1. Introduction
Cancer remains one of the most significant global health challenges, responsible for a substantial proportion of morbidity and mortality worldwide [
1,
2]. According to the World Health Organization (WHO), cancer accounted for approximately 10 million deaths in 2020, making it one of the leading causes of death globally [
3,
4]. Despite the development of advanced therapies such as targeted treatments, immunotherapies, and precision medicine, the personalization of cancer treatment is hampered by considerable challenges [
5,
6,
7,
8]. The complex and heterogeneous nature of cancer, with its diverse genetic, epigenetic, and phenotypic variations, demands individualized and precision treatment strategies [
9,
10,
11]. These challenges are further exacerbated when cancer patients present comorbid conditions that complicate the management of cancer can influence treatment outcomes.
Artificial intelligence (AI) has emerged as a transformative technology in medicine, offering new avenues to enhance cancer diagnosis, therapy and care by integrating and analyzing complex datasets [
12,
13]. Applying machine learning (ML), deep learning (DL), and natural language processing (NLP), AI has advanced rapidly and is increasingly being used in tackling challenges in healthcare [
14,
15,
16]. In the context of cancer treatment, AI's ability to process and analyze large volumes of data, including genomic sequences, medical imaging, and electronic health records (EHR), has the potential to revolutionize patient profiling [
12,
13]. This process involves identifying the unique characteristics of the primary pathology and comorbid conditions, which is critical for developing personalized treatment plans that are more likely to be effective and less likely to cause adverse effects.
Patient profiling in oncology involves a comprehensive analysis of various factors that influence the behavior of cancer, such as genetic mutations, the tumor microenvironment, and patient-specific factors including age, lifestyle, and the presence of other health conditions [
17,
18,
19,
20,
21]. A deep understanding of the specifics of the disease can assist in tailoring more effective and personalized treatment strategies that can significantly improve outcomes [
22,
23]. This is particularly relevant to patients with co-morbid conditions, that affect the trajectory and prognosis of cancer, adding a layer of complexity.
Migraines, which affect a substantial portion of the global population, are a common neurological disorder characterized by recurrent, severe headaches often accompanied by symptoms such as nausea, vomiting, and sensitivity to light and sound [
24,
25]. The relationship between migraines, cancer and chemotherapy are complex and not yet fully understood. Some studies suggest that migraines may be associated with an increased risk of certain types of cancer, such as breast cancer and glioma [
26,
27]. Additionally, the presence of migraines in cancer patients can complicate cancer management, particularly in terms of pain management and overall quality of life [
28]. Migraines may also impact the efficacy of cancer treatments, with chemotherapy increasing the frequency of headaches, further complicating the treatment landscape [
29,
30]. Given the high prevalence of migraines and their potential to influence cancer outcomes there is a growing interest in leveraging AI to improve profiling of cancer patients who also suffer from migraine [
31,
32].
AI based technologies enables analysis and cross-validation of large sets of multimodal data with a higher-level integration, assisting in generating more precise and individualized data for diagnosis, stratification and clinical decision making [
33]. Applied to cancer cases with co-morbidities such as migraine, this approach could enable better management of the primary pathology and co-morbid condition, improving outcomes for patients.
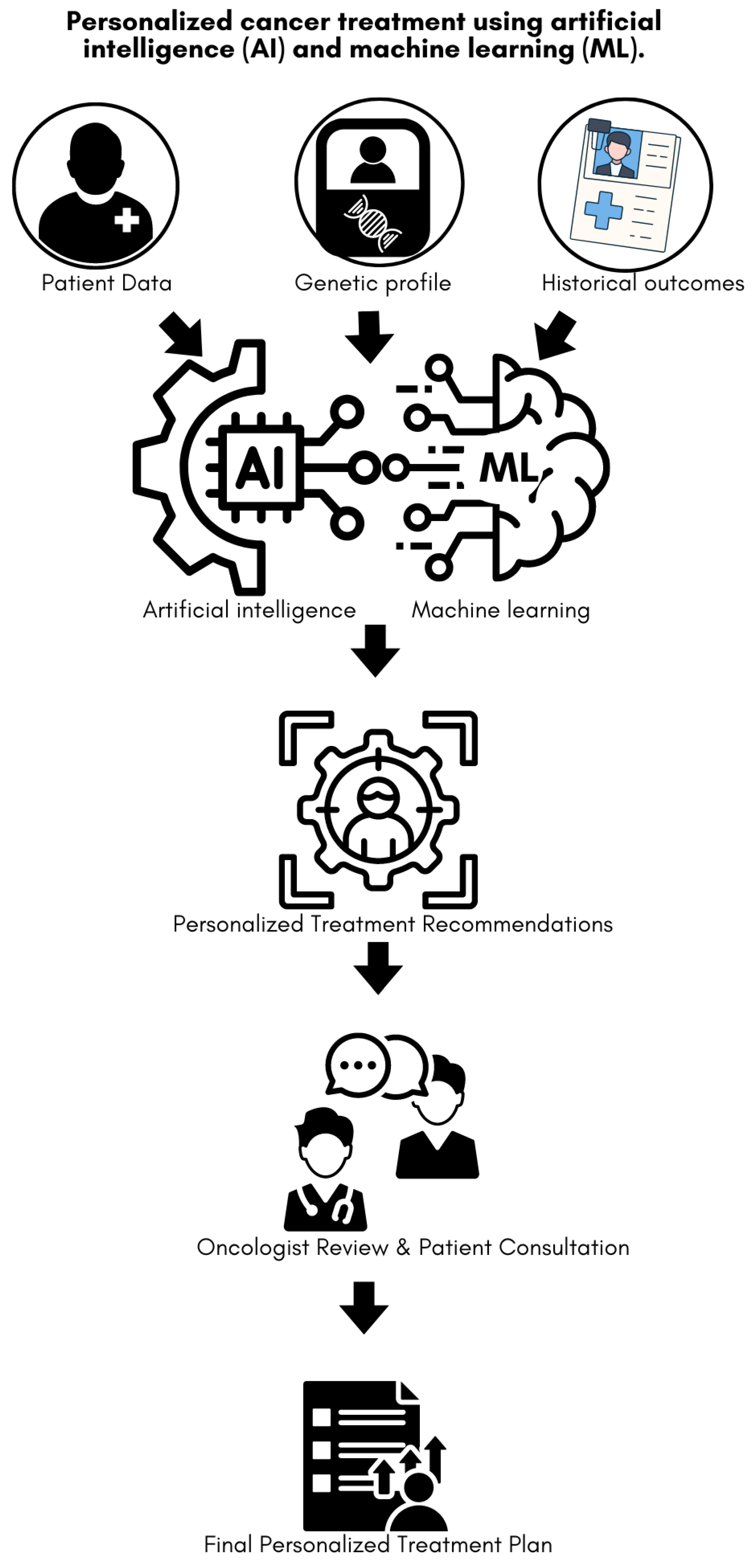
Figure 1 illustrates the workflow of personalized cancer treatment using AI and ML. Based on analysis of multi-modal proteomic, metabolomic, genomic and imaging data, the approach facilitates identification of shared molecular pathways and key mechanisms between migraines and cancer potentially uncovering new therapeutic targets. It can also help predict how migraines might influence the progression of cancer or the patient's response to treatment, allowing clinicians to adjust treatment strategies [
34].
This narrative review explores the potential of AI guided profiling of patients with integration of multimodal data from cancer patients with co-morbid migraine, to facilitate personalized and more effective interventions. The objective is to elucidate how AI can be leveraged to refine anticancer therapy of patients’ co-morbid migraine, to facilitate future research and clinical practice.
Workflow of personalized cancer treatment using AI and ML. Patient data, genetic profiles, and historical outcomes are integrated into AI/ML algorithms to generate personalized treatment recommendations. These recommendations are then reviewed by an oncologist, followed by a patient consultation, culminating in the development of a final personalized treatment plan tailored to the individual's specific characteristics and clinical history.
2. Methods
2.1. Literature Search Strategy
To conduct this narrative review, a comprehensive literature search was performed across multiple databases, including PubMed, Scopus, Web of Science, and Google Scholar. The search was conducted to identify relevant studies published up until August 2024. Keywords used in the search included combinations of the following terms: "artificial intelligence," "AI," "machine learning," "ML," "deep learning," "DL," "cancer therapy," "oncology," "patient profiling," "personalized medicine," "migraine," "comorbidities," and "cancer outcomes." Boolean operators such as "AND" and "OR" were used to refine the search results. Additionally, reference lists of selected articles were reviewed to identify any further relevant studies not captured in the initial search.
2.2. Inclusion and Exclusion Criteria
Studies were included in this review if they met the following criteria: (a) they focused on the application of AI in cancer therapy, particularly in the context of patient profiling or personalized medicine; (b) they addressed the impact of migraines as a comorbidity in cancer patients; (c) they were published in peer-reviewed journals; and (d) they were available in English. Exclusion criteria included (a) studies focusing solely on basic science or preclinical research without clinical implications; (b) articles that did not specifically address the intersection of AI, cancer therapy, and migraine comorbidities; and (c) studies with insufficient methodological rigor or unclear outcomes.
2.3. Data Extraction and Synthesis
Data from the selected studies were extracted and synthesized to provide a comprehensive overview of the current state of research in the application of AI in anticancer therapy, with a specific focus on migraine comorbidities. Key information extracted included the study design, AI methods used, types of cancer addressed, migraine-related data, and outcomes related to patient profiling and treatment personalization. The extracted data were then analyzed to identify common themes, gaps in the literature, and potential areas for future research.
2.4. Quality Assessment
All papers reviewed in this article have been scrutinised and selected based on (a) scientific rigor of presented data with carefully designed experiments and reference to controls, (b) adequate sample size with number of replicas and variability, (c) valid statistical analysis and test with significance of
p values, and (d) data quality and reproducibility, with discussions limited to the peer-reviewed papers only [
35].
2.5. Narrative Synthesis
The findings from the selected studies were synthesized narratively, with an emphasis on discussing how AI has been applied in the context of cancer therapy and patient profiling, particularly for patients with migraines. The narrative synthesis involved organizing the results into thematic sections, such as AI-driven genomic profiling, predictive modeling, and the integration of migraine data into treatment plans. The review also highlighted the novelty and implications of these findings for clinical practice and future research.
3. The Role of AI in Anticancer Therapy
AI has become a pivotal technology in the realm of anticancer therapy, transforming how patient care is approached by enabling more precise, data-driven decision-making. Recent advances in AI, particularly in ML and DL, have enhanced the ability of healthcare systems to analyze large volumes and complex datasets rapidly and accurately [
14,
16]. This capability is crucial in oncology, where the sheer volume of data—ranging from genomic sequences to real-time imaging and patient history—can overwhelm traditional analytical methods [
36,
37]. AI’s role in anticancer therapy extends beyond basic data processing; it is now integral to the development of personalized treatment plans, predictive modeling of disease progression, and even the identification of novel therapeutic targets.
3.1. AI in Personalized Medicine
In the last few years, AI has dramatically advanced personalized medicine, particularly in the context of cancer treatment. By leveraging AI, clinicians can now go beyond standard treatment protocols to develop highly individualized therapy plans tailored to the unique genetic and molecular profiles of cancer patients on case-by-case basis [
8]. Recent developments in AI, such as the use of neural networks to analyze multi-omics data (which includes genomics, transcriptomics, proteomics, and metabolomics), allow for the identification of specific biomarkers and genetic mutations that drive cancer in individual patients [
38,
39]. These insights enable the selection of targeted therapies that are more likely to be effective, reducing the trial-and-error approach that has traditionally characterized cancer treatment.
Moreover, AI's ability to integrate data from various sources—including EHR, wearable devices, and real-time monitoring tools—allows for the continuous adaptation of treatment plans [
40,
41]. For example, AI-driven platforms can analyze a patient’s response to a specific therapy in real-time, adjusting dosages or switching treatments as necessary to maximize efficacy and minimize side effects. This dynamic approach to treatment is increasingly recognized as a critical factor in improving patient outcomes, particularly in complex cases where standard treatments may fail.
3.2. AI in Predictive Modeling
Predictive modeling in cancer therapy has also seen significant advancements with the integration of AI. AI-driven predictive models have become more sophisticated, leveraging ML algorithms that can predict disease progression, potential metastasis, and patient survival with greater accuracy than traditional statistical methods [
42]. These models are trained on large datasets, which include not only clinical and genomic data but also lifestyle factors, treatment history, and the presence of comorbid conditions such as migraines.
Recent studies have shown that incorporating data on comorbid conditions into AI models can dramatically improve their predictive accuracy [
43]. For instance, a patient with a history of migraines might exhibit different patterns in treatment response or disease progression compared to a patient without such a history. AI can identify these subtle variations, enabling the development of more nuanced treatment strategies that account for the full spectrum of a patient's health profile [
44]. This level of precision is particularly important in oncology, where even small differences in treatment response can significantly impact survival rates.
Furthermore, AI is increasingly being used to predict the likelihood of adverse events or complications during cancer treatment, such as the exacerbation of migraines or other neurological conditions. By anticipating these risks, clinicians can preemptively modify treatment plans, such as by adjusting drug regimens or incorporating supportive care measures, to mitigate potential side effects. This proactive approach not only improves patient quality of life but also enhances the overall effectiveness of cancer therapy by ensuring that treatments are better tolerated and thus more likely to be completed.
4. Migraine as a Comorbid Condition in Cancer Patients
Migraine, a neurological disorder characterized by recurrent, often severe headaches and associated symptoms, is increasingly recognized as a common comorbid condition in cancer patients [
24,
45]. The coexistence of migraine and cancer presents unique challenges in clinical management, as the symptoms of migraine can exacerbate the physical and psychological burden of cancer, complicating treatment strategies and potentially impacting outcomes. The dual burden of these conditions demands a comprehensive approach to patient care, where both the oncological and neurological aspects of the patient’s health are addressed. As the understanding of migraine’s impact on cancer patients evolves, it becomes evident that tailored approaches to treatment and patient management are necessary to optimize both cancer therapy and the overall quality of life for these patients.
Table 1 provides an overview on how migraines affect cancer patients across various types of cancer, considering demographic factors, the nature of the migraines, potential biological mechanisms, and the impact on cancer treatment.
4.1. Epidemiology of Migraine and Cancer
Migraine is one of the most prevalent neurological disorders worldwide, affecting approximately 12-15% of the global population, with a higher prevalence among women [
45]. Recent epidemiological studies have explored the relationship between migraine and cancer, revealing potential associations that warrant further investigation [
47,
49,
50]. For instance, some research has suggested that women with a history of migraines may have a slightly increased risk of developing certain types of cancer, such as breast cancer. A study published in recent years has highlighted that the hormonal fluctuations associated with migraines, particularly in women, could be linked to mechanisms that influence cancer risk, although these findings warrant independent verification [
51].
Additionally, the role of inflammation, which is a common feature in both migraine pathophysiology and cancer development, has been proposed as a potential link between these two conditions [
52]. Chronic inflammation, which is known to play a role in cancer initiation and progression, may also exacerbate migraine severity, creating a feedback loop that could influence both conditions [
53,
54]. Understanding the prevalence and impact of migraine among cancer patients is crucial, as this knowledge can inform the development of more comprehensive treatment strategies that consider the interplay between these diseases. While the relationship between migraines and cancer remains complex and not fully understood, the growing body of evidence underscores the need for further research into how they may interact and influence each other.
4.2. Impact of Migraine on Cancer Treatment
The presence of migraines in cancer patients can significantly complicate the treatment process and impact the efficacy and tolerability of cancer therapies [
55]. Recent studies have shown that migraine sufferers often experience heightened sensitivity to pain and other sensory stimuli, which can exacerbate the side effects of cancer treatments, such as chemotherapy, radiation, and immunotherapy [
56,
57]. For example, the nausea and vomiting commonly associated with migraines may be worsened by chemotherapy, leading to more severe and prolonged episodes that are harder to manage. Additionally, the use of certain chemotherapeutic agents has been reported to trigger or worsen migraine episodes, complicating the overall management of both conditions [
55].
Moreover, the cognitive effects of migraines, including difficulty concentrating and memory problems, may interact with the cognitive side effects of cancer treatment, such as "chemo brain," potentially leading to more pronounced cognitive decline. This interaction can significantly affect a patient's ability to adhere to treatment protocols and maintain their quality of life during therapy [
58]. Recent research has also suggested that migraines might influence the overall prognosis of cancer patients, as the additional neurological burden could affect the patient's resilience and response to treatment. Given these complexities, there is an increasing recognition of the need for integrated care approaches that address both the oncological and neurological needs of patients. This might include more frequent monitoring of neurological symptoms, the use of tailored pain management strategies that consider the potential for migraine exacerbation, and the incorporation of supportive therapies such as cognitive behavioral therapy or biofeedback, which have been shown to help manage migraine symptoms. By addressing migraines as a critical component of cancer care, clinicians can better manage the dual burden of these conditions, potentially improving both treatment outcomes and patient quality of life.
5. AI in Patient Profiling for Migraine and Cancer
The integration of AI into patient profiling represents a key advancement in the management of complex conditions such as cancer, particularly when complicated by comorbidities like migraines [
32,
59]. By leveraging AI, clinicians can gain deeper insights into the genetic, molecular, and clinical factors that influence both diseases, enabling more personalized and effective treatment strategies [
60].
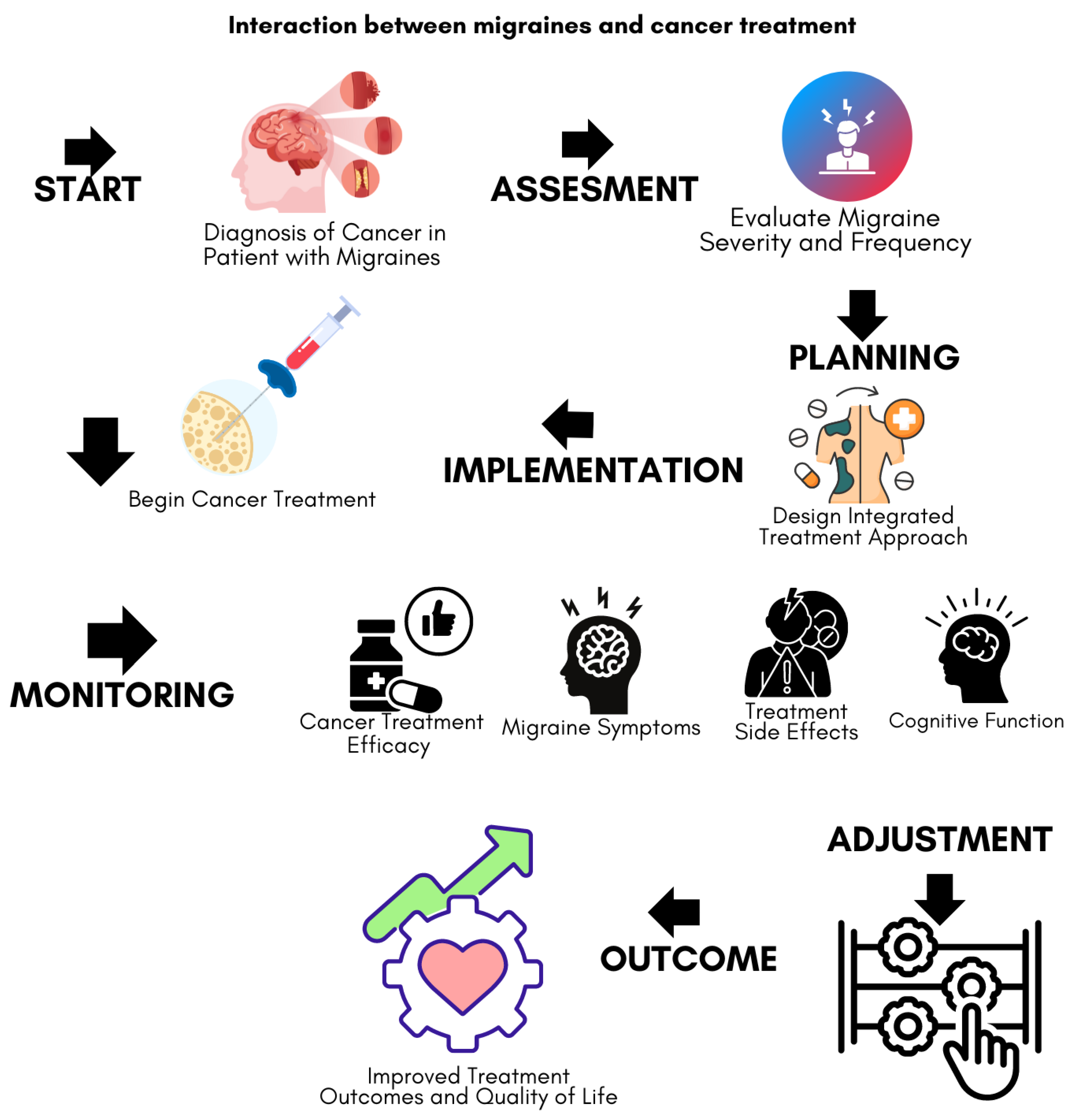
Figure 2 shows a simple flowchart diagram summarizing the interaction between migraines and cancer treatment. This approach not only facilitates a better understanding of the shared mechanisms between migraines and cancer but also enhances the precision of predictive analytics and treatment decision-making, ultimately leading to improved patient outcomes.
Table 2 provides an overview of how different AI technologies are being applied in the treatment of cancer and migraines, both separately and in combination.
A flowchart emphasizing the need for integrated care. It starts with diagnosis and assessment, moves through treatment planning and implementation, and includes regular monitoring and adjustments. The goal is to improve both treatment outcomes and quality of life by addressing both cancer and migraine-related issues throughout the care process.
5.1. Genetic and Molecular Profiling
AI can revolutionize genetic and molecular fingerprinting by sifting through vast datasets to identify genetic variants and molecular markers that may be common to both cancer and migraines. Recent advancements in AI, particularly in ML and DL, have enabled the identification of shared pathophysiological mechanisms that could underlie both conditions. For instance, certain genetic polymorphisms associated with inflammatory pathways, mitochondrial function, or hormonal regulation may predispose individuals to both cancer and migraines [
67,
68,
69]. AI-driven analyses can uncover these connections by analyzing large-scale genomic and proteomic data, revealing potential therapeutic targets that could be exploited to treat both conditions simultaneously [
70].
Moreover, AI can help in the identification of novel biomarkers that predict the onset or progression of migraines in cancer patients, offering opportunities for early intervention [
71]. By integrating data from various sources, including genomic sequences, transcriptomics, and even microbiome profiles, AI can provide a comprehensive view of the biological landscape shared between migraines and cancer [
71]. This information is invaluable for developing new drugs or repurposing existing therapies to target these shared pathways, potentially leading to more effective treatments with fewer side effects.
5.2. Predictive Analytics for Comorbid Conditions
AI-driven predictive analytics offer powerful tools for stratifying cancer patients based on their risk of developing migraines or the potential impact of pre-existing migraines on their cancer treatment outcomes. Through the analysis of historical data, including patient demographics, genetic information, treatment histories, and clinical outcomes, AI can generate predictive models that help clinicians assess the likelihood of migraine onset or exacerbation during cancer therapy [
72]. These models can also predict how migraines might influence cancer prognosis, allowing for more tailored treatment approaches.
For example, if a predictive model suggests that a patient with a specific genetic profile is at high risk for developing severe migraines during chemotherapy, clinicians can preemptively adjust the treatment regimen or incorporate prophylactic migraine therapies to mitigate this risk [
24]. Similarly, if the model indicates that a patient's existing migraines could complicate pain management or lead to increased cognitive side effects, alternative therapies or supportive care strategies can be employed to enhance the patient’s tolerance to cancer treatment. These predictive capabilities are particularly valuable in creating personalized treatment plans that not only address the cancer itself but also the broader context of the patient’s overall health, including the management of comorbid conditions like migraines.
5.3. AI in Treatment Decision Support
AI can be seamlessly integrated into clinical decision support systems (CDSS) to provide real-time, data-driven recommendations for managing patients with both cancer and migraines [
73]. These systems are designed to analyze patient data continuously, offering clinicians evidence-based suggestions on how to modify standard treatment protocols to better accommodate the unique needs of each patient. For instance, a CDSS powered by AI can take into consideration a patient’s migraine history, genetic predispositions, and response to previous treatments, and then recommend adjustments to the current cancer therapy plan.
These adjustments might include changes in medication dosages, the addition of migraine prophylaxis, or the use of alternative therapies that are less likely to trigger or worsen migraines. Furthermore, AI-driven CDSS can alert clinicians to potential drug interactions or contraindications that might arise when treating cancer patients who are also managing migraines, thereby reducing the risk of adverse effects.
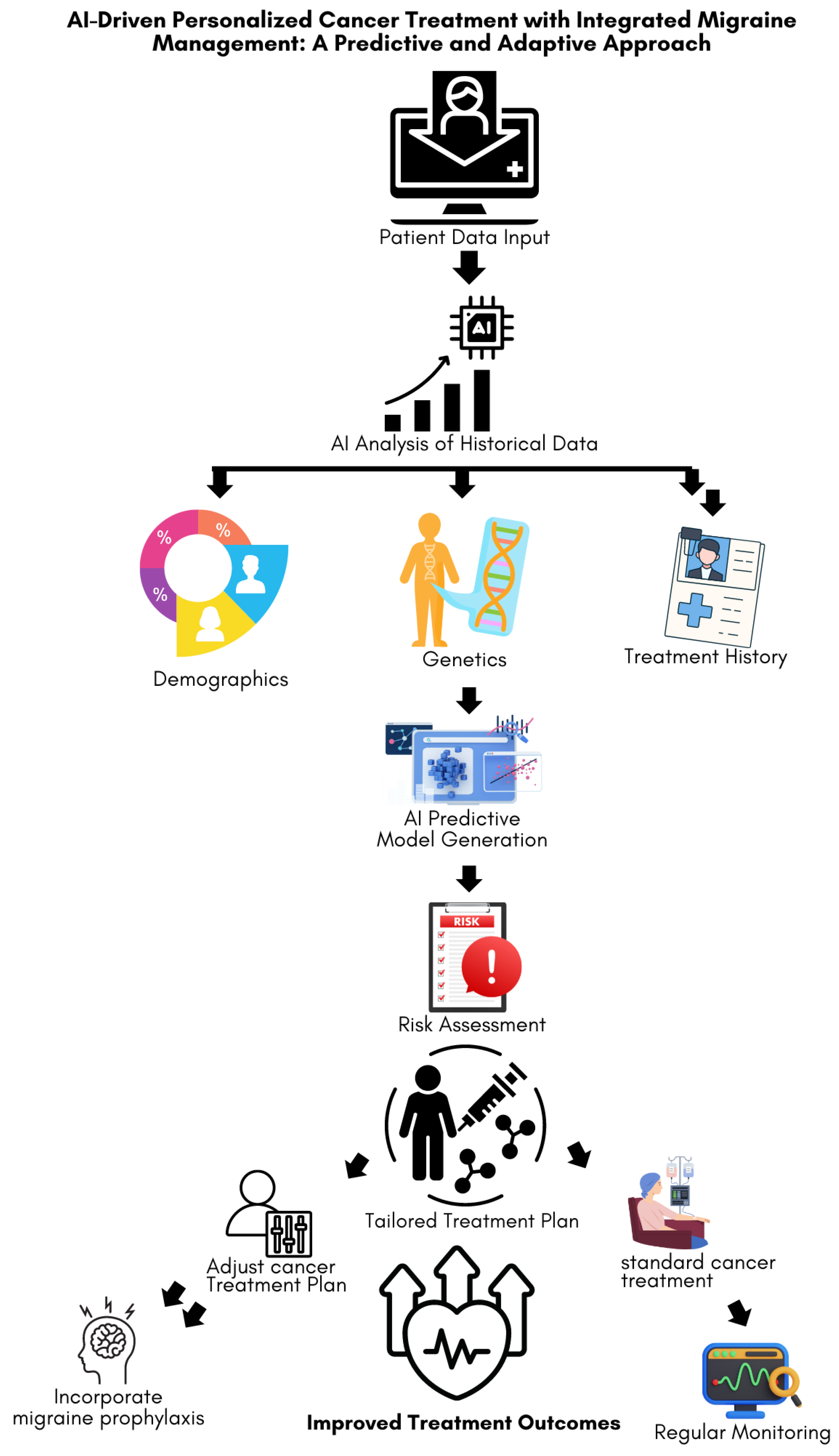
Figure 3 illustrates the process of using AI-driven predictive analytics to assess migraine risk in cancer patients and tailor treatment plans, accordingly, aiming to improve treatment outcomes through personalized patient care. By providing these insights in real-time, AI-enhanced CDSS ensures that both cancer and migraine are managed optimally, improving patient outcomes and quality of life.
Patient data, including demographics, genetics, and treatment history, is input into an AI system for analysis. The AI model generates predictive insights to assess patient-specific risks and guides the development of a tailored treatment plan. Adjustments are made to the standard cancer treatment to include migraine prophylaxis, when necessary, with ongoing monitoring to ensure improved treatment outcomes.6. Challenges and Future Directions
The integration of AI into patient profiling and anticancer therapy offers significant potential to revolutionize healthcare, particularly for complex cases involving comorbid conditions such as migraines. However, several challenges must be addressed to fully realize AI's potential in this field. These challenges encompass technical, clinical, and ethical dimensions that require careful consideration and ongoing research.
6.1. Data Integration and Privacy Concerns
One of the most significant challenges in applying AI to patient profiling is the integration of multi-modal data while maintaining the strictest standards of patient privacy [
74]. The data required for comprehensive profiling—such as genomic information, clinical records, imaging results, and patient-reported outcomes—are often scattered across different platforms and institutions. Integrating these distinct data types into a unified AI model requires sophisticated handling and processing strategies that can seamlessly combine structured and unstructured data from various sources. However, this process raises substantial concerns regarding data privacy and security. As patient data is aggregated and analyzed, the risk of breaches increases, potentially exposing sensitive personal information [
75]. Ensuring compliance with data protection regulations, such as the General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the United States, is essential [
76,
77]. Robust encryption methods, de-identification of patient data, and secure data-sharing protocols must be implemented to protect patient confidentiality while allowing for the sophisticated data integration necessary for AI-driven patient profiling [
78].
6.2. Clinical Implementation and Validation
While AI has demonstrated tremendous promise in research settings, translating these findings into clinical practice remains a significant challenge. AI models developed in controlled environments often struggle to maintain their performance when applied in real-world clinical settings where variability in patient populations, clinical workflows, and data quality can undermine their effectiveness [
79,
80]. Validating AI algorithms in these diverse and often unpredictable settings is crucial to ensure their robustness, reliability, and generalization [
81,
82]. This validation process involves extensive testing of AI models across different demographic groups, clinical environments, and disease conditions to confirm that they perform consistently and accurately. Furthermore, clinicians must be trained to interpret and act on AI-driven insights appropriately, which requires a deep understanding of both the AI technology and the clinical context in which, it is applied. The development of user-friendly interfaces and clear guidelines for AI use in clinical practice is essential to facilitate its adoption and ensure that AI enhances rather than complicates clinical decision-making.
6.3. Ethical Considerations
The integration of AI into healthcare also raises significant ethical concerns that must be addressed to ensure its responsible use [
83,
84]. One of the primary concerns is the potential for bias in AI algorithms [
85]. AI systems are only as good as the data they are trained on, and if the training data reflects existing biases—such as those related to race, gender, or socioeconomic status—the AI models may perpetuate or even exacerbate these biases in clinical decision-making [
86,
87]. Ensuring that AI systems are fair and unbiased requires careful attention to the data used in training, including the inclusion of diverse patient populations and the application of techniques to detect and mitigate bias. Additionally, the transparency of AI-driven decisions is a critical ethical issue. Clinicians and patients must be able to understand and trust the recommendations made by AI systems, which necessitates the development of explainable AI models that provide clear rationales for their decisions. Ensuring that AI systems are not only effective but also fair, transparent, and accountable is essential for their successful integration into clinical practice.
6.4. Future Directions
Looking ahead, the future of AI in patient profiling for cancer and migraine lies in overcoming these challenges and advancing the field through collaborative, interdisciplinary efforts. Future research should focus on developing more sophisticated AI models that can integrate multimodal data, including genomic, clinical, and environmental factors while preserving patient privacy through advanced encryption and data anonymization techniques. Additionally, ongoing efforts to validate AI models in diverse clinical settings are crucial to ensuring their reliability and generalizability.
Ethical considerations must continue to guide the development and deployment of AI in healthcare, with a focus on creating fair, unbiased, and explainable AI systems. This will involve not only technical advancements but also the establishment of clear regulatory frameworks and guidelines to govern the use of AI in clinical practice. Moreover, the involvement of patients, clinicians, and other stakeholders in the design and implementation of AI systems is vital to ensuring that these technologies meet the needs of all users and contribute positively to patient care [
88]. By addressing these challenges and focusing on responsible innovation, AI has the potential to transform patient profiling and anticancer therapy, particularly for complex cases involving comorbid conditions like migraines, leading to more personalized, effective, and equitable healthcare solutions.
7. Conclusions
AI holds significant promise in revolutionizing anticancer therapy, particularly through profiling individuals with comorbid conditions such as migraines. By leveraging AI's capabilities to integrate and analyze complex, multidimensional datasets—including genetic, molecular, clinical, and patient-reported data—clinicians are better equipped to develop personalized treatment strategies that cater to the unique needs of patients suffering from both cancer and migraines. This personalized approach has the potential to improve treatment efficacy, reduce adverse effects, and ultimately enhance patient outcomes.
However, realizing the full potential of AI in this domain is contingent upon overcoming several key challenges. The integration of diverse data sources while maintaining strict standards of patient privacy remains a critical hurdle. Furthermore, the clinical validation of AI models in real-world settings is essential to ensure their robustness, reliability, and applicability across diverse patient populations. Ethical considerations, particularly concerning bias, transparency, and fairness, must also be carefully managed to ensure that AI technologies contribute positively to healthcare without exacerbating existing disparities. Addressing these challenges will be crucial for the successful and widespread application of AI in anticancer therapy, particularly for complex cases involving comorbid conditions like migraines. As research and technology continue to advance, collaborative efforts across disciplines—including oncology, neurology, data science, and ethics—will be essential to harnessing AI's full potential, to unravel the Black Box of cancer with co-morbid migraine, to enable more personalized, effective, and equitable therapy and care.
References
- Fidler, M.M.; Bray, F.; Soerjomataram, I. The global cancer burden and human development: A review. Scand. J. Public. Health. 2018, 46, 27–36. [Google Scholar] [CrossRef]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Brown, J.E.; Royle, K.L.; Gregory, W.; Ralph, C.; Maraveyas, A.; Din, O.; Eisen, T.; Nathan, P.; Powles, T.; Griffiths, R.; et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet. Oncol. 2023, 24, 213–227. [Google Scholar] [CrossRef]
- Dey, A.; Mitra, A.; Pathak, S.; Prasad, S.; Zhang, A.S.; Zhang, H.; Sun, X.F.; Banerjee, A. Recent Advancements, Limitations, and Future Perspectives of the use of Personalized Medicine in Treatment of Colon Cancer. Technol. Cancer. Res. Treat. 2023, 22, 15330338231178403. [Google Scholar] [CrossRef]
- Eden, D.; Ghose, A.; Moschetta, M.; Pérez-Fidalgo, J.A.; Rassy, E.; Boussios, S. Immunotherapy Combined With Standard Therapies in Head and Neck Squamous Cell Carcinoma - A Meta-analysis. Anticancer. Res. 2024, 44, 861–878. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology. (Singap World Sci). 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Ganatra, H.; Tan, J.K.; Simmons, A.; Bigogno, C.M.; Khurana, V.; Ghose, A.; Ghosh, A.; Mahajan, I.; Boussios, S.; Maniam, A.; et al. Applying whole-genome and whole-exome sequencing in breast cancer: a review of the landscape. Breast. Cancer. 2024. [Epub ahead of print]. [Google Scholar] [CrossRef]
- Mathew, B.G.; Aliyuda, F.; Taiwo, D.; Adekeye, K.; Agada, G.; Sanchez, E.; Ghose, A.; Rassy, E.; Boussios, S. From Biology to Diagnosis and Treatment: The Ariadne’s Thread in Cancer of Unknown Primary. Int. J. Mol. Sci. 2023, 24, 5588. [Google Scholar] [CrossRef]
- Rassy, E.; Boussios, S.; Pavlidis, N. Genomic correlates of response and resistance to immune checkpoint inhibitors in carcinomas of unknown primary. Eur. J. Clin. Invest. 2021, 51, e13583. [Google Scholar] [CrossRef]
- Coccia, M. Artificial intelligence technology in oncology: a new technological paradigm. arXiv. 2019, 1905.06871. [Google Scholar]
- Kann, B.H.; Hosny, A.; Aerts, H.J.W.L. Artificial intelligence for clinical oncology. Cancer. Cell. 2021, 39, 916–927. [Google Scholar] [CrossRef]
- Alqahtani, T.; Badreldin, H.A.; Alrashed, M.; Alshaya, A.I.; Alghamdi, S.S.; Bin Saleh, K.; Alowais, S.A.; Alshaya, O.A.; Rahman, I.; Al Yami, M.S.; et al. The emergent role of artificial intelligence, natural learning processing, and large language models in higher education and research. Res. Social. Adm. Pharm. 2023, 19, 1236–1242. [Google Scholar] [CrossRef]
- Caglayan, A.; Slusarczyk, W.; Rabbani, R.D.; Ghose, A.; Papadopoulos, V.; Boussios, S. Large Language Models in Oncology: Revolution or Cause for Concern? Curr. Oncol. 2024, 31, 1817–1830. [Google Scholar] [CrossRef]
- Rubinger, L.; Gazendam, A.; Ekhtiari, S.; Bhandari, M. Machine learning and artificial intelligence in research and healthcare. Injury. 2023, 54, S69–S73. [Google Scholar] [CrossRef]
- Apte, V.; Ghose, A.; Linares, C.A.; Adeleke, S.; Papadopoulos, V.; Rassy, E.; Boussios, S. Paediatric Anatomical Models in Radiotherapy Applications. Clin. Oncol. (R Coll Radiol). 2024, 36, 562–575. [Google Scholar] [CrossRef]
- Guo, M.; Peng, Y.; Gao, A.; Du, C.; Herman, J.G. Epigenetic heterogeneity in cancer. Biomark. Res. 2019, 7, 23. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, J.; Zhong, X.; Selistre-de-Araujo, H.S.; Boussios, S.; Ma, Y.; Fang, H. A novel PD-1/PD-L1 pathway-related seven-gene signature for the development and validation of the prognosis prediction model for breast cancer. Transl. Cancer. Res. 2024, 13, 1554–1566. [Google Scholar] [CrossRef]
- Linares, C.A.; Varghese, A.; Ghose, A.; Shinde, S.D.; Adeleke, S.; Sanchez, E.; Sheriff, M.; Chargari, C.; Rassy, E.; Boussios, S. Hallmarks of the Tumour Microenvironment of Gliomas and Its Interaction with Emerging Immunotherapy Modalities. Int. J. Mol. Sci. 2023, 24, 13215. [Google Scholar] [CrossRef]
- Osseis, M.; Nehmeh, W.A.; Rassy, N.; Derienne, J.; Noun, R.; Salloum, C.; Rassy, E.; Boussios, S.; Azoulay, D. Surgery for T4 Colorectal Cancer in Older Patients: Determinants of Outcomes. J. Pers. Med. 2022, 12, 1534. [Google Scholar] [CrossRef]
- Boussios, S.; Devo, P.; Goodall, I.C.A.; Sirlantzis, K.; Ghose, A.; Shinde, S.D.; Papadopoulos, V.; Sanchez, E.; Rassy, E.; Ovsepian, S.V. Exosomes in the Diagnosis and Treatment of Renal Cell Cancer. Int. J. Mol. Sci. 2023, 24, 14356. [Google Scholar] [CrossRef]
- Pokorná, M.; Černá, M.; Boussios, S.; Ovsepian, S.V.; O’Leary, V.B. lncRNA Biomarkers of Glioblastoma Multiforme. Biomedicines. 2024, 12, 932. [Google Scholar] [CrossRef]
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: epidemiology and systems of care. Lancet. 2021, 397, 1485–1495. [Google Scholar] [CrossRef]
- Hansen, J.M.; Lipton, R.B.; Dodick, D.W.; Silberstein, S.D.; Saper, J.R.; Aurora, S.K.; Goadsby, P.J.; Charles, A. Migraine headache is present in the aura phase: a prospective study. Neurology. 2012, 79, 2044–2049. [Google Scholar] [CrossRef]
- Palmieri, A.; Valentinis, L.; Zanchin, G. Update on headache and brain tumors. Cephalalgia. 2021, 41, 431–437. [Google Scholar] [CrossRef]
- Radovanovic, I.; Zadeh, G. Cancer neurology in clinical practice, neurological complications of cancer and its treatment (2nd Edn). Br. J. Cancer. 2009, 100, 1020. [Google Scholar] [CrossRef]
- Savaliya, M.; Surati, D.; Surati, R.; Padmani, S.; Boussios, S. Posterior Reversible Encephalopathy Syndrome after Pazopanib Therapy. Diseases. 2023, 11, 76. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Pahapill, N. Successful Treatment of Chemotherapy-Induced Headaches With Occipital Nerve Stimulation: A Case Report. Neuromodulation. 2020, 23, 881–882. [Google Scholar] [CrossRef]
- Khan, R.B. Migraine-type headaches in children receiving chemotherapy and ondansetron. J. Child. Neurol. 2002, 17, 857–858. [Google Scholar] [CrossRef]
- Martelletti, P. Migraine in Medicine: A Machine-Generated Overview of Current Research. Springer Nature; 2022.
- Meier, T.A.; Refahi, M.S.; Hearne, G.; Restifo, D.S.; Munoz-Acuna, R.; Rosen, G.L.; Woloszynek, S. The Role and Applications of Artificial Intelligence in the Treatment of Chronic Pain. Curr. Pain. Headache. Rep. 2024, 28, 769–784. [Google Scholar] [CrossRef]
- Rainero, I.; Roveta, F.; Vacca, A.; Noviello, C.; Rubino, E. Migraine pathways and the identification of novel therapeutic targets. Expert. Opin. Ther. Targets. 2020, 24, 245–253. [Google Scholar] [CrossRef]
- An, Y.C.; Tsai, C.L.; Liang, C.S.; Lin, Y.K.; Lin, G.Y.; Tsai, C.K.; Liu, Y.; Chen, S.J.; Tsai, S.H.; Hung, K.S.; et al. Identification of Novel Genetic Variants Associated with Insomnia and Migraine Comorbidity. Nat. Sci. Sleep. 2022, 14, 1075–1087. [Google Scholar] [CrossRef]
- Snyder, H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Rehman, A.; Naz, S.; Razzak, I. Leveraging big data analytics in healthcare enhancement: trends, challenges and opportunities. Multimed. Syst. 2022, 28, 1339–1371. [Google Scholar] [CrossRef]
- Tapper, W.; Carneiro, G.; Mikropoulos, C.; Thomas, S.A.; Evans, P.M.; Boussios, S. The Application of Radiomics and AI to Molecular Imaging for Prostate Cancer. J. Pers. Med. 2024, 14, 287. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome. Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A.; et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Gupta, P.; Pandey, M.K. Role of AI for Smart Health Diagnosis and Treatment. In: Smart Medical Imaging for Diagnosis and Treatment Planning. Chapman and Hall/CRC; 2024. p. 23–45.
- Nazi, F.; Abbas, A. Personalized Medicine and Health Data Integration: Transforming Chronic Disease Monitoring with AI. Available online: https://www.researchgate.net/profile/Asad-Abbas-35/publication/382853245_Personalized_Medicine_and_Health_Data_Integration_Transforming_Chronic_Disease_Monitoring_with_AI/links/66af28aa299c327096ac1dc9/Personalized-Medicine-and-Health-Data-Integration-Transforming-Chronic-Disease-Monitoring-with-AI.pdf (accessed on 7 October 2024).
- Sufyan, M.; Shokat, Z.; Ashfaq, U.A. Artificial intelligence in cancer diagnosis and therapy: Current status and future perspective. Comput. Biol. Med. 2023, 165, 107356. [Google Scholar] [CrossRef]
- Majnarić, L.T.; Babič, F.; O'Sullivan, S.; Holzinger, A. AI and Big Data in Healthcare: Towards a More Comprehensive Research Framework for Multimorbidity. J. Clin. Med. 2021, 10, 766. [Google Scholar] [CrossRef]
- Stafie, C.S.; Sufaru, I.G.; Ghiciuc, C.M.; Stafie, I.I.; Sufaru, E.C.; Solomon, S.M.; Hancianu, M. Exploring the Intersection of Artificial Intelligence and Clinical Healthcare: A Multidisciplinary Review. Diagnostics. (Basel). 2023, 13, 1995. [Google Scholar] [CrossRef]
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front. Neurol. 2022, 12, 800605. [Google Scholar] [CrossRef]
- Winter, A.C.; Rexrode, K.M.; Lee, I.M.; Buring, J.E.; Tamimi, R.M.; Kurth, T. Migraine and subsequent risk of breast cancer: a prospective cohort study. Cancer. Causes. Control. 2013, 24, 81–89. [Google Scholar] [CrossRef]
- Chen, C.H.; Sheu, J.J.; Lin, Y.C.; Lin, H.C. Association of migraines with brain tumors: a nationwide population-based study. J. Headache. Pain. 2018, 19, 111. [Google Scholar] [CrossRef]
- Rice, M.S.; Rist, P.M.; Winter, A.C.; Kurth, T.; Tworoger, S.S. Migraine and invasive epithelial ovarian cancer risk in the Nurses' Health Study II and the Women's Health Study. Int. J. Cancer. 2018, 142, 534–539. [Google Scholar] [CrossRef]
- Hesari, E.; Ahmadinezhad, M.; Arshadi, M.; Azizi, H.; Khodamoradi, F. The association between migraine and breast cancer risk: A systematic review and meta-analysis. PLoS. One. 2022, 17, e0263628. [Google Scholar] [CrossRef]
- Peng, C.; Wu, K.; Chen, X.; Lang, H.; Li, C.; He, L.; Chen, N. Migraine and Risk of Breast Cancer: A Systematic Review and Meta-analysis. Clin. Breast. Cancer. 2023, 23, e122–e130. [Google Scholar] [CrossRef]
- Todd, C.; Lagman-Bartolome, A.M.; Lay, C. Women and Migraine: the Role of Hormones. Curr. Neurol. Neurosci. Rep. 2018, 18, 42. [Google Scholar] [CrossRef]
- Kursun, O.; Yemisci, M.; van den Maagdenberg, A.M.J.M.; Karatas, H. Migraine and neuroinflammation: the inflammasome perspective. J. Headache. Pain. 2021, 22, 55. [Google Scholar] [CrossRef]
- Conti, P.; D'Ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur. J. Pharmacol. 2019, 844, 87–94. [Google Scholar] [CrossRef]
- Sudershan, A.; Younis, M.; Sudershan, S.; Kumar, P. Migraine as an inflammatory disorder with microglial activation as a prime candidate. Neurol. Res. 2023, 45, 200–215. [Google Scholar] [CrossRef]
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Key, R.G.; Liebling, D.; Malhotra, V.T.; Passik, S.D.; Moryl, N, Breitbart WS. 2021. Cancer-related pain. In PsychoOncology,ed.Breitbart W, Butow P, Jacobsen P, Lam W, Lazenby M, Loscalzo M, pp.235–254.New York: Oxford Univ. Press. 4th ed.
- Nesbit, S.; Browner, I.; Grossman, S.A. (2019). Cancer-Related Pain. In Abeloff’s Clinical Oncology (pp. 581-592.e2). Elsevier.
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Woldeamanuel, Y.W.; Cowan, R.P. Computerized migraine diagnostic tools: a systematic review. Ther. Adv. Chronic. Dis. 2022, 13, 20406223211065235. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Zanzotto, F.M.; Scarpato, N.; Spila, A.; Fofi, L.; Egeo, G.; Rullo, A.; Palmirotta, R.; Barbanti, P.; Guadagni, F. Machine learning approach to predict medication overuse in migraine patients. Comput. Struct. Biotechnol. J. 2020, 18, 1487–1496. [Google Scholar] [CrossRef]
- Shah, D.; Zheng, W.; Allen, L.; Wei, W.; LeMasters, T.; Madhavan, S.; Sambamoorthi, U. Using a machine learning approach to investigate factors associated with treatment-resistant depression among adults with chronic non-cancer pain conditions and major depressive disorder. Curr. Med. Res. Opin. 2021, 37, 847–859. [Google Scholar] [CrossRef]
- Katsuki, M.; Narita, N.; Matsumori, Y.; Ishida, N.; Watanabe, O.; Cai, S.; Tominaga, T. Preliminary development of a deep learning-based automated primary headache diagnosis model using Japanese natural language processing of medical questionnaire. Surg. Neurol. Int. 2020, 11, 475. [Google Scholar] [CrossRef]
- Wei, H.L.; Wei, C.; Feng, Y.; Yan, W.; Yu, Y.S.; Chen, Y.C.; Yin, X.; Li, J.; Zhang, H. Predicting the efficacy of non-steroidal anti-inflammatory drugs in migraine using deep learning and three-dimensional T1-weighted images. iScience. 2023, 26, 108107. [Google Scholar] [CrossRef]
- Vandenbussche, N.; Van Hee, C.; Hoste, V.; Paemeleire, K. Using natural language processing to automatically classify written self-reported narratives by patients with migraine or cluster headache. J. Headache. Pain. 2022, 23, 129. [Google Scholar] [CrossRef]
- Nie, W.; Zeng, W.; Yang, J.; Zhao, L.; Shi, Y. Classification of Migraine Using Static Functional Connectivity Strength and Dynamic Functional Connectome Patterns: A Resting-State fMRI Study. Brain. Sci. 2023, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Facchetti, S.; De Icco, R.; Martinelli, D.; Allena, M.; Greco, R.; Tassorelli, C. Biomarkers of Migraine: An Integrated Evaluation of Preclinical and Clinical Findings. Int. J. Mol. Sci. 2023, 24, 5334. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Terwindt, G.M.; Al-Karagholi, M.A.; de Boer, I.; Lee, M.J.; Hay, D.L.; Schulte, L.H.; Hadjikhani, N.; Sinclair, A.J.; Ashina, H.; et al. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet. 2021, 397, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Salisbury, C.; Banks, J.; Whiting, P.; Hamilton, W. Predictive value of inflammatory markers for cancer diagnosis in primary care: a prospective cohort study using electronic health records. Br. J. Cancer. 2019, 120, 1045–1051. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Khan, R.; Sahu, P.; Ludhiadch, A.; Singh, G.; Munshi, A. Role of Omics in Migraine Research and Management: A Narrative Review. Mol. Neurobiol. 2022, 59, 5809–5834. [Google Scholar] [CrossRef]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef]
- Nagireddi, J.N.; Vyas, A.K.; Sanapati, M.R.; Soin, A.; Manchikanti, L. The Analysis of Pain Research through the Lens of Artificial Intelligence and Machine Learning. Pain. Physician. 2022, 25, E211–E243. [Google Scholar]
- Chen, E.; Prakash, S.; Janapa Reddi, V.; Kim, D.; Rajpurkar, P. A framework for integrating artificial intelligence for clinical care with continuous therapeutic monitoring. Nat. Biomed. Eng. 2023. [Epub ahead of print]. [Google Scholar] [CrossRef]
- Thapa, C.; Camtepe, S. Precision health data: Requirements, challenges and existing techniques for data security and privacy. Comput. Biol. Med. 2021, 129, 104130. [Google Scholar] [CrossRef]
- Balthazar, P.; Harri, P.; Prater, A.; Safdar, N.M. Protecting Your Patients' Interests in the Era of Big Data, Artificial Intelligence, and Predictive Analytics. J. Am. Coll. Radiol. 2018, 15, 580–586. [Google Scholar] [CrossRef]
- Forcier, M.B.; Gallois, H.; Mullan, S.; Joly, Y. Integrating artificial intelligence into health care through data access: can the GDPR act as a beacon for policymakers? J. Law. Biosci. 2019, 6, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Forti, M. The Deployment of Artificial Intelligence Tools in the Health Sector: Privacy Concerns and Regulatory Answers within the GDPR. Eur. J. Leg. Stud. 2021, 13, 29–44. [Google Scholar]
- Freymann, J.B.; Kirby, J.S.; Perry, J.H.; Clunie, D.A.; Jaffe, C.C. Image data sharing for biomedical research--meeting HIPAA requirements for De-identification. J. Digit. Imaging. 2012, 25, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Ahmed, M.; Basu, S.; Curtin, J.J.; Evans, B.J.; Matheny, M.E.; Nundy, S.; Sendak, M.P.; Shachar, C.; Shah, R.U.; et al. Advancing Artificial Intelligence in Health Settings Outside the Hospital and Clinic. NAM. Perspect. 2020, 2020, 10.31478/202011f. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC. Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Chander, B.; John, C.; Warrier, L.; Gopalakrishnan, K. Toward Trustworthy Artificial Intelligence (TAI) in the Context of Explainability and Robustness. ACM. Comput. Surv. 2024. [Google Scholar] [CrossRef]
- Winter, P.M.; Eder, S.; Weissenböck, J.; Schwald, C.; Doms, T.; Vogt, T.; Hochreiter, S.; Nessler, B. Trusted Artificial Intelligence: Towards Certification of Machine Learning Applications. arXiv 2021, 2103.16910. [Google Scholar]
- Khanna, S.; Srivastava, S.; Khanna, I.; Pandey, V. Ethical Challenges Arising from the Integration of Artificial Intelligence (AI) in Oncological Management. Int. J. Artif. Intell. 2020, 10, 34–44. [Google Scholar]
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef]
- Olawade, D.B.; David-Olawade, A.C.; Wada, O.Z.; Asaolu, A.J.; Adereni, T.; Ling, J. Artificial intelligence in healthcare delivery: Prospects and pitfalls. J. Med. Surg. Public. Health. 2024, 3, 100108. [Google Scholar] [CrossRef]
- Daneshjou, R.; Smith, M.P.; Sun, M.D.; Rotemberg, V.; Zou, J. Lack of Transparency and Potential Bias in Artificial Intelligence Data Sets and Algorithms: A Scoping Review. JAMA. Dermatol. 2021, 157, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M. The challenges of health inequities and AI. Intell-Based. Med. 2022, 6, 100067–100067. [Google Scholar] [CrossRef]
- Gore, M.N.; Olawade, D.B. Harnessing AI for public health: India's roadmap. Front. Public. Health. 2024, 12, 1417568. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).