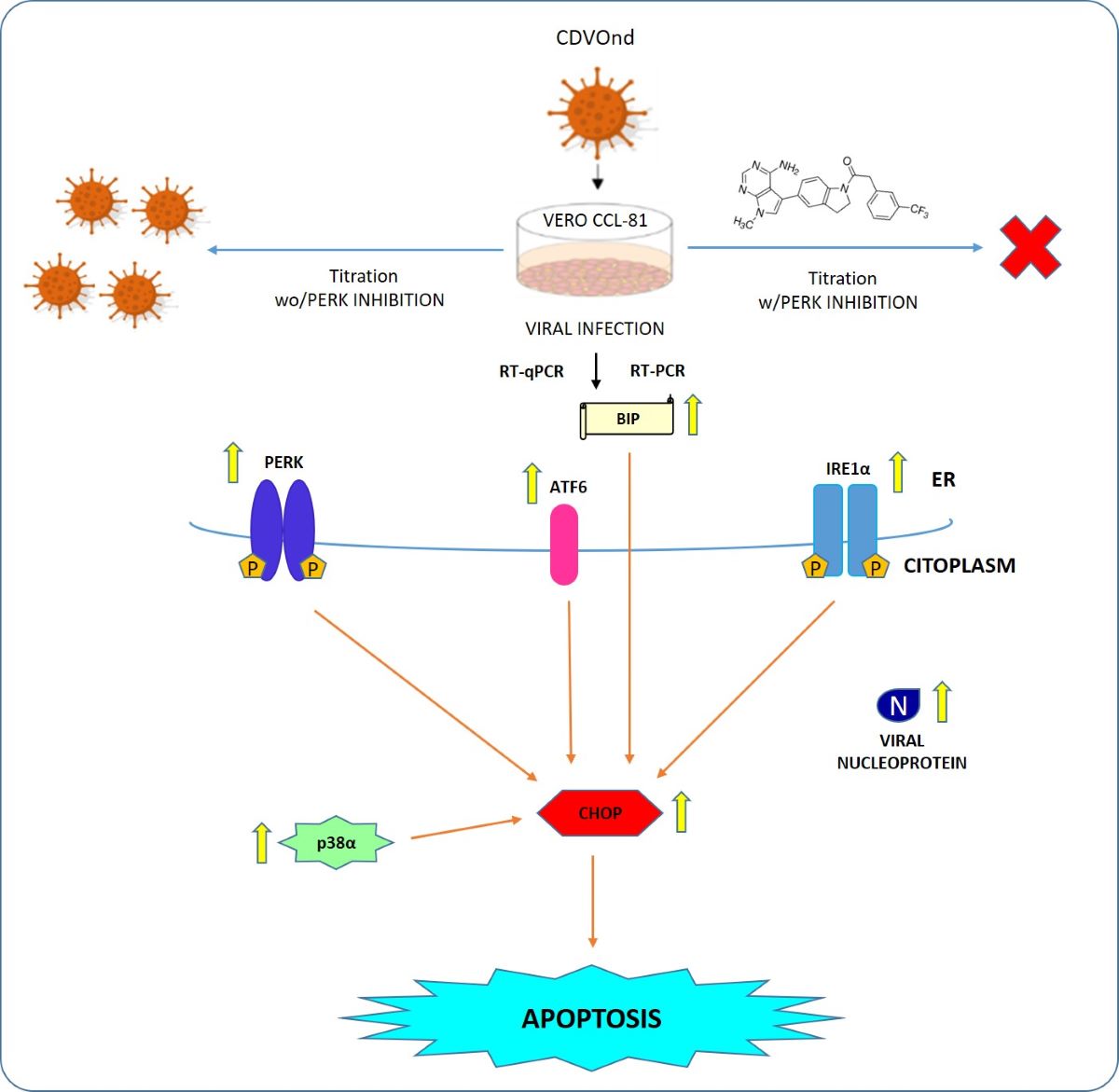
1. Introduction
Canine distemper virus (CDV) is the etiological agent of the disease known as canine distemper. The virus is classified within the Morbillivirus genus of the
Paramyxoviridae family, which also includes the measles virus and the peste des petits ruminants virus, among others. The viral particle comprises a non-segmented negative-sense RNA, covered by a nucleoprotein (N), along with a phosphoprotein (P) and a large polymerase protein (RdRp) (L). Collectively, these form a ribonucleoprotein complex which is enclosed in an envelope containing three main glycoproteins: the fusion (F), matrix (M), and hemagglutinin (H) proteins [
1,
2]. The disease affects individuals of all ages within the Canidae family and is characterised by fever, conjunctivitis, rash, respiratory and gastrointestinal signs, and leukocytopenia [
3,
4]. In dogs, neurological signs and digital hyperkeratosis producing hard pads are common manifestations of this disease.
Viral infections induce molecular changes that result in cell death through a variety of mechanisms, including apoptosis, necroptosis, and pyroptosis. The synthesis of substantial quantities of proteins by viruses disrupts the equilibrium of the endoplasmic reticulum (ER), thereby inducing stress and triggering a response known as the unfolded protein response (UPR) to attempt to restore ER homeostasis [
5]. In the event that this objective is not achieved, the UPR induces cell death through apoptosis in a caspase-12-dependent manner. [
6,
7,
8,
9]. The UPR comprises three distinct signaling pathways, each initiated by a transmembrane sensor protein: Protein Kinase RNA-like ER Kinase (PERK), Inositol-Requiring protein-1α (IRE1α) and Activation Transcription Factor 6 (ATF6). In normal conditions, the chaperone GRP78 (also known as Binding Immuno-globulin Protein, or BIP) is bound to the three sensors, thereby preventing the initiation of the UPR. However, when ER stress occurs, BIP separates from these proteins, thereby enabling the UPR to be initiated [
10].
The PERK pathway is initiated by the dimerisation and autophosphorylation of the sensor protein. The subsequent activation of PERK enables the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α), which in turn induces the transcription of the Activation Transcription Factor 4 (ATF4) gene, thereby stimulating the expression of various genes, including the transcriptional factor C/EBP homologous protein (CHOP). This process is involved in the induction of cell arrest and apoptosis [
10,
11]. The IRE1α pathway commences in a manner analogous to that of PERK. The sensor exhibits both protein kinase and endoribonuclease activities. The active form of IRE1α is capable of bonding and splicing different mRNAs, including the X-box binding protein 1 (XBP1) mRNA, also known as XBP1 unspliced (XBP1u). Consequently, this specific target is converted into a XBP1 spliced form (XBP1s), which enables the translation of an active XBP1 protein that activates genes involved in ER-assisted degradation (ERAD) to restore ER homeostasis [
10,
11]. The final UPR pathway involves the ATF6 sensor, which undergoes translocation to the Golgi apparatus in response to ER stress. This results in cleavage of the sensor, yielding two distinct domains: a cytosolic N-terminal domain and a transmembrane C-terminal domain. The cytosolic N-terminal domain then relocates to the nucleus, thereby inducing the activation of pro-survival genes [
10,
11].
CDV has been demonstrated to induce apoptosis in a range of cell lines, including the green monkey epithelial cell line (VERO) and the human colon cancer cell line (HCT-15) [
12,
13,
14]. Moreover, an attenuated CDV vaccine strain has been shown to exhibit oncolytic activity via the induction of apoptosis and the activation of the NF-κB pathway in a cell line originating from canine cancer [
15]. The role of ER stress in CDV pathogenesis has been the subject of considerable debate. Following infection with a recombinant CDV, Brunner and colleagues demonstrated that both the calreticulin chaperone and the CHOP transcription factor are upregulated, suggesting a potential involvement of the unfolded protein response pathway [
16]. Moreover, the expression of the H protein FLAG-tagged in a 293T cell line demonstrated the activation of the PERK pathway [
17].
The present study examines the role of ER stress, with a particular focus on the UPR pathways, and its implications in the context of in vitro CDV infection processes. The objective of this study is to examine the potential induction of ER stress and the subsequent activation of the three branches of the UPR, as well as the cell death that occurs following the induction of apoptosis. Restoration of homeostasis either clears the infection or leads to virus persistence without obvious signs of disease and immune reactions [
18].
2. Materials and Methods
2.1. Cell Culture, Virus and Reagents
The African green monkey (VERO) strain CCL-81 cell line, employed in all experiments, was obtained from ABAC. The cells were cultured in minimum essential medium (MEM) supplemented with 10% (v/v) of irradiated fetal bovine serum (FBS), gentamicin 0.04 mg/ml and amphotericin 1.25 mg/ml, and incubated at 37°C with 5% CO₂ in order to facilitate optimal cell growth. The attenuated CDV Onderstepoort strain (from now CDVOnd) employed in all assays was kindly provided by Dr. Marco Antonio Tizzano. Tunicamycin (Cell Signaling Technology Inc, #12819) was employed as a positive control for ER stress induction at a final concentration of 7.5 μg/μL. The PERK inhibitor, GSK2606414, was obtained from Thermo Fisher Scientific (#5165355MG) and used in a final concentration of 400 nM.
2.2. Viral Stock and Titration Assay
The CDVOnd strain was cultivated in VERO CCL-81 cells. The cells were incubated for one hour at 37°C to facilitate viral adsorption. Subsequently, the viral supernatant was removed, and the cells were supplemented with MEM and 2% FBS. The infected cells were maintained at 37°C until several cytopathic effects (CPE) were observed. Thereafter, the supernatant was collected, aliquoted and frozen at -70°C. Viral titration was conducted using tenfold serial dilutions, with the 50% tissue culture infectious doses (TCID50) on VERO CCL-81 cells assayed using the Reed and Muench method.
2.3. Infection Experiments
Vero CCL-81 cells were cultivated until they reached confluence in a 12-well plate. The infection was performed at a multiplicity of infection (MOI) of 1, and a variety of time points and conditions were subsequently analyzed. At 8, 16 and 24 hours post-infection (hpi). Briefly, the infected cells were incubated for one hour at 37°C with gentle agitation. Subsequently, the cells were supplemented with MEM and 2% FBS, and the plate was incubated at 37°C in a 5% CO₂ atmosphere. MOCK cells and cells induced with tunicamycin were prepared in the same way, at the maximum time point assayed, to serve as negative and positive controls, respectively.
2.4. RNA Extraction and Reverse Transcription Assay
Following each time point, the cells were harvested and total cellular RNA was extracted using the High Pure RNA Isolation Kit from Roche Sequencing Solutions, Inc., in accordance with the manufacturer's instructions. Subsequently, 1 μg of total RNA was retro-transcribed using a random hexamer from Biodynamic SRL and M-MLV Reverse Transcriptase from Promega Corporation. The cDNA obtained was stored at -20°C for subsequent analysis.
2.5. PCR Assay
Polymerase chain reactions (PCRs) were conducted for each condition using primers that enabled the differentiation of XBP1 mRNA splicing, as well as the detection of unspliced transcripts. This process involved the removal of a 26-nucleotide intron, thereby facilitating the translation of a functional protein [
10]. The sequence for each primer was as follows: XBP1-Fw: 5’-CCTTGTAGTTGAGAACCAGG-3’ and XBP1-Rv: 5’-GGGGCTTGGTATATATGTGG-3’ [
19]. The PCR mix reaction was conducted in a final volume of 20 μl utilizing the Kit T-Plus Free ADN Polimerasa Resistente a Inhibidores 500 U and the Kit dNTPs 100mM from Inbio Highway SRL. The concentration of Taq Polymerase was 0,125 U/μl, the dNTPs concentration was 250 nM and each primer was at 250 nM. A volume of 1 μl of cDNA was used as template. The incubation conditions comprised an initial denaturation step at 95°C for 5 minutes, 35 cycles of 94°C, 55°C and 72°C for 30 seconds each, and a final extension step at 72°C for 5 minutes. Band densitometry was performed using the Gel-Pro Analyzer software, with GAPDH used for normalization of the bands. The PCR products were separated on a 2% agarose gel and visualised by ultraviolet light after staining with ethidium bromide.
2.6. qPCR Assay
Quantitative polymerase chain reactions (qPCRs) were conducted with the use of iQ SYBR Green Supermix from Bio-Rad, in accordance with the instructions provided by the manufacturer. The final reaction volume was 10 μL. The primer pairs are presented in
Table 1, and GAPDH was employed as a housekeeping gene to normalise the transcript levels using the 2
-ΔΔCT method. All levels were relativised to the MOCK experiment. To validate the amplification results, the melting curves were analysed and the products were resolved by 1.5% agarose gel electrophoresis, as described above.
2.7. Inhibition of PERK Pathway
To corroborate the importance of the PERK pathway for viral progeny of CDVOnd, a classical selective inhibitor, GSK2606414 (GSK), was employed at a concentration of 400 nM. The VERO CCL-81 cells were incubated for one hour in the presence of GSK. Following the removal of the inhibitor, the cells were infected at a MOI of 1 with the CDVOnd strain for 1 hour. Finally, the viral inoculum was replaced with a maintenance medium in the presence of the inhibitor, and the supernatant was harvested at different time points. To enable comparison of the viral progeny with and without GSK, a TCID50 assay using the Reed and Muench method was performed for the titration.
2.8. DNA Fragmentation Assay
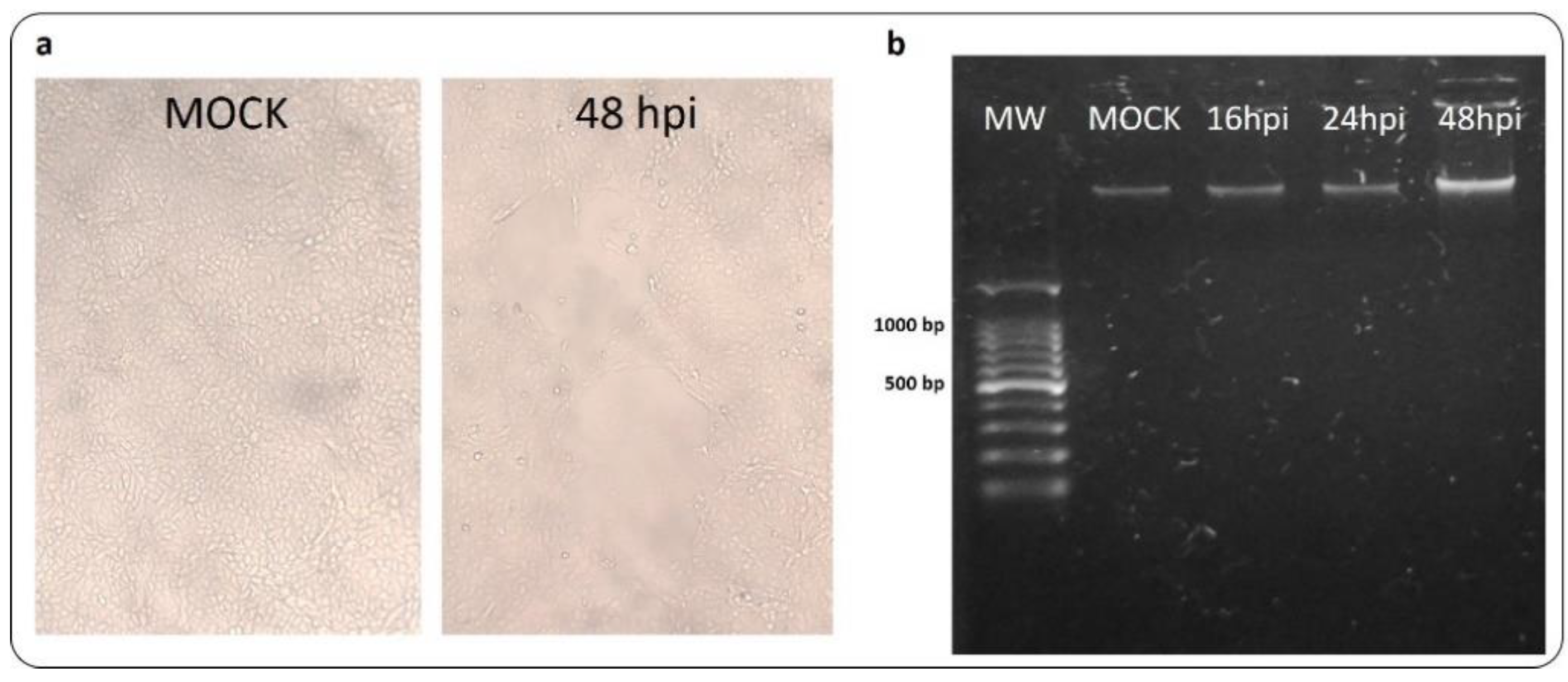
The induction of apoptosis can be evaluated through a specific process that involves the production of DNA fragments of a defined size. In the final stages of apoptosis, DNA is processed by an endonuclease, resulting in the generation of multiple fragments. These can be visualised in agarose gel as a ladder-like pattern [
25]. The production of DNA fragments was therefore evaluated following 16, 24 and 48 hpi with the CDVOnd strain. The MOCK cells were analysed at the final time point of 48 hours. The VERO CCL-81 cells were prepared in a 24-well plate. Following infection, genomic DNA was obtained by the phenol:chloroform:isoamyl alcohol extraction method and separated by agarose gel electrophoresis for visualisation, as previously described.
2.9. Statistical Analysis
The RT-PCR and qPCR experiments were conducted in triplicate and subsequently analyzed using the GraphPad Prism 8 software. The results are presented as the mean ± standard deviation (SD). In all cases, an unpaired Student's t-test was performed, with a statistical significance level of p < 0.05.
3. Results
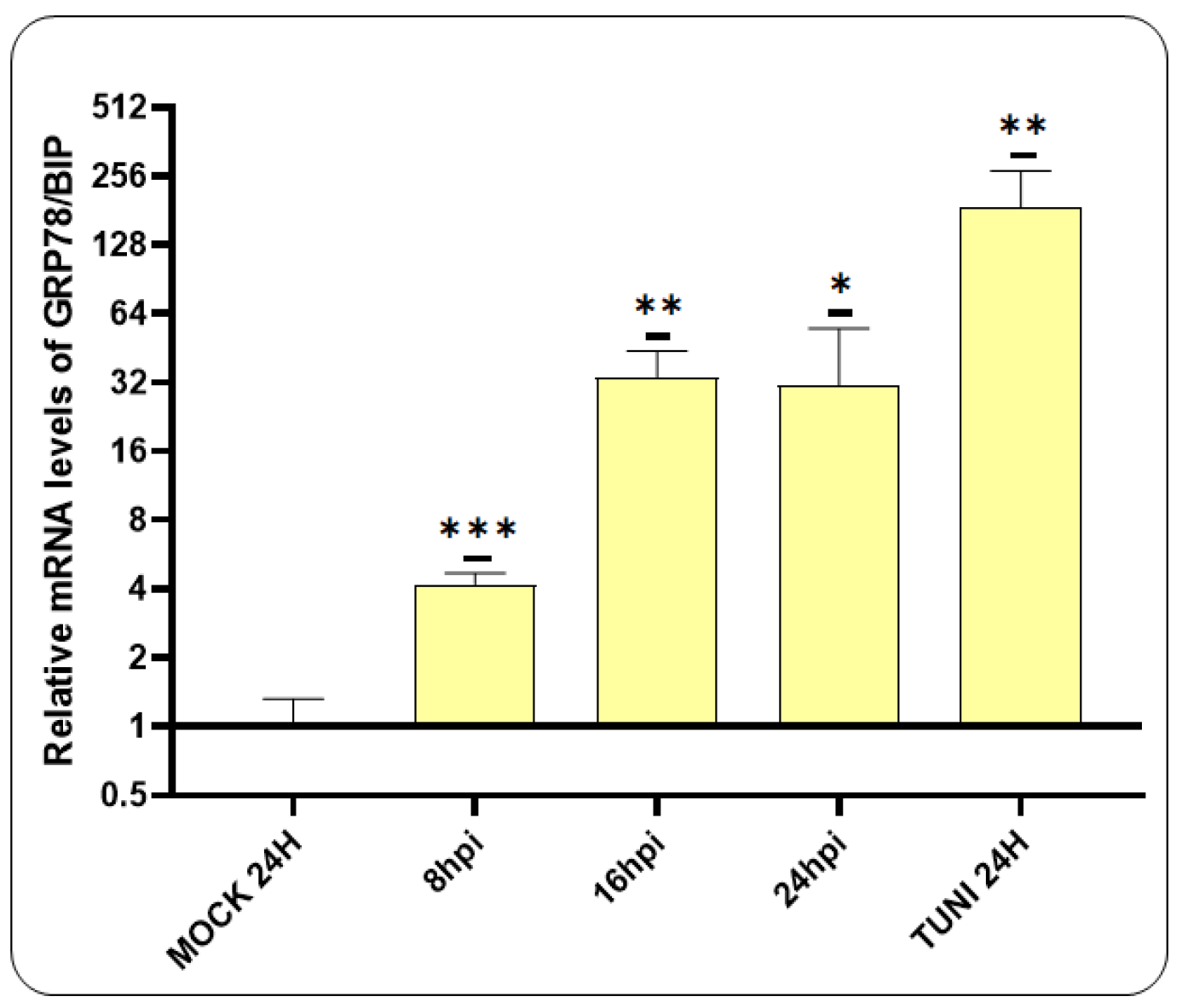
3.1. ER Stress Induced by CDV Infection
The titer of the CDVOnd strain used in our experiment was 10
4.5 TCID50/50 µl. An RT-qPCR assay was therefore performed to investigate the generation of ER stress following CDV infection, with an initial focus on GRP78/BIP transcript levels (
Figure 1).
Our findings revealed that the mRNA levels of BIP were significantly elevated following CDV infection. The initial detection occurred at 8 hpi, with a subsequent eight-fold increase at 16 hpi, which was maintained at 24 hpi.
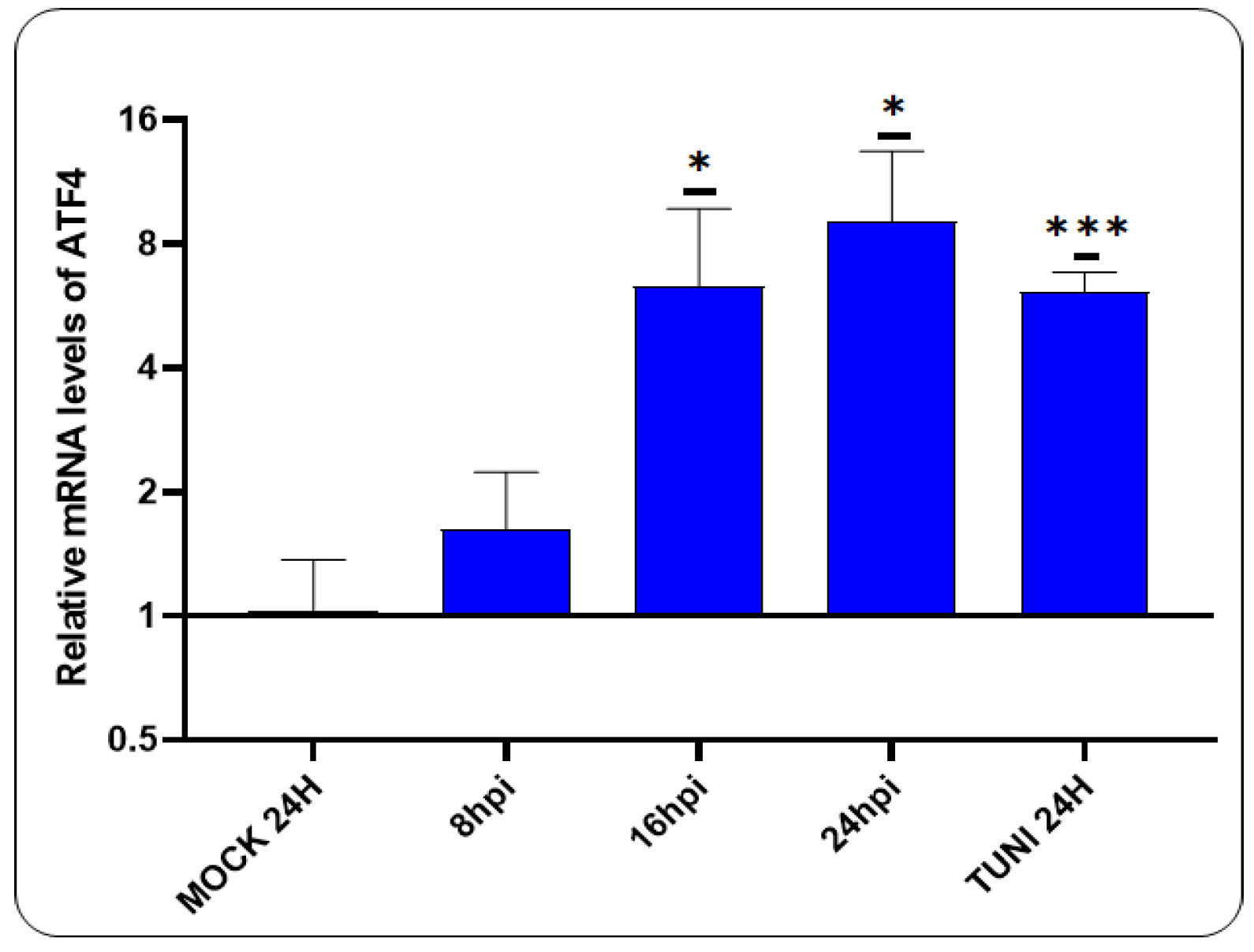
3.2. CDV Infection Activates PERK Pathway
Having previously demonstrated that CDVOnd infection induces ER stress, we now turn our attention to the PERK pathway (
Figure 2).
A notable elevation in ATF4 mRNA levels was discerned following 16 hpi, exhibiting a magnitude comparable to that observed in tunicamycin-induced ER stress cells.
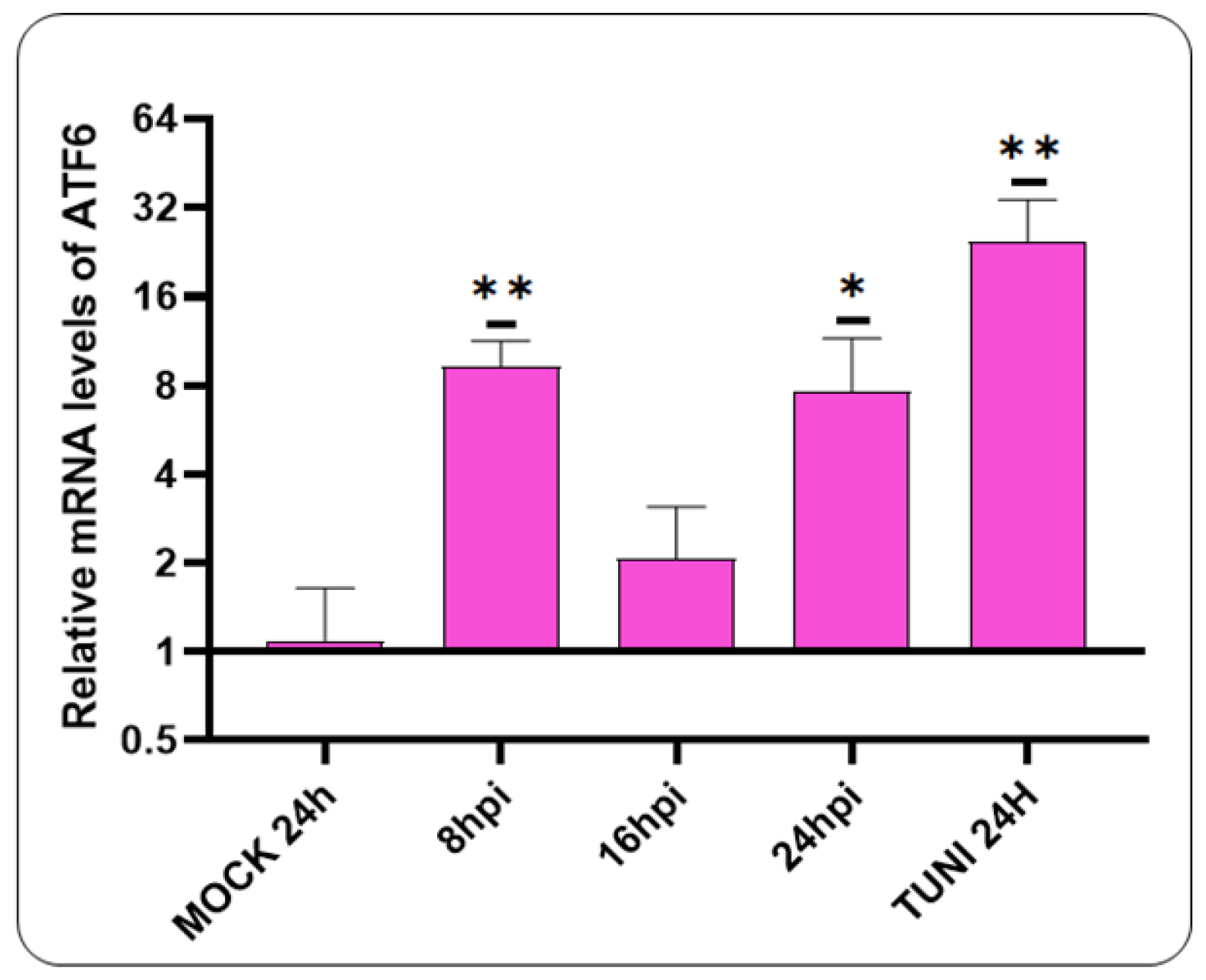
3.3. Activation of ATF6 Pathway after CDV Infection
The subsequent objective is to investigate the potential for ATF6 pathway activation following a CDVOnd infection (
Figure 3).
Our data indicates the activation of the ATF6 pathway at the earliest stages of CDV infection, specifically at 8 hpi. At 16 hpi, a decline in mRNA levels was observed. However, at 24 hpi, an elevation in mRNA levels was evident.
3.4. IRE1⍺ Pathway Is Activated by CDVOnd Infection
The splicing of the XBP1 transcript was evaluated through the use of retrotranscription-PCR and band densitometry assay (
Figure 4).
The results of this experiment demonstrate that the ratio of XBP1s levels was increased after 16 hpi in comparison with the MOCK control. In addition, a third band was visualised in our study, which corresponds to a hybrid between the two XBP1 forms [
22,
26]. Therefore, we can confirm that the IRE1α pathway is activated in vitro following CDV infection.
3.5. CDVOnd Infection Induces Apoptosis in VERO CCL-81
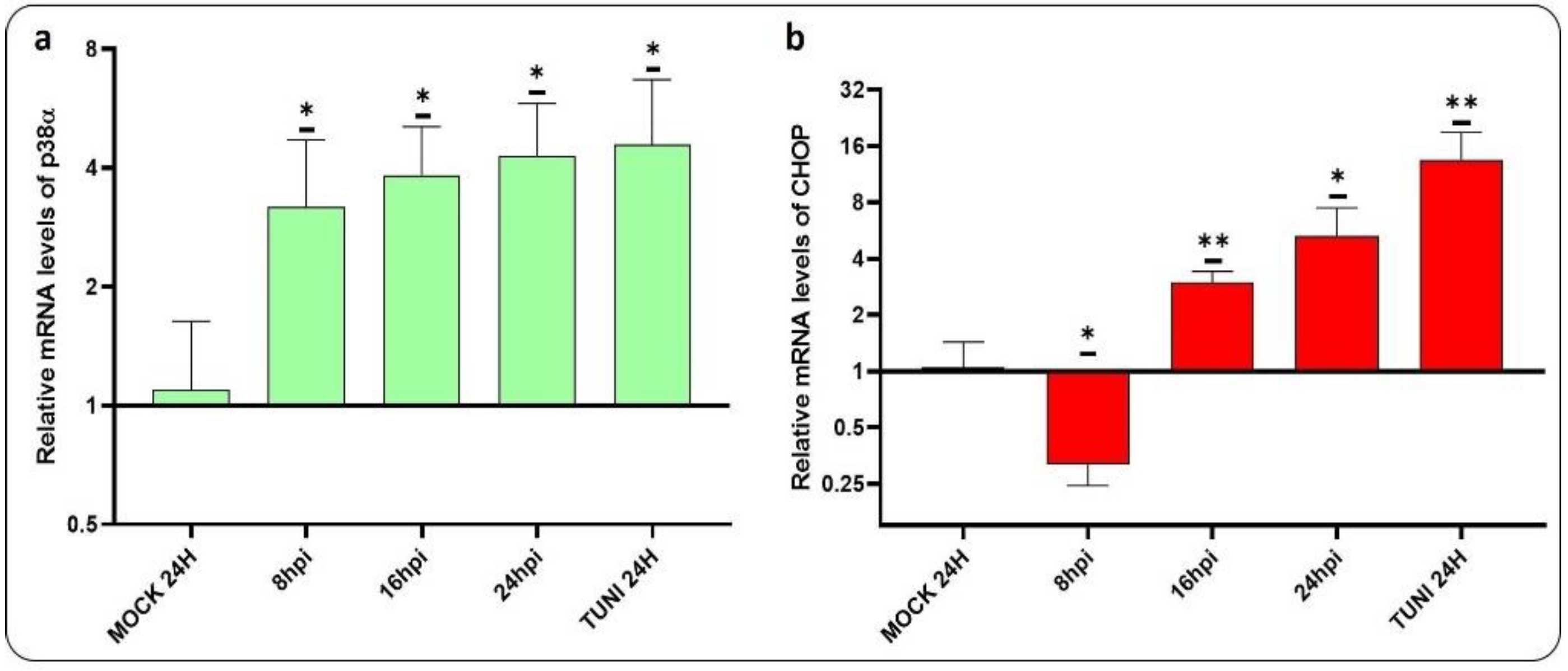
In order to gain insight into the mechanisms underlying apoptosis induced by CDV infection, we have conducted a study to assess the levels of p38α and CHOP mRNA transcript (
Figure 5).
Both transcripts involved in apoptosis induction were found to be upregulated. Activation of p38α could be observed at as early as 8 hpi, while activation of CHOP occurred at 16 hpi. Additionally, the progression of apoptosis was investigated via the DNA fragmentation assay (
Figure 6).
The infected-VERO CCL-81 monolayers exhibited a minimal CPE compared to mock control cells. Consequently, the experiment demonstrated that characteristic DNA laddering was not observed after 48 hpi.
3.6. Viral Replication after CDVOnd Infection
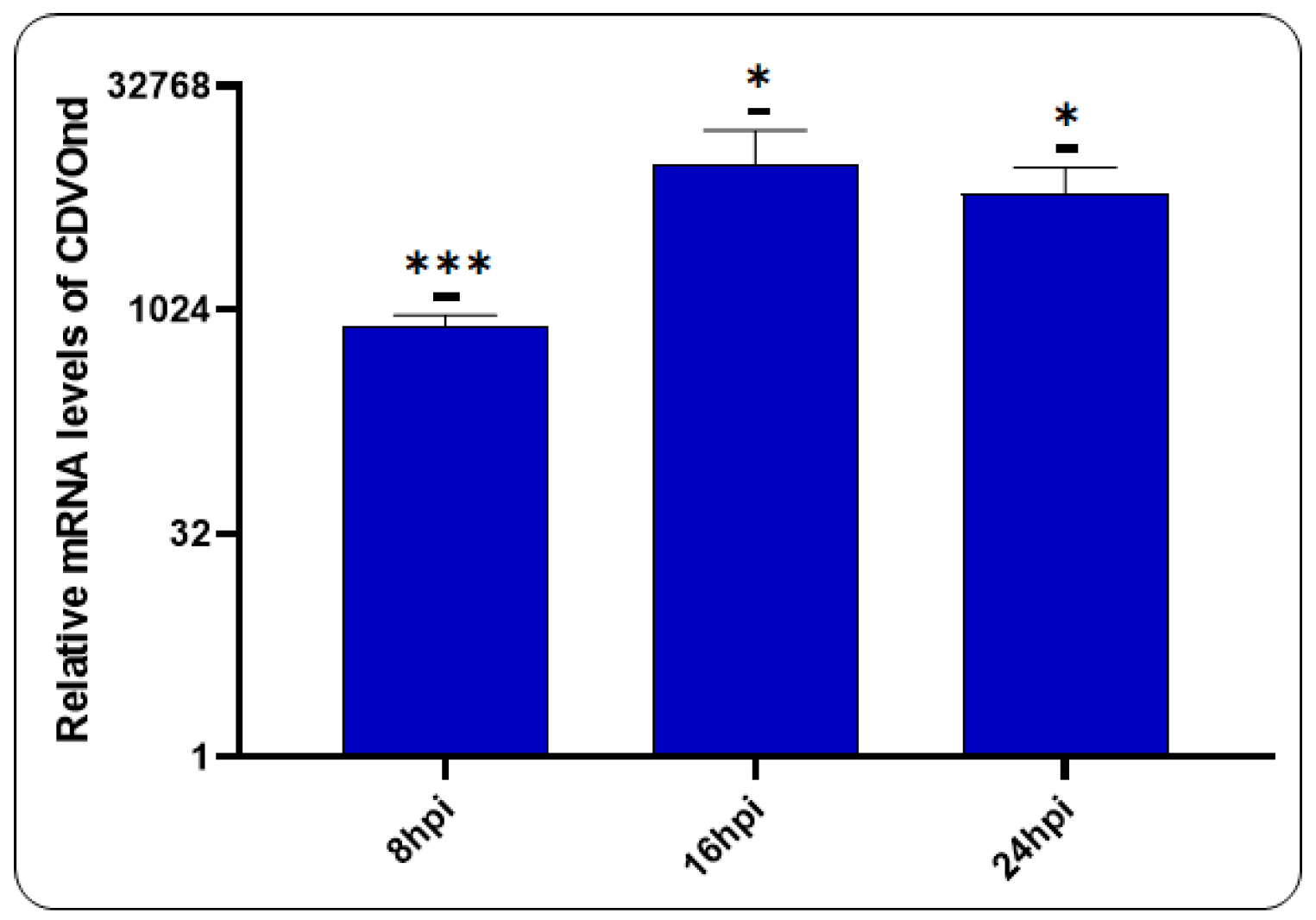
In order to evaluate the production of viral RNA following infection, a RT-qPCR assay was performed utilising specific primers to the N gene (
Figure 7).
The results presented herein demonstrate the production of viral RNA following CDV infection. The data demonstrate that the viral genome is augmented at 8 hpi, a process that continues at 16 and 24 hpi.
3.7. PERK Inhibition and Viral Progeny
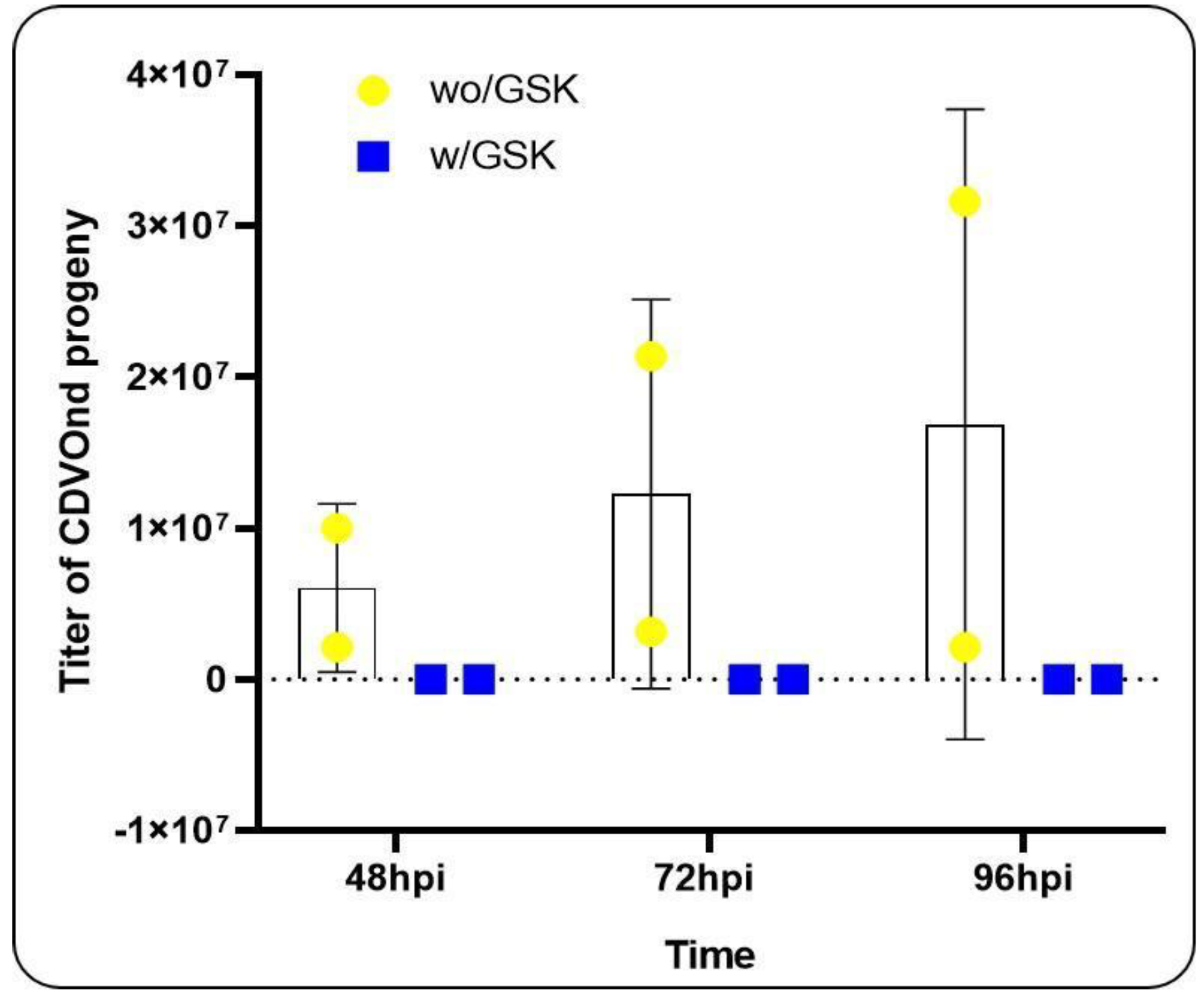
The subsequent objective was to evaluate the potential impact of inhibiting the UPR pathway on the viral progeny at different time points. To this end, we have chosen to inhibit the PERK pathway with the chemical inhibitor GSK2606414 and to compare the virus titration in the two conditions: with and without inhibition, at several time points (
Figure 8).
In all experiments, CPE was only observed when PERK pathway inhibition was avoided. This suggests that this pathway plays a significant role in viral progeny during CDVOnd infection. Furthermore, prior to these experiments, viral progeny was evaluated at 8, 16, and 24 hpi under the same conditions. However, a viral titer could not be obtained due to the absence of CPE (data not shown).
4. Discussion
It is well documented that the unfolded protein response pathways are involved in the pathogenesis of various diseases, including cancer, diabetes and viral infections [
27,
28]. Viral pathogens play an important role in the modulation of cell survival. With regard to RNA viruses, it is well documented that they interact with the endoplasmic reticulum during their replication processes [
5]. The ER chaperone protein GRP78/BIP plays a pivotal role in the activation of ER stress sensors and has recently been identified as a crucial protein in the context of viral infection [
29,
30]. The elevated expression of BIP was indicative of ER stress induction. Our investigation revealed that BIP expression is upregulated following CDVOnd infection, a phenomenon that has been observed in other paramyxovirus infections such as Newcastle Disease Virus (NDV) and Respiratory Syncytial Virus (RSV) and in this last case, in a dose-dependent manner [
31,
32]. Moreover, the solo expression of the H protein from simian parainfluenza virus 5 has been demonstrated to increase the levels of BIP by a factor of five at early times of infection [
33]. Furthermore, the siRNA-mediated knockdown of this ER stress sensor was demonstrated to suppress NDV replication [
32]. This emphasises the significance of this chaperone in paramyxovirus infection and in a wide range of other viral families [
34].
Following the induction of ER stress in cells, the UPR mechanism initiates a restoration of lost homeostasis through the action of three transmembrane proteins. A primary response involves the activation of the PERK pathway, whereby the translation initiation factor eIF2α is activated, promoting pro-adaptive signalling mechanisms that inhibit protein synthesis and activate the expression of the transcription factor ATF4 [
10]. In this study, we report the activation of the PERK pathway, as indicated by the increase in ATF4 mRNA levels. At 16 hpi, we observed a statistically significant upregulation in ATF4 mRNA in cells infected with CDV, comparable to the levels observed in cells treated with tunicamycin. Similar results were obtained with RSV, as evidenced by the activation of eIF2a [
32]. Furthermore, our findings revealed the activation of the ATF6 branch of the UPR as early as 8 hpi, which is in accordance with the observations made in prior studies where this pathway was also activated [
31]. Moreover, the implication of the ATF6 pathway was demonstrated in the induction of autophagy following infection with the Peste des Petits Ruminants Virus, a member of the Morbillivirus genus [
35].
In accordance with the proposed ER stress signalling cascade, the activation of the IRE1 branch results in the cleavage of XBP1 mRNA into its spliced form [
6,
10]. Here, the ratio of XBP1s/XBP1u indicated that the maximum rate of XBP1 splicing was reached at 16 hpi. Nevertheless, evidence suggest that ATF6 plays a role in XBP1 modulation, activating its transcription [
36,
37]. Therefore, the activation of ATF6 at 8 hpi may contribute to the transcription of XBP1, as evidenced by the higher level of XBP1u observed at later time points. It would be interesting to analyze the regulation of IRE1-dependent decay of mRNA (RIDD) after CDV infection to examine the sole activation of the IRE1 pathway.
The different branches of the UPR activated by ER stress have the capacity to regulate the equilibrium between viral infection and cellular fate. The death of cells following a prolonged ER stress response has been documented in the context of infection by several viruses [
5]. The transcription factor C/EBP homologous protein (CHOP), also known as growth arrest and DNA damage-inducible gene 153 (GADD153), has been demonstrated to mediate apoptosis in stressed ER cells [
38]. Furthermore, the IRE1 pathway has been implicated in MAPK activation and this pathway has been shown to participate in CHOP expression during tunicamycin-induced apoptosis, indicating that this interaction is crucial for innate immunity in mammals [
39,
40,
41]. The results presented here demonstrate that CDVOnd infection clearly activates p38α kinase, with the amount of mRNA detected after 24 hpi being equivalent to that obtained with tunicamycin. Furthermore, the induction of apoptosis following ER stress induced by the infection was evidenced by the upregulation of CHOP at 16 hpi. Although apoptotic signals are detected at early times of infection, such as 24 hpi, it is important to consider the potential involvement of alternative molecular pathways that may counteract this death effect. Our observations indicate that DNA fragmentation was not evident 24 hours after the apoptotic kinase and regulation factor were activated. However, it is noteworthy that a DNA laddering pattern was observed in VERO cells just after 72 hpi with the attenuated Onderstepoort strain by other authors [
12]. Furthermore, given the ultimate role of caspase cascades in apoptotic cells, additional studies are required to gain a deeper understanding of these effectors, such as caspase-12, which has been identified as a critical mediator between ER stress and cell death by apoptosis [
42,
43].
Given the established connection between CHOP activation and the PERK and ATF6 pathways, it is intriguing to hypothesize that the observed upregulation of CHOP transcript occurs in a PERK-dependent manner in our research. Additionally, the present study suggests that the PERK pathway plays an important role in the replication of the CDVOnd virus. Although our research was able to detect viral RNA at early stages of infection, infectious viruses could only be measured after 48 hpi. Furthermore, it was not possible to titrate them if the PERK pathway was inhibited in infected cells. It would be valuable to investigate the potential involvement of the other UPR pathways in CDVOnd replication.
5. Conclusions
The pathogenicity of CDV involves the infection of epithelial, neurological and lymphoid cells. For example, lymphocytes B and T, macrophages and dendritic cells are primary cells that facilitate the dissemination of viral infection in animals. Leukopenia may be a consequence of ER stress-induced apoptosis in response to CDV infection. The findings of this study demonstrate that the morbillivirus CDV Onderstepoort strain activates the three sensors of UPR following the induction of ER stress. Furthermore, the MAPK pathway, through the p38α protein and the CHOP transcription factor, which is involved in the apoptosis process, was demonstrated to be implicated in the infection events. Finally, the inhibition of the PERK pathway demonstrated the noteworthy involvement of this UPR branch in viral progeny at later stages of infection. The design of new molecular compounds against these pathways could be studied in an attempt to identify novel antiviral agents for the treatment of canine distemper disease, a pathology that continues to affect domestic and non-domestic animals globally.
Author Contributions
Conceptualization and methodology, G.E.M. and S.E.C.; validation, G.E.M. and M.A.T.; formal analysis, S.E.C.; investigation, S.E.C.; resources, M.A.T. and M.M.W.; data curation, G.E.M. and M.G.E.; writing—original draft preparation, G.E.M. and S.E.C.; writing—review and editing, G.E.M., M.S.S and M.G.E.; visualization, S.E.C. and G.E.M.; supervision, G.E.M.; project administration, G.E.M. and M.G.E.; funding acquisition, G.E.M. and M.G.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (Agencia I+D+i), PICT-2019-02510, and by Secretaría de Ciencia y Técnica UNLP, Proyecto de Incentivos Docentes UNLP: 11/V260 and 11/V301.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data can be requested from the corresponding authors.
Acknowledgments
We thanks to Antonela Ojeda and Martin Emanuel Mercapide-Cañas for their technical assistance in the preparation of materials used in the experiments.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- da Fontoura Budaszewski, R.; von Messling, V. Morbillivirus Experimental Animal Models: Measles Virus Pathogenesis Insights from Canine Distemper Virus. Viruses. 2016, 8, 274. [Google Scholar] [CrossRef]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine distemper virus. Vet. Clin. North. Am. Small. Anim. Pract. 2008, 38, 787-viii. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.J.; Summers, B.A. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet. Microbiol. 1995, 44, 187–191. [Google Scholar] [CrossRef]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R.J. Canine distemper in terrestrial carnivores: a review. J. Zoo. Wildl. Med. 2000, 31, 441–451. [Google Scholar] [CrossRef]
- Li, S.; Kong, L.; Yu, X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 150–164. [Google Scholar] [CrossRef]
- He, B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006, 13(3), 393–403. [Google Scholar] [CrossRef]
- Morishima, N.; Nakanishi, K.; Takenouchi, H.; Shibata, T.; Yasuhiko, Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 2002, 277, 34287–34294. [Google Scholar] [CrossRef]
- Szegezdi, E.; Fitzgerald, U.; Samali, A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann. N. Y. Acad. Sci. 2003, 1010, 186–194. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Chen, S. Liu, J.; Liu, L.; Liu, G.; Wang, F.; Jiang, W.; Zhang, C.; Wang, S.; Yuan, X. Caspase-12 is involved in stretch-induced apoptosis mediated endoplasmic reticulum stress. Apoptosis. 2016, 21, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Guo, A.; Lu, C. Canine distemper virus causes apoptosis of Vero cells. J. Vet. Med. B. Infect. Dis. Vet. Public Health. 2000, 47, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Katayama, H.; Murata, T.; Kai, C.; Hori, M.; Ozaki, H. Canine distemper virus induces apoptosis through caspase-3 and -8 activation in vero cells. J. Vet. Med. B. Infect. Di.s Vet. Public Health. 2006, 53, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Deka, D. Studies on the molecular mechanisms involved in apoptosis triggered by Canine Distemper Virus (CDV). I.J.B.B. 2023, 60, 525–544. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Chen, G.; Zhang, X.; Lin, D.; Zhou, Y.; Yu, Y.; Liu, W.; Zhang, D. Oncolytic activity of canine distemper virus in canine mammary tubular adenocarcinoma cells. Vet. Comp. Oncol. 2019, 17, 174–183. [Google Scholar] [CrossRef]
- Brunner, J.M.; Plattet, P.; Doucey, M.A.; Rosso, L.; Curie, T.; Montagner, A.; Wittek, R.; Vandelvelde, M.; Zurbriggen, A.; Hirling, H.; Desvergne, B. Morbillivirus glycoprotein expression induces ER stress, alters Ca2+ homeostasis and results in the release of vasostatin. PLoS One. 2012, 7, e32803. [Google Scholar] [CrossRef]
- Wang, W.; Bi, Z.; Liu, Y.; Xia, X.; Qian, J.; Tan, Y.; Zhao, J.; Song, S. The H protein of attenuated canine distemper virus is degraded via endoplasmic reticulum-associated protein degradation. Front. Vet. Sci. 2023, 10, 1214318. [Google Scholar] [CrossRef] [PubMed]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining chronic viral infection. Cell. 2009, 138, 30–50. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.R.; Sun, M.X.; Ni, B.; Huan, C.; Huang, L.; Li, C.; Fan, H.J.; Ren, X.F.; Mao, X. Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation. Antiviral Res. 2014, 106, 33–41. [Google Scholar] [CrossRef]
- Wolfson, J.J.; May, K.L.; Thorpe, C.M.; Jandhyala, D.M.; Paton, J.C.; Paton, A.W. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 2008, 10, 1775–1786. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Wang, X.; Wang, Y.; Guo, M.; Zhang, X.; Wu, Y. Avian reovirus infection activate the cellular unfold protein response and induced apoptosis via ATF6-dependent mechanism. Virus Res. 2021, 297, 198346. [Google Scholar] [CrossRef]
- Echavarría-Consuegra, L.; Cook, G.M.; Busnadiego, I.; Lefèvre, C.; Keep, S.; Brown, K.; Doyle, N.; Dowgier, G.; Franaszek, K.; Moore, N.A.; Siddell, S.G.; Bickerton, E.; Hale, B.G.; Firth, A.E.; Brierley, I.; Irigoyen, N. Manipulation of the unfolded protein response: A pharmacological strategy against coronavirus infection. PLoS Pathog. 2021, 17, e1009644. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, F.; Wang, L.; Gao, M.; Xie, Y.; Sun, Y.; Liu, H.; Yuan, Y.; Yi, W.; Huang, Z.; Yan, H.; Peng, K.; Wu, Y.; Cao, Z. Virus-induced p38 MAPK activation facilitates viral infection. Theranostics. 2020, 10, 12223–12240. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yan, X.; Chai, X.; Zhang, H., Zhao, J.; Wen, Y.; Wu, W. Differentiation of canine distemper virus isolates in fur animals from various vaccine strains by reverse transcription-polymerase chain reaction-restriction fragment length polymorphism according to phylogenetic relations in china. Virol. J. 2011, 8, 85. [CrossRef]
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Lehrman, M.A. Discordance of UPR signaling by ATF6 and Ire1p-XBP1 with levels of target transcripts. Biochem. Biophys. Res. Commun. 2004, 317, 390–396. [Google Scholar] [CrossRef]
- Choi, J.A.; Song, C.H. Insights Into the Role of Endoplasmic Reticulum Stress in Infectious Diseases. Front. Immunol. 2020, 10, 3147. [Google Scholar] [CrossRef]
- Park, S.W.; Ozcan, U. Potential for therapeutic manipulation of the UPR in disease. Semin. Immunopathol. 2013, 35, 351–373. [Google Scholar] [CrossRef]
- Ha, D.P.; Van Krieken, R.; Carlos, A.J.; Lee, A.S. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Toyoda, S.; Fukuhara, A.; Shimomura, I. GRP78, a novel host factor for SARS-coV-2: the emerging roles in COVID-19 related to metabolic risk factors. Biomedicines. 2022, 10, 1995. [Google Scholar] [CrossRef]
- Qiao, D.; Skibba, M.; Xu, X.; Garofalo, R.P.; Zhao, Y.; Brasier, A.R. Paramyxovirus replication induces the hexosamine biosynthetic pathway and mesenchymal transition via the IRE1α-XBP1s arm of the unfolded protein response. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L576–L594. [Google Scholar] [CrossRef]
- Han, C.; Xie, Z.; Lv, Y.; Liu, D.; Chen, R. Direct interaction of the molecular chaperone GRP78/BiP with the Newcastle disease virus hemagglutinin-neuraminidase protein plays a vital role in viral attachment to and infection of culture cells. Front. Immunol. 2023, 14, 1259237. [Google Scholar] [CrossRef] [PubMed]
- Watowich, S.S.; Morimoto, R.I.; Lamb, R.A. Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. J. Vir. 1991, 65, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Kohli, E.; Causse, S.; Baverel, V.; Dubrez, L.; Borges-Bonan, N.; Demidov, O.; Garrido, C. Endoplasmic Reticulum Chaperones in Viral Infection: Therapeutic Perspectives. Microbiol. Mol. Biol. Rev. 2021, 85, e0003521. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, H.; You, Q.; Zhang, Z.; Bai, L.; Chen, F.; Zhu, X.; Zhang, Z.; Li, Y. Peste des Petits Ruminants Virus Upregulates STING to Activate ATF6-Mediated Autophagy. J. Virol. 2022, 96, e0137522. [Google Scholar] [CrossRef]
- Bernales, S.; Papa, F.R.; Walter, P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006, 22, 487–508. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001, 107, 881–891. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Ryu, S.W.; Song, B.J. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006, 281, 21256–21265. [Google Scholar] [CrossRef]
- Lumley, E.C.; Osborn, A.R.; Scott, J.E.; Scholl, A.G.; Mercado, V.; McMahan, Y.T.; Coffman, Z.G.; Brewster, J.L. Moderate endoplasmic reticulum stress activates a PERK and p38-dependent apoptosis. Cell Stress Chaperones. 2017, 22, 43–54. [Google Scholar] [CrossRef]
- Guo, X.; Meng, Y.; Sheng, X.; Guan, Y.; Zhang, F.; Han, Z.; Kang, Y.; Tai, G.; Zhou, Y.; Cheng, H. Tunicamycin enhances human colon cancer cells to TRAIL-induced apoptosis by JNK-CHOP-mediated DR5 upregulation and the inhibition of the EGFR pathway. Anticancer Drugs. 2017, 28, 66–74. [Google Scholar] [CrossRef]
- Fujita, E.; Kouroku, Y.; Jimbo, A.; Isoai, A.; Maruyama, K.; Momoi, T. Caspase-12 processing and fragment translocation into nuclei of tunicamycin-treated cells. Cell Death Differ. 2002, 9, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Wang, Y.; Wang, H.; Huang, C.; Li, J. Endoplasmic reticulum stress-induced hepatic stellate cell apoptosis through calcium-mediated JNK/P38 MAPK and Calpain/Caspase-12 pathways. Mol. Cell. Biochem. 2014, 394, 1–12. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Activation of BIP in VERO CCL-81 cells. An RT-qPCR assay was conducted to evaluate the expression of the GRP78/BIP transcript following the infection with CDVOnd. The induction of ER stress was observed to commence at 8 hpi, reaching its maximum values at 16 hpi. The levels of BIP mRNA were represented as the mean ± standard deviation (SD) of three independent experiments. For a statistical significance *p < 0.05, **p < 0.005 and ***p < 0.0005.
Figure 1.
Activation of BIP in VERO CCL-81 cells. An RT-qPCR assay was conducted to evaluate the expression of the GRP78/BIP transcript following the infection with CDVOnd. The induction of ER stress was observed to commence at 8 hpi, reaching its maximum values at 16 hpi. The levels of BIP mRNA were represented as the mean ± standard deviation (SD) of three independent experiments. For a statistical significance *p < 0.05, **p < 0.005 and ***p < 0.0005.
Figure 2.
Activation of PERK pathway in CDVOnd infection. The relative levels of ATF4 transcript indicate the activation of the PERK pathway following 16 hpi with the CDVOnd strain. The data are presented as means ± SD from three independent experiments. Statistical significance *p < 0.05, ***p < 0.0005.
Figure 2.
Activation of PERK pathway in CDVOnd infection. The relative levels of ATF4 transcript indicate the activation of the PERK pathway following 16 hpi with the CDVOnd strain. The data are presented as means ± SD from three independent experiments. Statistical significance *p < 0.05, ***p < 0.0005.
Figure 3.
The ATF6 pathway is activated by the CDVOnd strain. The ATF6 mRNA levels were detected as early as 8 hpi. The values are presented as means ± SD of three independent experiments. Statistical significance values are indicated by *p˂0,05, **p˂0,005.
Figure 3.
The ATF6 pathway is activated by the CDVOnd strain. The ATF6 mRNA levels were detected as early as 8 hpi. The values are presented as means ± SD of three independent experiments. Statistical significance values are indicated by *p˂0,05, **p˂0,005.
Figure 4.
XBP1 spliced after CDVOnd infection. a. The images displayed are representative photographs of 2% agarose gels with GAPDH (left) and XBP1 (right) bands for each condition from the same experiment. The triangles indicate the XBP1 transcripts, XBP1u (green) and XBP1s (blue) with a size of approximately 440 bp and 414 bp, respectively. The star symbol denotes a hybrid between the two forms of XBP1 (XBP1H), with an apparent size of 466 base pairs. b and c. Bands densitometry assay. The expression levels of XBP1 species were relativised using a value of 1 for the GAPDH bands. In b, a comparison is made between the levels of XBP1u and XBP1s. In c, the ratio XBP1s/XBP1u is evaluated for each condition. Levels are represented as means ± SD of three independent experiments. Statistical significance is indicated by *p < 0.05 and **p < 0.005.
Figure 4.
XBP1 spliced after CDVOnd infection. a. The images displayed are representative photographs of 2% agarose gels with GAPDH (left) and XBP1 (right) bands for each condition from the same experiment. The triangles indicate the XBP1 transcripts, XBP1u (green) and XBP1s (blue) with a size of approximately 440 bp and 414 bp, respectively. The star symbol denotes a hybrid between the two forms of XBP1 (XBP1H), with an apparent size of 466 base pairs. b and c. Bands densitometry assay. The expression levels of XBP1 species were relativised using a value of 1 for the GAPDH bands. In b, a comparison is made between the levels of XBP1u and XBP1s. In c, the ratio XBP1s/XBP1u is evaluated for each condition. Levels are represented as means ± SD of three independent experiments. Statistical significance is indicated by *p < 0.05 and **p < 0.005.
Figure 5.
p38⍺ and CHOP induced by CDVOnd infection. The figure illustrates the relative levels of mRNA transcripts for two transcription factors involved in the activation of apoptotic genes. a. Levels of p38α mRNA transcript were found to be upregulated following CDV infection. b. Levels of CHOP transcript in response to CDV infection, illustrating the upregulation of this gene. The data are expressed as means ± SD from three independent experiments. Statistical significance was determined using the following criteria *p < 0.05, **p < 0.005.
Figure 5.
p38⍺ and CHOP induced by CDVOnd infection. The figure illustrates the relative levels of mRNA transcripts for two transcription factors involved in the activation of apoptotic genes. a. Levels of p38α mRNA transcript were found to be upregulated following CDV infection. b. Levels of CHOP transcript in response to CDV infection, illustrating the upregulation of this gene. The data are expressed as means ± SD from three independent experiments. Statistical significance was determined using the following criteria *p < 0.05, **p < 0.005.
Figure 6.
DNA laddering experiment after CDV infection. The study of apoptosis was conducted through the generation of DNA ladder-like patterns over a period of 16, 24 and 48 hours following the infection of CDVOnd. A 100 bp ladder (PM) was employed as a weight molecular marker. a. Photographs taken at 10X of the MOCK (left) and 48 hpi (right) wells. b. Electrophoretic analysis of genomic DNA in a 1% agarose gel.
Figure 6.
DNA laddering experiment after CDV infection. The study of apoptosis was conducted through the generation of DNA ladder-like patterns over a period of 16, 24 and 48 hours following the infection of CDVOnd. A 100 bp ladder (PM) was employed as a weight molecular marker. a. Photographs taken at 10X of the MOCK (left) and 48 hpi (right) wells. b. Electrophoretic analysis of genomic DNA in a 1% agarose gel.
Figure 7.
Production of viral RNA following CDV infection. The relative RNA level of the CDVOnd strain was investigated using primers to the N gene at the different time points. The levels of viral progeny are presented as means ± SD of three independent experiments. For a statistical significance: *p˂0,05, ***p˂0,0005.
Figure 7.
Production of viral RNA following CDV infection. The relative RNA level of the CDVOnd strain was investigated using primers to the N gene at the different time points. The levels of viral progeny are presented as means ± SD of three independent experiments. For a statistical significance: *p˂0,05, ***p˂0,0005.
Figure 8.
Inhibition of the PERK pathway affects viral progeny. The TCID50 assay utilising the Reed and Muench method was employed to quantify viral progeny after 48, 72 and 96 hpi in two distinct conditions, namely wo/GSK (without PERK inhibition) and w/GSK (with PERK inhibition). The experiment was conducted in duplicate over a five-day period.
Figure 8.
Inhibition of the PERK pathway affects viral progeny. The TCID50 assay utilising the Reed and Muench method was employed to quantify viral progeny after 48, 72 and 96 hpi in two distinct conditions, namely wo/GSK (without PERK inhibition) and w/GSK (with PERK inhibition). The experiment was conducted in duplicate over a five-day period.
Table 1.
List of primers used in RT-qPCR experiments.
Table 1.
List of primers used in RT-qPCR experiments.
| Use |
Gene |
Sequence (5’ to 3’) |
Size (bp) |
Reference |
| House- |
GAPDH-Fw |
AGGTCGGAGTCAACGGATTT |
112 |
[19] |
| keeping |
GAPDH-Rv |
TAGTTGAGGTCAATGAAGGG |
112 |
[19] |
| ER stress |
BIP-Fw |
ACCGCTGAGGCTTATTGGG |
147 |
[19] |
| activation |
BIP-Rv |
TGCCGTAGGCTCGTTGATG |
147 |
[19] |
| PERK |
ATF4-Fw |
CCAACAACAGCAAGGAGGAT |
143 |
[20] |
| pathway |
ATF4-Rv |
GTGTCATCCAACGTGGTCAG |
143 |
[20] |
| ATF6 |
ATF6-Fw |
CGAATAGCCCAGTGAA |
180 |
[21] |
| pathway |
ATF6-Rv |
ATCTCGCCTCTAACCC |
180 |
[21] |
| Apoptosis |
CHOP-Fw |
ACCAAGGGAGAACCAGGAAACG |
201 |
[22] |
| Apoptosis |
CHOP-Rv |
TCACCATTCGGTCAATCAGAGC |
201 |
[22] |
| MAPK |
p38α-Fw |
GCCCAAGCCCTTGCACAT |
156 |
[23] |
| pathway |
p38α-Rv |
TGGTGGCACAAAGCTGATGAC |
156 |
[23] |
| Viral |
CDV-N-Fw |
TTCTGAGGCAGATGAGTTCTTC |
829 |
[24] |
| genome |
CDV-N-Rv |
CTTGGATGCTATTTCTGACACT |
829 |
[24] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).