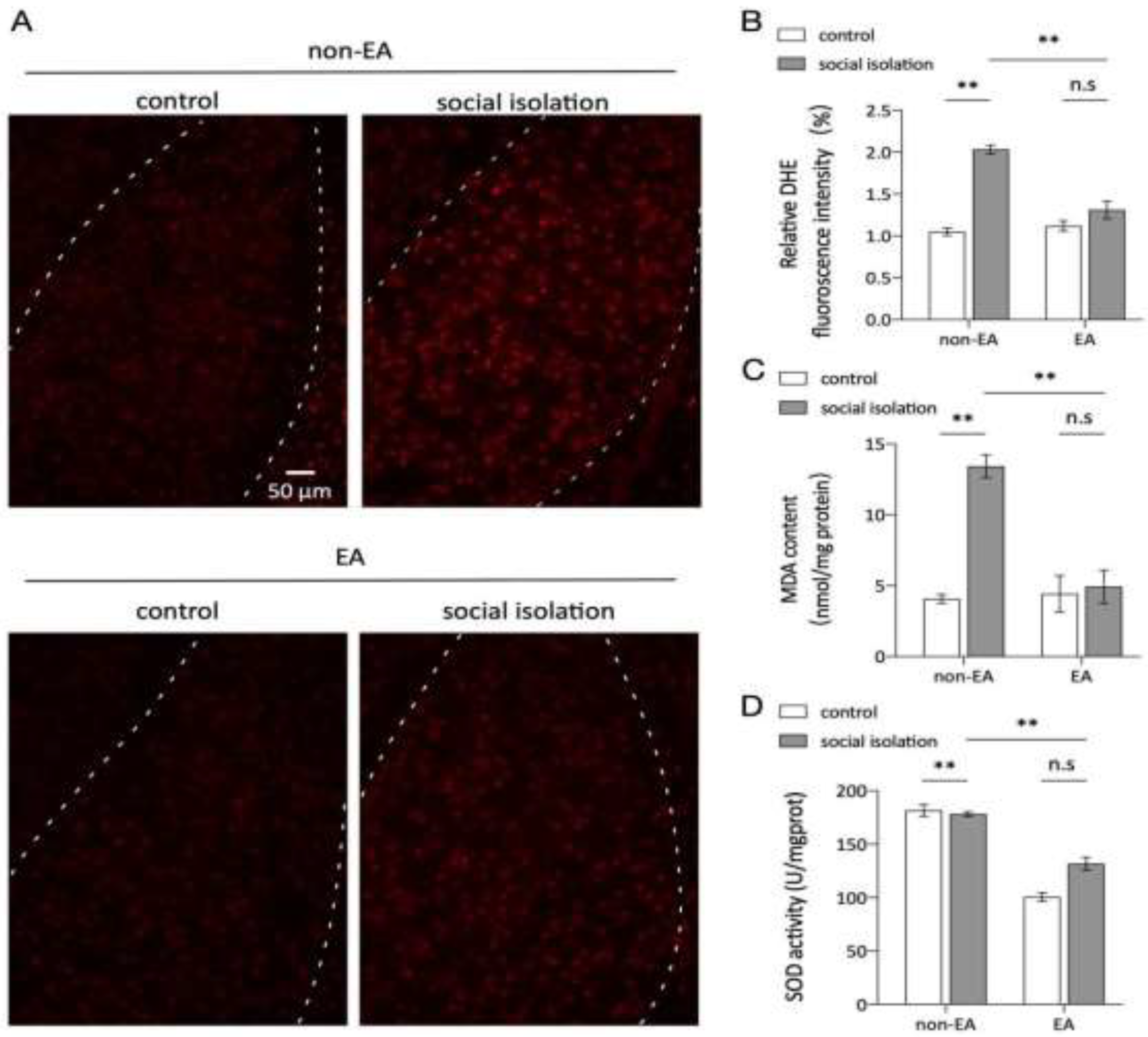
Figure 3.
EA reduced oxidative stress caused by social isolation in the amygdala. A. DHE fluorescence intensity in the amygdala region of the brain for each mouse group B. Mean fluorescence density in the amygdala for each group. Two-way ANOVA results: main effect of EA: F(1,8) =20.93, p=0.0018; main effect of SI: F(1,8) =68.58, p <0.0001; interaction effect: F(1,8) =30.91, p =0.0005; n=3 ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001. C. MDA content in the amygdala of each mouse group. Two-way ANOVA results: main effect of EA, F(1.8) =17.79, p =0.0029; main effect of SI, F(1,8) =26.09, p =0.0029; interaction effect, F(1,8) =21.01, p =0.001; n=3; n.s: not significant. D. Statistics of SOD content in the amygdala of each mouse group. Two-way ANOVA: Main effect of EA: F(1,8) =186.8 p <0.0001; Main effect of SI:F(1,8) =8.825 p =0.0179; Interaction effect: F(1.8) =13.58, p =0.0062 n=3;ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 3.
EA reduced oxidative stress caused by social isolation in the amygdala. A. DHE fluorescence intensity in the amygdala region of the brain for each mouse group B. Mean fluorescence density in the amygdala for each group. Two-way ANOVA results: main effect of EA: F(1,8) =20.93, p=0.0018; main effect of SI: F(1,8) =68.58, p <0.0001; interaction effect: F(1,8) =30.91, p =0.0005; n=3 ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001. C. MDA content in the amygdala of each mouse group. Two-way ANOVA results: main effect of EA, F(1.8) =17.79, p =0.0029; main effect of SI, F(1,8) =26.09, p =0.0029; interaction effect, F(1,8) =21.01, p =0.001; n=3; n.s: not significant. D. Statistics of SOD content in the amygdala of each mouse group. Two-way ANOVA: Main effect of EA: F(1,8) =186.8 p <0.0001; Main effect of SI:F(1,8) =8.825 p =0.0179; Interaction effect: F(1.8) =13.58, p =0.0062 n=3;ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 4.
EA inhibits oxidative stress responses in microglia linked to the basolateral amygdala. A. Confocal stack three-dimensional rendering showing NOX2 (green) and IBA1 (red) in the BLA region of the amygdala. White arrows indicate colocalization. Scale bar, 100µm. B-D. Quantitative analysis of IBA1 puncta density (d), NOX2 puncta density, and IBA1-NOX2 colocalization density (n=3 mice per group, one-way ANOVA). Representative protein blots for NOX2 and β-actin are shown. N=1/Group. E. Quantitative analysis of the protein expression ratios for NOX2 and β-actin F. Two-way ANOVA results: Main effect of EA: F(1,8)=5.845 p =0.0420 The Main effect of SI: F(1,8)=14.81,p =0.0049; Interaction effect: F(1,8)=7.910,p =0.0228; n=1 ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.F. ELISA measurements of total NOX activity in each group. Two-way ANOVA results: Main effect of EA: F(1,8)=0.0072 p =0.9345 The Main effect of SI: F(1,8)=67.86, p <0.01; interaction effect: F(1,8)=6.992, p <0.01; n=3,ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 4.
EA inhibits oxidative stress responses in microglia linked to the basolateral amygdala. A. Confocal stack three-dimensional rendering showing NOX2 (green) and IBA1 (red) in the BLA region of the amygdala. White arrows indicate colocalization. Scale bar, 100µm. B-D. Quantitative analysis of IBA1 puncta density (d), NOX2 puncta density, and IBA1-NOX2 colocalization density (n=3 mice per group, one-way ANOVA). Representative protein blots for NOX2 and β-actin are shown. N=1/Group. E. Quantitative analysis of the protein expression ratios for NOX2 and β-actin F. Two-way ANOVA results: Main effect of EA: F(1,8)=5.845 p =0.0420 The Main effect of SI: F(1,8)=14.81,p =0.0049; Interaction effect: F(1,8)=7.910,p =0.0228; n=1 ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.F. ELISA measurements of total NOX activity in each group. Two-way ANOVA results: Main effect of EA: F(1,8)=0.0072 p =0.9345 The Main effect of SI: F(1,8)=67.86, p <0.01; interaction effect: F(1,8)=6.992, p <0.01; n=3,ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.
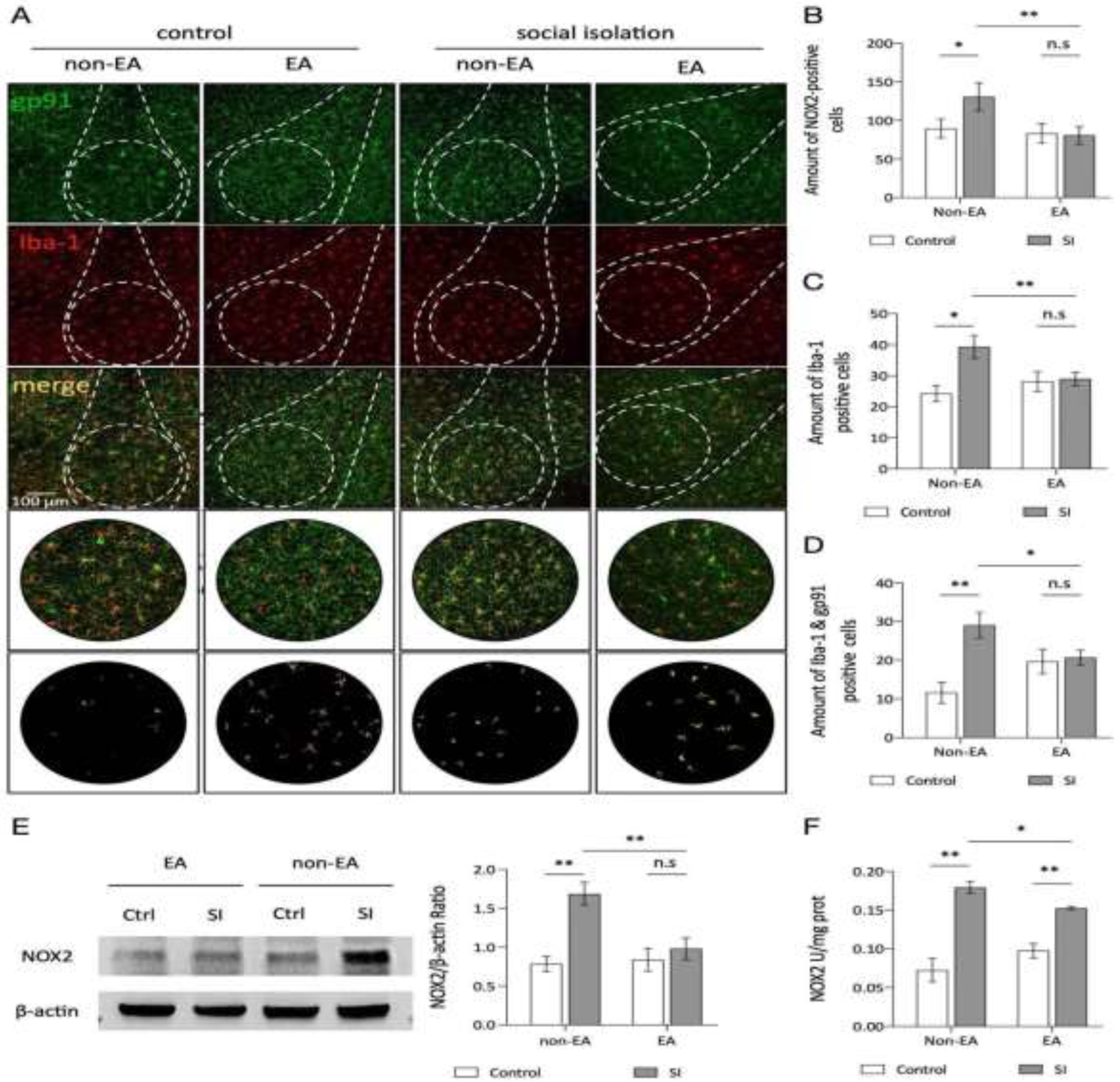
Figure 5.
EA ameliorates abnormal microglial morphology in the BLA induced by social isolation. Bone analysis of the micrographs. All bone analyses were performed on full-size micrographs (scale =50 µm) using a series of uniform ImageJ plug-in protocols, as detailed in the Methods section. The rules for identification and marking are as follows: the branch lengths are in orange, the endpoints are in blue, and the branch points are in purple. The labeled data were analyzed, summarized, and output. Original microglia and skeletonized images of the BLA region in each group of mice. C-D. Statistical analysis of the mean branch lengths and endpoints in the amygdala basolateral nucleus (BLA) region involved quantification of the number of microglia normalized per skeletal data analysis field. This analysis calculated the branch lengths per cell and endpoints per cell to assess morphological changes in microglia within this region. Statistical analysis also revealed a significant interaction effect of branch length between SI and EA (2way ANOVA: SI: F (1,38) =24.65, p <0.0001; EA: F (1,38) =0.7935, p=0.3786; Interaction: F (1,38) =23.33, p <0.0001). Interaction of endpoints between SI and EA (2way ANOVA: SI: F (1,38) =9.333, p=0.0041; EA: F (1,38) =0.9299, p=0.3410; Interaction: F (1,38) =5.089, p=0.0299). ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 5.
EA ameliorates abnormal microglial morphology in the BLA induced by social isolation. Bone analysis of the micrographs. All bone analyses were performed on full-size micrographs (scale =50 µm) using a series of uniform ImageJ plug-in protocols, as detailed in the Methods section. The rules for identification and marking are as follows: the branch lengths are in orange, the endpoints are in blue, and the branch points are in purple. The labeled data were analyzed, summarized, and output. Original microglia and skeletonized images of the BLA region in each group of mice. C-D. Statistical analysis of the mean branch lengths and endpoints in the amygdala basolateral nucleus (BLA) region involved quantification of the number of microglia normalized per skeletal data analysis field. This analysis calculated the branch lengths per cell and endpoints per cell to assess morphological changes in microglia within this region. Statistical analysis also revealed a significant interaction effect of branch length between SI and EA (2way ANOVA: SI: F (1,38) =24.65, p <0.0001; EA: F (1,38) =0.7935, p=0.3786; Interaction: F (1,38) =23.33, p <0.0001). Interaction of endpoints between SI and EA (2way ANOVA: SI: F (1,38) =9.333, p=0.0041; EA: F (1,38) =0.9299, p=0.3410; Interaction: F (1,38) =5.089, p=0.0299). ns: not significant. *p < 0.05, **p < 0.01, ***p < 0.001.