1. Introduction
Tumorigenesis is a multistep process that leads to the transformation of cells and the acquirement of malignant potential [
1]. The continual accumulation of genomic mutations is a key contributor to the transformation process. These mutations can be the result of DNA damage from exogenous genotoxic stresses, such as ultraviolet or ionizing radiation and carcinogenic chemicals. Cancer-promoting mutations can also arise from aberrations in cellular processes, such as DNA replication errors and errors in DNA double-strand break (DSB) repair [
2]. DNA can also be damaged during the export of mRNA from the nucleus to the cytoplasm [
3]. When mRNA export is hindered, the mRNAs hybridize with one strand of double-stranded DNA in the nucleus to form a structure known as an R-loop. This structure results in genomic instability and breaks in the DNA, which is called transcription-coupled DNA damage.
The transcription-export-2 (TREX-2) complex is a critical molecular complex that links gene expression with the nuclear mRNA export process. The TREX-2 complex consists of several molecules including GANP, PCID2, ENY2, centrin3/4, and DSS1. Each of these components also has unique functions in addition to an involvement in mRNA export. Studies have shown that each component of the TREX-2 complex is aberrantly expressed in various tumors. Defective function of the TREX-2 complex has been shown to augment the sensitivity of cancer cells towards chemotherapy [
4].
In this review, we focus on the relationship between deregulated mRNA export and cancer development, especially through the TREX-2 complex. We also describe recent evidence for a medical application that downregulation of the other components may be a good candidate for chemotherapeutic targets in terms of reducing the adverse effects.
2. Overview of the TREX-2 Complex in Yeast and Its Regulation of mRNA Export and Homologous Recombination
The mRNA nuclear export machinery has been extensively clarified over the past decades, especially in
Saccharomyces cerevisiae. Hurt and colleagues identified several molecules that were involved in mRNA export by proteomics techniques and reported that Sac3 was stably associated with Thp1, which was critical for transcription elongation [
5]. Sac3 was initially discovered as a complementary gene in a
S. cerevisiae act1-1 mutant [
6]. Sac3 is localized in the nucleus, and yeast deficient for Sac3 showed mitotic delay and chromosome instability [
7]. The molecular functions of Sac3 have remained unknown for over a decade. Hurt’s group demonstrated that
sac3- and
thp1-deficient yeast showed nuclear accumulation of mRNAs, suggesting that these two molecules are crucial for mRNA nuclear export [
5]. Both
sac3- and
thp1-deficienct yeast had a non-lethal phenotype. The authors subsequently identified additional molecules by proteomics, including Sus1, a component of the SAGA complex with histone acetylase activity, and found that Sus1 interacts with the Sac3-Thp1 complex [
8]. Other identified molecules included Cdc31 and Sem1, a yeast centrin and a component of the proteasome complex, respectively. Yeast deficient for each component showed impairment of mRNA nuclear export, suggesting that all components were indispensable for mRNA nuclear export. The complex consisting of these molecules was designated as the
transcription-
export-2 (TREX-2) complex.
Aguilera and colleagues identified the Sac3-Thp1 complex around the same time. The authors found that several
S. cerevisiae mutants exhibited hyper-recombination phenotypes using an artificial recombination substrate composed of a truncated
leucine-tandem-repeat construct; after a recombination process, functional leucine is produced, which is essential for yeast survival. Both
sac3-deficient and
thp1-deficient yeast showed a similar hyper-recombination phenotype [
9,
10]. Importantly, Aguilera proposed a DNA:RNA hybrid model that links transcription with recombination during mRNA metabolism [
11]. Upon disruption of mRNA export, mRNA accumulates in the nucleus and anneals with single-stranded DNA template to form a DNA:RNA hybrid, which disturbs the other DNA strand and leads to a dispersed single DNA strand. This unique structure of the DNA:RNA hybrid and dispersed single DNA strand, the so-called R-loop, is susceptible to DNA breaks, resulting in genomic instability. These DNA breaks are defined as transcription-coupled DNA damage, and repair is presumably mediated by homologous recombination (HR).
3. Cancer Development by Defects in HR
DNA DSBs caused by cytotoxic chemicals or ionizing radiation are precisely repaired by HR, which is a multistep DNA repair mechanism mediated by more than 20 molecules [
12]. DSBs are the most harmful lesion and are precisely repaired by HR, nonhomologous end joining, and other alternative pathways such as microhomology mediated end joining and single strand annealing. The choice of repair pathway depends on the cell cycle stage and cell type. In contrast to nonhomologous end joining, microhomology mediated end joining, and single strand annealing, HR requires intact DNA templates for more accurate repair. Several models of DNA repair by HR have been reported.
The most upstream molecules in the HR pathway are ataxia telangiectasia mutated (ATM) and ATM and Rad3-related (ATR), which mediate the phosphorylation of downstream effectors, including p53, PALB2, and RAD51, as well as histone H2AX, which acts as a marker for DSBs and triggers recruitment of chromatin remodelers and other end resection factors. PALB2 recruits BRCA2 and RAD51 to single-stranded DNA. ATM and ATR also phosphorylate checkpoint kinases CHK1 and CHK2, thus orchestrating signaling events that lead to checkpoint arrest while activating the machinery involved in HR [
13,
14].
Alteration of HR genes is widely observed among many cancer types. Biallelic loss of
ATM can cause ataxia-telangiectasia, which is associated with an increased cancer risk, especially lymphomas. Humans carrying ATR pathogenic variants (PVs) do not show cancer predisposition [
15]. However, inhibition of ATR resulted in an increase in chemosensitivity in cancer cells [
16]. 53BP1 acts as a DNA damage sensor, suppressing HR-mediated repair of DSBs and promoting other repair pathways; however, germline PVs in 53BP1 do not cause predisposition to cancers [
17]. Human carriers of biallelic
PALB2 PVs develop Fanconi anemia, whereas those with monoallelic
PALB2 PVs show increased cancer risk [
18,
19].
Germline PVs of
BRCA1 and
BRCA2 are strongly correlated with breast and ovarian cancer risk [
20]. While breast cancers from patients with
BRCA1 and
BRCA2 PVs frequently display the triple-negative phenotype, an aggressive subtype with poor survival rates, these tumors respond favorably to platinum-based chemotherapies and poly-ADP ribose polymerase inhibitors (PARPis). In addition to
BRCA1 and
BCRA2, other non-
BRCA HR genes may contribute to so-called “BRCAness,” which is a state of HR-deficiency irrespective of BRCA germline mutations [
21]. Whole-genome sequencing can provide direct evidence of BRCAness. In addition to PVs, copy number aberrations affect HR-deficiency. Therefore, easier and less expensive detections of HR-deficiency should be developed to expand the application of PARPis.
As described above, transcription-coupled DNA damage is repaired by HR. Whether the molecules associated with the repair of transcription-coupled DNA damage are identical or similar to ones in classical HR pathways is unknown.
4. Structural Components of the Human TREX-2 Complex
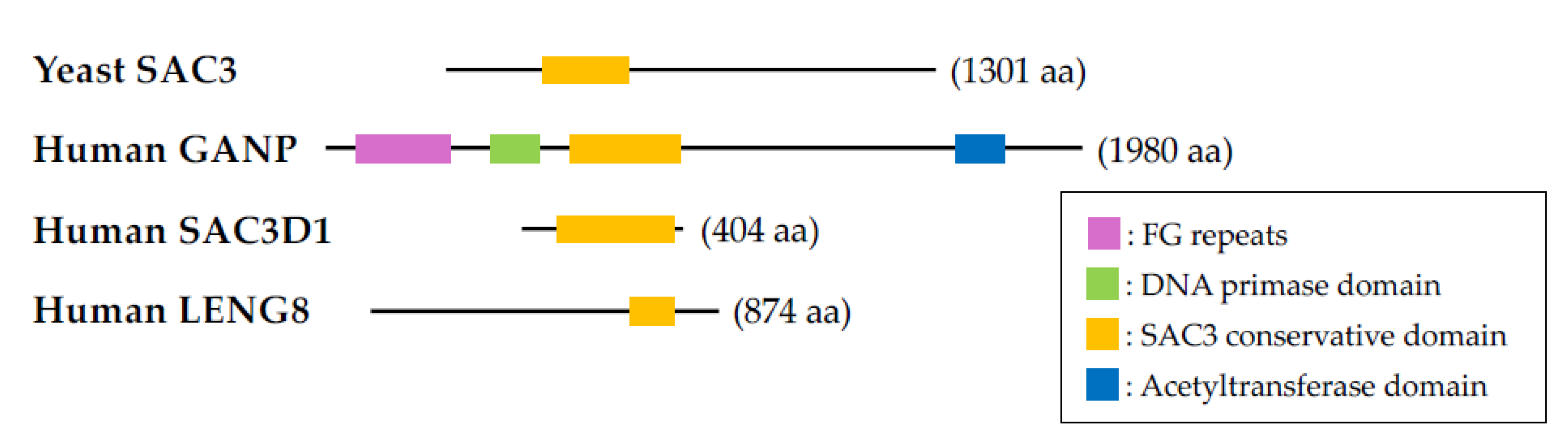
As described above, the human counterparts of the yeast TREX-2 complex have been identified, including GANP, PCID2, ENY2, centrin3/4, and DSS1 (the orthologues of
S. cerevisiae Sac3, Thp1, Sus1, Cdc31, and Sem1, respectively). Depletion of TREX-2 eliminates the connection of transcribed genes to the nuclear pore complex. TREX-2 is thus implicated as a crucial factor linking the mRNP export complexes in the nuclear interior to nuclear pore complexes. GANP was initially identified in a study searching for a novel molecule involved in mouse B-cell maturation and differentiation. A monoclonal antibody, 29-15, that recognized an upregulated molecule in germinal centers, was established. Using expression cloning method with a λgt11 phage library, a novel mouse gene was identified, designated as
germinal center-associated nuclear protein (
ganp), encoding a 210-kDa nuclear protein composed of 1,971 amino acids known as GANP. Further study revealed that the middle portion of GANP (approximately 600 amino acids) is homologous with that of the
S. cerevisiae Sac3 protein sequence (23% at the amino acid level) (
Figure 1), and this region is evolutionally conserved among several species (
Schizosaccharomyces pombe, Caenorhabditis elegans, Drosophila melanogaster, and
Xenopus laevis), indicating that GANP is the mammalian counterpart to yeast Sac3 [
22,
23]. This region was also observed in SAC3D1 [
24] and Leng8, and thus we propose these proteins as Sac3/GANP family members.
The carboxyl-terminal portion of mouse GANP is highly homologous to human MCM3AP (former name, Map80) [
25]. MCM3AP binds to and acetylates MCM3, which is essential for DNA replication [
26]. Comparison of the isolated human
ganp from chromosome 21q22.3 locus and this carboxyl-terminal portion showed this region was identical to human
mcm3ap. However, the relationship between GANP and MCM3AP is still controversial. Takei and Tsujimoto reported an 80-kDa band in immunoblotting of HeLa cell extract with an antibody recognizing the carboxy-terminal of GANP [
25]. In contrast, Northern blot analysis using
ganp- and
mcm3ap-specific probes revealed only a 6-kb band, and no shorter band was detected [
23], suggesting that MCM3AP might be an alternative spliced form of GANP or shorter form of GANP with post-translational modification. Another group demonstrated the existence of a unique promoter in
mcm3ap transcription [
27]. Mouse MCM3AP has not yet been reported. The functions of MCM3AP
in vivo remain elusive.
A 150 amino acid sequence in the amino-terminal region of GANP shows significant similarity to the DNA primase p49 [
28]. The amino-terminal region of GANP also contains FG repeats that bind to the NXF1 FG-binding domain, suggesting GANP’s involvement in NXF1-containing mRNP transfer to the nuclear pore complex [
29]. Lower species lack these functional domains and only harbor the Sac3 conserved domain (
Figure 1).
5. The Regulation of Recombination and mRNA Nuclear Export by the Mammalian TREX-2 Complex
In the event of transcription-coupled DNA damage, the damage should be repaired prior to the G1/S checkpoint. In yeast deficient for any component of the TREX/THO or TREX-2 complexes, genomic damage occurs. THO is a multimeric complex required for mRNA-protein biogenesis [
30]. Defects in TREX-2 lead to aberrations in replication or survival, signifying the importance of the function of individual components in the TREX-2 complex.
To examine whether TREX-2 orthologues in mammals have similar functions to those in
S. cerevisiae, a
β-galactosidase tandem-repeat construct was established to quantitatively measure cell recombination rate [
31]. Using a tandem
β-galactosidase reporter system, researchers found that GANP suppresses homology-mediated DNA recombination, particularly in rapidly proliferating cells or cells with high-grade DSBs, as in germinal center B cells, which is required for DNA repair. This regulatory effect on DNA recombination depended on the Sac-3 conserved region but was also affected by the C-terminal region of GANP, which contains a HAT domain [
31]. Thus, GANP might play distinct roles in DNA replication and repair mechanisms.
This sensor mechanism detects DNA damage during cell proliferation, thus regulating the cell cycle. GANP and Pcid2 selectively regulate mitotic spindle checkpoints, Shugoshin-1 and MAD2, to maintain the cellular genome [
32,
33]. DSS1 plays a role in BRCA2 stabilization by regulating its ubiquitin-dependent proteolytic degradation [
34]. This suggests a unique function for DSS1 in the organization of ribonucleoprotein complexes during transcription, nuclear to cytoplasmic export, and translation. Unlike GANP and Pcid2, whether DSS1 is involved in specific mRNA(s) export is unclear.
Functional and structural characterization of the mammalian TREX-2 complex has demonstrated how the complex links transcription/processing with nuclear mRNA export. A portion of TREX-2 was found to be located near the transcription site in the mammalian nucleus. After maturing into a transport-competent state, the mRNP particle was proposed to bind with TREX-2 through the interaction of GANP with NXF1, facilitating its delivery to the nuclear pore complex [
35].
Genome maintenance has been shown to be regulated by proteins involved in DNA replication or DNA damage response. Subsequent studies revealed that mutations in genes involved in pre-mRNA splicing and the biogenesis and export of mRNAs result in DNA damage and genome instability. The instability is frequently mediated by R-loops formed by DNA-RNA hybrids and displaced ssDNA. Mitotic HR protects the cellular genome by properly repairing transcription-coupled DNA damage and other lesions caused by exogenous insults. During proliferative stages in development and somatic cell renewal in adults, it is essential to repair DNA damage and protect cells against cell death and mutagenic outcomes [
36]. In yeast, HR primarily involves proteins within the RAD52 group. In mammalian cells, the BRCA2 tumor suppressor protein plays a central function in HR by regulating RAD51 binding DNA, which is required for DSB repair. BRCA2 forms a complex with DSS1 to promote the RAD51-loading activity of BRCA2. The binding of DSS1 masks the nuclear export signals of BRCA2 and regulates both BRCA2 and RAD51 nuclear localization [
37]. DSS1 was also shown to promote BRCA2-dependent HR by targeting replication protein A (RPA). DSS1 is thought to mimic DNA and reduce the affinity of RPA for ssDNA [
38].
Some studies reported that R-loops were not detected in TREX-2-depleted cells, but R-loop accumulation was observed in BRCA2-depleted cells [
39]. BRCA2 was shown to be associated with PCID2, which prevents R-loop formation and transcription-coupled DNA damage. These results indicate that BRCA2 is directly or indirectly required for R-loop formation and presumably transcription-coupled DNA damage, leading to carcinogenesis [
39].
6. Aberrant Expression of GANP Protein in Human Tumors
The expression levels of
ganp transcripts and GANP protein are extremely low in various tissues, except in normal mammary ducts. This evidence, together with GANP’s functions in DNA replication, suggests that GANP may be upregulated in cancer. Indeed, GANP upregulation was observed in various hematological disorders, including leukemias and lymphomas [
40]. Aberrant GANP expression was also observed in malignant melanomas [
41], liver fluke–associated cholangiocarcinomas [
42], and testicular postpubertal-type teratomas [
43]. Lymphomagenesis and teratomagenesis have also been linked to abnormal expression of GANP [
40,
43]. In contrast, GANP expression was decreased in some solid tumors, such as in glioblastomas [
44] and breast cancers [
45]. The expression of GANP was also associated with resistance to breast cancer development. However, the level of GANP expression did not affect tumor migratory and invasive properties [
45]. How GANP counteracts the oncogenic process in these tumors is yet to be elucidated. A further study discovered an association between polymorphisms in the
GANP locus, its expression, and breast cancer risk and prognosis [
46]. These results suggest that GANP may contribute differently to tumorigenesis depending on various associated molecules or tissue type.
7. Lymphomagenesis in GANP Transgenic Mice
Further investigation into the mechanism underlying GANP’s involvement in lymphomagenesis has revealed its multifaceted impact on cellular processes. GANP, through its targeting of Lyn-mediated signaling within germinal center B cells, orchestrates a delicate balance crucial for cell survival and differentiation [
47]. The PU.1 binding site in the
ganp promoter serves as a key regulatory mechanism, allowing PU.1 to modulate the dynamic reprogramming of B cells and macrophage differentiation [
48]. This regulatory network not only underscores the significance of GANP in maintaining the survival of mature germinal center B cells but also highlights its role in suppressing DNA damage, thus safeguarding cellular integrity [
49].
Abnormal overexpression of GANP has been documented in various human hematopoietic and lymphoid neoplasms, including Hodgkin and Reed-Sternberg (HRS) cells [
40,
50]. Furthermore, GANP is involved in the progression of phenotypic Hodgkinoid lymphoma, which displays features of B cells/macrophage phenotypic cells corresponding to human Hodgkin’s lymphoma.
GANP transgenic mice were generated under the immunoglobulin promoter and enhancer (
Ig-
ganpTg). In
Ig-
ganpTg mice, B-cell/macrophage bi-phenotypic Hodgkinoid lymphoma developed, showing genetic fingerprint evidence of
Ig gene rearrangement with expressions of Ig-μ/Ig-κ chains. GANP may also play a crucial role in ensuring the survival of HRS cells that stem from germinal center B cells in
Ig-
ganpTg mice [
50]. These results of GANP’s role in lymphomagenesis suggests that its actions are not confined to individual cell types but rather it orchestrates a synergistic interplay between germinal center B cells and macrophages. The survival and transdifferentiation facilitated by GANP may serve as critical drivers of Hodgkin lymphomagenesis, shedding light on potential therapeutic targets for this complex malignancy [
50]. Further elucidation of the molecular mechanisms underlying GANP’s function promises to deepen the understanding of lymphoma pathogenesis and may pave the way for more targeted therapeutic interventions.
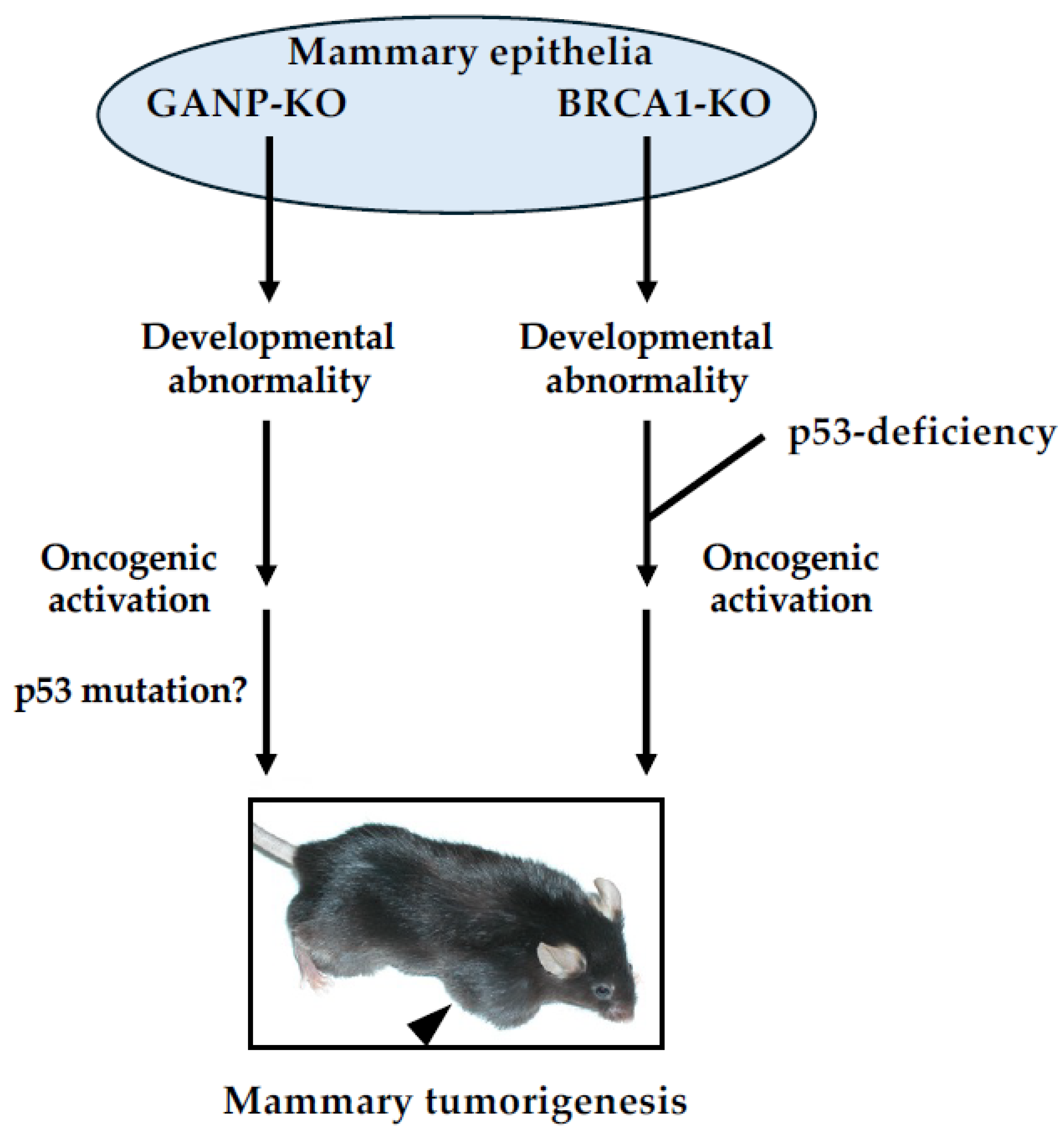
8. Mammary Tumorigenesis in ganp-Deficient Mice
In contrast to GANP overexpression in hematological malignancies, GANP expression tended to decrease in the malignant progression of breast cancers. In a study of over 400 breast cancer patients, the GANP-low group showed a worse prognosis compared with the GANP-high group in both breast cancer-specific and relapse-free survival [
45]. In normal mammary glands, GANP is highly expressed in the nuclei; GANP expression tended to downregulate in proportion to malignant progression. To examine the effects of downregulated GANP expression
in vivo, a study was conducted by Kuwahara and colleagues to explore tumorigenesis in mammary glands with GANP deficiency. The deficiency in GANP expression in the mammary gland was constructed using mammary-specific
ganp-deficient mice by crossing
ganp-floxed mice with whey acid protein (wap)-cre mice. During pregnancy, cre recombinase is translated and cleaves inserted loxP sequences in the
ganp gene, resulting in the induction of mammary-specific
ganp deletion. The mammary-specific
ganp-homodeficient mice were GANP deficient, as confirmed by immunohistochemistry analysis and real-time polymerase chain reaction. During lactation, mice exhibited distinct malformations in mammary glands; the development of glandular anatomy was abnormal, the epithelial lining of the mammary gland lumen was misaligned, and development of fibrous tissue was observed. After several gene alterations including mutations and translocations accumulated in mammary glands with abnormal morphology, mammary tumors developed. These phenotypes are similar to those observed in mammary-specific
brca1-deficient mice [
51] (
Figure 2). The mammary-specific
ganp-homodeficient mice also had metastases of tumor cells in the lung.
Conventional
ganp-homodeficient mice showed embryonic lethality [
30]. Further investigation of
ganp-heterodeficient (
ganp+/d) mice confirmed that the expression of GANP was lower in comparison to the wild-type mice. The
ganp+/d mice during multiparity developed mammary gland tumors with the growth of atypical cells, suggesting that two alleles of
ganp play a role in preventing mammary gland tumors. Tumors in
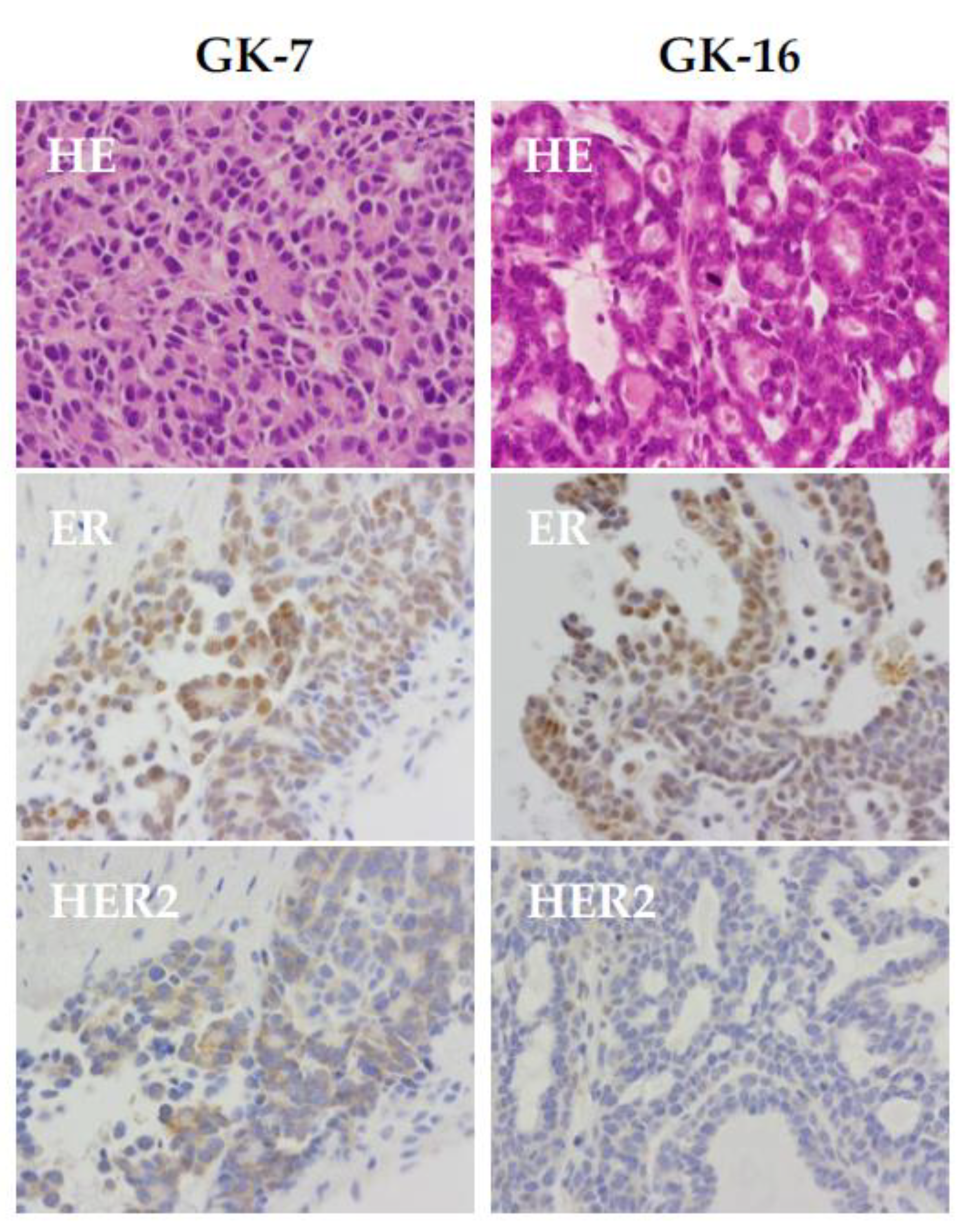
ganp+/d mice also displayed aberrant expression of several biomarkers detected in human breast cancer, such as ERα, PgR, Her2, and Ki67 (
Figure 3). Moreover, analysis of cultured mammary gland tumor cells revealed aneuploidy as it was further confirmed in the longer duration of culture exhibition of chromosomal instability at a high rate [
45]. Mouse embryonic fibroblasts (MEFs) derived from
ganp+/d mice partially demonstrated triradial chromosomes, whereas
ganp+/+-MEFs did not show any aberrant chromosomes (
Figure 4A). Sarcomatous tumors with severe nuclear atypia were observed in immunodeficient mice inoculated with
ganp+/d-MEFs [
52], suggesting that cells deficient for GANP have oncogenic potential (
Figure 4B). Together with
in vitro experiments, these results indicate that GANP suppresses the DNA damage induced by estrogen exposure and has an anti-oncogenic effect on breast carcinogenesis.
One way that GANP may suppress tumors is through its interaction with DNA-dependent protein kinase, catalytic subunit (DNA-PKcs), which inhibits DSB repair by the non-homologous end-joining pathway and promotes repair by HR [
49]. In addition to preventing tumor development, GANP is also required to maintain the lifespan of mice, as
ganp+/d mice were found to have a shorter lifespan than
ganp+/+ mice.
9. Functional Role of Other TREX-2 Components in Tumor Development
Studies in mice with conditionally targeted PCID2 in B cells demonstrated that PCID2 is essential for B-cell development, presumably through deregulated MAD2 [
32]. PCID2 was also reported to be associated with E1A-like inhibitor of differentiation one (EID1), which is involved in stemness in embryonic stem and induced pluripotent stem cells [
53]. EID1 inhibits the HAT activity of CBP/p300 to suppress developmental gene expression. Whether TREX-2 components are functionally related to embryonic stem cells to sustain their pluripotency is still unknown. Wap-cre-pcid2 deficient mice were generated, but no spontaneously developed mammary tumors were observed over a 2-year observation (unpublished observation). Whether aberrant expression of PCID2 is associated with mammary tumorigenesis is unknown. Rather, the unique pathway involved in mammary tumorigenesis might exist downstream of GANP.
The relationship of PCID2 with gastrointestinal tumors is more concrete, and the underlying pathway is not related to TREX-2. Clinical studies have shown that the level of PCID2 mRNA was significantly higher in colorectal cancer (CRC) tissues than in adjacent normal tissue, and its high expression was associated with recurrence [
54]. PCID2 may accelerate the G1-S cell cycle transition and inhibit apoptosis in CRC cells. The upregulation of cyclin D1 and the downregulation of p21Cip1 are linked to the PCID2-induced G1-S transition [
55]. Aberrant expression of PCID2 also contributes to degradation of the tumor suppressor promyelocytic leukemia (PML) through ubiquitin proteasome system-dependent degradation [
56]. Under normal conditions, PML suppresses oncogenic Wnt/β-catenin signaling while enhancing tumor suppressive β-catenin signaling. If PML is suppressed, such as in the case of aberrant PCID2 expression, oncogenic Wnt/β-catenin signaling is activated, thus contributing to CRC tumorigenesis. When cells with elevated PCID2 expression were implanted in an
in vivo model, an increased tumor weight and incidence of metastasis were observed, which further proved that PCID2 is an important factor in the tumorigenesis and progression of CRC [
54].
Other TREX-2 components like ENY-2 are also markedly elevated in many types of cancers. Deregulation of mRNA export by ENY2 and cell cycle disruption is a key to the development of cancers [
57]. ENY2 may facilitate immune evasion by modifying and disrupting the balance between MHC, immune suppressors, and immune stimulators [
56]. Furthermore, ENY2 was found to facilitate breast cancer cell migration and metastasis both
in vitro and
in vivo [
58]. This indicates ENY2 is also an important molecule in tumor development and progression. Direct evidence of the role of other components of the TREX-2 complex, centrin 3/4 and DSS1, in tumorigenesis has not been reported.
10. Molecular Targets of Increased Chemosensitivity
Several components of the TREX-2 complex regulate the chemosensitivity of cancer cells. DSS1, a molecule of the TREX-2 complex that binds to BRCA2, has been associated with the occurrence of several types of tumors and sensitivity to chemotherapy drugs. In cervical cancer, DSS1 was reported as an up-regulated molecule in cancerous lesions compared with normal cervix [
59]. A clinical study in female breast cancer patients revealed that those with elevated levels of DSS1 tended to experience poorer prognosis or shorter survival times compared with those with lower levels of DSS1 [
60]. This may be caused by the interaction between DSS1 and the RPN3/S3 proteasomal subunit, leading to enhanced degradation of ubiquitinated p53 [
61]. A recent report suggested that DSS1 is critically involved in the proliferation, apoptosis, invasion, and migration of glioma cells via the Akt signaling pathway [
62].
dss1-deficient mice have not yet been reported, and thus whether DSS1 is associated with tumorigenesis remains unclear. DSS1 depletion causes BRCA2 destabilization even in cells without BRCA1/2 germline mutation, leading to impaired HR repair in cancers (our unpublished results). Another study showed that DSS1 and PCID2 contribute to changes in the sensitivity threshold for anticancer drugs
in vitro. PARPis may be effective in BRCA wild-type and DSS1-depleted breast cancer patients [
4].
ENY-2, another component of the TREX-2 complex, may also be associated with decreased sensitivity to anti-cancer drugs, leading to chemoresistance. Proteomics was performed using the trastuzumab-pertuzumab-resistant HER-2
+ breast cancer cell line. Among more than 600 proteins involved in important biological processes like metabolism, ribosome formation, and mitochondrial activity, ENY-2 was found to be upregulated or present in SK-BR3.rTP (resistant) cells, but not in SK-BR3 (sensitive) parental cells. Upregulation of ENY-2 could also contribute to the development of resistant HER-2
+ breast cancer cells, although the detailed mechanism is still unclear [
63]. As chemotherapy is often administered alongside other breast cancer treatments like surgery, radiation, or hormone therapy, these findings could be leveraged to develop additional chemotherapy preparation strategies, potentially enhancing clinical outcomes.
11. Conclusion and Perspective
Transcription-coupled DNA damage has been extensively analyzed in
S. cerevisiae. Recent evidence demonstrated that deregulation of the mammalian TREX-2 component GANP affects tumorigenesis, especially lymphomagenesis and breast carcinogenesis [
64]. Whether mRNA export
per se is critical for carcinogenesis and/or cancer development remains to be elucidated. Germline mutations of
ganp have not yet been identified in cancers. Nevertheless, GANP is an important gatekeeper to prevent the development of tumors. Mammary-specific
ganp-deficient mice showed abnormal ductal development leading to the formation of mammary tumors, as observed in mammary-specific
brca1-deficient mice [
51]. The TREX-2 complex, including GANP, is functionally associated with BRCA2 [
38]. GANP may be a BRCA-like molecule or function in concert with BRCA1 and/or BRCA2 in sporadic breast carcinogenesis (
Figure 2).
Other TREX-2 components like PCID-2, ENY-2, and DSS1 are also related to tumorigenesis. Elevation of these proteins are associated with tumor progression, metastases, and chemoresistance, which is often associated with poor outcome in patients. Further studies are needed to elucidate the complex relationships between TREX-2 components and cancer in the hope that the results might identify a target for novel treatment that can save the lives of many patients.
Author Contributions
Conceptualization, A.R. and K.K.; formal analysis, A.R., N.G., Y.N., S.P., S.O. and K.K.; funding acquisition, K.K.; investigation, A.R., N.G., Y.S., Y.N., S.P., T.T., S.O. and K.K.; project administration, A.I. and K.K.; resources, A.R., N.G., T.T., S.O. and K.K.; supervision, K.K.; validation, Y.S., T.T., S.O. and K.K.; visualization, Y.S., T.T., S.O. and K.K.; writing—original draft, A.R. and K.K.; writing—review and editing, A.R., A.I. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grants-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science grant number 23K06436 and partly supported by the Aichi Cancer Research Foundation, the 24th General Assembly of the Japanese Association of Medical Sciences, and the Aichi Health Promotion Foundation (K.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Kumamoto University (C23-220 and C23-223 approved on March 31, 2011) and Aichi Cancer Center (2508 approved on April 30, 2014).
Data Availability Statement
The raw data supporting the conclusions of this article are available by the corresponding author (K.K.) on request.
Acknowledgments
The authors thank Masahisa Tsuji for the chromosomal analysis in MEFs. We also thank Mika Ito and Rie Miura for technical assistance. We thank Gabrielle White Wolf, PhD, from Edanz (
https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rogozin, I.B.; Pavlov, Y.I.; Goncearenco, A.; De, S.; Lada, A.G.; Poliakov, E.; Panchenko, A.R.; Cooper, D.N. Mutational Signatures and Mutable Motifs in Cancer Genomes. Brief. Bioinform. 2018, 19, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.S. The Molecular Biology of Cancer. Mol. Aspects Med. 2000, 21, 167–223. [Google Scholar] [CrossRef]
- Sakai, Y.; Phimsen, S.; Okada, S.; Kuwahara, K. The Critical Role of Germinal Center-Associated Nuclear Protein in Cell Biology, Immunohematology, and Hematolymphoid Oncogenesis. Exp. Hematol. 2020, 90, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gondo, N.; Sakai, Y.; Zhang, Z.; Hato, Y.; Kuzushima, K.; Phimsen, S.; Kawashima, Y.; Kuroda, M.; Suzuki, M.; Okada, S.; et al. Increased Chemosensitivity via BRCA2-Independent DNA Damage in DSS1- and PCID2-Depleted Breast Carcinomas. Lab. Invest. 2021, 101, 1048–1059. [Google Scholar] [CrossRef]
- Fischer, T.; Strässer, K.; Rácz, A.; Rodriguez-Navarro, S.; Oppizzi, M.; Ihrig, P.; Lechner, J.; Hurt, E. The mRNA Export Machinery Requires the Novel Sac3p-Thp1p Complex to Dock at the Nucleoplasmic Entrance of the Nuclear Pores. EMBO J. 2002, 21, 5843–5852. [Google Scholar] [CrossRef]
- Novick, P.; Osmond, B.C.; Botstein, D. Suppressors of Yeast Actin Mutations. Genetics 1989, 121, 659–674. [Google Scholar] [CrossRef]
- Bauer, A.; Kölling, R. The SAC3 Gene Encodes a Nuclear Protein Required for Normal Progression of Mitosis. J. Cell Sci. 1996, 109, 1575–1583. [Google Scholar] [CrossRef]
- González-Aguilera, C.; Tous, C.; Gómez-González, B.; Huertas, P.; Luna, R.; Aguilera, A. The THP1-SAC3-SUS1-CDC31 Complex Works in Transcription Elongation-mRNA Export Preventing RNA-Mediated Genome Instability. Mol. Biol. Cell 2008, 19, 4310–4318. [Google Scholar] [CrossRef]
- Gallardo, M.; Luna, R.; Erdjument-Bromage, H.; Tempst, P.; Aguilera, A. Nab2p and the Thp1p-Sac3p Complex Functionally Interact at the Interface between Transcription and mRNA Metabolism. J. Biol. Chem. 2003, 278, 24225–24232. [Google Scholar] [CrossRef]
- Gallardo, M.; Aguilera, A. A New Hyperrecombination Mutation Identifies a Novel Yeast Gene, THP1, Connecting Transcription Elongation With Mitotic Recombination. Genetics 2001, 157, 79–89. [Google Scholar] [CrossRef]
- Huertas, P.; Aguilera, A. Cotranscriptionally Formed DNA:RNA Hybrids Mediate Transcription Elongation Impairment and Transcription-Associated Recombination. Mol. Cell 2003, 12, 711–721. [Google Scholar] [CrossRef]
- Gartner, A; Engebrecht, J. DNA Repair, Recombination, and Damage signaling. Genetics 2022, 220, iyab178. [Google Scholar] [CrossRef]
- Cortez, D.; Guntuku, S.; Qin, J.; Elledge, S.J. ATR and ATRIP: Partners in Checkpoint Signaling. Science 2001, 294, 1713–1716. [Google Scholar] [CrossRef]
- Reinhardt, H.C.; Yaffe, M.B. Kinases That Control the Cell Cycle in Response to DNA Damage: Chk1, Chk2, and MK2. Curr. Opin. Cell Biol. 2009, 21, 245–255. [Google Scholar] [CrossRef]
- Lecona, E.; Fernandez-Capetillo, O. Targeting ATR in Cancer. Nat. Rev. Cancer 2018, 18, 586–595. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, J.; He, K.; Zhang, J. Ataxia Telangiectasia and Rad3-Related Inhibitors and Cancer Therapy: Where We Stand. J. Hematol. Oncol. 2019, 12, 43. [Google Scholar] [CrossRef]
- Ward, I.M.; Difilippantonio, S.; Minn, K.; Mueller, M.D.; Molina, J.R.; Yu, X.; Frisk, C.S.; Ried, T.; Nussenzweig, A.; Chen, J. 53BP1 Cooperates with P53 and Functions as a Haploinsufficient Tumor Suppressor in Mice. Mol. Cell. Biol. 2005, 25, 10079–10086. [Google Scholar] [CrossRef]
- Fewings, E.; Larionov, A.; Redman, J.; Goldgraben, M.A.; Scarth, J.; Richardson, S.; Brewer, C.; Davidson, R.; Ellis, I.; Evans, D.G.; et al. Germline Pathogenic Variants in PALB2 and Other Cancer-Predisposing Genes in Families with Hereditary Diffuse Gastric Cancer without CDH1 Mutation: A Whole-Exome Sequencing Study. Lancet Gastroenterol. Hepatol. 2018, 3, 489–498. [Google Scholar] [CrossRef]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef]
- Paul, A.; Paul, S. The Breast Cancer Susceptibility Genes (BRCA) in Breast and Ovarian Cancers. Front. Biosci. (Landmark Ed). [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness Revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- Kuwahara, K.; Yoshida, M.; Kondo, E.; Sakata, A.; Watanabe, Y.; Abe, E.; Kouno, Y.; Tomiyasu, S.; Fujimura, S.; Tokuhisa, T.; et al. A Novel Nuclear Phosphoprotein, GANP, Is up-Regulated in Centrocytes of the Germinal Center and Associated with MCM3, a Protein Essential for DNA Replication. Blood 2000, 95, 2321–2328. [Google Scholar] [CrossRef]
- Abe, E.; Kuwahara, K.; Yoshida, M.; Suzuki, M.; Terasaki, H.; Matsuo, Y.; Takahashi, E.I.; Sakaguchi, N. Structure, Expression, and Chromosomal Localization of the Human Gene Encoding a Germinal Center-Associated Nuclear Protein (GANP) That Associates with MCM3 Involved in the Initiation of DNA Replication. Gene 2000, 255, 219–227. [Google Scholar] [CrossRef]
- Khuda, S.E.; Yoshida, M.; Xing, Y.; Shimasaki, T.; Takeya, M.; Kuwahara, K; Sakaguchi, N. The Sac3 Homologue shd1 Is Involved in Mitotic Progression in Mammalian Cells. J. Biol. Chem. 2004, 279, 46182–46190. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Tsujimoto, G. Identification of a Novel MCM3-Associated Protein That Facilitates MCM3 Nuclear Localization. J. Biol. Chem. 1998, 273, 22177–22180. [Google Scholar] [CrossRef]
- Takei, Y.; Swietlik, M.; Tanoue, A.; Tsujimoto, G.; Kouzarides, T.; Laskey, R. MCM3AP, a Novel Acetyltransferase That Acetylates Replication Protein MCM3. EMBO Rep. 2001, 2, 119–123. [Google Scholar] [CrossRef]
- Wickramasinghe, V.O.; McMurtrie, P.I.; Marr, J.; Amagase, Y.; Main, S; Mills, A.D.; Laskey, R.A.; Takei, Y. MCM3AP Is Transcribed from a Promoter within an Intron of the Overlapping Gene for GANP. J. Mol. Biol. 2011, 406, 355–361. [Google Scholar] [CrossRef]
- Kuwahara, K.; Tomiyasu, S.; Fujimura, S.; Nomura, K.; Xing, Y.; Nishiyama, N.; Ogawa, M.; Imajoh-Ohmi, S.; Izuta, S.; Sakaguchi, N. Germinal Center-Associated Nuclear Protein (GANP) Has a Phosphorylation-Dependent DNA-Primase Activity That Is up-Regulated in Germinal Center Regions. Proc. Natl. Acad. Sci. 2001, 98, 10279–10283. [Google Scholar] [CrossRef]
- Wickramasinghe, V.O.; McMurtrie, P.I.A.; Mills, A.D.; Takei, Y.; Penrhyn-Lowe, S.; Amagase, Y.; Main, S.; Marr, J.; Stewart, M.; Laskey, R.A. mRNA Export from Mammalian Cell Nuclei Is Dependent on GANP. Curr. Biol. 2010, 20, 25–31. [Google Scholar] [CrossRef]
- Luna, R.; Rondon, A.G.; Aguilera, A. New Clues to Understand the Role of THO and Other Functionally Related Factors in mRNP Biogenesis. Biochim. Biophys. Acta 2012, 1819, 514–520. [Google Scholar] [CrossRef]
- Yoshida, M.; Kuwahara, K.; Shimasaki, T.; Nakagata, N.; Matsuoka, M.; Sakaguchi, N. GANP Suppresses DNA Recombination, Measured by Direct-repeat b-galactosidase Gene Construct, but Does Not Suppress the Type of Recombination Applying to Immunoglobulin Genes in Mammalian Cells. Genes Cells 2007, 12, 1205–1213. [Google Scholar] [CrossRef]
- Okamoto, N.; Kuwahara, K.; Ohta, K.; Kitabatake, M.; Takagi, K.; Mizuta, H.; Kondo, E.; Sakaguchi, N. Germinal Center-Associated Nuclear Protein (GANP) Is Involved in mRNA Export of Shugoshin-1 Required for Centromere Cohesion and in Sister-Chromatid Exchange. Genes Cells 2010, 15, 471–484. [Google Scholar] [CrossRef]
- Nakaya, T.; Kuwahara, K.; Ohta, K.; Kitabatake, M.; Toda, T.; Takeda, N.; Tani, T.; Kondo, E.; Sakaguchi, N. Critical Role of Pcid2 in B Cell Survival through the Regulation of MAD2 Expression. J. Immunol. 2010, 185, 5180–5187. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Bai, Y.; Wazer, D.E.; Band, V.; Gao, Q. DSS1 Is Required for the Stability of BRCA2. Oncogene 2006, 25, 1186–1194. [Google Scholar] [CrossRef]
- Jani, D.; Lutz, S.; Hurt, E.; Laskey, R.A.; Stewart, M.; Wickramasinghe, V.O. Functional and Structural Characterization of the Mammalian TREX-2 Complex That Links Transcription with Nuclear Messenger RNA Export. Nucleic Acids Res. 2012, 40, 4562–4573. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Jasin, M. Mitotic Homologous Recombination Maintains Genomic Stability and Suppresses Tumorigenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 196–207. [Google Scholar] [CrossRef]
- Jeyasekharan, A.D.; Liu, Y.; Hattori, H.; Pisupati, V.; Jonsdottir, A.B.; Rajendra, E.; Lee, M.; Sundaramoorthy, E.; Schlachter, S.; Kaminski, C.F.; et al. A Cancer-Associated BRCA2 Mutation Reveals Masked Nuclear Export Signals Controlling Localization. Nat. Struct. Mol. Biol. 2013, 20, 1191–1198. [Google Scholar] [CrossRef]
- Zhao, W.; Vaithiyalingam, S.; San Filippo, J.; Maranon, D.G.; Jimenez-Sainz, J.; Fontenay, G.V.; Kwon, Y.; Leung, S.G.; Lu, L.; Jensen, R.B.; et al. Promotion of BRCA2-Dependent Homologous Recombination by DSS1 via RPA Targeting and DNA Mimicry. Mol. Cell 2015, 59, 176–187. [Google Scholar] [CrossRef]
- Bhatia, V.; Barroso, S.I.; García-Rubio, M.L.; Tumini, E.; Herrera-Moyano, E.; Aguilera, A. BRCA2 Prevents R-Loop Accumulation and Associates with TREX-2 mRNA Export Factor PCID2. Nature 2014, 511, 362–365. [Google Scholar] [CrossRef]
- Fujimura, S.; Xing, Y.; Takeya, M.; Yamashita, Y.; Ohshima, K.; Kuwahara, K.; Sakaguchi, N. Increased Expression of Germinal Center-Associated Nuclear Protein RNA-Primase Is Associated with Lymphomagenesis. Cancer Res. 2005, 65, 5925–5934. [Google Scholar] [CrossRef]
- Kageshita, T.; Kuwahara, K.; Oka, M.; Ma, D.; Ono, T.; Sakaguchi, N. Increased Expression of Germinal Center-Associated Nuclear Protein (GANP) Is Associated with Malignant Transformation of Melanocytes. J. Dermatol. Sci. 2006, 42, 55–63. [Google Scholar] [CrossRef]
- Chan-On, W.; Kuwahara, K.; Kobayashi, N.; Ohta, K.; Shimasaki, T.; Sripa, B.; Leelayuwat, C.; Sakaguchi, N. Cholangiocarcinomas Associated with Long-Term Inflammation Express the Activation-Induced Cytidine Deaminase and Germinal Center-Associated Nuclear Protein Involved in Immunoglobulin V-Region Diversification. Int. J. Oncol. 2009, 35, 287–295. [Google Scholar] [PubMed]
- Sakai, Y.; Yoshinaga, K.; Yoshida, A.; Rezano, A.; Shiogama, K.; Kawashima, Y.; Yoshizawa, T.; Yoshizawa, A.; Hatakeyama, S.; Ohyama, C.; et al. Testicular Teratomagenesis from Primordial Germ Cells with Overexpression of Germinal Center-associated Nuclear Protein. Cancer Sci. 2022, 114, 1729–1739. [Google Scholar] [CrossRef]
- Ohta, K.; Kuwahara, K.; Zhang, Z.; Makino, K.; Komohara, Y.; Nakamura, H.; Kuratsu, J.; Sakaguchi, N. Decreased Expression of Germinal Center-Associated Nuclear Protein Is Involved in Chromosomal Instability in Malignant Gliomas. Cancer Sci. 2009, 100, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, K.; Yamamoto-Ibusuki, M.; Zhang, Z.; Phimsen, S.; Gondo, N.; Yamashita, H.; Takeo, T.; Nakagata, N.; Yamashita, D.; Fukushima, Y.; et al. GANP Protein Encoded on Human Chromosome 21/Mouse Chromosome 10 Is Associated with Resistance to Mammary Tumor Development. Cancer Sci. 2016, 107, 469–477. [Google Scholar] [CrossRef]
- Kotani, H.; Ito, H.; Kuwahara, K.; Kuzushima, K.; Iwata, H.; Tsunoda, N.; Nagino, M.; Matsuo, K. Impact of Germinal Center-Associated Nuclear Protein Polymorphisms on Breast Cancer Risk and Prognosis in a Japanese Population. Breast Cancer 2019, 26, 562–572. [Google Scholar] [CrossRef]
- Kuwahara, K.; Nakaya, T.; Phimsen, S.; Toda, T.; Kitabatake, M.; Kaji, T.; Takemori, T.; Watanabe, T.; Sakaguchi, N. Lyn Signaling to Upregulate GANP Is Critical for the Survival of High-Affinity B Cells in Germinal Centers of Lymphoid Organs. J. Immunol. 2012, 189, 3472–3479. [Google Scholar] [CrossRef]
- EL-Gazzar, M.A.; Maeda, K.; Nomiyama, H.; Nakao, M.; Kuwahara, K.; Sakaguchi, N. PU. 1 Is Involved in the Regulation of B Lineage-Associated and Developmental Stage-Dependent Expression of the Germinal Center-Associated DNA Primase GANP. J. Biol. Chem. 2001, 276, 48000–48008. [Google Scholar] [CrossRef]
- Eid, M.M.A.; Maeda, K.; Almofty, S.A.; Singh, S.K.; Shimoda, M.; Sakaguchi, N. GANP Regulates the Choice of DNA Repair Pathway by DNA-PKcs Interaction in AID-Dependent IgV Region Diversification. J. Immunol. 2014, 192, 5529–5539. [Google Scholar] [CrossRef]
- Sakai, Y.; Rezano, A.; Okada, S.; Ohtsuki, T.; Kawashima, Y.; Tsukamoto, T.; Suzuki, M.; Kohara, M.; Takeya, M.; Sakaguchi, N.; et al. A Novel Cytological Model of B-Cell/Macrophage Biphenotypic Cell Hodgkin Lymphoma in Ganp-Transgenic Mice. Cancers 2020, 12, 204. [Google Scholar] [CrossRef]
- Xu, X.; Wagner, K.U.; Larson, D.; Weaver, Z.; Li, C.; Ried, T.; Hennighausen, L.; Wynshaw-Boris, A.; Deng, C.X. Conditional Mutation of Brca1 in Mammary Epithelial Cells Results in Blunted Ductal Morphogenesis and Tumour Formation. Nat. Genet. 1999, 22, 37–43. [Google Scholar] [CrossRef]
- Ono, A.; Hattori, S.; Kariya, R.; Iwanaga, S.; Taura, M.; Harada, H.; Suzu, S; Okada, S. Comparative Study of Human Hematopoietic Cell Engraftment into BALB/c and C57BL/6 Strain of Rag-2/Jak3 Double-deficient Mice. J. Biomed. Biotechnol. 2011, 2011, 539748. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Dai, Z.; Liu, B.; Wang, R.; Li, C.; Huang, G.; Wng, S.; Xia, P.; Yang, X.; Kuwahara, K. Pcid2 Inactivates Developmental Genes in Human and Mouse Embryonic Stem Cells to Sustain Their Pluripotency by Modulation of EID1 Stability. Stem Cells 2014, 32, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhai, J.; Wong, C.C.; Chen, H.; Wang, X.; Ji, J.; Yu, J. A Novel Amplification Gene PCI Domain Containing 2 (PCID2) Promotes Colorectal Cancer through Directly Degrading a Tumor Suppressor Promyelocytic Leukemia (PML). Oncogene 2021, 40, 6641–6652. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A.M. Control of Cell Cycle Transcription during G1 and S Phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Chen, J.; Chang, C.-W.; Jen, J.; Huang, T.-Y.; Chen, C.-M.; Shen, R.; Liang, S.-Y.; Cheng, I.-C.; Yang, S.-C.; et al. Ubiquitination of Tumor Suppressor PML Regulates Prometastatic and Immunosuppressive Tumor Microenvironment. J. Clin. Invest. 2017, 127, 2982–2997. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, X.; Bao, Y.; Sun, G.; Wu, S.; Chen, Y. An Integrative Analysis of Enhancer of Yellow 2 Homolog (ENY2) as a Molecular Biomarker in Pan-Cancer. Funct. Integr. Genomics 2023, 23, 72. [Google Scholar] [CrossRef]
- Atanassov, B.S.; Mohan, R.D.; Lan, X.; Kuang, X.; Lu, Y.; Lin, K.; Mclvor, E.; Li, W.; Zhang, Y.; Florens, L.; et al. ATXN7L3 and ENY2 Coordinnate Activity of Multiple H2B Deubiquitinases Important for Cellular Proliferation and Tumor Growth. Mol. Cell 2016, 62, 558–571. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Lin, H.; Chang, F.-M.; Chang, T.-C.; Trieu, T.; Pridgen, H.I.; Zhang, Y.; Huang, J.; Patiño-Guzman, K.; Diab, N.; et al. Identification of the Deleted in Split Hand/Split Foot 1 Protein as a Novel Biomarker for Human Cervical Cancer. Carcinogenesis 2013, 34, 68–78. [Google Scholar] [CrossRef]
- Rezano, A.; Kuwahara, K.; Yamamoto-Ibusuki, M.; Kitabatake, M.; Moolthiya, P.; Phimsen, S.; Suda, T.; Tone, S.; Yamamoto, Y.; Iwase, H.; et al. Breast Cancers with High DSS1 Expression That Potentially Maintains BRCA2 Stability Have Poor Prognosis in the Relapse-Free Survival. BMC Cancer 2013, 13, 562. [Google Scholar] [CrossRef]
- Wei, S.-J.; Williams, J.G.; Dang, H.; Darden, T.A.; Betz, B.L.; Humble, M.M.; Chang, F.-M.; Trempus, C.S.; Johnson, K.; Cannon, R.E.; et al. Identification of a Specific Motif of the DSS1 Protein Required for Proteasome Interaction and P53 Protein Degradation. J. Mol. Biol. 2008, 383, 693–712. [Google Scholar] [CrossRef]
- Li, C.; Chen, B.; Zhang, J.; Yang, J.; Guo, M.; Ren, Y.; Zhou, Z.; Fung, K.M.; Li, M.; Zhang, L.; et al. SEM1 Promotes Tumor Progression of Glioblastoma via Activating the Akt Signaling Pathway. Cancer Lett. 2023, 577, 216368. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Álvarez, M.; Luque, M.; Morales-Gallego, M.; Cristóbal, I.; Ramírez-Merino, N.; Rangel, Y.; Izarzugaza, Y.; Eroles, P.; Albanell, J.; Madoz-Gúrpide, J.; et al. Generation and Characterization of Trastuzumab/Pertuzumab-Resistant HER2-Positive Breast Cancer Cell Lines. Int. J. Mol. Sci. 2024, 25, 207. [Google Scholar] [CrossRef]
- Sakai, Y.; Kuwahara, K. Carcinogenesis Caused by Transcription-coupled DNA Damage through GANP and Other Components of the TREX-2 Complex. Pathol. Int. 2024, 74, 103–118. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).