1. Introduction
Rice (
Oryza sativa L.) is one of the world’s major food crops and a staple food for over 50% of the global population. It is widely planted in various regions and holds significant social and economic importance. With the rapid population growth, the demand for food crops has greatly increased. According to the Food and Agriculture Organization (FAO) of the United Nations, the global demand for agricultural products is expected to grow by about 70% by 2050. Therefore, the annual grain production needs to increase from the current 2.1 billion tons to approximately 3 billion tons to feed the predicted world population[
1]. The biotic stresses caused by field diseases and pests affect crop yields to varying degrees. Diseases such as rice blast, bacterial leaf blight, and sheath blight significantly limit rice yield. The brown planthopper is a huge challenge in rice production worldwide, causing serious yield losses in rice. Extreme climatic conditions such as high temperatures, droughts, and floods occur frequently worldwide, and these abiotic stresses reduce crop yields by nearly 50%, further threatening human food security[
2]. Therefore, cultivating high-yielding, high-quality, and environmentally adaptable crop varieties has currently become the goal pursued by breeders, and improving plants’ tolerance to biotic and abiotic stresses is one of the focuses of agricultural research[
3]. However, traditional crop breeding methods require processes such as crossing, backcrossing, and selection, which involve long breeding cycles, complex steps, and low efficiency, severely limiting the speed of breeding. Random mutagenesis, naturally occurring mutations, and classical breeding techniques require prolonged periods and sometimes do not generate individuals with the desired phenotype. Gene editing is a simple and efficient technique for inserting, deleting, or replacing gene bases at the genomic level. Previous studies have shown that gene editing can be used for crop trait improvement while reducing the effects of abiotic stresses[
4,
5]. The emergence and development of gene editing technologies such as CRISPR/Cas9 have provided new approaches for efficient germplasm innovation and genetic improvement in crops such as rice.
2. The Emergence and Development of CRISPR/Cas9
2.1. The Emergence of CRISPR/Cas System
In 1987, Ishino et al. discovered regular repeats of DNA sequences in
Escherichia coli[
6]. Mojica also discovered similar repetitive sequences in 2000 when analyzing archaeal DNA sequences[
7]. In 2005, Mojica discovered that prokaryotes contain short repetitive DNA sequences called Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), which form the prokaryotic adaptive immune system together with CRISPR-associated (Cas) genes. This system can recognize and perform targeted cleavage on the specific sequences of bacteriophages that have previously invaded the bacteria[
8]. Another study further demonstrated that CRISPR/Cas is part of the adaptive defense system of bacteria and archaea against invasion by phages and exogenous plasmid DNA and that it includes CRISPR/Cas9-related Cas enzymes[
9]. Based on phylogenetics, comparative genomics, and structural analysis, CRISPR/Cas systems can be divided into three types: type I systems containing Cas3 protein, type II systems containing Cas9 protein, and type III systems containing Cas10 protein[
10,
11].
CRISPR/Cas is an important genome editing tool developed based on the CRISPR system and widely used in various organisms, such as bacteria, yeast, and plants. CRISPR/Cas9 consists of two basic components: gRNA (guide RNA), which identifies the target gene sequences, and Cas9 (CRISPR-associated protein 9), which is an endonuclease that can create double-stranded DNA breaks in the genome. The double-strand breaks (DSBs) of chromosomes can be repaired through two DNA repair mechanisms, i.e., homology-directed repair (HDR) and non-homologous end-joining (NHEJ) [
12]. The DSBs generated by Cas9 induce NHEJ repair, which produces small random insertions or deletions at the cleavage site[
13]. These DSBs can also be repaired in a targeted manner through HDR, producing precise genomic modifications at the cleavage sites[
14].
2.2. The Development of CRISPR/Cas System
CRISPR technology was first applied to mammalian cells in 2013, with the successful achievement of Cas9-induced DSBs in both mouse and human cells[
15]. Due to its simple design, low vector construction cost, and high editing efficiency, CRISPR/Cas9 gene editing technology has been widely used to study gene functions in animals, plants, and microorganisms, making it the mainstream gene editing technology in a short period[
16]. The functions of the CRISPR/Cas9 components have also been explored and optimized, leading to the development of base-editors capable of performing single-base editing and guided-editing techniques capable of conducting precise genome modification[
17,
18]. Different types of genome-editing tools can mediate DNA DSBs, triggering HDR and NHEJ repair mechanisms to achieve base deletion or insertion of the target gene. Compared with techniques such as ZFNs (zinc-finger guided-editing techniques) and TALENs (transcription activator-like effector nucleases), CRISPR/Cas9 exhibits higher gene editing efficiency and accuracy, reducing off-target effects while enhancing editing efficiency[
19].
2.3. Trends in the Publishing of CRISPR/Cas-Related Papers
Scientific and technological journals are important sources for knowledge transmission and academic communication [
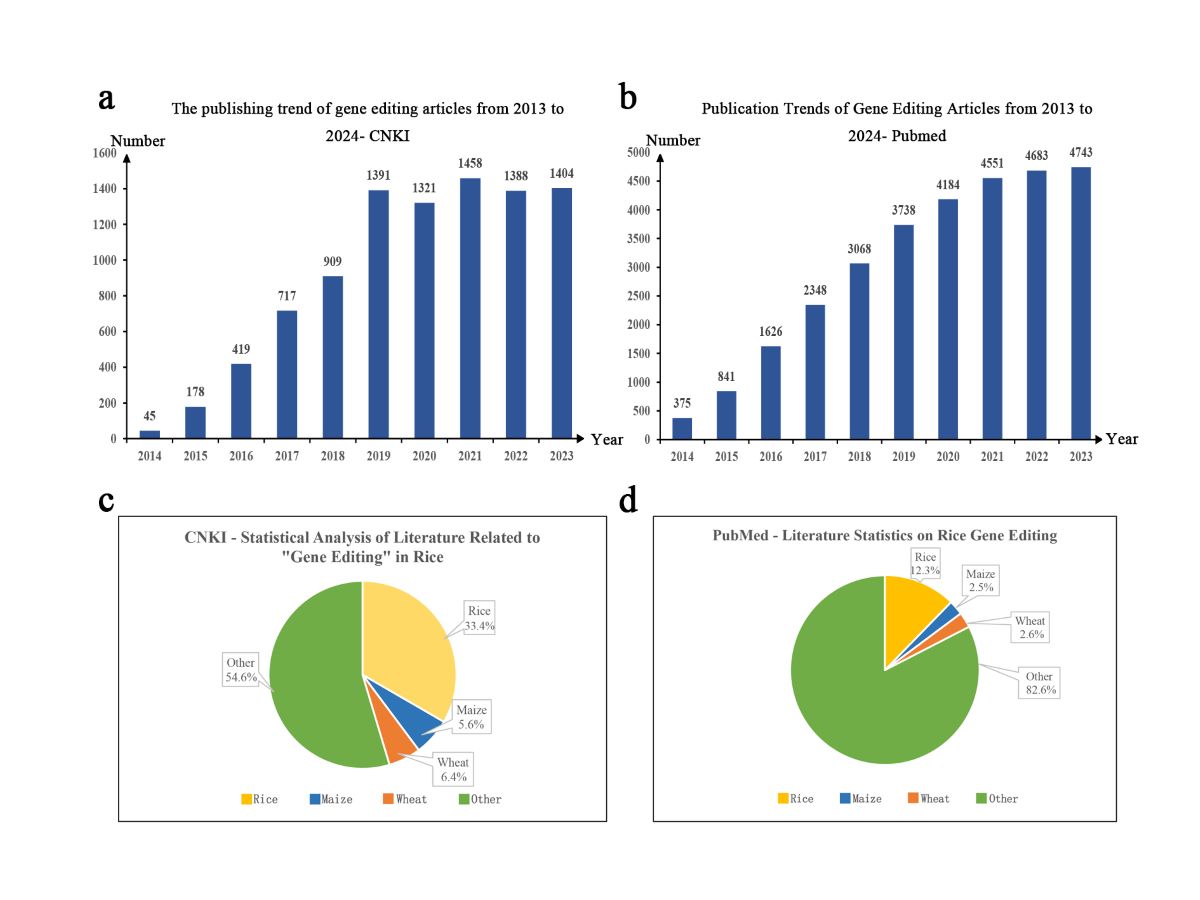
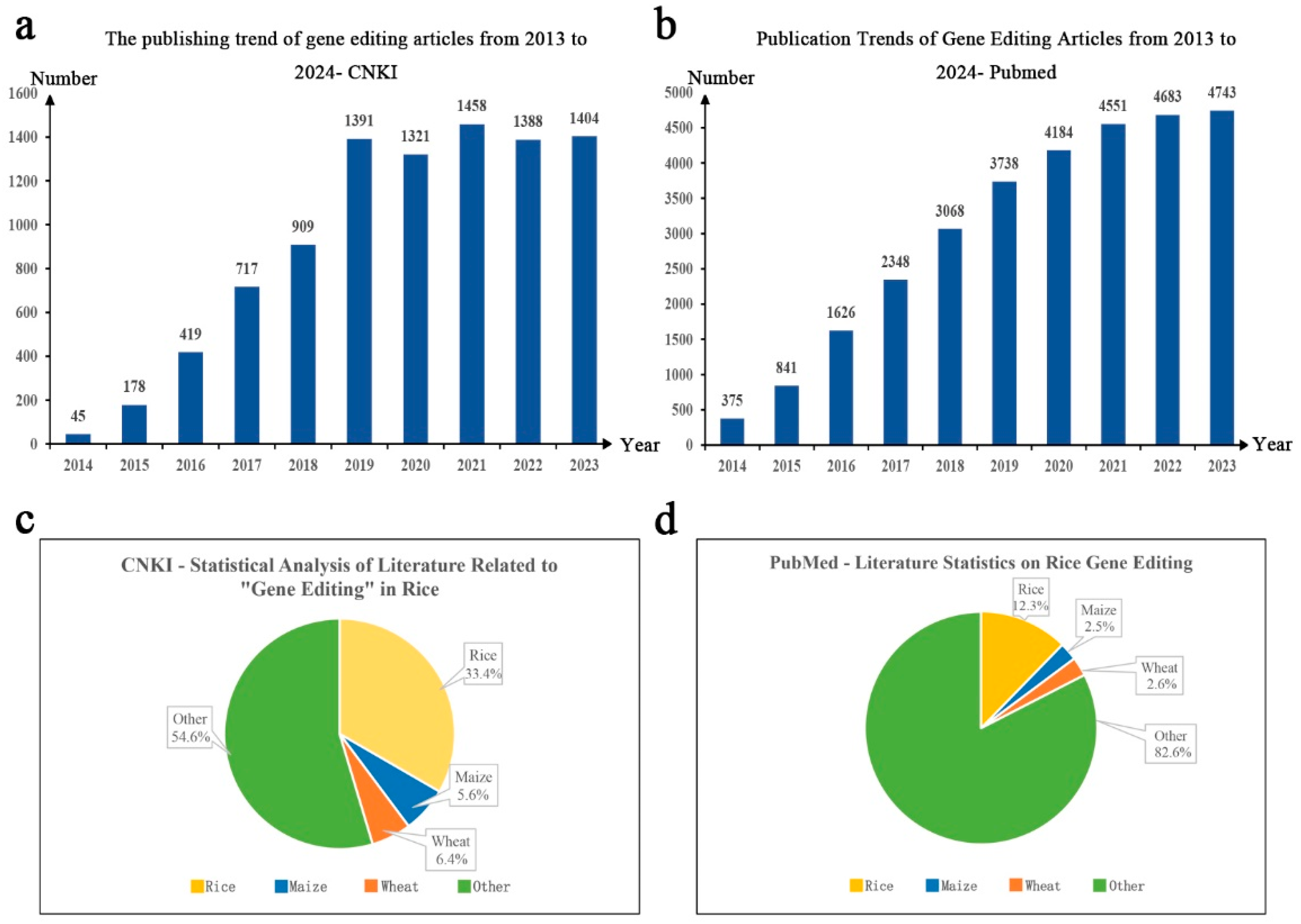
20], and the quantitative analysis of journal literature can effectively reflect the development trend of a certain research field. China National Knowledge Infrastructure (CNKI) and PubMed are well-known literature review platforms, with massive amounts of literature data available for reference and analysis. This article aimed to understand the publication status of gene editing papers in China and internationally and accurately determine the development trends and progress in gene editing technology. Using "gene editing" as the search term, 9230 and 30,157 papers related to gene editing-related papers published between 2014 and 2023 were retrieved from CNKI and PubMed, respectively, with an increasing trend of publication in subsequent years (
Figure 1a and
Figure 1b). To further analyze the research progress in staple crop gene editing, we used "gene editing" and "CRISPR/Cas9" as search terms and "crops" as the discipline category to download and summarize the literature published between 2014 and 2023, obtaining a total of 2163 publications on CNKI. Using text analysis methods, the "titles" of 2,163 papers were extracted for further analysis. After comparison and deduplication, 1,631 valid papers were obtained. Among them, 544, accounting for 33.4%, were related to rice gene editing, 91, accounting for 5.6%, were related to maize (
Zea mays L.) gene editing, and 105, accounting for 6.4%, were related to wheat (
Triticum aestivum L.) gene editing (
Figure 1c).
Similarly, by summarizing and analyzing literature related to "gene editing" and "plant CRISPR/Cas9" on the English literature indexing website PubMed, 4,995 valid studies were obtained, with studies on rice, maize, and wheat accounting for 12.3%, 2.5%, and 2.6%, respectively (
Figure 1d). According to the results obtained from the two databases, gene editing studies in rice involved both basic and applied research compared to those in maize and wheat, indicating that rice gene editing plays a dominant role as the basis for gene editing application in crops. Rice is a major cereal crop and an important model crop species. CRISPR/Cas9 technology not only promotes gene functional studies in rice but also provides an efficient approach for variety improvement.
3. Application of CRISPR/Cas9 in Rice Genetic Improvement
3.1. Biotic Stress Resistance
3.1.1. Rice Blast Resistance
Rice blast is among the major diseases that threaten rice growth, reducing the total global rice yield by 10% to 30% annually[
21]. At present, chemical control is the main approach used to prevent and control rice blast; however, large-scale chemical usage causes land pollution and pesticide resistance in the pathogen. In recent years, CRISPR/Cas9 gene editing technology has been effectively employed to improve the disease resistance of rice by editing disease-resistant genes to generate new disease-resistant varieties. Wang et al. conducted functional knockout of the
Pi21 gene in Nanjing 9108 rice variety using CRISPR/Cas9 technology to obtain mutants, laying the foundation for developing new rice lines with broad-spectrum resistance to rice blast[
22]. Similarly, Zhou et al. created new hybrid rice germplasm with broad-spectrum resistance to rice blast by knocking out the blast disease susceptibility genes
Bsr-d1,
Pi21, and
ERF922 in the sterile line Longke 638S[
23]. Wu and Peng et al used gene editing technology to knock out the
pid3 and
pi21 genes of the rice variety Dalixiang, creating high-quality materials resistant to rice blast disease without genetic modification or changes in rice quality[
24,
25].
3.1.2. Bacterial Blight Resistance
Bacterial blight is a vascular disease caused by the Gram-negative bacterium
Xanthomonas oryzae pv. Oryzae (Xoo). It can reduce rice yield by 20% to 30%, or up to 50% in severe cases, and even result in no grain harvest[
26]. Zhu et al. used a single Cas9/gRNA to target the homologous sequences of the
Xa13 and
Xa25 genes, disrupting the function of the target genes and inducing bacterial blight resistance in five rice varieties, including Yuxiang Youzhan, indica restorer line Shuhui 143, and indica sterile line Zhinong S. This provided a promising germplasm resource for developing rice varieties with lasting resistance to bacterial blight[
27]. Zeng et al. used CRISPR/Cas9 to modify the corresponding coding region of
OsSWEET14 in the rice variety Zhonghua 11 and generated 9 different
OsSWEET14 mutant alleles. The mutants exhibited not only broad-spectrum resistance to Asian bacterial strains but also strong resistance to African bacterial strain
AXO1947[
28].
3.1.3. Brown Planthopper Resistance
The frequent occurrence of pests in rice fields is a huge danger affecting the world’s food security. Currently, the biological methods for agricultural pest control mainly involve mining and utilizing insect-resistant genes, as well as breeding rice varieties with strong disease resistance. The brown planthopper is a typical monophagous herbivore that feeds on the phloem and poses a significant threat to rice production and global food security. Lu et al. conducted an integrated transcriptome and metabolome analysis and found that the
OsSPL10 knockout could enhance direct and indirect resistance to
BPH, and this can serve as a potential target for rice genetic improvement for resistance to brown planthopper[
29]. Tang et al. used the rice line Xiushui 11 to construct a homozygous
OsLRR-RLK18 knockout line and found that the egg production of brown planthoppers on this line was significantly reduced compared to that on un-edited Xiushui 11, indicating that
OsLRR-RLK18 plays an essential role in regulating rice resistance to brown planthoppers[
30]. Lu et al. knocked out the
CYP71A1 gene in the Xidao 1 rice variety, generating a mutant with significant insect resistance[
31].
3.2. Tolerance to Abiotic Stresses
3.2.1. Salt Tolerance
Salt stress is one of the unfavorable factors caused by global climate change that affects the growth of crops. Rice is very sensitive to salt stress, especially during the seedling stage. Identifying salt-tolerance genes and developing genetically modified germplasm for rice breeding are effective ways to reduce the impact of salt stress on rice production. Zhang et al. obtained two T2 homozygous mutant lines with significantly enhanced salt tolerance using Cas9-OsRR22-gRNA, thereby improving the salt tolerance of the japonica rice variety WPB106 [
32]. Sheng et al. improved the salt tolerance of next-generation hybrid rice lines by combining CRISPR/Cas9-mediated targeted editing of
OsRR22 with hybrid vigor utilization in varieties 733-3B and Huazhan[
33]. Furthermore, Hang et al. used CRISPR/Cas9 technology to create a new
OsRR22 mutant germplasm with improved salt tolerance from the three-line restorer line R192, providing a reference for improving salt tolerance in rice[
34]. Kumar et al. used CRISPR/Cas9 for targeted editing of the
DST gene of indica rice variety MTU1010 and found that the mutant had a higher tolerance to salt stress, with increased yield[
35].
3.2.2. Herbicide Resistance
Weeds are an important factor affecting rice production since they compete with rice for resources such as nutrients, water, and sunlight. In addition, the presence of weeds also increases the occurrence of pests and diseases and can reduce rice yield by more than 40%[
36]. Appropriate use of herbicides can reduce the impact of weeds on rice yield to a certain extent. At present, most herbicides that can kill weeds also cause certain damage to rice plants. Using CRISPR/Cas9 technology to quickly and accurately edit relevant genes regulated by herbicides and cultivating herbicide-resistant rice varieties are the most promising means to control weeds. Jiang et al. used optimized base-editing techniques ePE3max and ePE5max to edit the
EPSPS gene in japonica rice variety, generating homozygous mutations (T173I and A174V) and a heterozygous mutation P177S (TAP-IVS), thus laying a solid foundation for non-transgenic breeding of glyphosate resistance in rice[
37]. Li et al. used the CRISPR/Cas9-mediated NHEJ pathway to insert endogenous
EPSPS genes in rice to obtain rice materials containing glyphosate-resistant genes[
38]. Furthermore, Liu et al. generated two new single-mutation sites, I1879V and W2125S, in the coding region of the herbicide-resistant gene
OsACC through CRISPR-mediated base screening of rice callus, generating the same aryloxyphenoxypropionic acid herbicide resistance previously reported in the C2186R site[
39,
40]. Sun et al. used a CRISPR/Cas9 plasmid vector with two gRNAs to modify the
ALS sequence in the rice genome through directed insertion and substitution of bases and obtained herbicide-resistant rice varieties containing a discontinuous point mutation in
ALS[
41]. Kuang et al. used base-editing-mediated gene evolution (BEMGE) to artificially change the
OsALS1 gene in rice cells, thereby generating two sites with varying degrees of tolerance to the herbicide sodium dipyridamole. The study also edited the P171F site of the
OsALS gene in Nanjing 46 using base-editing to obtain mutant materials resistant to bispyribac-sodium, demonstrating the enormous potential of the BEMGE method in creating important genetic variations in target genes for crop improvement[
42]. Wu et al. used CRISPR/Cas9 and CRISPR/Cas12a to edit the 3'UTR region of the
OsHPPD gene and created a rice line resistant to HPPD herbicide in the background of the Yangjing 3012 variety[
43]. Using an optimized adenine base editor (ABE) TadA9, Yan et al. simultaneously edited four endogenous herbicide-resistant genes in the commercial rice variety Nanjing 46, and generated SNPs for multiple herbicide-resistant genes, providing a potential approach for controlling weeds in rice fields[
44]
3.3. Improving Rice Quality
3.3.1. Grain Shape
Currently, cultivating rice with suitable grain shapes and low chalkiness is important for improving the appearance quality of rice. Slender grain rice has lower chalkiness and better appearance quality but lower whole-grain rate and grain weight. Zhao et al. used gene editing technology to knock out the
GS9 gene in the Yandao 8 rice variety. The
gs9 mutant had increased grain length, a significantly reduced chalkiness rate and degree, and a significantly improved appearance quality[
45]. Usman et al. used CRISPR/Cas9 technology to edit the
GS3 gene in the japonica rice variety TP309 and obtained stable long-grain rice mutants[
46].
3.3.2. Chalkiness Degree and Chalky Grain Rate
Liu et al. simultaneously edited
Chalk5,
Gn1a,
GW2, and
TGW3 genes in the high-quality variety Nanjing 9108 using CRISPR/Cas9 technology, obtaining different allelic variation combinations that significantly reduced chalkiness and improved grain weight and yield[
47]. Tan et al. used CRISPR/Cas9 technology to edit the region near the ACII motif in the promoter region of the gene encoding the slender grain type
SLG7/GL7/GW7 and created three new
SLG7 allelic variations that significantly reduced chalkiness in the background of Wuyunjing 30. Another study indicated that the
AH2 transcription factor could bind the
SLG7 promoter and inhibit its expression. Knocking out the ACII element or adjacent fragments of the
SLG7 promoter can also weaken the binding ability of
AH2 to the
SLG7 promoter, thereby enhancing the expression level of
SLG7, increasing the length-width ratio of grain, and reducing rice chalkiness[
48].
GWD1/LSE1 encodes a glucan-hydrating kinase that regulates starch metabolism in rice leaves. CRISPR/Cas9 technology was used to edit the promoter region of
GWD1, creating two down-regulated mutants,
gwd1-1 and
gwd1-2. The chalky rate and chalkiness degree of these two lines significantly decreased, with improved appearance quality and no significant changes in other traits such as plant type and grain weight[
49,
50].
3.3.3. Rice Fragrance
Fragrance is one of the important indicators that determine the edibility of rice grain. 2-acetyl-1-pyrroline (2-AP) is the main source of rice fragrance, and its biosynthesis is mainly controlled by the recessive gene
OsBADH2 encoding betaine aldehyde dehydrogenase. Qi et al. edited exons 2 and 7 of the
OsBADH2 gene of non-aromatic japonica rice varieties Jia 58 and Xiushui 134 using CRISPR/Cas9 technology and generated multiple mutants. The 2-AP content was significantly increased in four T2-edited materials in the background of Xiushui 134[
51]. Zhu et al. performed targeted aroma improvement for Ningjing 50 using CRISPR/Cas9. The grain of the mutant had increased aroma, with no significant changes in the chalky rate and chalkiness degree[
52].
3.3.4. Low Content of Amylose Starch
The taste quality directly reflects consumers’ acceptance of rice and is an important indicator for evaluating rice quality. The taste quality of rice is determined by various characteristics, from its production to processing. Rice with good taste quality is characterized by a good appearance, round grains, strong aroma, and moderate softness and hardness. Zeng et al. created new allelic variations conferring varying degrees of reduced amylose content, such as
Wxa-dA,
Wxa-dC1,
Wxa-dC2, and
Wxa-dC3, by editing the promoter region of the
Wx gene in indica rice variety TFB[
53]. The
Du13 gene can regulate the splicing efficiency of rice endosperm gene
Wxb by activating the C2H2 zinc-finger protein, thereby affecting the amylose content in rice. Cai et al. used gene-editing technology to perform a knockout experiment on the japonica rice variety Koshihikari and indica rice variety Nanjing 11 to create a mutant
Y1 with a decreased amylose content and an increased taste value[
54]. Transcription factors can bind to the promoter sequence of target genes to regulate its gene expression. Many reports have shown that the amylose content in rice can be regulated by editing transcription factors to regulate the expression of the
Wx gene [
55,
56,
57].
3.4. Nutrition and Safety of Rice
3.4.1. Low Glutelin Content
The endosperm is the main edible component of rice, composed of starch (80%~90%), protein ( 7%~10%), and lipids ( 1%). The content and composition of each component in the endosperm directly affect the edibility and taste quality of rice[
58]. Genetic improvement of the regulatory genes of each component in the endosperm can improve the edibility and taste quality of rice. Yang et al. used CRISPR/Cas9 technology to simultaneously knock out 8 highly expressed genes from 4 subgroups (GluA, GluB, GluC, and GluD) of rice glutenin genes. The mutant rice showed varying degrees of reduced protein content[
59]. Chen et al. designed three sgRNAs to target nine glutenin genes and created nine homozygous lines without T-DNA fragments in the background of Wuyunjing 7, among which most lines showed varying degrees of reduced glutenin content[
60]. Wang et al. used gene editing technology to edit two amino acid transporter genes,
OsAAP6 and
OsAAP10, in multiple lines, such as Yangjing 158 and Nanjing 9108, significantly reducing glutenin content[
61].
3.4.2. Reducing Heavy Metal Accumulation in Rice
Heavy metals, even in trace amounts, pose a huge threat to human health. Plants can absorb heavy metals, and long-term consumption of foods with excessive heavy metal content can increase the risk of diseases[
62]. Excessive levels of heavy metals in rice, such as rice with excessive cadmium, not only affect its quality but also seriously impact human health. Cultivating low-cadmium rice varieties and reducing cadmium content in rice has become a new breeding goal for rice breeders. Studies have shown that the loss-of-function of the cadmium uptake and transport gene OsNramp5 can effectively reduce the accumulation of cadmium in rice. Hu et al. used CRISPR/Cas9 technology to knock out the cadmium uptake and transport gene OsNramp5 in Chuanhui 491 material and created new rice germplasm with low cadmium accumulation, providing new genetic resources for accelerating the cultivation of suitable rice varieties that can be planted in cadmium-contaminated areas[
63]. Similarly, Yang et al. knocked out the OsNRAMP5 gene in two japonica rice varieties, Nanjing 46 and Huaidao 5, using CRISPR/Cas9 and obtained three OsNRAMP5 mutants without transgenic markers. Compared with wild-type plants, the accumulation of cadmium and manganese was significantly reduced in the roots, shoots, and grains of the mutants[
64]. Chang et al. knocked out the OsNRAMP2 gene in Zhonghua 11, significantly reducing the distribution of cadmium in rice leaves, straw, and grains[
65]. Xu et al. used CRISPR/Cas9 technology to edit the promoter region of OsLsi1 and the C-terminal coding sequences of OsLsi1/OsLsi2 in Zhongjiazao 17 and Jiahe 212 varieties, thereby providing a method of reducing arsenic accumulation in rice grains without affecting grain yield[
66].
4. Future Prospects
After more than a decade of continuous development and innovation of gene-editing technology, particularly CRISPR/Cas9, rapid, targeted improvement of traits such as stress resistance, quality, nutrition, and safety have been achieved in various rice varieties. The technology greatly improved the efficiency of germplasm innovation and became an important genetic improvement method for rice. In addition, cutting-edge biological breeding technologies such as de novo domestication, non-fusion reproduction, and haploid induction developed through gene-editing technology also provide unlimited possibilities for the molecular improvement of crops. However, some challenges still limit the application of gene editing, including editing efficiency, intellectual property rights of gene-edited germplasm, and transgenic safety of the process of germplasm innovation:
(1) CRISPR/Cas9 technology is currently the most widely used method to edit target genes and improve crops by knocking out functional genes that negatively regulate target traits. However, the number of such major functional genes with negative regulatory effects is limited, and most traits are regulated synergistically by multiple genes. Therefore, there is a need for further functional genomics studies of rice to explore gene resources with important breeding value and iteratively upgrade the CRISPR system to enhance the ability and efficiency of site-directed mutagenesis, large fragment knock-in and multi-gene editing.
(2) Protecting intellectual property is protecting breeding innovation. Currently, commercially promoted rice varieties are mainly developed through traditional hybrid breeding with balanced, comprehensive traits. Gene-edited germplasms based on these varieties are more easily accepted in the market and can achieve industrialization, which also better reflects the applicability of gene editing technology. However, the edited germplasm and original basic varieties belong to substantial derived varieties. The revised version of the 2022 Seed Law in China specifically emphasizes the establishment of a substantial derived variety system, with the aim of acknowledging and encouraging original innovation and establishing a reasonable system for distributing benefits.
(3) Self-segregation can eliminate exogenous fragments in gene-edited progeny materials, which also ensures gene editing without introducing exogenous genes. Some countries, such as the United States, Japan, and Australia, have adopted relatively flexible regulatory policies for gene-edited crops[
67]. The Chinese Ministry of Agriculture and Rural Affairs issued the Guidelines for Safety Evaluation of Agricultural Gene-Edited Plants (Trial) and the Detailed Rules for Evaluation of Agricultural Gene-Edited Plants in 2022 and 2023, respectively. In 2023, Shandong BellaGen Biotechnology Co., Ltd. obtained China's first safety certificate of agricultural gene editing for high-oleic soybeans generated through gene editing, further accelerating the industrial application of gene editing technology in crops.
Thus, the breakthroughs in biological breeding technologies, such as gene editing, could advance rice breeding in China towards the Breeding 4.0 generation, ultimately achieving precise editing and breeding of crops and providing technical support for strengthening China’s seed industry.
Author Contributions
Conceptualization, L.L. and Y.L.; writing—original draft preparation, J.C.; formal analysis, Z.M. and D.K.; data curation, A.Z., F.W. and G.L; validation, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shanghai Municipal Commission of Science and technology (Grant No. 23N11900100), the Bill and Melinda Gates Foundation (INV-033236-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.
Acknowledgments
We would like to thank MogoEdit (
https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Romero, F.M.; Gatica-Arias, A. CRISPR/Cas9: Development and Application in Rice Breeding. Rice Science 2019, 26, 265–281. [Google Scholar] [CrossRef]
- Rahman, M.U.; Zulfiqar, S.; Raza, M.A.; Ahmad, N.; Zhang, B. Engineering Abiotic Stress Tolerance in Crop Plants through CRISPR Genome Editing. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Delorge, I.; Janiak, M.; Carpentier, S.; Van Dijck, P. Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front Plant Sci 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usadel, B.; Salts, Y.; Barg, R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant biotechnology journal 2017, 15, 634–647. [Google Scholar] [CrossRef]
- Dalla Costa, L.; Malnoy, M.; Gribaudo, I. Breeding next generation tree fruits: technical and legal challenges. Horticulture research 2017, 4, 17067. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Mojica, F.J.; Diez-Villasenor, C.; Soria, E.; Juez, G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol 2000, 36, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Mojica, F.J.; Diez-Villasenor, C.; Garcia-Martinez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Haft, D.H.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Horvath, P.; Moineau, S.; Mojica, F.J.; Wolf, Y.I.; Yakunin, A.F.; et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 2011, 9, 467–477. [Google Scholar] [CrossRef]
- Makarova, K.S.; Koonin, E.V. Annotation and Classification of CRISPR-Cas Systems. Methods Mol Biol 2015, 1311, 47–75. [Google Scholar] [CrossRef]
- Chapman, J.R.; Taylor, M.R.; Boulton, S.J. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 2012, 47, 497–510. [Google Scholar] [CrossRef]
- Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 2010, 79, 181–211. [Google Scholar] [CrossRef] [PubMed]
- San Filippo, J.; Sung, P.; Klein, H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem 2008, 77, 229–257. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Wang, H.; La Russa, M.; Qi, L.S. CRISPR/Cas9 in Genome Editing and Beyond. Annu Rev Biochem 2016, 85, 227–264. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Base editing a CRISPR way. Nat Methods 2018, 15, 767–770. [Google Scholar] [CrossRef]
- Marzec, M.; Braszewska-Zalewska, A.; Hensel, G. Prime Editing: A New Way for Genome Editing. Trends Cell Biol 2020, 30, 257–259. [Google Scholar] [CrossRef]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Qing, M.T.; Wang, Y.T.; Zhou, R.T. Visual communication of graphic information in the cover of scentific jorunals: Taking Scientific American (international edition) as example. Chinese Journal of Scientifc and Technical Periodicals 2018, 29, 211–220. [Google Scholar]
- Fernandez, J.; Orth, K. Rise of a Cereal Killer: The Biology of Magnaporthe oryzae Biotrophic Growth. Trends Microbiol 2018, 26, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Q.; Fan, F.J.; Li, W.Q.; Zhu, J.Y.; Wan, J.; Zhong, W.G.; Yang, J. Knock-out Efficiency Analysis of Pi21 Gene Using CRISPR/Cas9 in Rice. Chinese Journal OF Rice Science 2016, 30, 469–478. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, S.; Jiang, N.; Zhao, X.; Bai, Z.; Liu, J.; Yao, W.; Tang, Q.; Xiao, G.; Lv, C.; et al. Engineering of rice varieties with enhanced resistances to both blast and bacterial blight diseases via CRISPR/Cas9. Plant biotechnology journal 2022, 20, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Li, J.L.; Xu, H.F.; Zhang, X.C.; Zhang, D.S.; Zhu, S.S. Editing Pi21 Gene to Improve the Blast Resistance of Cultivated Rice dalixiang by Using CRISPR/Cas9 System. Molecular Plant Breeding 2022, 1–12. [Google Scholar]
- Wu, X.; Li, J.L.; Zeng, Q.H.; Zhang, X.C.; Zhang, D.S.; Xu, H.F.; Xue, J.; Song, L.; Zhu, S.S. Improvement of Blast Resistance of Rice Variety Dalixiang by CRISPR/Cas9 Gene Editing Technology. Seed 2021, 40, 50–55. [Google Scholar] [CrossRef]
- Yu, J.L.; Zhang, G.L.; Ding, X.W.; Gao, Y.; Xie, Y.F. Progress in Identification and Application of Resistance Genes to Bacterial Blight. Plant Physiology Journa 2012, 48, 223–231. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Luo, P.; Yan, J.; Cao, X.; Qian, H.; Zhu, X.; Fan, Y.; Mei, F.; Fan, M.; et al. Creation of Bacterial Blight Resistant Rice by Targeting Homologous Sequences of Xa13 and Xa25 Genes. Agronomy 2024. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, Y.; Vu, N.T.Q.; Shen, S.; Xia, K.; Zhang, M. CRISPR/Cas9-mediated mutation of OsSWEET14 in rice cv. Zhonghua11 confers resistance to Xanthomonas oryzae pv. oryzae without yield penalty. BMC Plant Biol 2020, 20, 313. [Google Scholar] [CrossRef]
- Lu, L.; Sun, Z.; Wang, R.; Du, Y.; Zhang, Z.; Lan, T.; Song, Y.; Zeng, R. Integration of transcriptome and metabolome analyses reveals the role of OsSPL10 in rice defense against brown planthopper. Plant Cell Rep 2023, 42, 2023–2038. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Peng, K.; Qiu, C.; Lu, J.; Lou, Y.G. Knocking out OsLRR-RLK18 in rice enhances the resistance to brown planthopper Nilaparvata lugens. Journal of Plant Protection 2024, 51, 383–393. [Google Scholar] [CrossRef]
- Lu, H.P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.Y.; Gatehouse, A.M.R.; Lou, Y.G.; et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nature plants 2018, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Molecular Breeding 2019, 39, 47. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Ai, Z.; Tan, Y.; Hu, Y.; Guo, X.; Liu, X.; Sun, Z.; Yu, D.; Chen, J.; Tang, N.; et al. Novel Salinity-Tolerant Third-Generation Hybrid Rice Developed via CRISPR/Cas9-Mediated Gene Editing. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, Z.; Li, P.; Xu, H.; Liu, K.; Zha, W.; Li, S.; Chen, J.; Yang, G.; Huang, J.; et al. Development of Novel Rice Germplasm for Salt-Tolerance at Seedling Stage Using CRISPR-Cas9. Sustainability 2022, 14, 2621. [Google Scholar] [CrossRef]
- Santosh Kumar, V.V.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao, M.V.; Chinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol Mol Biol Plants 2020, 26, 1099–1110. [Google Scholar] [CrossRef]
- Burgos, N.R.; Singh, V.; Tseng, T.M.; Black, H.; Young, N.D.; Huang, Z.; Hyma, K.E.; Gealy, D.R.; Caicedo, A.L. The impact of herbicide-resistant rice technology on phenotypic diversity and population structure of United States weedy rice. Plant Physiol 2014, 166, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chai, Y.; Qiao, D.; Wang, J.; Xin, C.; Sun, W.; Cao, Z.; Zhang, Y.; Zhou, Y.; Wang, X.-C.; et al. Optimized prime editing efficiently generates glyphosate-resistant rice plants carrying homozygous TAP-IVS mutation in <em>EPSPS</em>. Molecular plant 2022, 15, 1646–1649. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Zong, Y.; Chen, K.; Zhang, H.; Liu, J.; Li, J.; Gao, C. Gene replacements and insertions in rice by intron targeting using CRISPR-Cas9. Nature plants 2016, 2, 16139. [Google Scholar] [CrossRef]
- Li, C.; Zong, Y.; Wang, Y.; Jin, S.; Zhang, D.; Song, Q.; Zhang, R.; Gao, C. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biology 2018, 19, 59. [Google Scholar] [CrossRef]
- Liu, X.; Qin, R.; Li, J.; Liao, S.; Shan, T.; Xu, R.; Wu, D.; Wei, P. A CRISPR-Cas9-mediated domain-specific base-editing screen enables functional assessment of ACCase variants in rice. Plant biotechnology journal 2020, 18, 1845–1847. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X.; Du, W.; Zhao, Y.; Xia, L. Engineering Herbicide-Resistant Rice Plants through CRISPR/Cas9-Mediated Homologous Recombination of Acetolactate Synthase. Molecular plant 2016, 9, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, S.; Ren, B.; Yan, F.; Spetz, C.; Li, X.; Zhou, X.; Zhou, H. Base-Editing-Mediated Artificial Evolution of OsALS1 In Planta to Develop Novel Herbicide-Tolerant Rice Germplasms. Molecular plant 2020, 13, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, N.; Cai, Y.; Yang, Q.; Yu, L.; Chen, Z.; Shi, W.; Liu, J.; Pan, C.; Li, Y.; et al. CRISPR-Cas9-mediated editing of the OsHPPD 3' UTR confers enhanced resistance to HPPD-inhibiting herbicides in rice. Plant communications 2023, 4, 100605. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ren, B.; Liu, L.; Yan, F.; Li, S.; Wang, G.; Sun, W.; Zhou, X.; Zhou, H. High-efficiency and multiplex adenine base editing in plants using new TadA variants. Molecular plant 2021, 14, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.S.; Li, Q.F.; Zhang, C.Q.; Zhang, C.; Yang, Q.Q.; Pan, L.X.; Ren, X.Y.; Lu, J.; Gu, M.H.; Liu, Q.Q. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nature communications 2018, 9, 1240. [Google Scholar] [CrossRef]
- Usman, B.; Zhao, N.; Nawaz, G.; Qin, B.; Liu, F.; Liu, Y.; Li, R. CRISPR/Cas9 Guided Mutagenesis of Grain Size 3 Confers Increased Rice (Oryza sativa L. ) Grain Length by Regulating Cysteine Proteinase Inhibitor and Ubiquitin-Related Proteins. 2021, 22, 3225. [Google Scholar]
- Liu, P.; He, L.; Mei, L.; Zhai, W.; Chen, X.; Ma, B. Rapid and Directional Improvement of Elite Rice Variety via Combination of Genomics and Multiplex Genome Editing. J Agric Food Chem 2022, 70, 6156–6167. [Google Scholar] [CrossRef]
- Tan, W.; Miao, J.; Xu, B.; Zhou, C.; Wang, Y.; Gu, X.; Liang, S.; Wang, B.; Chen, C.; Zhu, J.; et al. Rapid production of novel beneficial alleles for improving rice appearance quality by targeting a regulatory element of SLG7. Plant biotechnology journal 2023, 21, 1305–1307. [Google Scholar] [CrossRef]
- Hirose, T.; Aoki, N.; Harada, Y.; Okamura, M.; Hashida, Y.; Ohsugi, R.; Akio, M.; Hirochika, H.; Terao, T. Disruption of a rice gene for α-glucan water dikinase, OsGWD1, leads to hyperaccumulation of starch in leaves but exhibits limited effects on growth. Front Plant Sci 2013, 4, 147. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, K.; Xiong, M.; Wang, J.D.; Zhang, C.Q.; Fan, X.L.; Huang, L.C.; Zhao, D.S.; Liu, Q.Q.; Li, Q.F. Glucan, Water-Dikinase 1 (GWD1), an ideal biotechnological target for potential improving yield and quality in rice. Plant biotechnology journal 2021, 19, 2606–2618. [Google Scholar] [CrossRef]
- Qiu, Y.B.; Zhang, L.X.; Wang, L.Y.; Song, J.; Wang, J.J. CRISPR/Cas9 Targeted Editing for the Fragrant Gene Badh2 in Rice. Scientia Agricultura Sinica 2020, 53, 1501–1509. [Google Scholar]
- Zhu, Y.S.; Shen, L.H.; Ma, J.; Wei, Y.D.; Chen, L.P.; Jiang, J.H.; Wu, F.X.; Zhang, J.F. Using CRISPR/Cas9 Technology to Edit BADH2 for Targeted Improvement of the Fragrance of Ningjing 50. Fujian Science and Technology 2023, 41, 18–22. [Google Scholar]
- Zeng, D.; Liu, T.; Ma, X.; Wang, B.; Zheng, Z.; Zhang, Y.; Xie, X.; Yang, B.; Zhao, Z.; Zhu, Q.; et al. Quantitative regulation of Waxy expression by CRISPR/Cas9-based promoter and 5'UTR-intron editing improves grain quality in rice. Plant biotechnology journal 2020, 18, 2385–2387. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, W.; Fu, Y.; Shan, Z.; Xu, J.; Wang, P.; Kong, F.; Jin, J.; Yan, H.; Ge, X.; et al. Du13 encodes a C(2) H(2) zinc-finger protein that regulates Wx(b) pre-mRNA splicing and microRNA biogenesis in rice endosperm. Plant biotechnology journal 2022, 20, 1387–1401. [Google Scholar] [CrossRef]
- Jin, S.K.; Xu, L.N.; Leng, Y.J.; Zhang, M.Q.; Yang, Q.Q.; Wang, S.L.; Jia, S.W.; Song, T.; Wang, R.A.; Tao, T.; et al. The OsNAC24-OsNAP protein complex activates OsGBSSI and OsSBEI expression to fine-tune starch biosynthesis in rice endosperm. Plant biotechnology journal 2023, 21, 2224–2240. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Zhang, Q.; Meng, S.; Wei, C. The NAC Transcription Factors OsNAC20 and OsNAC26 Regulate Starch and Storage Protein Synthesis. Plant Physiol 2020, 184, 1775–1791. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, X.L.; Wang, Z.Y.; Hong, M.M. An interaction between a MYC protein and an EREBP protein is involved in transcriptional regulation of the rice Wx gene. J Biol Chem 2003, 278, 47803–47811. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.H.; Lu, J.; Zhang, C.Q.; Liu, Q.Q.; Li, Q.F. Genes and Their Molecular Functions Determining Seed Structure, Components, and Quality of Rice. Rice (New York, N.Y.) 2022, 15, 18. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, Z.; Li, Y.; Xu, C.; Xia, H.; Zhuang, H.; Sun, S.; Guo, M.; Yan, C. Rapid improvement of rice eating and cooking quality through gene editing toward glutelin as target. Journal of integrative plant biology 2022, 64, 1860–1865. [Google Scholar] [CrossRef]
- Chen, Z.; Du, H.; Tao, Y.; Xu, Y.; Wang, F.; Li, B.; Zhu, Q.H.; Niu, H.; Yang, J. Efficient breeding of low glutelin content rice germplasm by simultaneous editing multiple glutelin genes via CRISPR/Cas9. Plant Sci 2022, 324, 111449. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Guo, M.; Zhong, C.; Yan, C.; Sun, S. Targeted mutagenesis of amino acid transporter genes for rice quality improvement using the CRISPR/Cas9 system. The Crop Journal 2020, 8, 457–464. [Google Scholar] [CrossRef]
- Zhao, F.J.; Xie, W.Y.; Wang, P. Soil and Human Health. Acta Pedologica Sinica 2020, 57, 1–11. [Google Scholar] [CrossRef]
- Hu, S.H.; Pu, Z.G.; He, Z.Y.; Wang, P.; Bai, Y.L.; Li, G.M.; Zhang, T.; Jiang, K.F.; Yang, L. Generating Low Cadmium Accumulation New Rice Germplasms by Editing OsNramp5 using CRISPR/Cas9 Technology. 2024, 1–11. [Google Scholar] [CrossRef]
- Yang, C.-h.; Zhang, Y.; Huang, C.-f. Reduction in cadmium accumulation in japonica rice grains by CRISPR/Cas9-mediated editing of OsNRAMP5. Journal of Integrative Agriculture 2019, 18, 688–697. [Google Scholar] [CrossRef]
- Chang, J.-D.; Xie, Y.; Zhang, H.; Zhang, S.; Zhao, F.-j.J.P.; Soil. The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germination and affects Cd distribution to rice grain. Plant and Soil 2022, 476, 79–95. [Google Scholar] [CrossRef]
- Xu, X.; Sun, S.K.; Zhang, W.; Tang, Z.; Zhao, F.J. Editing Silicon Transporter Genes to Reduce Arsenic Accumulation in Rice. Environmental science & technology 2024, 58, 1976–1985. [Google Scholar] [CrossRef]
- Raman, R. The impact of Genetically Modified (GM) crops in modern agriculture: A review. GM crops & food 2017, 8, 195–208. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).