1. Introduction
Aneurysms of the pancreaticoduodenal arcades (PDA) are uncommon, estimated to be less than 2% among all visceral artery aneurysms. However, they could be life-threatening if left untreated [
1]. Evidence suggests that, unlike other visceral aneurysms, the risk of PDA aneurysm rupture is not significantly affected by age, aneurysm size, number of aneurysms, or underlying cause. Rupture rates for untreated aneurysms can be as high as 50% and reported mortality rates following rupture range from 20% to 50%, underscoring the critical importance of comprehensive identification and treatment of PDA aneurysms [
2,
3,
4,
5,
6].
The pathophysiology of these aneurysms often involves the compensatory hypertrophy of the pancreaticoduodenal arteries secondary to celiac trunk occlusion or significant stenosis, commonly caused by median arcuate ligament syndrome, which affects approximately 4% of the general population, but also more rarely by atherosclerosis, or inflammatory diseases [
1]. In such cases, the increased blood flow and pressure through collateral pathways lead to heightened arterial wall stress and aneurysm formation [
7,
8,
9,
10].
The treatment of PDA aneurysms lacks consensus, particularly between surgical management with revascularization of the celiac trunk and endovascular embolization alone. Revascularization traditionally aims to restore celiac trunk flow to prevent splanchnic ischemia by addressing the underlying cause. However, this approach may be overly dogmatic. Recent evidence suggests that revascularization might not be necessary, as embolization alone can effectively exclude the aneurysm without increasing the risk of ischemic complications, thanks to multiple collateral pathways supplying the splanchnic territory. These findings challenge the need for surgical intervention in all cases and underscores the potential effectiveness of embolization alone [
11,
12].
The present study aims to evaluate the effectiveness and safety of retrograde embolization of high-flow PDA aneurysms without celiac trunk revascularization.
2. Materials and Methods
2.1. Study Design and Population
The local institutional review board approved this bicentric retrospective observational study (CERIM 10-2024). Because of its retrospective design, written informed consent was waived.
From January 2015 to July 2024, all adult patients with high-flow PDA aneurysms secondary to celiac trunk occlusion or significant stenosis (>50%), treated exclusively by image-guided trans-arterial embolization, were included. The interventions were performed either in an emergency context or in a scheduled manner without clinical symptoms.
Non-inclusion criteria were patients with false aneurysms of PDA and the absence of significant celiac trunk stenosis on computed tomography (CT).
2.2. Pre Interventional Imaging
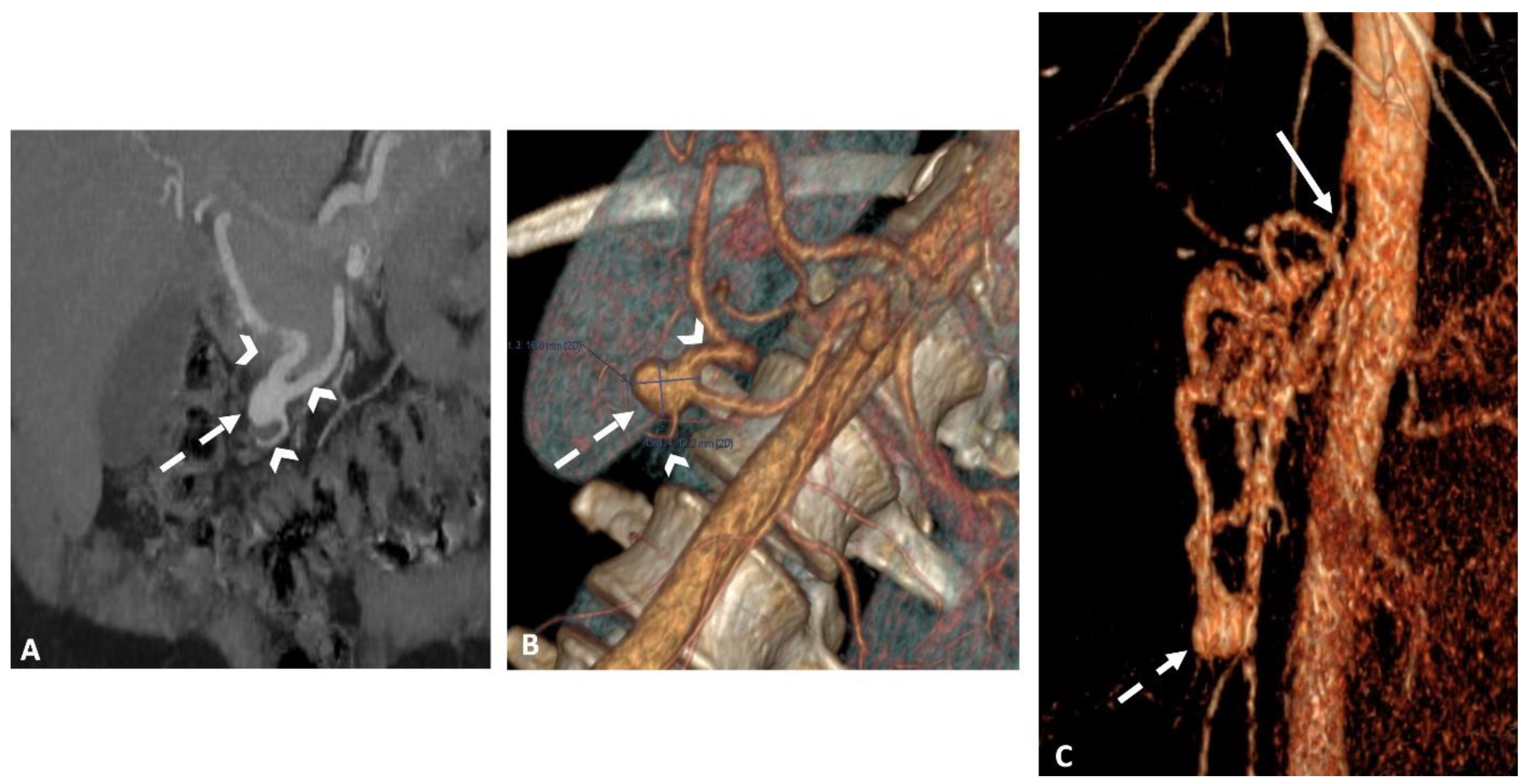
All patients underwent an abdominal dynamic contrast-enhanced CT scan using a multi-detector row CT (MDCT) (Revolution CT 256; GE Healthcare, Waukesha - WI, USA or Somatom Definition AS+128; Siemens Healthineers, Erlangen - Germany). The imaging protocol included unenhanced, arterial, and portal phase acquisitions following the intravenous administration of 1.5 mL/kg of iodinated contrast agent (Iomeron, 350-Iomeprol; Bracco Imaging, Milan, Italy) at a rate of 3–4 mL/s. A bolus-tracking technique with automated scan triggering was used, placing an elliptic region of interest in the descending thoracic aorta at the diaphragm level, with an enhancement threshold set at 100 HU. CT acquisition and reconstruction settings included: a tube current range of 150–650 mA (mean: 455), a tube voltage of 120 kVp, a rotation time of 0.7 s, and a pitch of 1.375. Automatic exposure control was managed using Auto mA-Smart mA with a noise index of 25, and the field of view was set to large body. Using model-based iterative reconstruction, images were reconstructed with a standard reconstruction kernel at a section thickness of 0.625 mm. The reconstructed images were archived in the institutional picture archiving and communication system (PACS) (
Figure 1).
2.3. Embolization Interventions
All interventions were performed in three angiographic suites with a flat panel detector C-arm angiographic system (Allura Clarity®/Azurion Clarity®; Phillips Healthcare, Best, The Netherlands).
Patients were placed in a supine position on the angiographic table. All procedures were performed under local anesthesia using either common femoral or brachial endovascular access, with the insertion of a long 5F sheath of 55 or 90 cm, depending on the access (Flexor®, Cook Medical, Bloomington, Indiana, USA). In the case of femoral access with challenging catheterization, the sheath was stabilized in the superior mesenteric artery (SMA) using the anchoring technique with a 0.014" stiff guidewire (Spartacore®, Abbot, Illinois, USA) in a non-target branch of SMA [
13].
All embolization interventions were performed using a retrograde approach via the SMA.
Catheterization was performed using 5F catheters: USL2 (Cordis, Miami Lakes, USA) / COBRA 2 (Terumo Europe, Leuven, Belgium) and 4F catheters: BER (Cordis, Miami Lakes, USA), along with 2.7F, 2.4F, and 2.0 F Progreat® microcatheters (Terumo Corporation, Tokyo, Japan).
Controlled and/or uncontrolled release microcoils (0.018") were used through two main techniques: the sandwich technique and the packing of the aneurysm sac. The sandwich technique involves embolization of the inflow and the outflow vessels of the aneurysm to completely exclude it from circulation, preventing side-branch blood flow back into the aneurysm. In the packing technique, coils are densely inserted into the aneurysm sac to fill it, thereby promoting thrombosis and excluding blood flow.
Liquid agents, cyanoacrylate glue (n-butyl cyanoacrylate metacryloxysulfolane, Glubran 2®; GEM, Viareggio, Italy) and Onyx® 18 (LES, Covidien, Plymouth, MN), were used in a critic salvage setting of hemorrhagic shock with disseminated intravascular coagulopathy, allowing fast hemodynamic status control.
Procedures were conducted under fluoroscopic or hybrid guidance using fusion between cone beam CT and fluoroscopy. Cone beam CT was used when fluoroscopy was insufficient to analyze vasculature anatomy and to guide catheterization in complex cases. The cone beam CT protocol involved a single rotation acquisition (C-arm at a head position with breath holding) following a selective injection of 30 ml of iodinated contrast agent into the SMA at the rate of 3ml/s. Cone beam CT acquisition was performed after a 5-second delay with a tube rotation time of 5 seconds. Images were automatically transferred to a dedicated 3D workstation (XtraVision Release 8®; Philips Healthcare, Best, The Netherlands).
A final digital subtracted angiogram was performed in all patients to ensure the exclusion of the aneurysm and the retrograde injection of the splanchnic territory via collateral arteries.
For all patients, no endovascular or surgical revascularization of the celiac trunk was attempted.
2.4. Outcomes and Follow-Up Assessment
According to the Interventional Radiology Standards Board Guidelines [
14], technical success was defined as the complete exclusion of the aneurysm on final angiographic control. Clinical success refers to the 30-day clinical outcomes assessed through clinical and imaging data. It was defined as the resolution of the signs or symptoms that initially prompted the embolization procedure, the absence of significant symptom development in initially asymptomatic patients, and the exclusion of the aneurysm on follow-up imaging.
All patients were monitored similarly in the two institutions, clinically by the interventional radiologist and avascular surgeon in the unit where the patients were hospitalizedand by imaging. Follow-up imaging was conducted at one month after embolization, using either a 1.5 or 3-T MRI system (Avanto® and Skyra®; Siemens Healthcare, Erlangen, Germany and Signa TM Artist ; GE Healthcare, Waukesha - WI, USA), or a MDCT. The protocol included mainly a MR angiography sequence with intravenous contrast injection (Dotarem ®, Guerbet, Villepinte, France).
Safety was assessed by monitoring both minor and major complications [
14]. Minor complications were defined as those that did not require additional therapy and did not impact the length of hospital stay (including asymptomatic non-target embolization). Major complications were identified by the need for escalated care, such as hospital admission or prolonged hospitalization for more than 24 hours, transfer from a regular floor to the intensive care unit (ICU), or complications requiring surgical intervention.
2.5. Statistical Analysis
The Kolmogorov-Smirnov test was used to assess the normality of quantitative variables. Continuous variables were presented as means ± standard deviations (SDs), and ranges in cases of normal distribution and as median, Q1 (25%), and Q3 (75%) in other cases. Categorical variables are reported as absolute numbers and percentages.
All statistical analyses were conducted using Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA) and SPSS Version 25.0 (SPSS, Inc., Chicago, IL, USA).
A two-sided p-value of 0.05 was considered statistically significant for all tests.
3. Results
3.1. Demographics and Clinical Data
Over the study period, 23 patients met the inclusion criteria (mean age of 65 years ± 14 years [34-87 years], 12 males).
The embolization intervention was performed in an emergency setting in 12 patients (52%). Of these, 10 (43%) were treated for hemorrhagic complications after aneurysm rupture and two (9%) for acute abdominal pain. The 11 remaining patients (48%) underwent elective procedures: 9 of these patients (39%) were asymptomatic, with aneurysms discovered incidentally during imaging, and the remaining two patients (9%) presented with abdominal discomfort.
An average number of 1.4 ± 0.65[
1,
2,
3] was detected with a mean size, of 16 mm ±9 mm [4-45 mm]. All patients had celiac stenosis/occlusion caused by the median arcuate ligament.
All clinical and demographic data are reported in
Table 1.
3.2. Procedure Details
All procedures were performed under local anesthesia. Vascular access was through the right common femoral artery with the insertion of a 55 cm-long 5F sheath in 22 patients (95.5%). In one patient, the left brachial artery was approached using a 90 cm-long 5F sheath because of severe bilateral iliac stenosis.
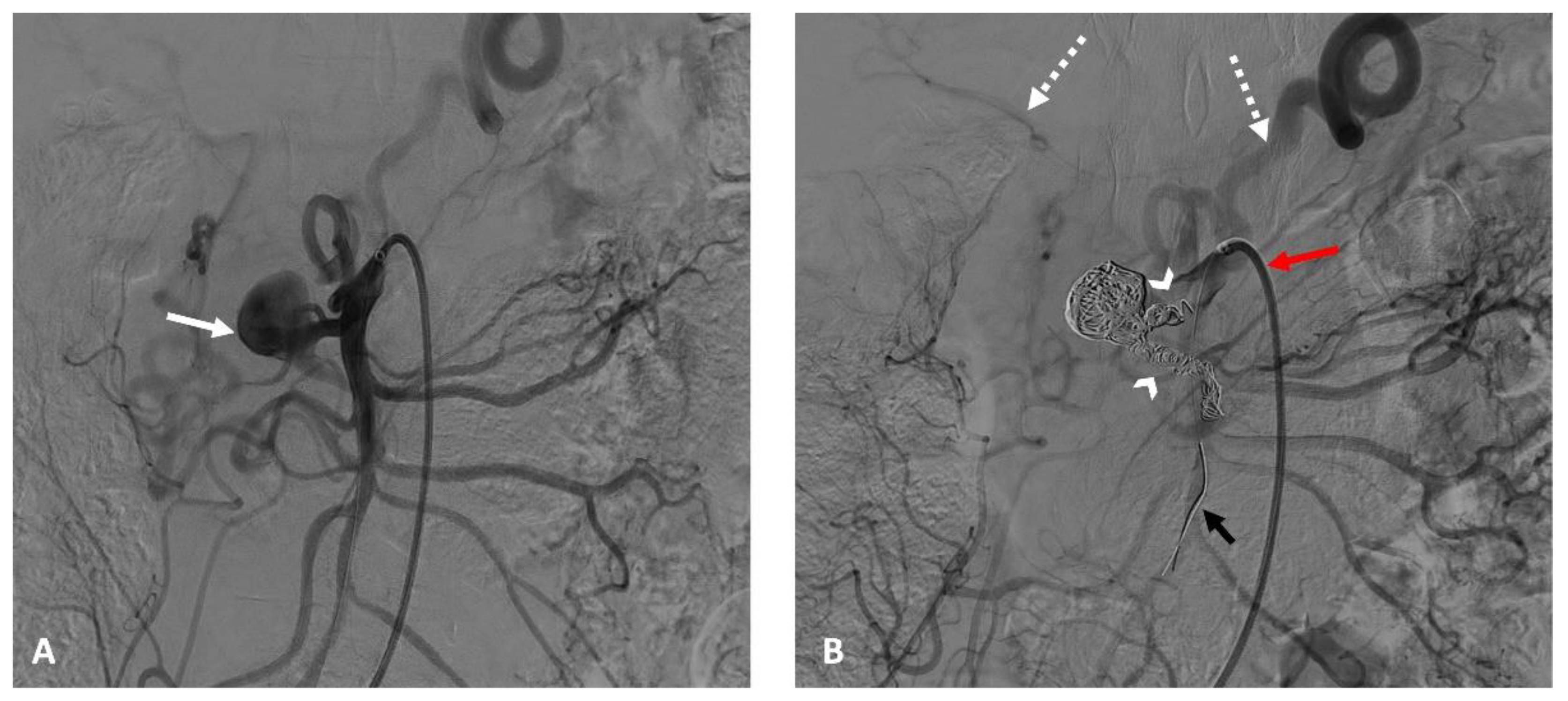
In 10 cases (43%), the anchoring technique in another non-target branch of the SMA was used, enabling stability for long sheath, catheter, microcatheter, and guidewire placements and overcoming tension in the coaxial equipment (
Figure 2).
The procedures were conducted under fluoroscopic guidance in 18 patients (78%), and under hybrid guidance (fluoroscopy-cone beam CT image fusion) in the remaining five patients (22%).
Microcoils were used as the principal embolization agent in 21 patients (91%): 19 were treated using the sandwich technique, and 2 had aneurysmal sac packing. Liquid agents were utilized in two cases of extreme hemorrhagic shock (Glubran 2® in one patient and Onyx®18 in the other).
3.3. Technical and Clinical Success
The technical success rate was 100%, and all embolized aneurysms were excluded entirely on the final angiography.
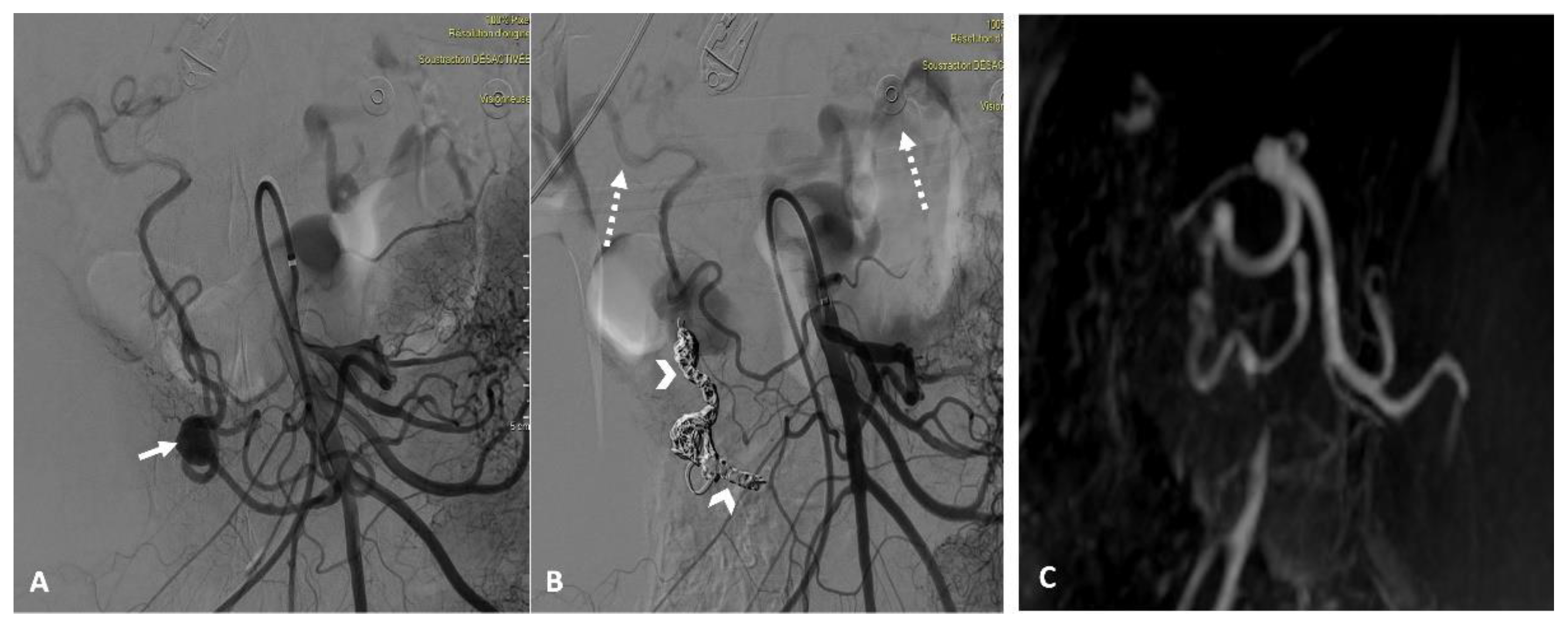
Patients were followed for a median of 16 months [2-48 months]. The clinical success rate was 100%. At the one-month follow-up, imaging confirmed the complete exclusion of the embolized aneurysm in all patients (
Figure 3).
During follow-up, all patients showed no signs of hemorrhagic recurrence (in cases with an initial rupture), experienced resolution of abdominal pain, and had no new symptoms.
3.4. Embolization Safety
Three complications (14.2%) were documented, including two minor (9.5%) and one major (5%), all secondary to the embolization agent's migration into a non-target artery within the splanchnic territory.
The two minor complications were due to the migration of microcoils, one into the splenic artery in one patient and the other into the common hepatic artery in another patient. No splenic infarction or hepatic dysfunction was noted during clinical follow-up or on imaging with complete arterial patency. Both patients remained utterly asymptomatic. One major complication occurred in a patient who underwent glue embolization for a ruptured aneurysm in a salvage context from severe hemorrhagic shock. This non-target embolization was complicated by a total splenic infarction, leading to infection, necessitating admission to the intensive care unit and a splenectomy.
Technical details, outcomes, and follow-up data are reported in
Table 2.
4. Discussion
This study suggests that embolization of PDA aneurysms via retrograde SMA approach, without celiac trunk revascularization, is an effective and safe treatment modality.
Endovascular techniques offer numerous advantages over traditional surgical treatment of aneurysms with celiac trunk revascularization, including reduced morbidity, shorter recovery time, and lower complication rates. These benefits make the endovascular approach preferable for most patients, as evidenced in our study and supported by existing literature [
1,
15,
16,
17].
The present study's data demonstrate a 100% technical success rate, with complete aneurysm exclusion confirmed on final angiographic control and one-month follow-up imaging. The clinical success rate was also 100%, with all patients experiencing resolution of symptoms or remaining asymptomatic. These outcomes align with previous studies, which report similarly high success rate for endovascular interventions in PDA aneurysms [
18,
19,
20,
21]. Achieving these outcomes requires meticulous anatomical study prior to embolization and careful procedural planning. High-resolution imaging modalities, such as dynamic CT with 3D reconstruction, are crucial for pre-procedural planning. These modalities allow for precise mapping of vascular anatomy and facilitate safe and effective interventions [
1].
Despite the high success rates, specific technical challenges must be addressed, particularly during catheterization in ruptured aneurysms requiring rapid management. In the present study, the use of a long 5F sheath in all patients and the relatively frequent application of the anchoring technique (43% of patients) effectively mitigated these challenges. This approach provided better stability during the procedure, ensuring that catheters, microcatheters, and guidewires remained securely positioned, even in the most challenging vascular anatomies [
13]. Additionally, cone-beam CT, used in 22% of our patients, not only aids in better planning and endovascular navigation but also allows for a more detailed anatomical study. This guidance modality significantly improves the accuracy and safety of these procedures, which is crucial for successful embolization in complex cases [
22,
23].
The low incidence of ischemic complications underscores the safety of endovascular embolization via the retrograde SMA approach. This low complication rate observed in the present study aligns with the literature, confirming the safety of this technique when properly implemented [
18,
19,
20]. The safety of the endovascular approach is attributed to the extensive collateral network in the splanchnic circulation, including multiple pancreaticoduodenal arcades that can compensate for the occlusion of the primary arteries. These collateral pathways, observed in 87% of our patients on final angiography, ensure adequate perfusion even after embolization, thereby mitigating the risk of ischemic damage [
24,
25,
26].
In the present cohort, the only patient who had an ischemic complication of the spleen was initially in severe hemorrhagic shock and was embolized with glue. This ischemic complication can be mainly attributed to the non-target migration of glue into the splenic artery and partly to vasoconstriction in the splanchnic territory secondary to shock, which makes supplementation via the collaterals less effective.
This study has some limitations that need to be acknowledged. First, its retrospective design with a relatively small patient sample may limit the robustness of our results. However, this should be interpreted in context, as this is a rare pathology. To our knowledge, the present series represents the largest published on endovascular embolization of PDA aneurysms via a retrograde SMA approach. Moreover, the absence of a control group in which celiac trunk recanalization was performed limits the robustness of our results. However, the median clinical and radiological follow-up of 16 months may ensure the effectiveness and the safety of the approach based uniquely on the aneurysms embolization, without celiac trunk recanalization.
5. Conclusions
Endovascular embolization of high-flow PDA aneurysms via a retrograde SMA approach is sufficient, effective, and safe, even without complementary recanalization of the celiac trunk. This technique can be proposed as the first-line approach for managing these high-risk bleeding aneurysms. It should be scheduled in nearly asymptomatic patients to prevent aneurysm rupture independently of their size. Meanwhile, accurate intervention planning based on high-quality pre-interventional imaging and appropriate embolization techniques are crucial to ensuring technical and clinical success.6. Patents
Author Contributions
Conceptualization, H.D, H.K and M.EH; methodology, H.D, S.J, H.K and M.EH; validation, H.K and H.D; formal analysis, S.J, H.D and M.EH; investigation, S.J, H.D, M.G, Y.Z and M.EH; resources, S.J, H.D, M.G, Y.Z, A.G, M.EH, V.T, P.D, R.A and H.K; data curation, S.J and H.D; writing—original draft preparation, S.J and H.D; writing—review and editing, H.K, M.EH, H.D and V.T.; visualization, H.D, H.K and M.EH; supervision, H.D and H.K; project administration, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The retrospective observational study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the College of Radiology Academics of France (CERIM 10-2024).
Informed Consent Statement
According to the retrospective design and the non-interventional nature of the study, written informed consent was waived.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- R Rossi, M.; Krokidis, M.; Kashef, E.; Peynircioglu, B.; Tipaldi, M.A. CIRSE Standards of Practice for the Endovascular Treatment of Visceral and Renal Artery Aneurysms and Pseudoaneurysms. CardioVascular and Interventional Radiology 2024, 47, 26–35. [Google Scholar] [CrossRef] [PubMed]
- De Perrot, M.; Berney, T.; Deléaval, J.; Bühler, L.; Mentha, G.; Morel, P. Management of True Aneurysms of the Pancreaticoduodenal Arteries. Annals of Surgery 1999, 229, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Kim, H.J.; Kim, B.; Lee, J.H.; Kim, J.; Kim, J.K. Clinical Impact of Collateral Circulation in Patients with Median Arcuate Ligament Syndrome. Diagnostic and Interventional Radiology 2018, 24, 181. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Gushimiyagi, M.; Takara, H.; Mototake, H. True Aneurysm of the Pancreaticoduodenal Arteries: A Single Institution Experience. Journal of Gastrointestinal Surgery 2010, 14, 1409–1413. [Google Scholar] [CrossRef]
- Flood, K.; Nicholson, A.A. Inferior Pancreaticoduodenal Artery Aneurysms Associated with Occlusive Lesions of the Celiac Axis: Diagnosis, Treatment Options, Outcomes, and Review of the Literature. CardioVascular and Interventional Radiology 2013, 36, 578–587. [Google Scholar] [CrossRef]
- Kalva, S.P.; Athanasoulis, C.A.; Greenfield, A.J.; Fan, C.M.; Curvelo, M.; Waltman, A.C.; Wicky, S. Inferior Pancreaticoduodenal Artery Aneurysms in Association with Celiac Axis Stenosis or Occlusion. European Journal of Vascular and Endovascular Surgery 2007, 33, 670–675. [Google Scholar] [CrossRef]
- Horton, K.M.; Talamini, M.A.; Fishman, E.K. Median Arcuate Ligament Syndrome: Evaluation with CT Angiography. Radiographics 2005, 25, 1177–1182. [Google Scholar] [CrossRef]
- Kim, E.N.; Lamb, K.; Relles, D.; Moudgill, N.; DiMuzio, P.J.; Eisenberg, J.A. Median Arcuate Ligament Syndrome—Review of This Rare Disease. JAMA Surgery 2016, 151, 471–477. [Google Scholar] [CrossRef]
- Kallamadi, R.; Marc, A.D.; Kalva, S.P. Inferior Pancreaticoduodenal Artery Aneurysms in Association with Celiac Stenosis/Occlusion. Seminars in Interventional Radiology 2009, 26, 215–223. [Google Scholar] [CrossRef]
- Kobayashi, T.; Uenoyama, S.; Isogai, S. Successful Transcatheter Arterial Embolization of an Inferior Pancreaticoduodenal Artery Aneurysm Associated with Celiac Axis Stenosis. Journal of Gastroenterology and Hepatology 2004, 19, 599–601. [Google Scholar] [CrossRef]
- Siauve, V.; Chevallier, O.; Mazit, A.; Falvo, N.; Comby, P.O.; Loffroy, R. Interventional Radiology for High-Flow Aneurysm of the Pancreaticoduodenal Arcades with Median Arcuate Ligament Syndrome: Review of 14 Patients. Journal of Clinical Medicine 2023, 12, 4692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Zhou, W.; Zhou, W. Endovascular Treatment of Ruptured Pancreaticoduodenal Artery Aneurysm with Celiac Axis Stenosis. Annals of Vascular Surgery 2019, 57, 273.e1–273.e5. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, I.; Derbel, H.; Chiaradia, M.; Deprez, F.; Vitellius, M.; Kobeiter, H.; Tacher, V. Parallel Guidewire for Catheter Stabilization in Interventional Radiology: The Anchoring Wire Technique. Journal of the Belgian Society of Radiology 2020, 104. [Google Scholar] [CrossRef] [PubMed]
- Dariushnia, S.R.; Redstone, E.A.; Heran, M.K.; Cramer, H.R., Jr.; Ganguli, S.; Gomes, A.S.; Lewis, C.A. Society of Interventional Radiology Quality Improvement Standards for Percutaneous Transcatheter Embolization. Journal of Vascular and Interventional Radiology 2021, 32, 476.e1. [Google Scholar] [CrossRef]
- Secco, G.; Chevallier, O.; Falvo, N.; Guillen, K.; Comby, P.O.; Mousson, C.; Loffroy, R. Packing Technique with or without Remodeling for Endovascular Coil Embolization of Renal Artery Aneurysms: Safety, Efficacy, and Mid-Term Outcomes. Journal of Clinical Medicine 2021, 10, 326. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Reeves, J.G.; Dayama, A.; Perez, S.D.; Debus, E.S.; Ricotta, J.J., II. Endovascular vs Open Repair of Renal Artery Aneurysms: Outcomes of Repair and Long-Term Renal Function. Journal of the American College of Surgeons 2013, 217, 263–269. [Google Scholar] [CrossRef]
- Hislop, S.J.; Patel, S.A.; Abt, P.L.; Singh, M.J.; Illig, K.A. Therapy of Renal Artery Aneurysms in New York State: Outcomes of Patients Undergoing Open and Endovascular Repair. Annals of Vascular Surgery 2009, 23, 194–200. [Google Scholar] [CrossRef]
- Abdulmalak, G.; Chevallier, O.; Falvo, N.; Di Marco, L.; Bertaut, A.; Moulin, B.; Abi-Khalil, C.; Gehin, S.; Charles, P.E.; Latournerie, M.; Midulla, M.; Loffroy, R. Safety and Efficacy of Transcatheter Embolization with Glubran®2 Cyanoacrylate Glue for Acute Arterial Bleeding: A Single-Center Experience with 104 Patients. Abdominal Radiology 2018, 43, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Takao, H.; Amemiya, S.; Ohtomo, K. Perioperative Hemodynamic Monitoring of Common Hepatic Artery for Endovascular Embolization of a Pancreaticoduodenal Arcade Aneurysm with Celiac Stenosis. CardioVascular and Interventional Radiology 2017, 40, 465–469. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chang, C.C.; Liou, J.M.; Jaw, F.S.; Liu, K.L. Transcatheter Arterial Embolization with N-Butyl Cyanoacrylate for Nonvariceal Upper Gastrointestinal Bleeding in Hemodynamically Unstable Patients: Results and Predictors of Clinical Outcomes. Journal of Vascular and Interventional Radiology 2014, 25, 1850–1857. [Google Scholar] [CrossRef]
- Sarad, N.; Basilious, M.; Nag, U.; Jethmalani, N.; Agrusa, C.; Ellozy, S.; Connolly, P. Presentation and Management of True Aneurysms of the Pancreaticoduodenal Arcade with Concomitant Celiac Artery Stenosis Using the Endovascular Approach. Journal of Vascular Surgery Cases, Innovations and Techniques 2024, 10, 101499. [Google Scholar] [CrossRef]
- Zaarour, Y.; Kobeiter, H.; Derbel, H.; Vitellius, M.; Ridouani, F.; You, K.; Tacher, V. Immediate and 1-Year Success Rate of Type 2 Endoleak Treatment Using Three-Dimensional Image Fusion Guidance. Diagnostic and Interventional Imaging 2020, 101, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Tacher, V.; Radaelli, A.; Lin, M.; Geschwind, J.F. How I Do It: Cone-Beam CT during Transarterial Chemoembolization for Liver Cancer. Radiology 2015, 274, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Guo, D.; Xu, X.; Chen, B.; Shi, Z.; Luo, J.; Fu, W. Early and Intermediate Results of Endovascular Treatment of Symptomatic and Asymptomatic Visceral Artery Aneurysms. Journal of Vascular Surgery 2016, 64, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.D.; Kabutey, N.K.; Krishnam, M.; Fujitani, R.M. Pancreaticoduodenal Artery Aneurysms Secondary to Median Arcuate Ligament Syndrome May Not Need Celiac Artery Revascularization or Ligament Release. Annals of Vascular Surgery 2015, 29, 122.e1–122.e1227. [Google Scholar] [CrossRef]
- Sugaya, T.; Suzuki, T.; Wada, J.; Shimizu, H.; Uchihara, D.; Yokogawa, Y.; Ichii, O.; Tai, M.; Ejiri, Y.; Ohira, H. Transarterial Embolization for Ruptured Pancreaticoduodenal Artery Aneurysm Due to Segmental Arterial Mediolysis Combined with Median Arcuate Ligament Syndrome: A Case Report. Clinical Journal of Gastroenterology 2023, 16, 859–863. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).