Introduction
The androgen receptor (AR) signaling pathway is pivotal in the development of prostate cancer (PCa) [
1]. Several innovative therapies targeting AR have shown substantial overall survival benefits in both hormone-sensitive and castration-resistant PCa [2-8]. Despite advances in androgen receptor signaling inhibitors, resistance to these agents inevitably develops.
Polo-like kinase 1 (PLK1), a serine/threonine kinase crucial for cell-cycle progression, is overexpressed in PCa [
9]. Its association with higher-grade tumors suggests its involvement in tumorigenesis and progression. Mounting evidence indicates that PLK1 activity is linked to resistance to various therapies and plays an important role in therapy resistance in PCa [10-11]. PLK1 phosphorylates CLIP-170 and p150Glued, two microtubules plus end-binding proteins, enhancing microtubule dynamics and resulting in docetaxel resistance [
12]. PLK1-mediated activation of the PI3K/AKT/mTOR pathway leads to AR signaling activation, counteracting the effects of abiraterone and enzalutamide [
13]. Thus, PLK1 elevation/activation appears to be a general mechanism for treatment resistance in PCa. Inhibition of PLK1 could enhance anti-neoplastic activity [14, 15]. Notably, a new orally bioavailable PLK1 inhibitor, onvansertib, is currently undergoing early-phase clinical trials in select tumors [16-18]. There is an ongoing phase II clinical trial (NCT03414034) testing the combination of onvansertib and abiraterone acetate in men with metastatic castration-resistant PCa who have early resistance to abiraterone [
19].
In light of this, we evaluated mRNA-PLK1 expression as a prognostic marker by correlating PLK1 expression with clinicopathological characteristics and survival, and assessed the correlation between PLK1 mRNA expression and clinical outcomes in prostate cancer patients treated with abiraterone/enzalutamide or docetaxel. In this study, we used RNA-seq PLK1 expression from patients with all stages of prostate adenocarcinoma using the Oncology Research Information Exchange Network (ORIEN) and correlated it with survival outcomes. We hypothesized that high PLK1 overexpression by RNA-seq is associated with high Gleason grade and negatively impacts overall survival, leading to poor survival outcomes in patients treated with abiraterone/enzalutamide and/or docetaxel.
Methods
Study Population and Design
The Oncology Research Information Exchange Network (ORIEN) stands as a pioneering cancer precision medicine initiative [
20]. Over time, it has grown into a collaborative research consortium network of nineteen prominent cancer centers across the United States. Patients were enrolled in the Total Cancer Care (TCC) protocol across 19 cancer centers within ORIEN. TCC is a prospective cohort study of unparalleled scope, encompassing whole-exome tumor sequencing, RNA sequencing, germline sequencing, and lifetime follow-up.
Patients with all stages of prostate adenocarcinoma diagnosed between 2014 and 2020 from nine participating members of ORIEN were included in the study. Clinical data such as age at diagnosis, race, stage at diagnosis, Gleason grade at the time of diagnosis, prostatic specific antigen (PSA) at diagnosis, type of hormone therapy, type of chemotherapy, and survival data were extracted from the ORIEN AVATAR Database. The duration of treatment was defined as the time from the start of the treatment to its end for any reason. Overall survival (OS) was calculated from the time of PCa diagnosis until death for any cause. RNA-seq was performed on tumor samples following the RSEM pipeline, enabling the quantification of gene expressions in terms of Transcript Per Million (TPM).
In our study, tumor RNA-seq was analyzed for PLK1 expression and associated with clinical data. PLK1 expression was stratified into high expression (≥75th percentile of PLK1 mRNA expression) vs. low expression (<75th percentile) as defined in a previous breast cancer study [
21]. The study was approved by the local Institutional Review Boards (IRBs) at the University of Kentucky (approval number: 64688).
Statistics and Analysis
Descriptive statistics of clinical and prognostic variables including age, race, stage, PSA, and Gleason grade were reported by PLK1 level (high versus low). The Fisher’s exact test was used to compare the distribution of a categorical variable, and the Wilcoxon rank-sum test was used to compare the distribution of a continuous variable between PLK1 high and PLK1 low groups. Kaplan-Meir curves and the log-rank test were used to compare overall survival and duration of treatment between PLK1 high and PLK1 low groups. The univariable Cox proportional hazards model was used to calculate the hazard ratio (HR). The multivariable Cox proportional hazards model was used to adjust for clinical factors including age, race, PSA level, and stage. Two-sided p values <0.05 was considered as statistically significant for all tests.
Results
Study Population
A total of 452 primary tumors were evaluated for PLK1 expression. Of them, 241 (53.3%) met our definition of high PLK1 expression, and 211 (46.7%) were defined as low PLK1 expression (
Table 1). The median age at diagnosis for high and low PLK1 expression was 65 years (range 43–85). Most patients were Caucasian in both groups: 218 (91%) in the PLK1 high group and 186 (89.4%) in the PLK1 low group. In the high PLK1 group, 93 (39%) had localized high risk/very high-risk disease, 27 (11%) had localized intermediate risk, and 20 (8%) had metastatic disease (
Table 1). In the low PLK1 group, 97 (46%) had localized high risk/very high-risk disease, 38 (18%) had localized intermediate risk, and 18 (8%) had metastatic disease. Gleason ≥ 8 and PSA <20 were seen slightly more in the high PLK1 group compared to the low PLK1 group; 9% vs. 6%and 89% vs. 83%, respectively (
Table 1).
Survival Analysis
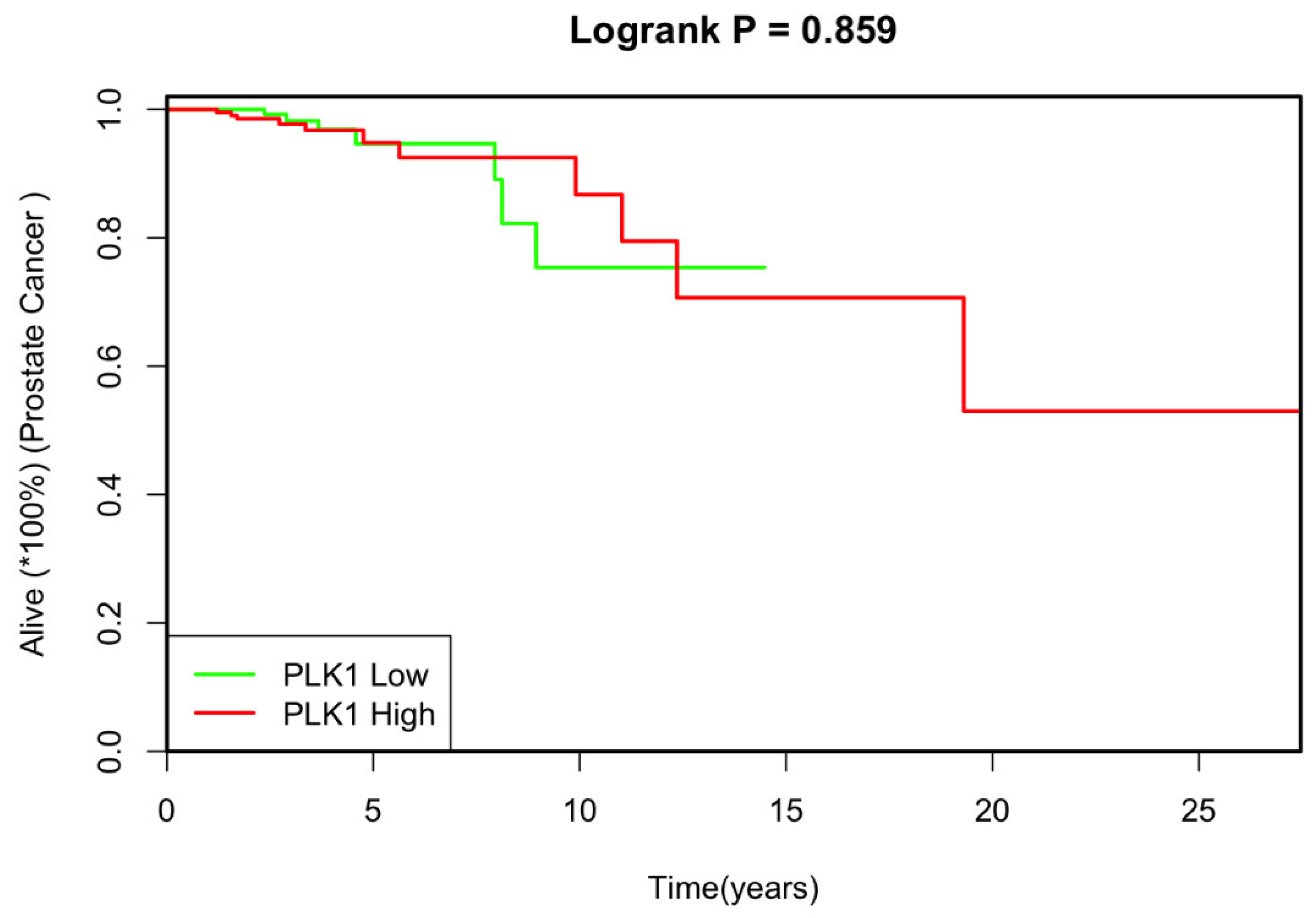
The median follow-up duration was 2.74 years in the low PLK1 group and 2.71 years for the high PLK1 group. Univariate analysis revealed no significant correlation between PLK1 expression and the studied covariates. Additionally, there was no notable difference in overall survival between patients with low and high PLK1 expression (
p=0.86; HR 1.09, 95% CI: 0.41-2.88) (
Figure 1).
Furthermore, the study found no statistically significant differences in PLK1 expression or overall survival among different racial groups, including Whites, African Americans, and others (
Table 2). Likewise, there was no significant variation in overall survival observed among different age groups and PSA levels (
Table 2). These results suggest that PLK1 expression does not serve as a significant prognostic marker in this context.
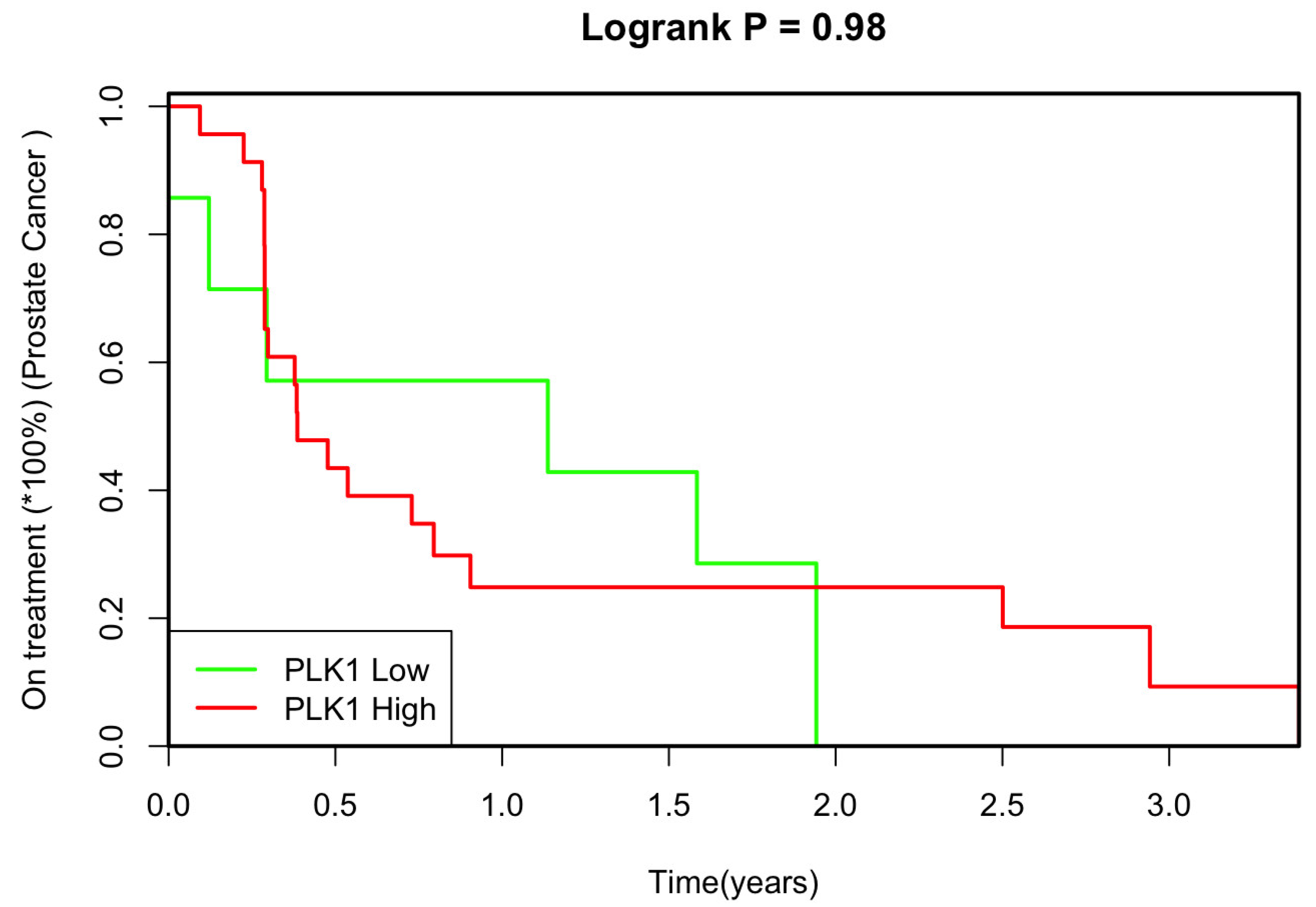
We also explored the relationship between PLK1 expression and the duration of treatment to determine whether PLK1 expression could potentially serve as a marker for treatment resistance. The analysis revealed no significant statistical correlation between PLK1 expression levels and time to treatment response (
p=0.98, HR 1.00, 95% CI 0.39-2.56) (
Figure 2).
Upon conducting a multivariate analysis and adjusting for various factors, age and PLK1 expression exhibited a positive correlation concerning treatment response (
p=0.02, HR=0.91, 95% CI 0.85-0.98;
p=0.5, HR =0.69, 95% CI 0.24 – 2.0, respectively) (
Table 3). However, no significant correlations were observed between PLK1 expression level, PSA level, race, and the duration to treatment response. These findings suggest that while PLK1 expression levels alone may not directly impact time to treatment response, when considered alongside age, there appears to be a nuanced relationship warranting further investigation.
Discussion
High PLK1 expression has been reported as a potential prognostic marker for poor response in solid tumors such as ovarian cancer [
22], non-small cell lung cancer [
23], and breast cancer [
24]. We investigated the role of PLK1 expression in PCa prognosis by examining RNA-seq data from a large cohort of patients with PCa. Despite previous immunohistochemical evidence suggesting a link between high PLK1 expression and higher Gleason grades [
9], our findings did not demonstrate a significant association between PLK1 mRNA expression and overall survival in PCa. It also did not serve as a reliable marker for treatment resistance when patients were stratified by PLK1 expression levels.
The lack of a significant association between PLK1 expression and clinical outcomes suggests that PLK1 might not be a standalone prognostic biomarker for PCa. This finding contrasts with previous preclinical studies that highlighted PLK1’s role in tumor progression and treatment resistance [10-15]. It is possible that the complexities of in vivo tumor environments and the multifactorial nature of treatment resistance cannot be fully captured by PLK1 expression alone.
Several factors may explain the discrepancy between our results and earlier studies that implicated PLK1 in PCa prognosis and treatment resistance. First, the method of PLK1 measurement varies significantly between studies. Immunohistochemistry (IHC) evaluates protein expression and localization within the tissue context, which may reflect the functional state of the protein more accurately than mRNA levels [25-26]. In contrast, RNA-seq provides a quantitative measure of mRNA expression but does not account for post-transcriptional modifications, protein stability, or activity [26-27]. The lack of correlation between PLK1 mRNA and protein levels could be attributed to these post-transcriptional regulatory mechanisms, as well as potential differences in sample handling and analysis techniques [28-29].
Our study cohort included a large and diverse sample of 452 patients with PCa, with a balanced distribution of high and low PLK1 expression cases. Despite the robust sample size, we found no significant differences in key clinical and pathological features, such as age, race, stage, PSA levels, and Gleason grade, between patients with high and low PLK1 expression. This uniformity suggests that PLK1 mRNA expression does not vary significantly across these parameters, further challenging its utility as a prognostic biomarker.
Moreover, our survival analysis, both univariate and multivariate, revealed no significant association between PLK1 mRNA expression and overall survival. The hazard ratios close to 1 (HR 1.09 for univariate and HR 1.12 for multivariate analysis) indicate a lack of prognostic impact, which aligns with the absence of significant differences in clinical features between the two groups. These findings contrast with preclinical studies that identified PLK1 as a key player in PCa treatment resistance, emphasizing the potential limitations of translating preclinical findings directly to clinical outcomes.
The observed inconsistency between PLK1 IHC and RNA-seq data might also reflect the complexity of tumor biology and the influence of the tumor microenvironment [
30]. Factors such as hypoxia and other microenvironmental conditions can differentially affect mRNA and protein expression, potentially explaining the divergent findings [
30]. Additionally, the technical variability between IHC and RNA-seq, including differences in sensitivity and specificity, may contribute to these conflicting results.
There are several limitations to this study. First, the majority of cases were localized disease, with only 14% representing metastatic disease. The retrospective nature of the study and the small sample sizes in cohorts undergoing hormone treatment and chemotherapy limit the generalizability of the findings. Additionally, treatment duration data was limited to start and stop dates, which may not accurately reflect the actual duration of therapy, and the relatively short median follow-up time of approximately 2.7 years may not be sufficient to capture long-term outcomes and late-onset resistance mechanisms. Future studies with longer follow-up periods and larger, more diverse patient cohorts are necessary to validate our findings and explore potential interactions between PLK1 expression and other molecular pathways involved in PCa progression and treatment resistance.
Conclusions
In conclusion, our study provides important insights into the role of PLK1 expression in PCa. While PLK1 does not appear to serve as a prognostic marker based on RNA-seq data, the complexity of its role in cancer biology warrants further research.
Conflicts of interest: Nothing to disclosed for all authors except EAS: Aura Biosciences data safety monitoring board member.
Author Contributions
Responsible for conceptualization and funding acquisition: SN and ZWM. Responsible for methodology: all authors. Responsible for formal analysis, software and visualization: CW and AXL. Responsible writing – original draft: AD and ZWM. Responsible for writing – review & editing: all authors.
Funding
The study was supported by the University of Kentucky Internal Pilot Grant. Responsible for supervision: ZWM.
Informed Consent Statement
Patient consent was waived due to retrospective nature.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical considerations.
Acknowledgments
he Biostatistics and Bioinformatics Shared Resource Facility provided statistical support which is supported by the NCI Cancer Center Support Grant (P30 CA177558).
References
- Ferraldeschi R, Welti J, Luo J, Attard G, de Bono JS. Oncogene. Vol 34. Macmillan Publishers Limited; 2015. Targeting the androgen receptor pathway in castration-resistance prostate cancer: progresses and prospects; pp. 1745-1757.
- Smith MR, Hussain M, Saad F, Fizazi K, Sternberg C, Crawford D, Kopyltsov E, Park CH, Alekssev B, Montesa-Pino A et al. Darolutamide and survival in metastatic, hormone sensitive prostate cancer. N Engl J Med 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, Flechon A, Tombal B, Supiot S, Berthold D, Ronchin P et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicenter, open-label, randomized, phase 3 study with a 2x2 factorial design. Lancet. 2022, 399, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de AJ, Gomes S, Given R, Juarez Soto A, Merseburger AS, Ozguroglu M et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Gartrell BA, Saad F. Abiraterone in the management of castration-resistant prostate cancer prior to chemotherapy. Ther Adv Urol. 2015, 7, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vida A, galazi M, Rudman S, Chowdhury S, Sternberg CN. Enzalutamide for the treatment of metastatic castration resistant prostate cancer. Drug Des Devel Ther. 2015, 9, 3325–3339. [Google Scholar]
- Davis DI, Martin AJ, Stockler MR, Begbie S, Chid KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG et al. Enzalutamide with standard first line therapy in metastatic prostate cancer. N Engl,J Med 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Ozguroglu M, Ye D, Feyerabend S, Protheroe A, et al. Abiraterone plus prednisone in metastatic castration sensitive prostate cancer. N Engl J Med 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Weichert W, Schmidt M, Gekeler V, Denkert C, Stephan C, Jung K, Loening S, Dietel M, Kristiansen G. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate. 2004, 60, 240–245. [Google Scholar] [CrossRef]
- Song B, Liu XS, Rice SJ, Kuang S, Elzey BD, Konieczny SF, Ratliff TL, Hazbun T, Chiorean EG, Liu X. Plk1 phosphorylation of orc2 and hbo1 contributes to gemcitabine resistance in pancreatic cancer. Mol Cancer Ther. 2013, 12, 58–68. [Google Scholar] [CrossRef]
- Rodel F, Keppner S, Capalbo G, Bashary R, Kaufmann M, Rodel C, Strebhardt K, Spankuch B. Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol. 2010, 177, 918–929. [Google Scholar] [CrossRef]
- Hou X, Li Z, Huang W, Li J, Staiger C, Kuang S, Ratliff T, Liu X. Plk1-dependent microtubule dynamics promotes androgen receptor signaling in prostate cancer. Prostate. 2013, 73, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhang Z, Hou X, Shao C, li J, Cheng JX, Kuang S, Ahmad N, Ratliff T, Liu X. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res. 2014, 74, 6635–6647. [Google Scholar] [CrossRef] [PubMed]
- Li J, Karki A, Hodges KB, Ahmad N, Zoubeidi A, Strebhardt K, Ratliff TL, Konieczny SF, Liu X. Co-targeting Polo-like kinase 1 (Plk1) and the Wnt/betacatenin signaling pathway in castration resistant prostate cancer. Mol Cell Biol. 2015. [Google Scholar]
- Shao C, Ahmad N, Hodges K, Kunag S, Ratliff T, Liu X. Inhibition of polo-like kinase (Plk1) enhances the antineoplastic activity of metformin in prostate cancer. J Biol Chem. 2015, 290, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Casolaro A, Golay J, Albanese C, Ceruti R, Patton V, Cribioli S, Pezzoni A, Losa M, Texido G, Giussani U et al. The Polo-like kinase 1 (PLK1) inhibitor NMS-P937 is effective in a new model of disseminated primary CD56+ acute monoblastic leukemia. PLoS One. 2013, 8, e58424. [Google Scholar]
- Weiss GJ, Jameson G, Von Hoff DD, Valsasina B, Davite C, Di Giulio C, Fiorentini F, Alzani R, Carpinelli P, Di Sanzo A et al. Phase I dose escalation study of NMS-1286937, an orally available Polo-Like Kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Invest New Drugs. 2018, 36, 85–95. [Google Scholar] [CrossRef]
- Chiappa M, Petrella S, Damia G, Broggini M, Guffanti F, Ricci F. Present and Future Perspective on PLK1 inhibition in cancer treatmen. Front Oncol. 2022, 12, 903016. [Google Scholar] [CrossRef]
- Einstein DJ, Choudhury AD, Saylor PJ, Patterson JC, Croucher P, Ridinger M, Erlander MG, Yaffe MB, Bubley G. A phase 2 study of onvansertib in combination with abiraterone and prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol (suppl 6; abstr TPS219). 2022, 40.
- Fenstermacher DA, Wenham RM, Rollison DE, Dalton WS. Implementing personalized medicine in a cancer center. Cancer J, 2011, 17, 528–36. [Google Scholar] [CrossRef]
- Takeshita T, Asaoka M, Katsuta E, Photiadis SJ, Narayanan S, Yan L, Takabe K. High expression of polo-like kinase 1 is associated with TP53 inactivation, DNA repair deficiency, and worse prognosis in ER positive HER2 negative breast cancer. Am J Transl Res. 2019, 11, 6507–6521. [Google Scholar]
- Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Kobel M, Dietel M, Hauptmann S. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer. 2004, 90, 815–21. [Google Scholar] [CrossRef]
- Wolf G, Elez R, Doermer A, Holtrich U, Ackermanna H, Stutte HJ, Altmannsberger HM, Rubsamen-Waigmann H, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997, 14, 543–9. [Google Scholar] [CrossRef] [PubMed]
- Lashen AG, Toss MS, Wootton L, Green AR, Mongan NP, Madhusudan S, Rakha E. Characteristics and prognostic significance of polo-like kinase-1 (PLK1) expression in breast cancer. Histopathology. 2023, 83, 414–425. [Google Scholar] [CrossRef]
- Rizzardi AE, Johnson AT, Vogel RI, Pambuccian SE, Henriksen J, Skubitz APN, Metzger GJ, Schmechel SC. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagnostic Pathology 2012, 7, 42. [Google Scholar] [CrossRef]
- Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep. 2015, 5, 10775. [Google Scholar] [CrossRef] [PubMed]
- Kukurba KR and Montgomery, SB. RNA sequencing and analysis. Cold Spring Harb Protoc. 2015, 2015, 951–969. [Google Scholar]
- Sorokin M, Ignatev K, Poddubskaya E, Vladimirova U, Gaifullin N, Lantsov D, Garazha A, Allina D, Suntsova M, Barbara V, Buzdin A. RNA sequencing in comparison to immunohistochemistry for measuring cancer biomarkers in breast cancer and lung cancer specimens. Biomedicines. 2020, 8, 114. [Google Scholar]
- De Vries NL, Mahfouz A, Koning F, de Miranda NFCC. Unraveling the complexity of the cancer microenvironment with multidimensional genomic and cytometric technologies. Front Oncol. 2020, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Bauer R, Meyer SP, Kloss AK, Guerrero Ruiz VM, Reuscher S, Zhou Y, Fuhrmann DC, Zarnack K, Schmid T, Brune B. Functional RNA dynamics are progressively governed by RNA destabilization during the adaptation to chronic hypoxia. Int J Mol Sci. 2022, 23, 5824. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).