1. Introduction
Mushrooms are favoured due to their good taste and their nutritive and healthy values.
Agrocybe cylindracea species have culinary, nutritional food, and medicinal properties and are grown worldwide, and it is easy and cheaper to implement[
1].
A. cylindracea is an edible mushroom that belongs to the family of basidiomycete and in the order of Agaricales. Its gills range from pink to dark brown[
2].
For instance, Chen et al explored the nutritional properties of
A. cylindracea from different regions. They discovered many nutritional properties, including protein, dietary fiber, amino acids, fat, ash, and moisture[
3]. Recently, [
4] extensively reviewed and reported the effects of substrate chemistry, strain, developmental stage, and ecological interactions of mycelial cells and fruiting bodies. These authors identified a wide variation of the concentrations of metabolites (e.g., ergosterol and phenolic compounds, etc.) in both mycelial cells and fruiting bodies. A related investigation revealed that physically modifying
A. Cylindracea fruiting bodies exhibits enhanced antioxidant activities, attributed mainly to ultra-high pressure treatment and superfine grinding, indicating potential health benefits from their antioxidant properties [
5]. Meanwhile, different cultural media can affect the
A. cylindracea fruiting body growth, development, yield, and physicochemical composition.
Mushrooms, including
A. cylindracea, can be grown in different culture media [
6]. For example, agro-industrial wastes such as giant grass[
7,
8], tea leaves [
9], cottonseed hulls [
10] etc., have gained traction among researchers in recent decades as ideal culture media for mushroom cultivation, primarily due to their cost effectiveness and supply vital nutrients.
Buba et al [
11]focused on cultivating oyster mushrooms using sugarcane bagasse as a substrate. They found that mushrooms had high vitamin (A, E, B1, B6) content, protein (28.45 mg/g), and potassium Liang et al [
2]investigated the efficacy of korshinsk pea shrub on
Agrocybe aegerita [
12]. It was pointed out that the polysaccharide and crude protein contents were 4.46% and 26.60%, respectively, indicating an increase of 4.51% and 12.34% over the control. Additionally, related work proved that different substrates (e.g., cacao shells, etc.) notably impacted triterpenoid and fatty acid production in
Pleurotus fungi [
13]. Despite these findings, our fundamental understanding of how different growth stages of
A. cylindracea respond to the syngeneic effects of different culture media contributing to carbon dioxide emissions remains largely undocumented. Furthermore, there is a lack of comprehensive literature concerning the response of different growth stages of
A. cylindracea to the response impacts on the yield and physicochemical properties of
A. cylindracea. The different growth stages of
A. cylindracea grown in various substrates giant grass, tea leaves, and cottonseed hulls) were evaluated. We also deciphered the physicochemical composition such as bioactive compounds, proteins, etc.
2. Material and Methods
2.1. Fungal Strain Identification and Cultivation
A fresh fungi mycelium sample of
A. cylindracea was frozen using liquid nitrogen, and that nitrogen liquid was left to evaporate. Deoxyribonucleic acid (DNA) was isolated from
A. cylindracea with a fungi DNA kit following the instruction of a manufacturing company called Mybio. We used a pestle to grind fresh mushrooms into powder as a DNA sample and 100 mg of DNA samples were weighed using an electric scale. The samples were placed in 1.5 mL tubes, and 400 μL of Lys (pyrolysis liquid) was added. After 2 min, the samples were centrifuged at 10.000 rpm for 30 s, and the supernatant was moved into a DNA absorbance test tube. Later, we mixed the same quantity of supernatant and isopropyl alcohol and centrifuged for 1 min. The absorbance tube was washed by adding 600 µL Wash Buffer (WB) diluted with absolute (96% - 100%) ethanol centrifuged at ≥ 10,000 rpm for 1 min. The DNA adsorption column was moved into the new 1.5 eppendorf tube (EP), and the lid was opened for 2 min to evaporate ethanol residues after adding 30 μL of preheated Elution Buffer (EB) at 65℃. We performed centrifugation after 2 min at 12,000 rpm for 2 min. The DNA samples were collected and stored at -20℃. Later, the internal transcribed spacer region was amplified and sequenced using ITS 4 and 5 primers. Detailed information regarding the PCR reaction and procedure can be found in Supplementary Table, Sheets 3 and 4. We analyzed the amplified products using 1.5 % agarose gel by running in 20 X 50 TAE buffer at 120 V for 40 min. We leveraged a UV transilluminator to visualize and photograph the gels after staining with ethidium bromide[
14]. The bright bands were extracted and sent to the company for sequencing. The results from BLAST indicated that the strain belonging to
A. cylindracea has 96%, and the query length is 706 bp.
2.2. Primary Spawn Preparation
The Potato Dextrose Agar (PDA) was prepared using 200 g of potatoes. The potatoes were peeled and cut into small pieces, and 1000 mL of pure water was added in and boiled for about 15 min in a pressure boiler. Then, the starched liquid was filtered and mixed with 20 g of Dextrose and 20 g of Agar. We discharged 15 mL of PDA into the test tubes and covered them. The solution was sterilized in the autoclave at 121℃ for 30 min. The test tubes cooled at temperature after sterilization. The inoculation for primary spawn involved extracting a small fragment, approximately 10 mm in size, from a cultured strain of A. cylindracea. Subsequently, the inoculated tubes were transferred into tubes and placed within a darkened environment in an incubating machine set at a temperature of 25°C.
2.3. Substrate Preparation, and Cultivation
The formulations of the culture media were performed as follows: The first substrate comprised 48% giant Junco grass (GJ) combined with 30% Dicranopteris dichotoma (DD), the second substrate contained 48% wasted tea leaves (WTL) mixed with 30% sawdust (SD), and the third substrate consisted of 78% cottonseed hulls supplemented with 20% wheat bran and 2% lime (CSH). Before cultivation, we thoroughly mixed the culture materials with a mixture machine, and water was added to reach a moisture content of approximately 60%. Polyethylene bags were filled with about 1000g to 1220 g of substrates. The substrates were sterilized separately in an autoclave machine at 121℃ for 120 minutes and cooled for 24 h. The inoculated polyethylene bags were transferred at the room temperature of 25℃ and 85% relative humidity for cultivation. The different formulated culture materials mentioned above were considered as the treatments. That is, the giant Juncao grass combined with Dicranopteris dichotoma (GJ+DD), wasted tea leaves combined with sawdust (WTL+SD), and cottonseed hulls (Supplementary Table, Sheet 1) supplemented with wheat bran and lime (CSH) were inoculated with A. cylindracea spawn. Three groove cultivation boxes were prepared and injected with water to form a liquid surface. Each treatment in every cultivation box contained six fungi polyethylene bags, replicated thrice. The total number of polyethylene bags in groove cultivation boxes was 18 and inoculated with A. cylindracea spawn. Additional information regarding the properties of the substrates was documented in (Supplementary Table, Sheets 2 and 10).
2.4. Sampling of the Mycelium Running Stage and Measurement of A. cylindracea Yield and Biological Efficiency
The samples were collected from the first developmental stages of the mycelium running stage up to the fruiting body stage in the cultural materials in three replicates on January 20, 2023. We sampled the cultured material at a ten-day interval to investigate many enzyme activities during the growing and development stages of A. cylindracea mycelium. We generated a total of 54 samples at each time point. The samples of the same replicate were homogenized and mixed accordingly, forming one sample. The fruiting bodies of A. cylindracea in each polyethylene bag were harvested in April 2023 (maturity stage) and weighed. We used the accumulated data to calculate the biological efficiency (BE). The BE is the ratio of the weight of the A. cylindracea fresh fruiting body (g)/dry weight of substrate (g), expressed as a percentage.
2.5. Determination of Carbon Dioxide and Nitrogen of A. cylindracea Cultivated with Different Substrates
We collected samples from the first developmental stages of the mycelium running stage up to the fruiting body stage to investigate the carbon and nitrogen contents. The spent mushroom substrates and the fruit bodies of
A. cylindracea from each medium were dried, weighed, crushed, mixed, and measured using a TruMac machine (LECO, America) [
15]. The total quantity of nitrogen and carbon was calculated by referring to the mixed dry quantity of fruiting bodies in three substrates. The total amount of nitrogen and carbon was calculated based on the dry weight of the fungi residue. The total amount of carbon and nitrogen in the initial mushroom substrate was calculated based on the dry weight of the initial fungi substrate.
2.6. Determination of Carboxymethylcellulose Activity
We mixed 1 g of the sample with 1 mL of the extract and centrifuged at 8000 rpm at 4℃ for 10 min. After that, we poured the supernatant into ice. The content of reducing sugar produced by carboxymethyl cellulase catalyzed cellulose degradation was calculated by the 3,5-dinitrosalicylic acid method. The spectrophotometer was preheated for 30 min, the wavelength was adjusted to 550 nm, and the distilled water was at zero. We added 50 μL of reagent one, 200 μL of reagent two, and 50 μl of pure water in each test tube, followed by 50 μL of the sample. Boiled water (50 μL) was added to the control samples, mixed properly, and incubated in the water at 40℃ for 30 min and subsequently placed in a boiling water bath for 15 min before collecting the saccharifying solution. The 15 μL of saccharified solutions and 35μL of reagent three were added in 1.5 mL Eppendorf tubes (EP), mixed properly, and incubated in the boiling water for 15 min and cooled. After that, 250 μL of pure water was added to all the tubes to facilitate the cooling of the samples. Finally, 200μl of the final solution was taken to a trace quartz cuvette, and absorbance was measured at 550 nm.
2.7. Determination of Xylanase Activity
The D-xylose mother liquor was accurately weighed (1 g). The volume of D-xylose was fixed in a 100 mL bottle with pure water. The concentration of D-xylose mother liquor was 10.0 mg/mL. Six 100 ml volumetric flasks were taken from the mother liquor, 1-6 mL from the gradient, and 100 mL from distilled water. We generated a D-xylose standard solution with a 100-600 ug/mL concentration. The standard solution of D-xylose with different concentrations was 1 uL and was mixed with a DNS reagent of 1.5 mL. The water was quickly boiled in an electromagnetic oven for 7 min and cooled at normal temperature, containing a volume of 10 mL. The absorbance was measured at 550 nm by an ultraviolet spectrophotometer. Eighteen 10 mL test tubes were filled with 750 µL, and 1% xylan solution was added. We treated 9 test tubes with a 250 µL crude solution. The rest were treated with the same quantity of inactivated crude enzyme solution. Eighteen test tubes were placed in a water bath at 50℃ for 30 min. After taking out the samples, we added 1.5 µL DNS reagents, placed them in boiling water, took them out after 7 min, and immediately cooled the reaction with cold water. After cooling the samples, we adjusted the volume to10 µL by adding distilled water and placed them at 540 nm of the enzyme label to measure the absorbance.
2.8. Determination Laccase Activity
We mixed about 1g of the sample with 1 mL of the extraction solution, homogenized in an ice bath, and centrifuged 10000 xg for 10 min at the temperature of 4℃. The supernatant was stored on ice for testing. The control sample was prepared by pipetting 25 μL of boiled samples and 25 μl test sample into a 2 mL EP tube, and 150 μL of working fluid was added (containing ABTS) mixed with the test sample. Another 150 μL of working fluid was added to control and incubated in the water bath at 60 for 3 min. The samples were analyzed using a spectrophotometer /a micro quartz cuvette/96-well plate / at room temperature. The oxidation of the ABTS was measured by determining the increased absorbance at 420 nm, which was recorded every 30 s for 180 seconds. The following formula was used to calculate laccase activity: Enzyme activity definition: The quantity of enzyme required to oxidize 1 nmol of substrate ABTS per gram of sample per minute is one unit of enzyme activity.
Laccase activity (nmol/min/g) = △ A ÷ ε ÷ d × V total ÷ (V sample × W ÷ V sample total) ÷T=130 × △ A ÷ W [
16]
2.9. Determination Amylase Activity
We mixed 0.1g of the sample with 0.8 mL pure water in a pot and slowed it to homogenate. The mixture was later poured into 50 mL of the centrifuge tube and extracted at ambient temperature for 15 min. The sample was well shaken once a time for 5 min and centrifuged at 6000 rmp in the ambient temperature for 10 min. The supernatant was taken and diluted with water to a volume of 10 mL. Then, it was shaken well to get an amylase stock solution. Amylase crude solution was mixed with 4 mL of double-pure water and shaken well to measure the total activity of alpha and beta amylase. The released reducing sugar was determined by the spectrophotometer/microplate reader that was preheated for 40 min. The wavelength was adjusted to 540 nm, and the distilled water was zero. The glucose was diluted to 1, 0.5, 0.25, 0.125, 0.0625, 0.03125, 0.01562, 0.0078, and 0.0039 mg/mL with pure water.
In the alpha-amylase activity assay, 75 μL of amylase crude solution was transferred in 18 tubes (1.5 mL), and the standard curve was prepared by taking 75 μL of pure water and mixed with 75 μL of standard solution. All the tubes were heated at 70℃ in a water bath for 15 min and cooled down. The total amylase activity was determined by adding 75 μL of amylase solution.
2.10. Measurement of Organic Compounds and Carbon Dioxide Emission during A. cylindracea in Different Growth Stages
The traditional Van Soest method was adopted to determine the number of organic compounds, including lignin, hemicellulose, and cellulose contents, using 0.50 g mushroom powder [
17]. We used a square plexiglass cover with a total volume of 0.125 m
2 to track gas. The cultivation box was coved, and the control gas was tracked immediately after placing the glass cover. The test gas was sampled 1 h later in three parallel treatments. We measured the gas in the airbag using a gas chromatograph R1778A, Agilent Technologies[
18]. The peak area and blank of three sets of parallel data were measured by gas chromatography (ppm value of carbon dioxide emissions)/(average value of peak area of carbon dioxide emissions). The peak area of the control was subtracted, and the resulting value was multiplied by the above data to give the ppm of carbon dioxide for the three sets of parallel data.
2.11. Measurement of A. cylindracea Nutritional Composition Cultivated in Different Substrates
Agrocybe cylindracea's nutritional composition was investigated following the method documented in Dimopoulou et al. [
19] work, established by the Association of Official Analytical Chemists (AOAC) [
20]with some modification. The
A. cylindracea fruiting body was collected and dried at 75℃, pulverized in the mill, and sieved. We used 20g to determine the crude fat, ash content, crude protein, fiber content, polysaccharide content. The Kjeldahl method was leveraged to determine protein using a 6.25 correction factor. The Kjeldahl method, AOAC method 950.36, was used to determine the total nitrogen[
21]. The fat content was measured using continuous “Soxhlet” tool type extraction. The ash content was measured following the method adopted in the Ivanov et al.[
22] study. In brief, we accurately weighed 1 g of sample and transferred it into a crucible. The sample was placed on a clay pipe triangle, heated over a low flame until all the material was completely charred, and heated in a muffle furnace (5~6 h) at 600℃. Then, the sample was cooled in a desiccator and weighed. We heated the crucible in the muffle furnace (1 h) and cooled it before weighing it. The process was repeated to obtain two identical consecutive weights, and the ash exhibited a greyish-white or almost white colour. To determine the total ash, we used the following approach: Ash content (g/100 g sample) = weight of ash × 100/weight of sample taken.
2.12. Screening of Metabolites/Bioactive Compounds during A. cylindracea Maturity Stage
We leveraged the method documented in Tsiaka et al.[
23] work to screen the bioactive compounds within the mushrooms. In brief, 3 mg of extract was thoroughly mixed with 90% dichloromethane (DCM) and 10% methanol and homogenized. Shimadzu mass spectrometer (GC-MS) solutions system, consisting of chromatography interfaced with GC-MS, which had Elitel and a fused silica capillary column (30 mm × 0.25 mm 1D X1 μMdf, consisting of 100% Dimethyl poly siloxane) was employed to conduct the GC-MS analysis. We used an electron ionization system with an ionizing energy of 70 eV for the GCMS identification. Helium (99.999%) was utilized at a constant flow rate of 1 mL/min, with an injection volume of 8.00 μL, and a 10: 1.250°C split ratio was the injector temperature and 28°C being the ion source temperature. Later, we automated the oven at 110°C (2 min isothermal) and increased it to 10°C/min - 200°C and 5°C/min - 280°C. This process ended with 9 min isothermal, with a temperature of 280°C. For the mass spectra with a scanning interval of 0.5 s, we used 70 eV, containing fragments ranging from 45 - 450 Da. The GC had a total running time of 36 min, at which the relative percentage of each component was measured using a comparison of the average peak area with the total areas. Finally, we interpreted the GC-MS mass spectrum using the National Institute Standard Technology (NIST), which has over 62000 patterns in its library. We adopted the inductively coupled plasma tandem quadrupole mass spectrometry (ICP-QMS/QMS) with an octopole reaction/collision cell (ORC) to determine the sulfur content.
3. Statistical Analysis
We employed principal coordinate analysis (PCoA) with Bray-Curtis distance to explore and visualize similarities or dissimilarities in metabolite communities. Later, we carried out a Permutational Multivariate Analysis of Variance (PERMANOVA) and paired PERMANOVA by using the “adonis” command in vegan at 999 permutations and
α = 0.05 to understand the changes in communities of metabolites. Later, the overlap and unique metabolites were visualized using Venn diagrams (
http://bioinfogp.cnb.csic.es/tools/venny/index.html). A volcano plot was conducted using an R language-based ggtern and grid to detect the enriched and depleted metabolites in the
A. cylindracea is subjected to various culture media. We performed hierarchical clustering analysis using the “heatmap.2” function from the “gplots” R package. Bubble plot of top-20 statistical analysis KEGG pathways was conducted using R software. Pearson’s correlation coefficient was performed to test the association between metabolites and various essential elements/parameters identified during the growth and development of the
A. cylindracea using R-software [
24]. We performed redundancy analysis (RDA) to evaluate the association between metabolite composition and various essential elements/parameters identified during the growth and development of the
A. Cylindracea. We used 999 permutations by employing the ‘vegan’ package to perform the significance difference. ANOVA was adopted to evaluate the test data. Later, we displayed the results using DPS software (version 7.05,
www.dpssoftware.co.uk). Lastly, the changes between the mean values of each culture medium were explored using Tukey’s HSD test (
p < 0.05).
4. Results
4.1. Enzyme Activities and Organic Compounds Detected during A. cylindracea Different Growing Stages Subjected to the Various Substrates
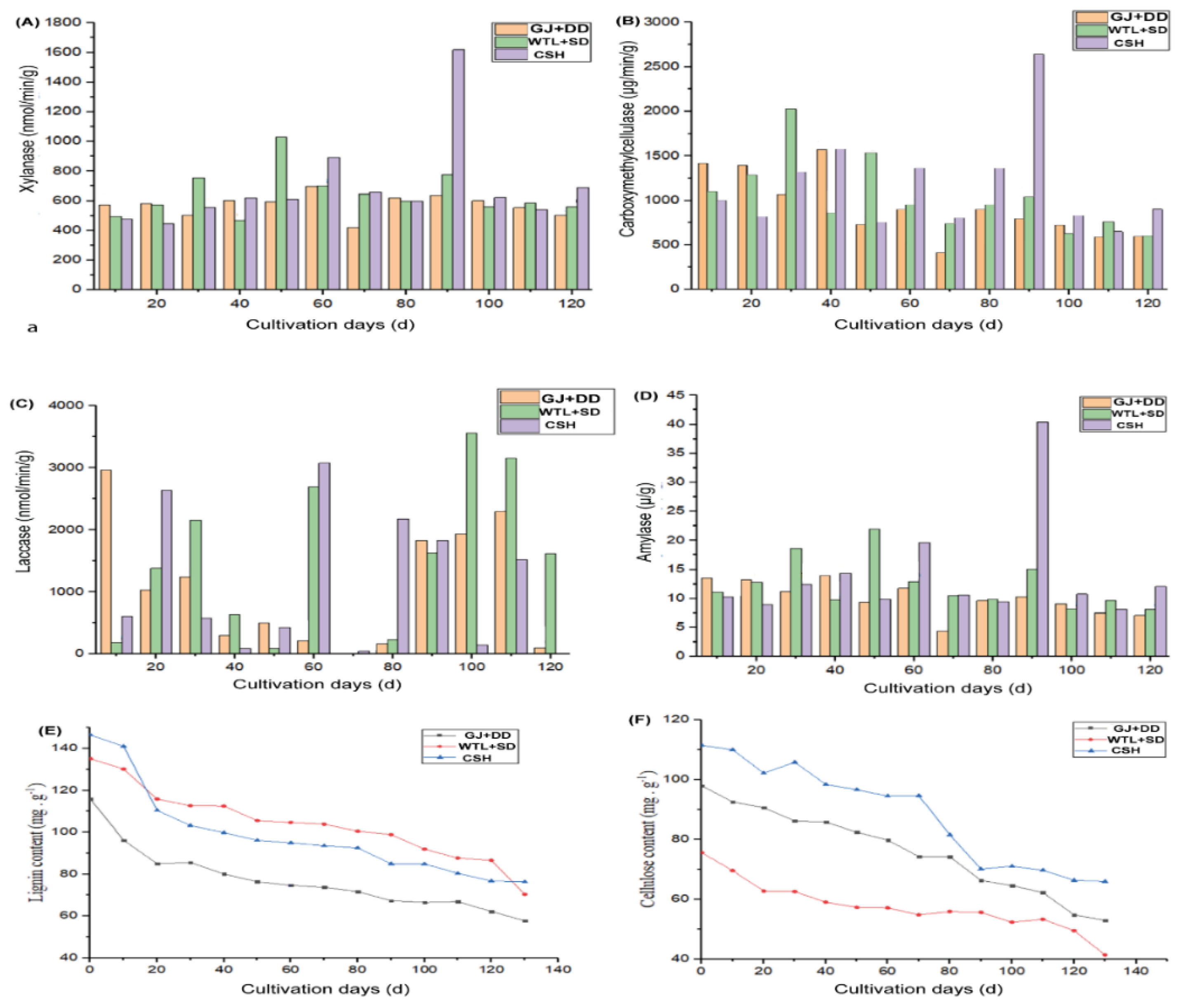
A. cylindracea enzyme activities at various growing stages cultivated under different substrates exhibited distinct patterns. For instance, xylanase enzyme activity was more pronounced in the WTL+SD substrate, 30 and 50 days after
A. cylindracea cultivation. A similar pattern was observed in the CSH substrate 60 days after
A. cylindracea cultivation. Noticeably, xylanase enzyme activity peaked significantly (1618.6 nmol/min/mg) in the CSH treatment during
A. cylindracea primordial formation compared with the WTL+SD and GJ+DD substrates (
Figure 1A).
Furthermore, CMCase activity was significantly high (
p < 0.05) in the GJ+DD substrate 10, 20, and 40 days after
A. cylindracea cultivation. Similarly, CMCase activity demonstrated a similar trend in the WTL+SD 20, 30, and 50 days after
A. cylindracea was cultivated. Moreover, CMCase activity exhibited a similar phenomenon in the CSH substrates 30, 40, and 60 days of
A. cylindracea cultivation compared with the WTL+SD and GJ+DD substrates. It is worth noting that CMCase activity significantly peaked (
p < 0.05) (2642.5μg/min/g) in the CSH substrates during the early stages of fruiting body development (primordial formation), occurring 90 days after
A. cylindracea cultivation. We noticed a similar pattern during the maturity stage of the fruiting body (120 days). Overall, CMCase activity decreased from
A. cylindracea cultivation through its maturity stage (
Figure 1B).
Figure 1C showed that GJ+DD exhibited the advantage of significantly increasing (
p < 0.05) laccase activity 10 d after
A. cylindracea cultivation compared with CSH and WTL+SD treatments. Laccase activity in the CSH treatment marked a significant increase (
p < 0.05) 20 and 60 d after
A. cylindracea cultivation, followed by the early stages of fruiting body development (primordial formation, i.e., 80 days after
A. cylindracea cultivation) compared with GJ+DD and WTL+SD. We also found that laccase activity significantly increased (
p < 0.05) in the WTL+SD substrate 30 and 60 days after
A. cylindracea cultivation compared with the GJ+DD and CSH treatments. A similar pattern was noticed during primordia formation (i.e., 100 days after
A. cylindracea cultivation) and maturity stage (i.e., 120 days after
A. cylindracea cultivation).
We also explored
A. cylindracea amylase activity and found that amylase activity was significantly high (
p < 0.05) in WTL+SD treatment 30 and 50 days after
A. cylindracea cultivation compared with the GJ+DD and CSH treatments. Amylase activity significantly peaked (
p < 0.05) in the CSH treatment, especially during primordia formation, followed by the maturity stage of
A. cylindracea fruiting body (i.e., 120 d after
A. cylindracea cultivation) (
Figure 1D).
Meanwhile, a number of organic compounds were determined during
A. cylindracea different growing stages subjected to the various substrates. For example, lignin and cellulose exhibited a descending pattern from the period of
A. cylindracea cultivation to the maturity in the different substrates. This phenomenon became more prevalent 20 d after
A. cylindracea cultivation, with the primordial formation and the maturity stage of the fruiting body exhibiting the lowest lignin and cellulose. It is worth noting that this behavior was more pronounced in the GJ+DD compared with the WTL+SD and CSH treatments. Cellulose demonstrated a similar trend in the WTL+SD treatment, followed by the GJ+DD treatment (
Figure 1E and F).
4.2. Organic Compound and Carbon Dioxide Emissions Were Detected during A. cylindracea Different Growing Stages Subjected to the Various Substrates
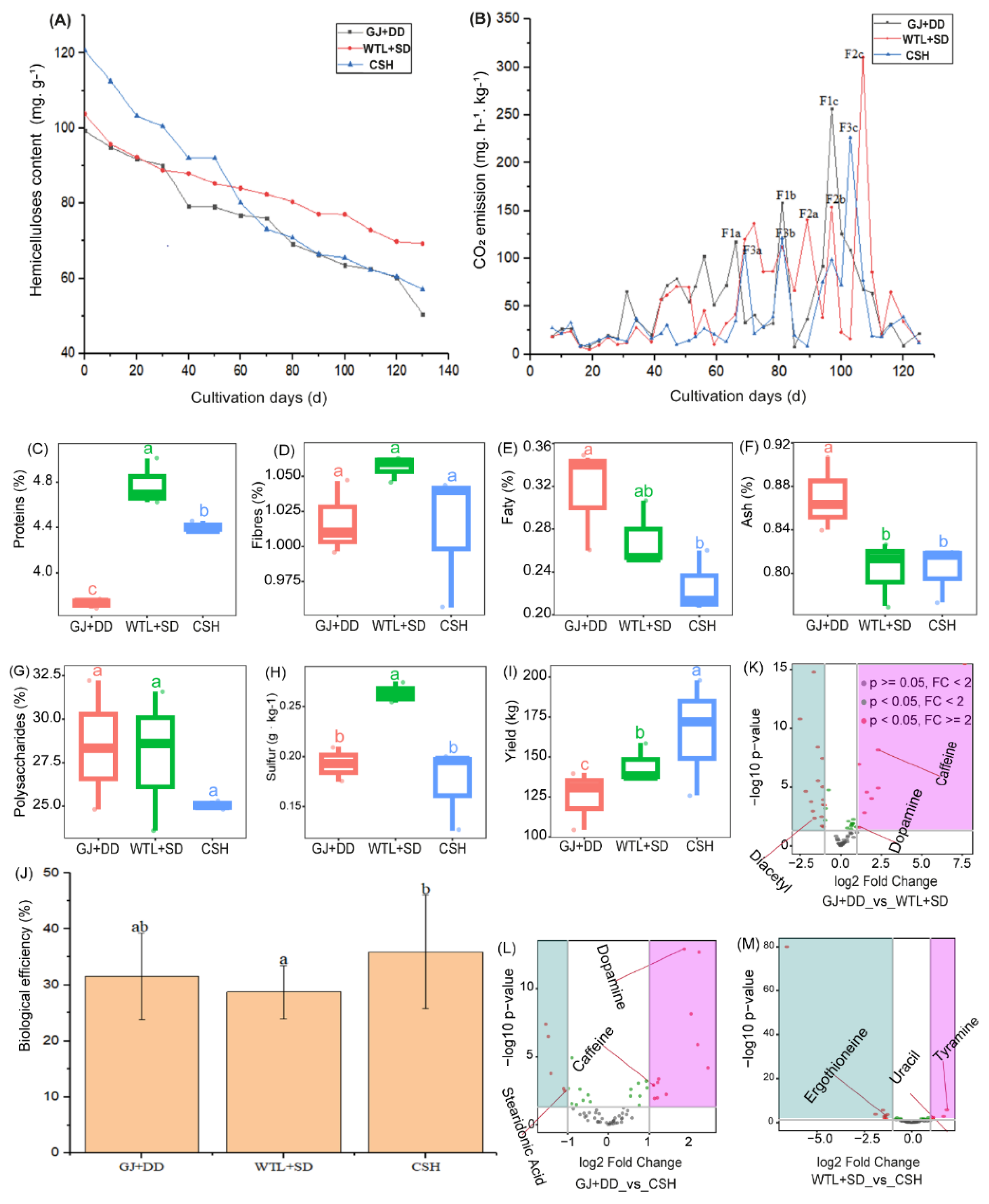
Our results further showed that hemicellulose exhibited a descending trend from the period of
A. cylindracea cultivation to the maturity stage. This phenomenon was telling 20 d after
A. cylindracea cultivation, with the primordial formation and the maturity stage of the fruiting body exhibiting a significantly low amount of hemicellulose in the different culture media (
Figure 2A).
Figure 2B showed that exhibited carbon dioxide emissions were slightly high when
A. cylindracea was initially cultivated and decreased 20 d after cultivation. We also noticed that carbon dioxide emissions in the different substrates fluctuated, exhibiting an increasing trend, especially in the GD+DD and WTL+SD culture media. This pattern was more pronounced during the maturity stage of the fruiting body, followed by the primordia formation stage. However, carbon dioxide emissions sharply plummeted 110 d after
A. cylindracea cultivation (
Figure 2B). Moreover, the total carbon content significantly diminished in the
A. cylindracea fruiting subjected to the WTL+SD and CSH culture media compared with the GD+DD culture medium.
4.3. Nutritional Components, and Yield, Biological Efficiency of A. cylindracea Cultivated in the Different Substrates
We also observed that the protein content was significantly higher (
p < 0.05) in the WTL+SD, followed by the CSH treatment, compared with the GD+DD culture medium (
Figure 1C). It was also revealed that the dietary fibers and polysaccharides content of the
A. cylindracea fruiting bodies subjected to the WTL+SD exhibited no significant difference (
Figure 2D, G).
Figure 1E illustrates that the fat content in the
A. cylindracea fruiting bodies subjected to the GD+DD culture medium peaked, while the opposite was in the WTL+SD, followed by the CSH culture medium. We also noticed that the ash content of the
A. cylindracea fruiting body marked a significant increase in the GJ+DD culture medium compared with the WTL+SD and CSH culture media (
Figure 2F). The nitrogen content significantly decreased in the
A. cylindracea fruiting subjected to the WTL+SD culture medium compared with the GD+DD and CSH culture media. The total nitrogen revealed a similar trend in the
A. cylindracea fruiting subjected to the WTL+SD culture medium, followed by the CSH culture medium compared with the GD+DD culture medium (Supplementary Table, Sheet 6). Further analysis revealed that the sulfur in the
A. cylindracea fruiting subjected to the WTL+SD culture medium marked a significant increase (
p < 0.05) compared with the GD+DD and CSH culture media (
Figure 1H).
Figure 2I also demonstrated that the yield of the
A. cylindracea subjected to the CSH treatment marked a significant increase (
P < 0.05), followed by the WTL+SD culture medium relative to the GD+DD. We also investigated the biological efficiency (BE) during the entire growth period of the
A. cylindracea. We observed that the
A. cylindracea BE was significantly high (
p < 0.05) in CSH treatment, accounting for 35.8%, followed by the GD+DD (31.5%) and WTL+SD (28.7%) culture medium (
Figure 2J).
4.4. Metabolites Detected during A. cylindracea Different Growing Stages Subjected to the Various Substrates
We evaluated the performance of metabolites (Supplementary Table, Sheet 7) in each culture medium relative to those in another treatment (
Figure 2K-M, Supplementary Table, Sheets 8-10). The analysis revealed that some essential metabolites, including dopamine and caffeine, were significantly enriched (
p < 0.05) in the
A. cylindracea subjected to the WTL+SD and CSH culture media compared with the GJ+DD culture medium. However, diacetyl and stearidonic acid depleted considerably in the
A. cylindracea subjected to WTL+SD compared with the
A. cylindracea subjected to the GJ+DD (
Figure 2K and M, Supplementary Table, Sheets S8 and 9). Moreover, tyramine and uracil were significantly enriched (
p < 0.05) in the
A. cylindracea subjected to the CSH compared with those subjected to the WTL+SD, while ergothioneine demonstrated the opposite (
Figure 2L, Supplementary Table, Sheet 10).
4.5. Composition and Abundance of Metabolites Detected during A. cylindracea Different Growing Stages Subjected to the Various Substrates
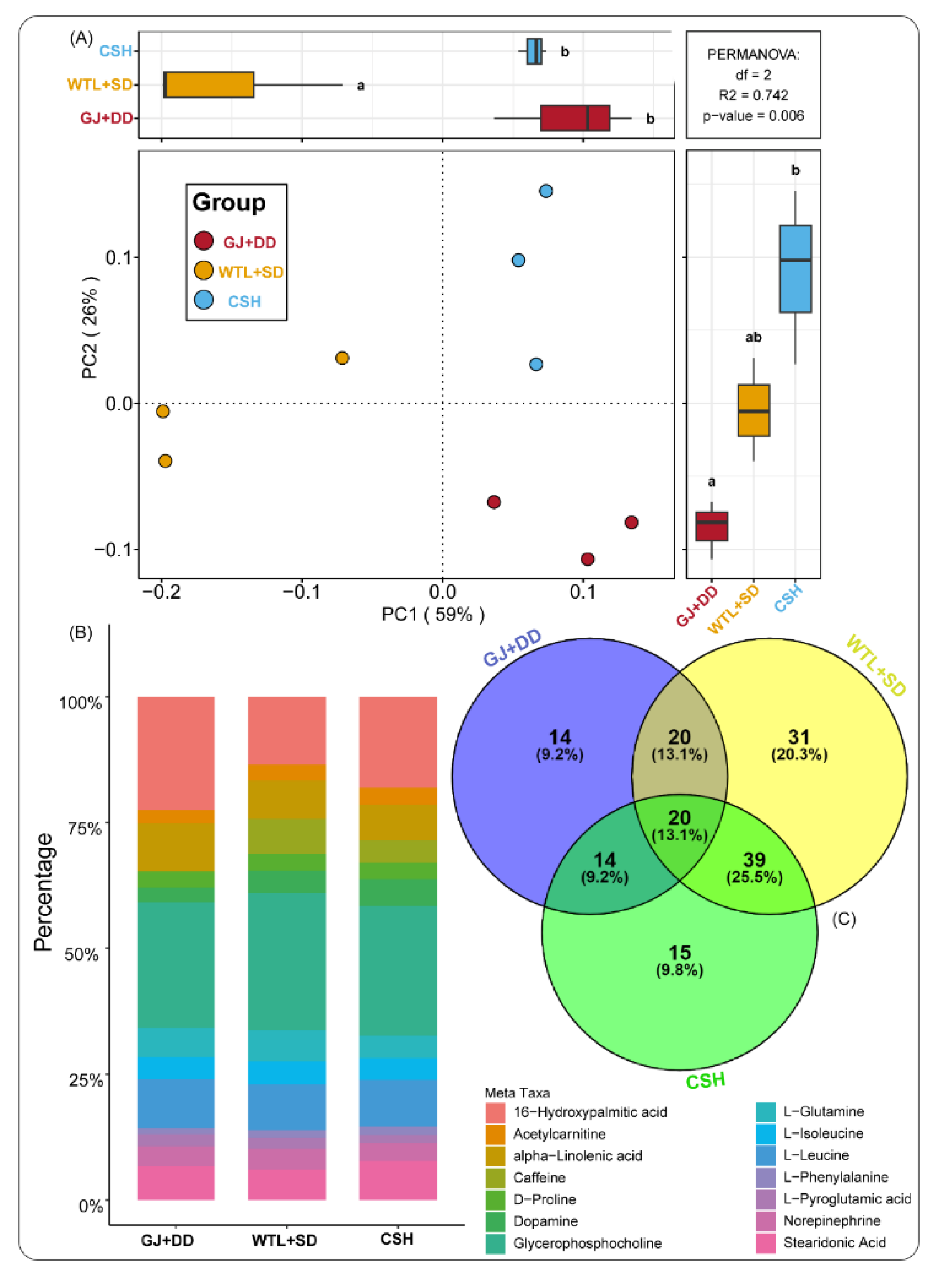
We leveraged PCoA analysis to explore metabolite compositions identified during the maturity stage of the
A. cylindracea fruiting body subjected to the different substrates. We found that the distribution pattern of metabolite compositions was distinctly separated from each other, implying that the distribution pattern of these metabolite compositions was substrate-specific (
Figure 3A). We also investigated the relative abundance of these metabolites and observed that glycerophosphocholine (25.94%), 16-hydroxy palmitic acid (16.47%), alpha-linolenic acid (7.59%), stearidonic acid (6.80%), dopamine (5.57%), L-Glutamine (5.29%), and caffeine (4.44%) were abundant. Others include l-isoleucine (4.38%), norepinephrine (3.83), acetylcarnitine (3.51%), D-Proline (3.35%), L-Pyroglutamic acid (1.89%), and L-Phenylalanine (1.84%) (
Figure 3B).
Venn diagram analysis further revealed that 14 (9.2%), 31 (20.3%), and 15 (9.8%) of metabolites identified during the maturity stage of the
A. cylindracea fruiting bodies were unique to the GJ+DD, WTL+SD, and CSH treatments, respectively. We also noticed that 20 (13.1%), 39 (25.5%), and 14 (9.2%) metabolites were common in both GJ+DD and WTL+SD, WTL+SD and CSH, and GJ+DD and CSH, respectively (
Figure 3C).
Figure 4 illustrated that metabolite composition was largely substrate-specific, which reinforced the pattern observed in
Figure 3A.
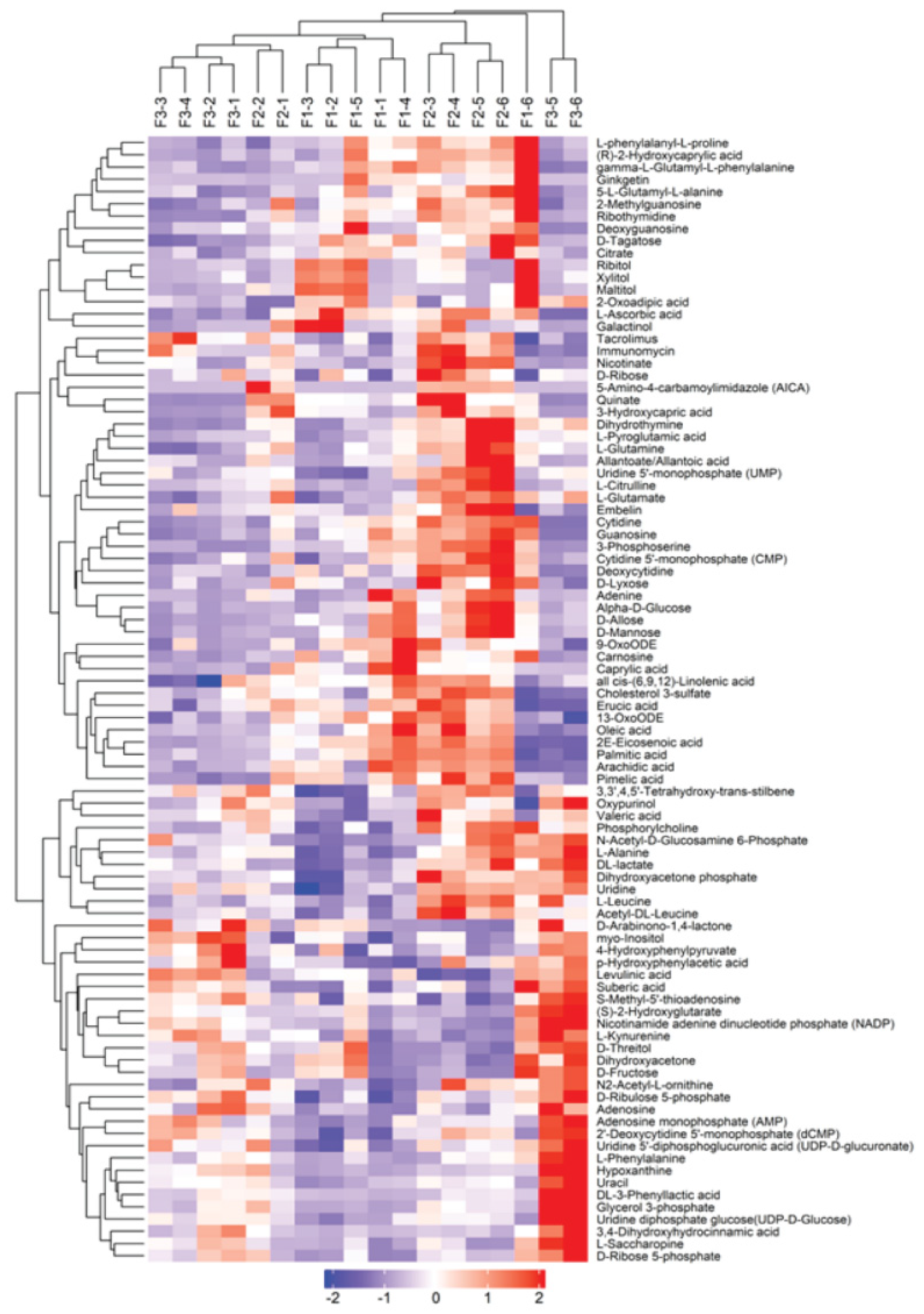
Figure 4 demonstrates that a significant number of these metabolites were detected during the maturity stage of the
A. cylindracea fruiting bodies and were significantly expressed, especially those subjected to the WTL+SD and CSH culture media compared with the GJ+DD treatment. For instance, N-Acetyl-D-Glucosamine 6-PhosphateL-Alanine, DL-lactate, Dihydroxyacetone phosphate, Uridine, L-Leucine, D-Arabinono-1.4-lactone, myoinositol, 4-Hydroxyphenylpyruvate, p-Hydroxyphenylacetic acid, Levulinic acid, Suberic acid, S-Methyl-5'-thioadenosine, (S)-2-Hydroxyglutarate, Nicotinamide adenine dinucleotide phosphate (NADP), L-Kynurenine, D-Threitol, Dihydroxyacetone, and D-Fructose, N2-Acetyl-L-ornithine were more pronounced in the
A. cylindracea fruiting bodies under the WTL+SD compared culture medium with the CSH and GJ+DD treatments. We also observed that D-Ribulose 5-phosphate, Adenosine, Adenosine monophosphate (AMP), 2'-Deoxycytidine 5'-monophosphate (CMP), Uridine 5'-diphosphoqlucuronic acid (UDP.D-glucuronate), L-Phenylalanine, Hypoxanthine, Uracil, DL-3-Phenyllactic acid, Glycerol 3-phosphate,Uridine diphosphate glucose(UDP-D-Glucose), 3.4-Dihydroxyhydrocinnamic acid, L-Saccharopine, and D-Rose 5-phosphate demonstrated a similar trend in the
A. cylindracea fruiting bodies subjected to the WTL+SD compared with the CSH and GJ+DD treatments. Moreover, Quinate, 3-Hydroxycapric acid, Dihydrothymine, L-Pyroglutamic acid, L-Glutamine, Allantoate/Allantoic acid, Uridine 5-monophosphate (UMP), L-Citrulline, L-Glutamate, Embelin, Cytidine, Guanosine, 3-Phosphoserine, Cytidine 5'monophosphate (CMP), Deoxycytidine, D-Lyxose, Adenine, Alpha-D-Glucose, D-Allose, and D-Mannose were more telling in the
A. Cylindracea fruiting bodies subjected to the CSH treatment compared with the WTL+SD and GJ+DD.
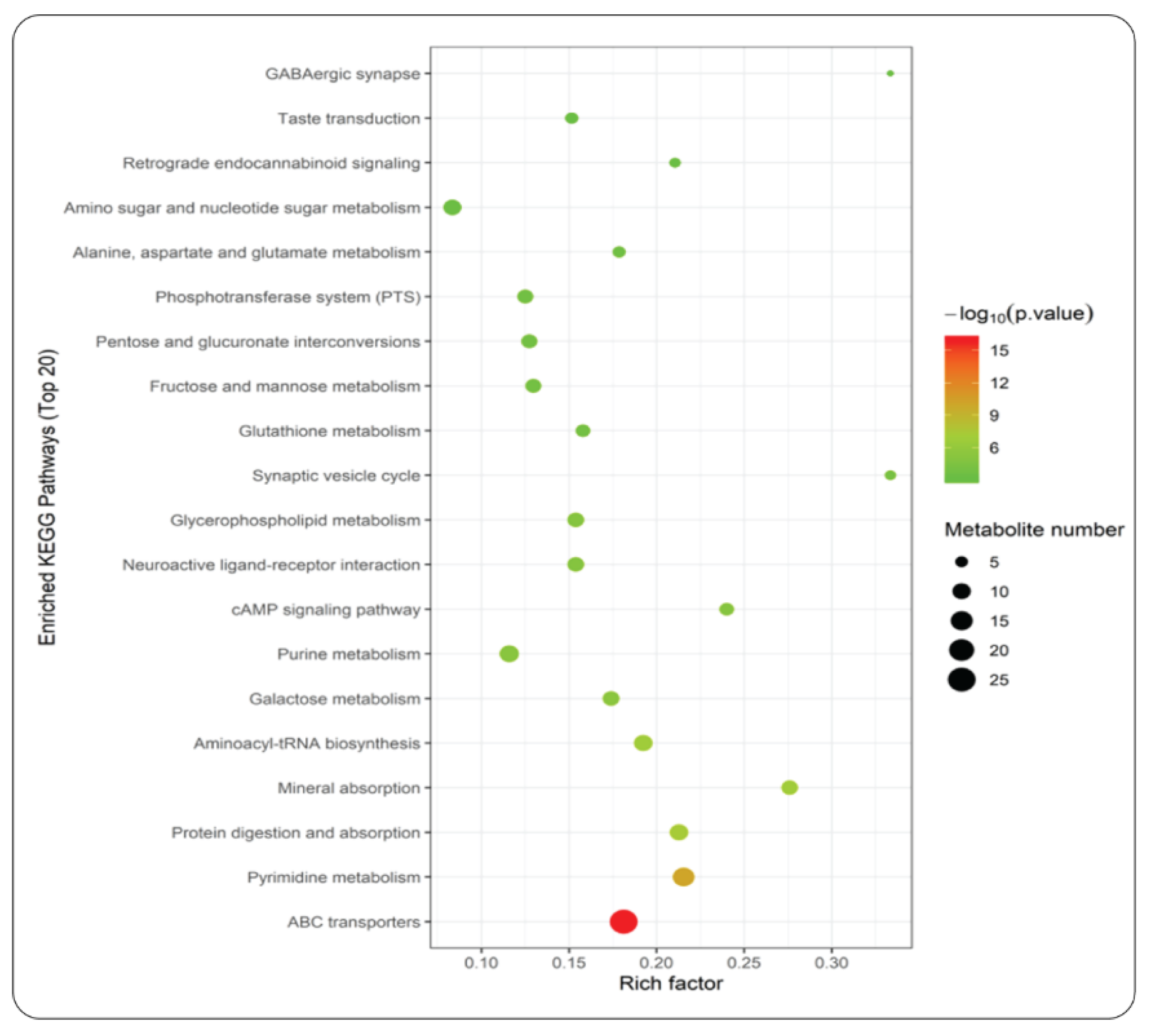
4.6. Metabolites Enriched KEGG Pathways Identified during the A. cylindracea Cultivated in the Different Substrates
An enriched KEGG pathways analysis was performed further among the top 20 metabolites detected during the maturity stage of the
A. cylindracea fruiting body. It was revealed that ABC transporters, pyrimidine metabolism, protein digestion and absorption, aminoacyl-RNA biosynthesis, mineral absorption, galactose metabolism, etc., enriched significantly in the
A. Cylindracea fruiting bodies under the WTL+SD culture medium compared with the GJ+DD (
Figure 5).
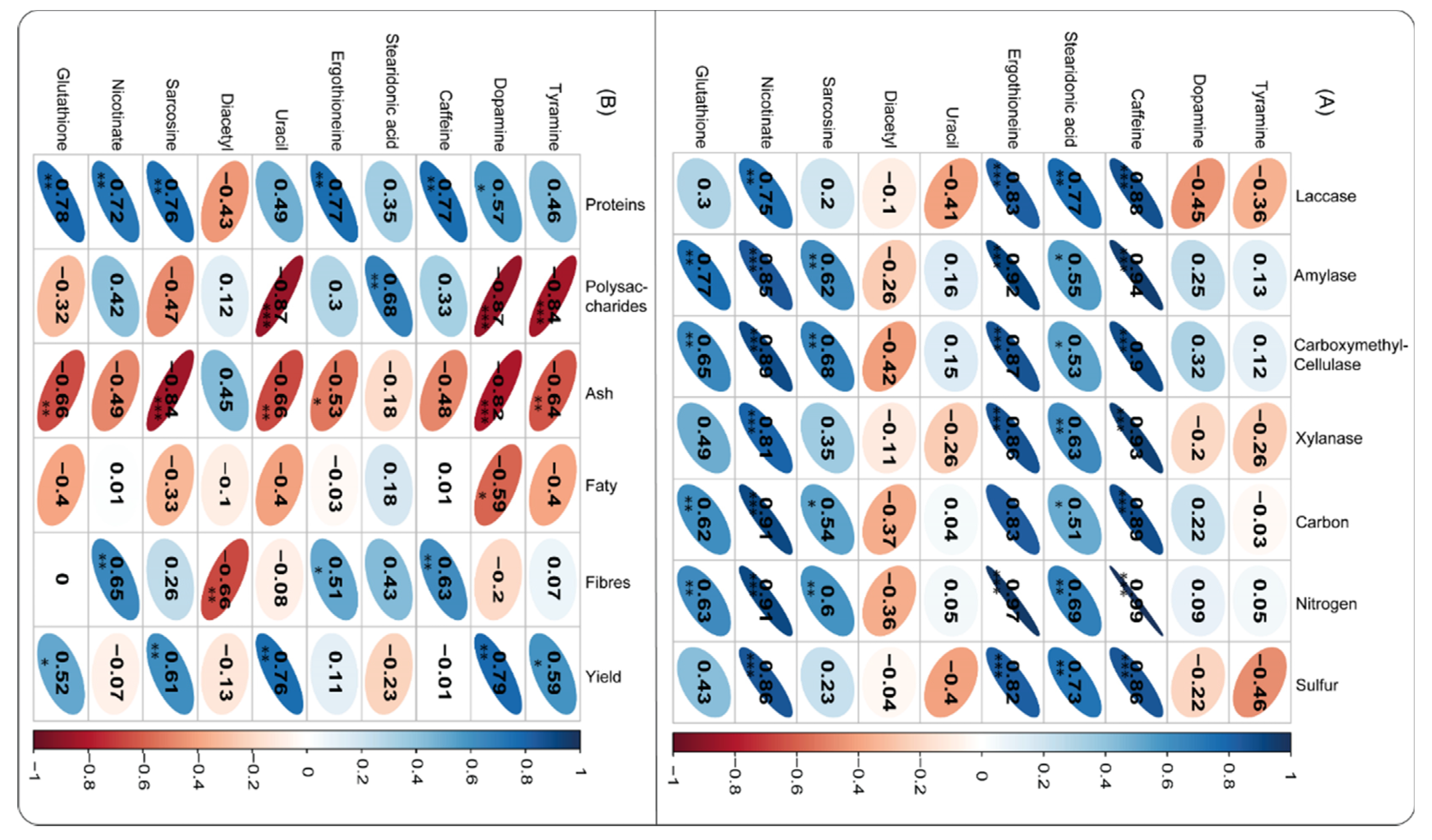
4.7. Metabolites Association with the Different Parameters Explored during A. cylindracea Maturity Stage
The association among some essential metabolites, enzyme activities, and vital
A. cylindracea nutrients was established. It was noticed that metabolites, including caffeine, exhibited a significant positive association with xylanase, CMCase, amylase, laccase, and vital
A. cylindracea nutrients, namely sulfur, nitrogen, and carbon dioxide. Correspondingly, stearidonic acid demonstrated a similar pattern with laccase and xylanase, followed by CMCase and amylase. Stearidonic acid positively correlated with sulfur, nitrogen, and carbon dioxide. Furthermore, sarcosine had a significant positive relationship with CMCase, amylase, and other nutrients, namely nitrogen and carbon dioxide. Nicotinate was significantly and positively associated with enzymes, namely xylanase, CMCase, and amylase, followed by nicotinate and vital
A. cylindracea nutrients, including sulfur, nitrogen, and carbon dioxide. Glutathione exhibited a similar phenomenon with amylase, CMCase, and
A. cylindracea nutrients, namely nitrogen and carbon dioxide (
Figure 6A).
Besides, we tested the association of metabolites and the
A. cylindracea's physicochemical properties and productivity. The analysis demonstrated that tyramine exhibited a strong positive correlation with the
A. cylindracea yield. However, polysaccharides and ash revealed the opposite. It was also found that dopamine had a significant positive correlation with the
A. cylindracea yield. Caffeine was significantly and positively correlated with dietary fibers and proteins, whereas stearidonic acid exhibited the same trend with polysaccharides. In addition, ergothioneine was considerably and positively associated with proteins, followed by dietary fibers. On the other hand, ash demonstrated a contrary trend. Uracil was significantly and positively associated with the
A. cylindracea yield, whereas uracil exhibited a strong negative relationship with ash and polysaccharides, while diacetyl demonstrated a similar pattern with dietary fibers. Additionally, sarcosine displayed a significant and positive association with the
A. cylindracea yield and proteins. Nicotinate significantly and positively correlated dietary fibers and proteins. Glutathione exhibited a similar trend with yield and proteins, while ash revealed the opposite (
Figure 6B).
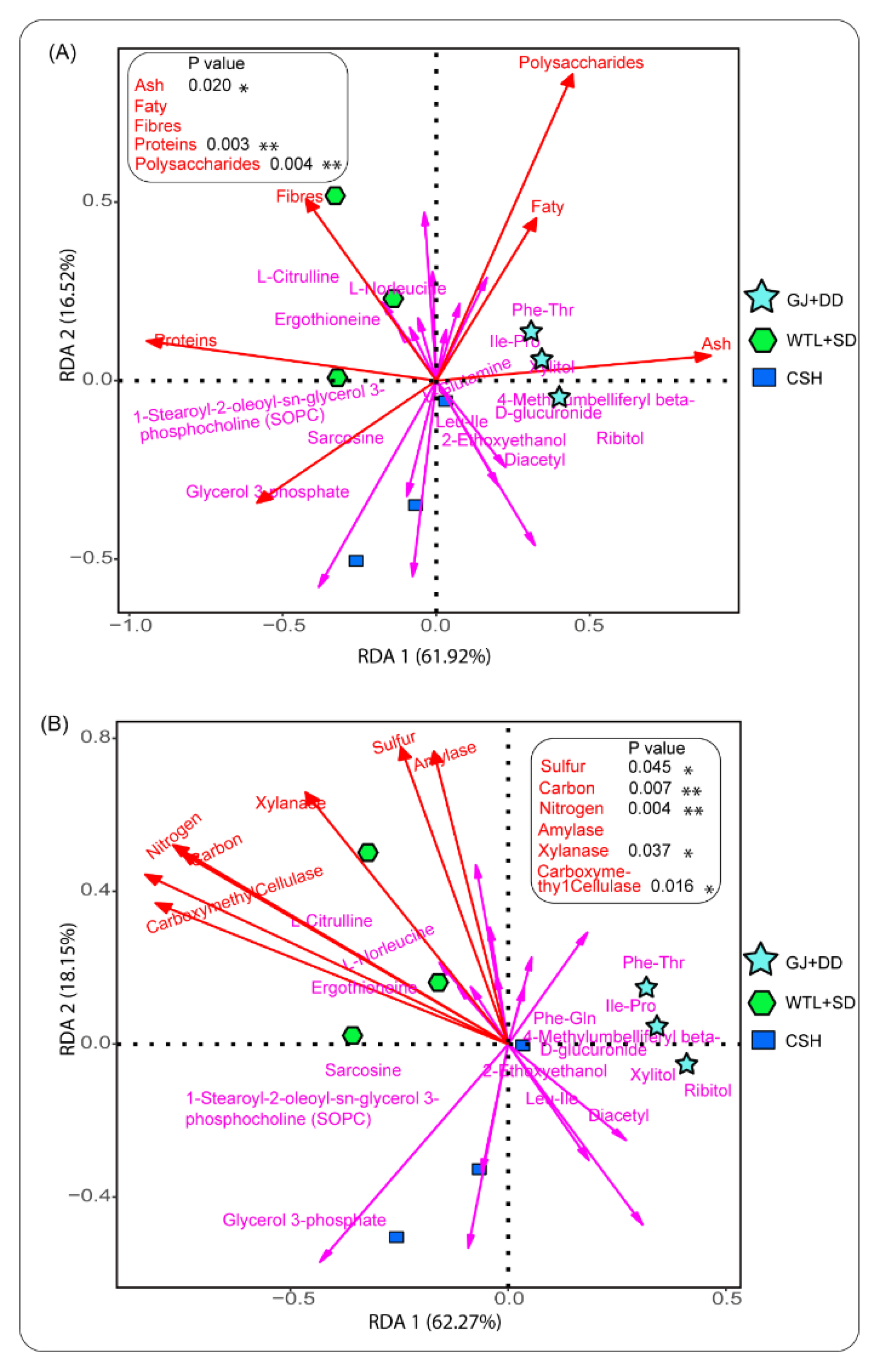
We explored the relationships between metabolites, biomolecular, and biochemical properties (
Figure 7A), followed by metabolites and physicochemical properties (
Figure 7B) detected in the
A. cylindracea fruiting bodies to understand the role these different variables play in shaping metabolite compositions. It was revealed that metabolites, including L-Norleucine, ergothioneine, and L-Citrulline tended to favour dietary fibers and proteins, especially in the
A. cylindracea fruiting bodies subjected to the WTL+SD culture media. Further analysis using permutation test analysis demonstrated that proteins had a significant positive correlation with these metabolites. Similar behaviour was observed between xylitol and ash (
Figure 7A). It was demonstrated that several metabolites, including ergothioneine, norleucine, and L-Citrulline tended to favour all the physicochemical properties detected in the
A. cylindracea fruiting bodies, especially those subjected to the WTL+SD culture medium. Further analysis proved that carbon dioxide and nitrogen, followed by sulfur, xylanase, and carboxyme thy1cellulase exhibited a significant positive relationship with these metabolites (
Figure 7B).
5. Discussion
Growing evidence has established that mushrooms' growth, development, and productivity are responsive to various cultural media. Indeed, Adams. et al.[
25] pointed out that different waste materials, including wheat bran and sawdust, significantly influenced
Agrocybe cylindracea yield. Ahmed et al. also found that combining wasted tea leaves, giant Juncao grass, sawdust, and cottonseed hulls with wood waste exhibited the advantage of boosting mushroom production, especially with a 3:1 ratio of wood waste to tea leaves. Here, we found that the synergetic effects of the CSH culture medium (i.e., the cottonseed hulls supplemented with wheat bran and lime) marked a significant in
A. cylindracea yield, followed by the WTL+SD growth medium (i.e., the wasted tea leaves combined with sawdust) compared with the GJ+DD culture medium (i.e., the giant Junco grass combined with Dicranopteris dichotoma). Several theories underpin this phenomenon: Sawdust's fibrous structure supports mushroom mycelium growth and development and facilitates the formation of fruiting bodies[
26]. Combining sawdust with wasted tea leaves adds additional structural support and nutrients. The resulting substrate provides an ideal environment for
A. cylindracea propagation (Ahmed et al., 2024). Moreover, the effects of the CSH culture medium provide a balanced substrate with structural integrity and nutrients.
Mycelium and fruiting bodies of mushrooms have gained traction among many researchers, largely due to their nutritional and health benefits [
28]. For instance, a study explored 11 species of fresh and dried, medicinal, and edible macrofungi and found that carbohydrates and proteins were more pronounced in the different species of mushrooms [
29]. Chen et al. investigation also revealed that 43
A. cylindracea samples from 13 provinces in China exhibited differences in nutritional components of
A. cylindracea. These authors documented that
A. cylindracea had high protein and insoluble dietary fiber but low soluble dietary fiber and fat [
3]. Our findings demonstrated that the ash content significantly peaked in the fruiting body of
A. cylindracea was subjected to the synergetic effects of the GJ+DD culture medium, while the faty of
A. cylindracea fruiting body exhibited the same behaviour subjected to the GJ+DD culture medium, followed by the WTL+SD culture medium. Moreover, proteins in the
A. cylindracea fruiting body cultivated in the WTL+SD culture medium, followed by the CSH culture medium, significantly increased. This finding parallels Li et al. [
30] investigation, wherein the efficacy of the Korshinsk pea shrub on
A. Aegerita triggered a peak in polysaccharide and crude protein contents by 4.46% and 26.60%, respectively, indicating an increase of 4.51% and 12.34% over the control. The marked increase in the nutritional composition of
A. cylindracea subjected to the various effects of the different culture media could be ascribed to these substrate compositions and nutrient-rich.
Extensive investigations have reported on the decisive role different culture media play (e.g., giant Juncao grass, sawdust, wasted tea leaves, and cottonseed hulls) in promoting the nutritional composition of mushrooms[
31]. However, our fundamental understanding of how
A. cylindracea different growth stages respond to the syngeneic effects of different culture media contributes to carbon dioxide emissions remains undocumented.
Figure 2B showed that the carbon dioxide emissions were slightly high when
A. cylindracea was initially inoculated in the various substrates and decreased 20 d after cultivation. We also noticed that carbon dioxide emissions in the different culture media fluctuated, exhibiting an increasing trend, especially in the GD+DD and WTL+SD culture media. This pattern was more pronounced during the maturity stage of the fruiting body, followed by the primordia formation stage. However, carbon dioxide emissions sharply plummeted 110 d after
A. cylindracea cultivation. This finding corroborates Li et al. work, revealing that spent mushroom substrate slightly increased methane emission but reduced the global warming potential of methane and nitrous oxide by 33.95%.[
32]. We believe that the ability of mycelium of
A. cylindracea undergoes cellular respiration, utilising oxygen and releasing carbon dioxide as a byproduct, which contributed to this phenomenon, largely due to mycelium's ability to colonize the substrate and grow, its metabolic activity contributes to carbon dioxide emissions[
33].
Studies have revealed that the bioactive active composition of living organisms, including plants [
34] and eukaryotic organisms (mushrooms, yeasts, etc.) [
35] is responsive to substrate amendments. Claude. I et al.2024 findings documented that substrates, especially sawdust, influenced the bioactive components of oyster mushrooms, including flavonoids, saponins, triterpenoids, polyphenols, and steroids. Volcano plot analysis revealed that the syngenetic effect of different culture media significantly enriched some essential metabolites. For example, essential metabolites, including dopamine and caffeine, were significantly enriched in the
A. cylindracea fruiting body subjected to the WTL+SD and CSH culture media. Moreover, tyramine and uracil were significantly enriched in the
A. cylindracea fruiting body subjected to the CSH culture medium.
6. Conclusions
This work deciphered the physicochemical composition of A. cylindracea has different growth stages subjected to various culture media. The investigation proved the effects of different culture media on the growth stages of A. cylindracea can trigger carbon dioxide emissions, promote yield, and boost the physicochemical properties of A. cylindracea. The study is very important to researchers and mushroom growers who intend to use agricultural waste and giant grasses to increase the yield and nutrition of mushrooms and preserve the environment. There are few studies on enzymatic activity in various development stages and the carbon utilization of edible fungi. Based on that we suggest more studies on fungi enzymatic activities on the growth of fungi in different substrates.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
HM: NA, IC, and NF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing, original draft, Writing – review & editing, Software; MA, JB, YH, LZ, HZ Conceptualization, Data curation, Software, Visualization, Writing – review & editing, Writing, original draft LJ, PL, LZ and LD: Project administration, Supervision, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
This study was supported by the Special Project for the Protection and Utilization of Agricultural Resources of the Department of Agriculture and Rural Affairs of Fujian Province, "Research and Application of Key Technologies for Innovation and Industrialized Utilization of Juncao Grass and Juncao Mushroom" (22001XA).
Data Availability Statement
The datasets presented in this study will be made available upon request.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
We would like to thank the Academy of Juncao Science and Technology, at Fujian Agriculture and Forestry University for funding this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Díaz-Godínez, G.; Téllez-Téllez, M. (2021). Mushrooms as Edible Foods. In X. Dai, M. Sharma, & J. Chen (Eds.), Fungi in Sustainable Food Production (pp. 143–164). Cham: Springer International Publishing. [CrossRef]
- Liang, Y.; Lu, D.; Wang, S.; Zhao, Y.; Gao, S.; Han, R.; Hu, S. Genome Assembly and Pathway Analysis of Edible Mushroom Agrocybe Cylindracea. Genomics, Proteomics & Bioinformatics 2020, 18, 341–351. [Google Scholar]
- Chen, L.; Chen, Y.; Wang, J.; Zhiqiang Wang, & Huang, X. Study on nutritional components evaluation and origin differences of Agrocybe cylindracea from different regions. eFood 2023, 4, e81. [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Lu GuoYing, L.G.; Zhang ZuoFa, Z.Z.; Pan HuiJuan, P.H.; Fan LeiFa, F.L. (2011). Antioxidant activities of extracts from the physically modified fruiting bodies of Agrocybe cylindracea in vitro.
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Wu, N.; Tian, F.; Moodley, O.; Song, B.; Jia, C.; Ye, J.; Li, C. Optimization of agro-residues as substrates for Pleurotus pulmonarius production. Amb Express 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Claude, I.; Aimable, N.; Mediatrice, H.; Zhou, H.; Lin, D.; Liu, P.; Lin, Z. Evaluation of the Influence of Varied Juncao Grass Substrates on Physiological and Enzymatic Reactions of Pleurotus ostreatus. Current Issues in Molecular Biology 2024, 46, 9493–9502. [Google Scholar] [CrossRef]
- Yang, D.; Liang, J.; Wang, Y.; Sun, F.; Tao, H.; Xu, Q.; Wan, X. Tea waste: an effective and economic substrate for oyster mushroom cultivation. Journal of the Science of Food and Agriculture 2016, 96, 680–684. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, H.; Zhang, J.; Li, F.; Li, C.; Zhang, X.; Ma, F. The potential of cottonseed hull as biorefinery substrate after biopretreatment by Pleurotus ostreatus and the mechanism analysis based on comparative proteomics. Industrial Crops and Products 2019, 130, 151–161. [Google Scholar] [CrossRef]
- Buba, F.; Sadiq Said, A.; Hassan, A.; Ali Abdulrahman, A.; Musa, H.; Bello, H.; Adamu Milala, M. Cultivation and Determination of Protein, Elements and Vitamin Content of Oyster Mushroom. Arid Zone Journal of Basic and Applied Research 2022, 1, 70–78. [Google Scholar] [CrossRef]
- Zou, Y.; Du, F.; Zhang, H.; Hu, Q. Evaluation of Korshinsk Peashrub (Caragana korshinskii Kom.) as a Substrate for the Cultivation of Pleurotus eryngii. Waste and Biomass Valorization 2019, 10, 2879–2885. [Google Scholar] [CrossRef]
- Nieto, I.J.; Chegwin, A.C. The effect of different substrates on triterpenoids and fatty acids in fungi of the genus Pleurotus. Journal of the Chilean Chemical Society 2013, 58, 1580–1583. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.-Y.; Kim, Y.H. Agarose Gel Electrophoresis for the Separation of DNA Fragments. Journal of Visualized Experiments 2012, 62, 3923. [Google Scholar] [CrossRef]
- Eongprkornkeaw, A. (2006). Determination of carbon, hydrogen, and nitrogen in biomass fuels by using an elemental gas-chromatographic analyzer. In 32nd congress on science and technology of Thailand.
- Lulea, A.C.; Ruginescu, R.; Banciu, R.M.; Pantazi, C.; Brinduse, E.; Ion, M.; Villarán, M.C. Fast Electrochemical Measurement of Laccase Activity for Monitoring Grapes’ Infection with Botrytis cinerea. Processes 2022, 10, 575. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass and Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Luong, J.; Yang, X.; Hua, Y.; Yang, P.; Gras, R. Gas Chromatography with In Situ Catalytic Hydrogenolysis and Flame Ionization Detection for the Direct Measurement of Formaldehyde and Acetaldehyde in Challenging Matrices. Analytical Chemistry 2018, 90, 13855–13859. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional composition and biological properties of sixteen edible mushroom species. Applied Sciences 2022, 12, 8074. [Google Scholar] [CrossRef]
- Ihnat, M. A survey of methods of analysis for minerals in feedstuffs1,2. Journal of Animal Science 2003, 81, 3218–3225. [Google Scholar] [CrossRef]
- Stilinović, N.; Čapo, I.; Vukmirović, S.; Rašković, A.; Tomas, A.; Popović, M.; Sabo, A. Chemical composition, nutritional profile and in vivo antioxidant properties of the cultivated mushroom Coprinus comatus. Royal Society Open Science 2020, 7, 200900. [Google Scholar] [CrossRef]
- Ivanov, D.; Čolović, R.; Bera, O.; Lević, J.; Sredanović, S. Supercritical fluid extraction as a method for fat content determination and preparative technique for fatty acid analysis in mesh feed for pigs. European Food Research and Technology 2011, 233, 343–350. [Google Scholar] [CrossRef]
- Tsiaka, T.; Sinanoglou, V.J.; Zoumpoulakis, P. (2017). Extracting Bioactive Compounds From Natural Sources Using Green High-Energy Approaches: Trends and Opportunities in Lab- and Large-Scale Applications. In Ingredients Extraction by Physicochemical Methods in Food (pp. 307–365). Elsevier. [CrossRef]
- Team, C. (2014). Team RDC.R: A Language And Environment For Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. R: A Language and Environment for Statistical Computing.
- Adams, A.; Seecharran, D.; Ansari, A. Growth of oyster mushroom using sawdust and agriculture waste as substrates. Mushroom Research 2022, 31, 73–80. [Google Scholar] [CrossRef]
- On, K.L.-. , & Sangsila, A. Development of Rice Straw, Maize, and Giant Mimosa for Growing Mushrooms Instead of Sawdust. International Journal of Membrane Science and Technology 2023, 10, 324–331. [Google Scholar] [CrossRef]
- Ahmed, R.; Niloy, M.A.H.M.; Islam, M.S.; Reza, M.S.; Yesmin, S.; Rasul, S. Bin, & Khandakar, J. Optimizing tea waste as a sustainable substrate for oyster mushroom (Pleurotus ostreatus) cultivation: a comprehensive study on biological efficiency and nutritional aspect. Frontiers in Sustainable Food Systems 2024, 7, 1308053. [Google Scholar]
- Holt, R.R.; Munafo, J.P.; Salmen, J.; Keen, C.L.; Mistry, B.S.; Whiteley, J.M.; Schmitz, H.H. Mycelium: A Nutrient-Dense Food To Help Address World Hunger, Promote Health, and Support a Regenerative Food System. Journal of Agricultural and Food Chemistry 2024, 72, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Y.-Q.; Xiao, X.-W.; Zhong, R.-T.; Yang, C.-F.; Liu, B.; Zhao, C. Nutrient Properties and Nuclear Magnetic Resonance-Based Metabonomic Analysis of Macrofungi. Foods 2019, 8, 397. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Zhao, G.; Xu, C.; Pan, J.; Li, H.; Zou, Y. Impacts of Yield, Nutritional Value, and Amino Acid Contents during Short-Term Composting for the Substrate for Agrocybe aegerita. Horticulturae 2024, 10, 234. [Google Scholar] [CrossRef]
- Niyimbabazi, O.; Nsanzinshuti, A.; Hatungimana, M.; Lin, H.; Zhang, L.; Lin, D.; Zhanxi, L. Ability of Three Pleurotus Species for Effective use of Giant Grass Compost. Journal of Horticultural Research 2022, 30, 67–76. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Li, J.; Li, Y.; Li, G.; Zang, B.; Li, Y. Effect of spent mushroom substrate as a bulking agent on gaseous emissions and compost quality during pig manure composting. Environmental Science and Pollution Research 2018, 25, 12398–12406. [Google Scholar] [CrossRef]
- Meilleur, M.-A.; Bastien, D.; Monfet, D. Modeling Mushrooms’ Carbon Dioxide Emission and Heat Exchange Rates for Synergistic Cultivation with Leafy Greens. Sustainability 2023, 15, 16740. [Google Scholar] [CrossRef]
- Fallah, N.; Pang, Z.; Lin, Z.; Lin, W.; Mbuya, S.N.; Abubakar, A.Y.; Zhang, H. Plant growth and stress-regulating metabolite response to biochar utilization boost crop traits and soil health. Frontiers in Plant Science 2023, 14, 1271490. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Cedric Simelane, M.B.; Mongalo, I.N.; Pooe, O.J. The Effect of Supplementing Mushroom Growing Substrates on the Bioactive Compounds, Antimicrobial Activity, and Antioxidant Activity of Pleurotus ostreatus. Biochemistry Research International 2022, 2022, 1–10. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).