Submitted:
15 October 2024
Posted:
16 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Biochar
2.3. Determination of Biochar Characteristics
2.4. Sorption Experiments
2.5. Isotherm and Kinetic Model
3. Results and Discussion
3.1. Physicochemical Characteristics of Biochar
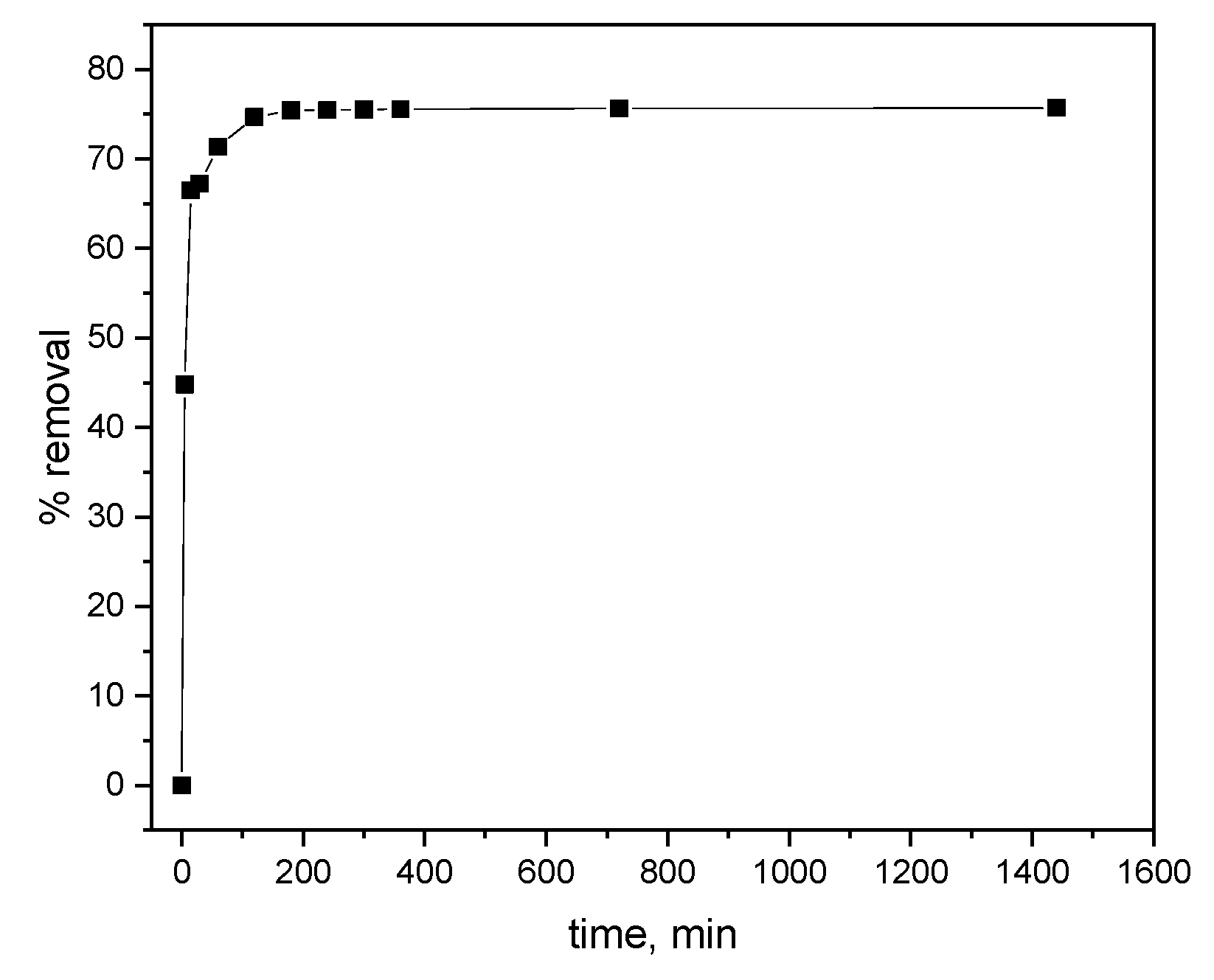
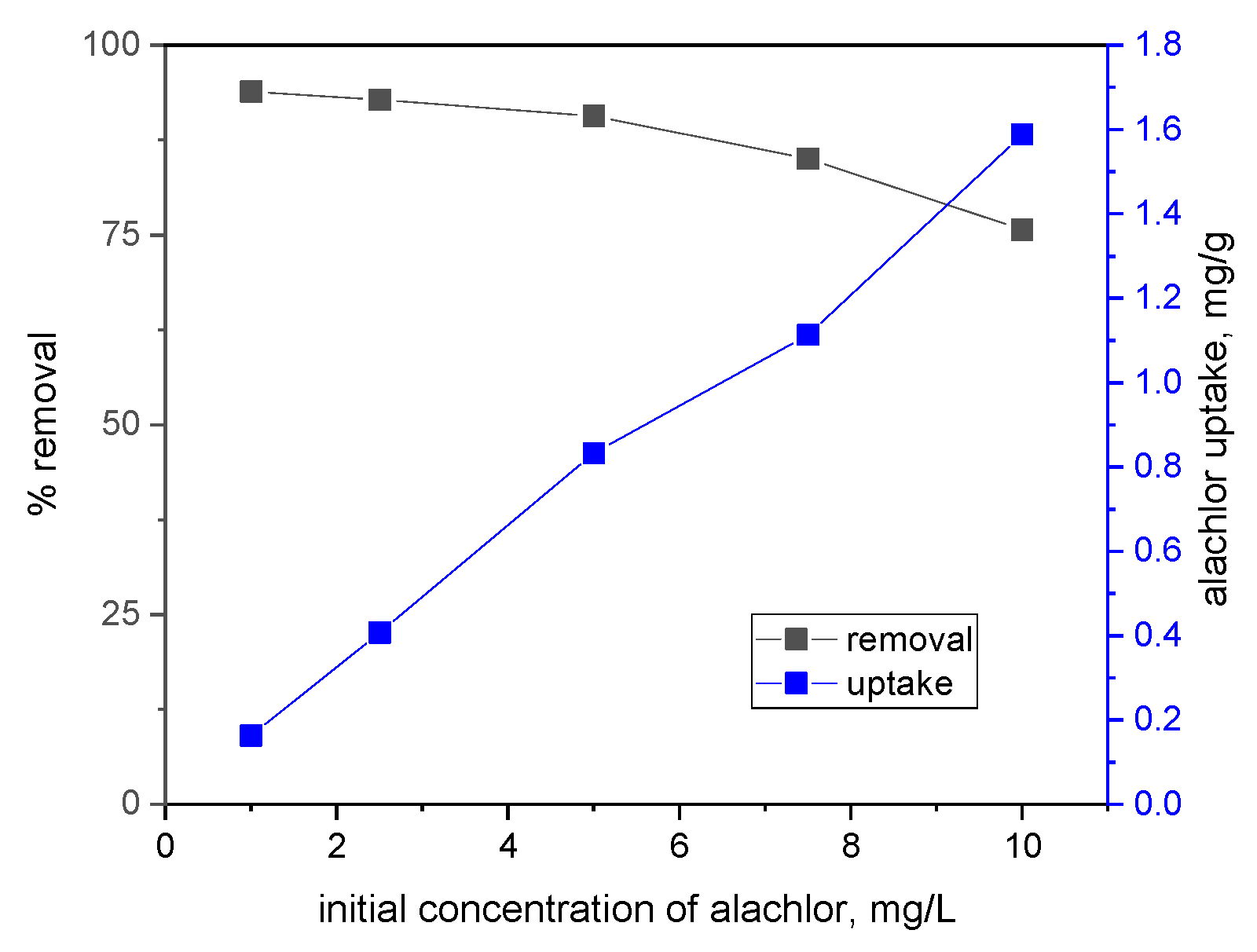
3.2. Performance of Wheat Grains Biochar in Removing of Alachlor from Water
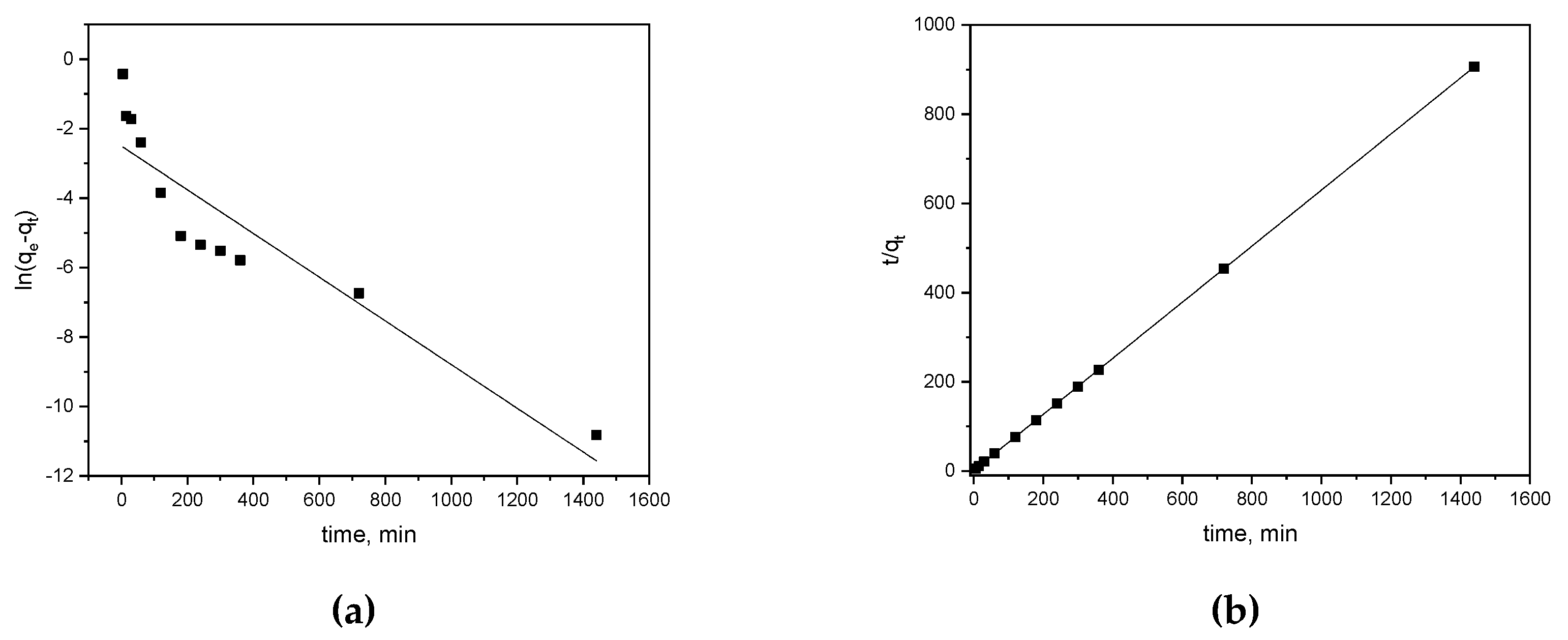
3.3. Isotherm and Kinetic Models
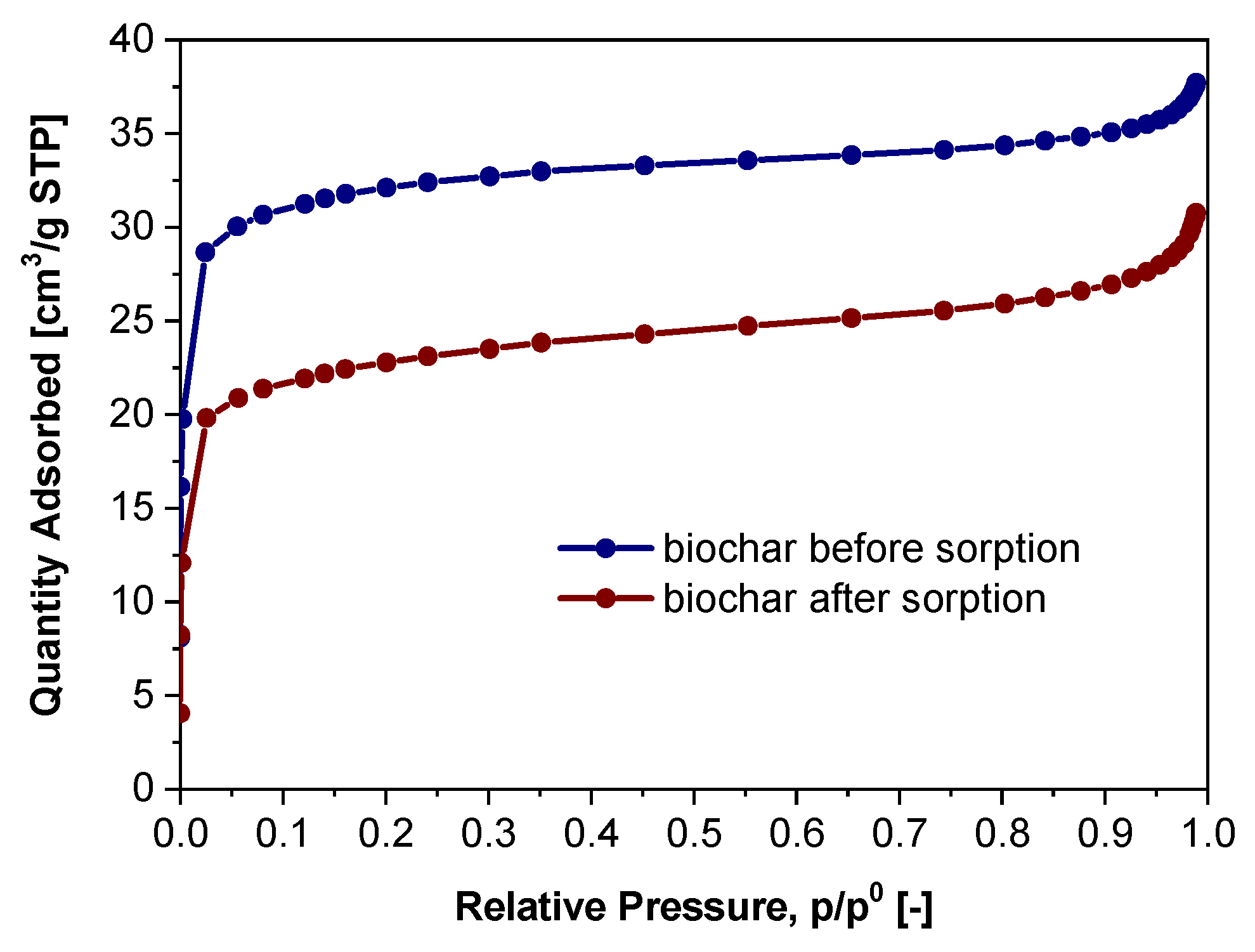
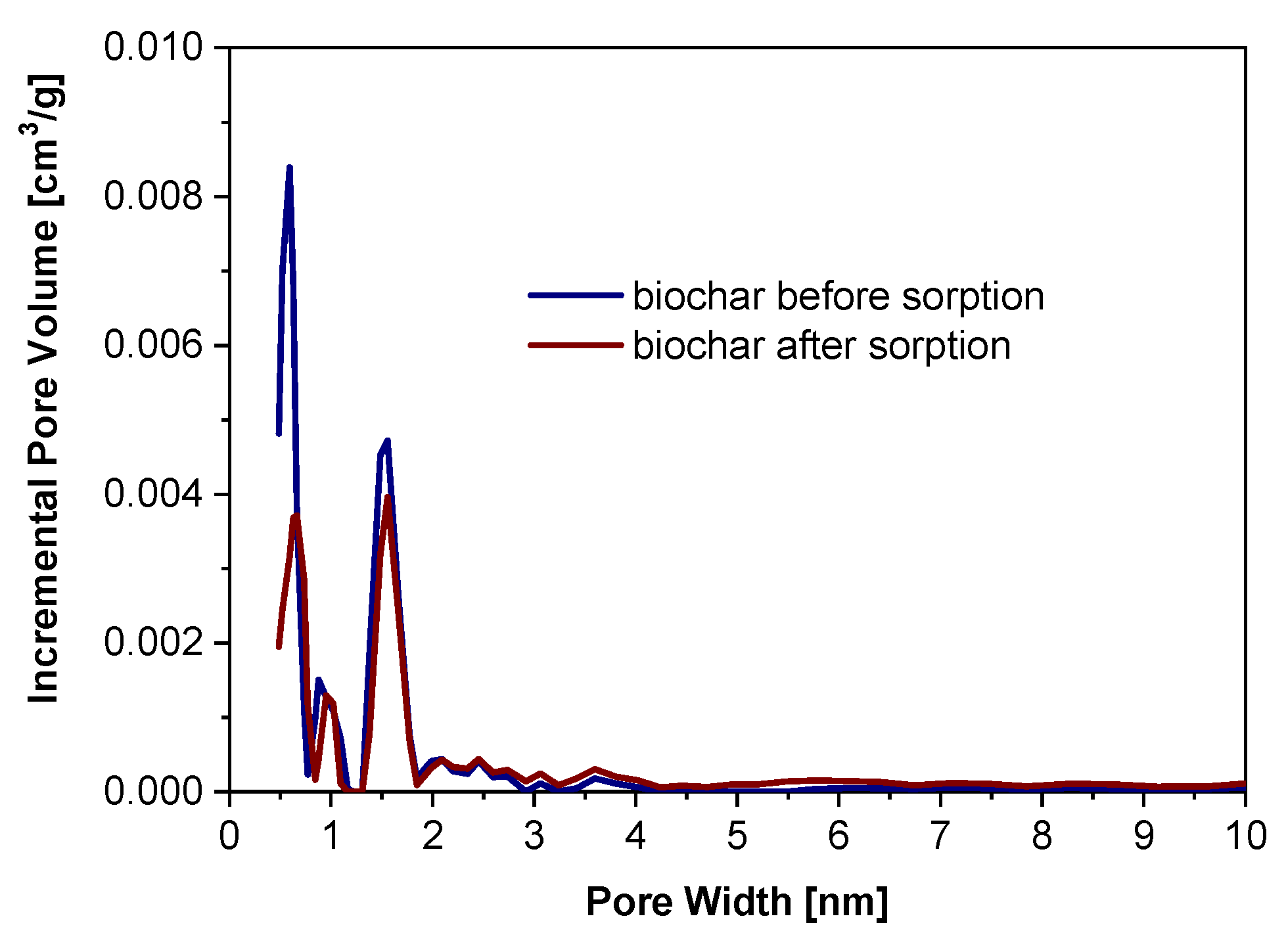
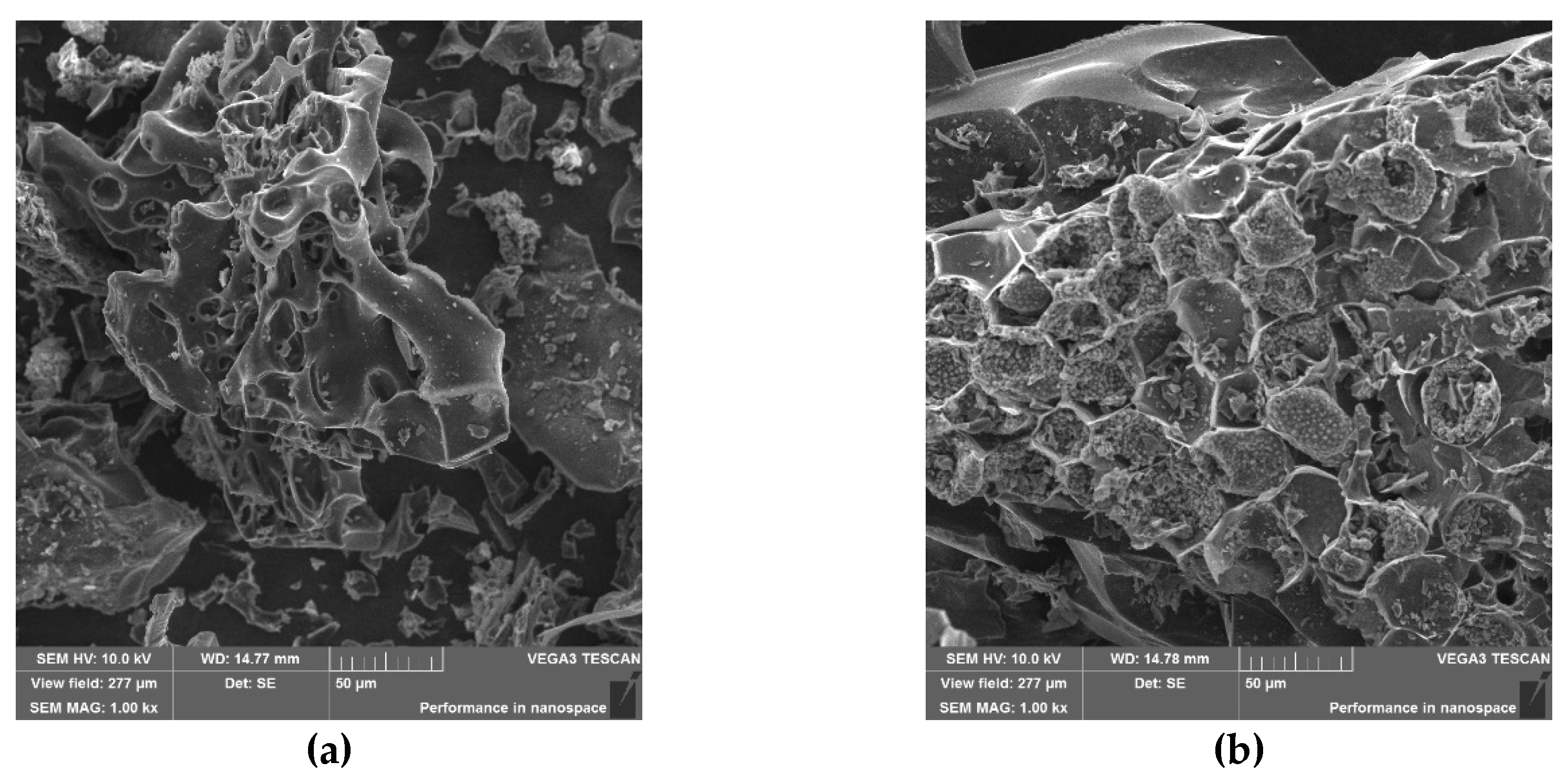
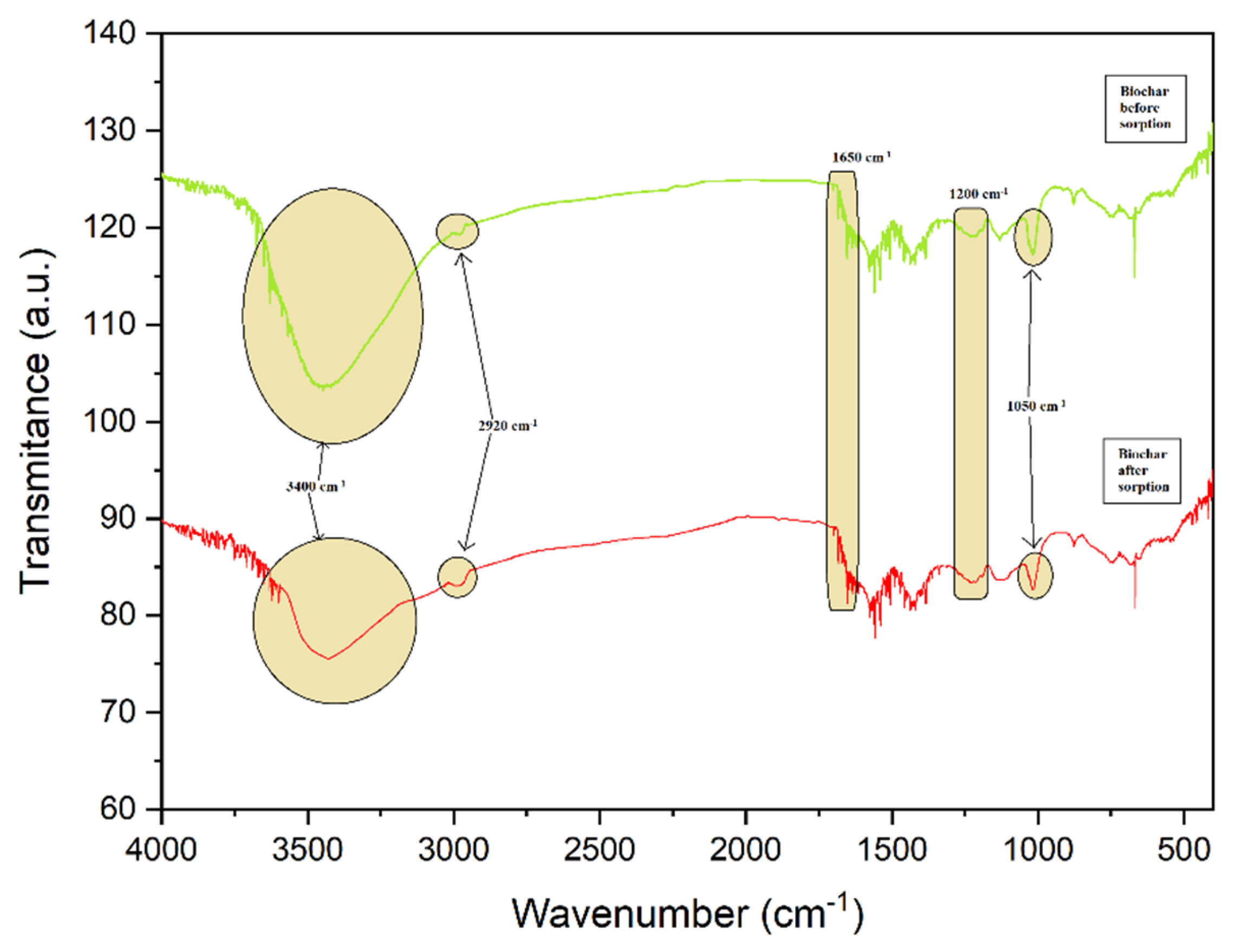
3.4. The Alteration of Biochar Properties as a Result of Alachlor Sorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Khan, M.S.; Othmani, A.; Khanday, W.A.; Gökkuş, Ö.; Osagie, C.; Ahmaruzzaman, Md.; Mishra, S.R.; Lima, E.C.; Mubarak, N.M.; et al. Sustainable Remediation Technologies for Removal of Pesticides as Organic Micro-Pollutants from Water Environments: A Review. Applied Surface Science Advances 2024, 19, 100558. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Gonzalez-Rey, M.; Tapie, N.; Le Menach, K.; Dévier, M.H.; Budzinski, H.; Bebianno, M.J. Occurrence of pharmaceutical compounds and pesticides in aquatic systems. Mar. Pollut. Bull. 2015, 96, 384–400. [Google Scholar] [CrossRef]
- Ccanccapa, A.; Masiá, A.; Navarro-Ortega, A.; Picó, Y.; Barceló, D. Pesticides in the Ebro River basin: Occurrence and risk assessment. Environ. Pollut. 2016, 211, 414–424. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Rodríguez-Cruz, M.S.; Pose-Juan, E.; Sánchez-González, S.; Andrades, M.S.; Sánchez-Martín, M.J. Seasonal distribution of herbicide and insecticide residues in the water resources of the vineyard region of La Rioja (Spain). Sci. Total Environ. 2017, 609, 161–171. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. 2017. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Fernando, M.; Pereira, R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Szöcs, E.; Brinke, M.; Karaoglan, B.; Schäfer, R.B. Large Scale Risks from Agricultural Pesticides in Small Streams. Environ. Sci. Technol. 2017, 51, 7378–7385. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, B.; Qiu, X.; Chen, M.; Ma, Z.; Yu, X. Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China. Chemosphere 2016, 144, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- McManus, S.L.; Coxon, C.E.; Mellander, P.E.; Danaher, M.; Richards, K.G. Hydrogeological characteristics influencing the occurrence of pesticides and pesticide metabolites in groundwater across the Republic of Ireland. Sci. Total. Environ. 2017, 601–602, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Moschet, C.; Wittmer, I.; Simovic, J.; Junghans, M.; Piazzoli, A.; Singer, H.; Stamm, C.; Leu, C.; Hollender, J. How a complete pesticide screening changes the assessment of surface water quality. Environ. Sci. Technol. 2014, 48, 5423–5432. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, J.M.; Montuelle, B.; Mouchet, F.; Gauthier, L.; Julien, F.; Sauvage, S.; Teissier, S.; Dedieu, K.; Destrieux, D.; Vervier, P.; et al. Role of the hyporheic heterotrophic biofilm on transformation and toxicity of pesticides. Ann. Limnol.—Int. J. Limnol. 2013, 49, 87–95. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wator, K. Fate of selected neonicotinoid insecticides in soil-water systems: Current state of the art and knowledge gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.U.; Asghar, H.N.; Nadeem, H.; Shahid, M.; Zeshan, M.A.; Niaz, A.; Hussain, S.; Hussain, S. Processes Governing the Environmental Fates of Alachlor in Soil and Aqueous Media: A Critical Review. Int J. Environ. Sci. Technol. 2022, 19, 8043–8060. [Google Scholar] [CrossRef]
- The pesticide manual, 9th ed.; Worthing, C.R., Ed.; Farnham, British Crop Protection Council, 1991. [Google Scholar]
- US Environmental Protection Agency. Alachlor. Reviews in environmental contamination and toxicology 1988, 104, 9–20. [Google Scholar]
- Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; 2010.
- Environmental risk analysis for chemicals; Conway, R.A., Ed.; New York, NY, Van Nostrand Reinhold, 1982. [Google Scholar]
- Chesters, G.; Simsiman, G.V.; Levy, J.; Alhajjar, B.J.; Fathulla, R.N.; Harkin, J.M. Environmental fate of alachlor and metolachlor. Rev. Environ. Contam. Toxicol. 1989, 110, 1–74. [Google Scholar] [CrossRef]
- Mangipudy, R.S.; Mehendale, H.M. Alachlor. In Encyclopedia of Toxicology, 2nd ed.; Elsevier, 2005. [Google Scholar] [CrossRef]
- Toxicology Data Bank, Bethesda, MD, National Library of Medicine.
- Neera Singh, Y.B.K. Removal of Atrazine, Metribuzin, Metolachlor and Alachlor by Granular Carbon. J. Environ. Anal. Toxicol. 2013, 03. [Google Scholar] [CrossRef]
- Ponnuchamy, M.; Kapoor, A.; Senthil Kumar, P.; Vo, D.-V.N.; Balakrishnan, A.; Mariam Jacob, M.; Sivaraman, P. Sustainable Adsorbents for the Removal of Pesticides from Water: A Review. Environ. Chem. Let.t 2021, 19, 2425–2463. [Google Scholar] [CrossRef]
- Bocșa, M.; Pintea, S.; Lung, I.; Opriș, O.; Stegarescu, A.; Humayun, M.; Bououdina, M.; Soran, M.-L.; Bellucci, S. Biochar-Based Adsorbents for Pesticides, Drugs, Phosphorus, and Heavy Metal Removal from Polluted Water. Separations 2023, 10, 533. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research Status, Trends, and Mechanisms of Biochar Adsorption for Wastewater Treatment: A Scientometric Review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Szara, E.; Thornton, S.; Fenton, O.; Malina, G. Efficacy of Woodchip Biochar and Brown Coal Waste as Stable Sorbents for Abatement of Bioavailable Cadmium, Lead and Zinc in Soil. Water Air Soil Pollut. 2020, 231, 515. [Google Scholar] [CrossRef]
- Caban, M.; Folentarska, A.; Lis, H.; Kobylis, P.; Bielicka-Giełdoń, A.; Kumirska, J.; Ciesielski, W.; Stepnowski, P. Critical Study of Crop-Derived Biochars for Soil Amendment and Pharmaceutical Ecotoxicity Reduction. Chemosphere 2020, 248, 125976. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Folentarska, A.; Lis, H.; Kobylis, P.; Kumirska, J.; Stepnowski, P.; Ciesielski, W. Valuable Polar Moieties on Cereal-Derived Biochars. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 561, 275–282. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Fenton, O.; Szara, E.; Thornton, S.F.; Malina, G. Holistic Assessment of Biochar and Brown Coal Waste as Organic Amendments in Sustainable Environmental and Agricultural Applications. Water Air Soil Pollut. 2021, 232, 106. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of Soil Quality Using Biochar and Brown Coal Waste: A Review. Science of The Total Environment 2020, 722, 137852. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. Journal of Analytical and Applied Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Use of biochar as a low-cost adsorbent for removal of heavy metals from water and wastewater: A review. Journal of Environmental Chemical Engineering 2023, 11, 110986. [Google Scholar] [CrossRef]
- Ye, Q.; Li, Q.; Li, X. Removal of heavy metals from wastewater using biochars: adsorption and mechanisms. Environmental Pollutants and Bioavailability 2022, 34, 385–394. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and Inorganic Contaminants Removal from Water with Biochar, a Renewable, Low Cost and Sustainable Adsorbent—A Critical Review. Bioresource Technology 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Applications. Applied Sciences 2020, 10, 3492. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review. Toxics 2023, 11, 117. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. Journal of Hazardous Materials Advances, 2023, 9, 100226. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Separation and Purification Technology 2022, 300, 121925. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, J.; Saleem, M.; Yang, Y.; Zhang, Q. Enhanced Adsorptive Removal of Carbendazim from Water by FeCl3-Modified Corn Straw Biochar as Compared with Pristine, HCl and NaOH Modification. Journal of Environmental Chemical Engineering 2022, 10, 107024. [Google Scholar] [CrossRef]
- Ćwieląg-Piasecka, I.; Jamroz, E.; Medyńska-Juraszek, A.; Bednik, M.; Kosyk, B.; Polláková, N. Deashed Wheat-Straw Biochar as a Potential Superabsorbent for Pesticides. Materials 2023, 16, 2185. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Singh, N. Optimization of Atrazine and Imidacloprid Removal from Water Using Biochars: Designing Single or Multi-Staged Batch Adsorption Systems. International Journal of Hygiene and Environmental Health 2017, 220, 637–645. [Google Scholar] [CrossRef]
- Okoya, A.A.; Adegbaju, O.S.; Akinola, O.E.; Akinyele, A.B.; Amuda, O.S. Comparative Assessment of the Efficiency of Rice Husk Biochar and Conventional Water Treatment Method to Remove Chlorpyrifos from Pesticide Polluted Water. CJAST 2020, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Shen, Q.; Zhang, D.; Mei, X.; Ran, W.; Xu, Y.; Yu, G. Functional Groups Determine Biochar Properties (pH and EC) as Studied by Two-Dimensional 13C NMR Correlation Spectroscopy. PLoS ONE 2013, 8, e65949. [Google Scholar] [CrossRef]
- Langmuir, I. THE ADSORPTION OF GASES ON PLANE SURFACES OF GLASS, MICA AND PLATINUM. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Seaton, N.A.; Walton, J.P.R.B.; Quirke, N. A New Analysis Method for the Determination of the Pore Size Distribution of Porous Carbons from Nitrogen Adsorption Measurements. Carbon 1989, 27, 853–861. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Ding, C.; Gan, Y.; Luo, J.; Cui, Y. Wheat Straw Biochar and Its Performance in Treatment of Phenanthrene Containing Water and Microbial Remediation of Phenanthrene Contaminated Soil. Front. Environ. Sci. 2022, 10, 1039603. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure and Applied Chemistry 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J. , Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Yang, T.; Meng, J.; Jeyakumar, P.; Cao, T.; Liu, Z.; He, T.; Cao, X.; Chen, W.; Wang, H. Effect of Pyrolysis Temperature on the Bioavailability of Heavy Metals in Rice Straw-Derived Biochar. Environ. Sci. Pollut. Res. 2021, 28, 2198–2208. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Li, X.; Jia, C.Q.; Jiang, W. Preparation of High-Yield N-Doped Biochar from Nitrogen-Containing Phosphate and Its Effective Adsorption for Toluene. RSC Adv. 2018, 8, 30171–30179. [Google Scholar] [CrossRef]
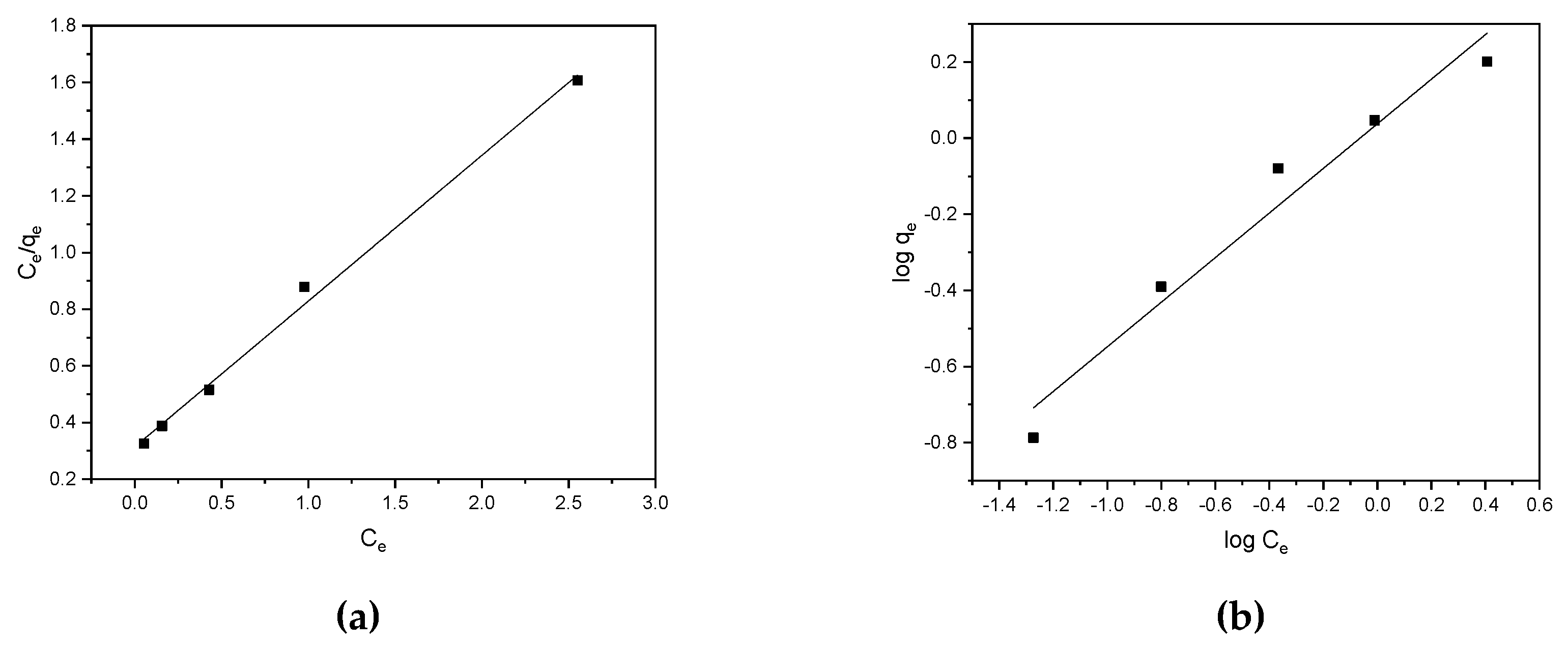
| Isotherm models | Parameters | Values |
|---|---|---|
| Langmuir | qmax Kl R2 |
1.94 1.64 0.996 |
| Freundlich | n Kf R2 |
1.71 1.09 0.962 |
| Kinetic model | Parameters | Values |
|---|---|---|
| Pseudo-second order | qe2 k2 R2 |
1.592 0.271 0.999 |
| Pseudo-first order | qe1 k1 R2 |
0.082 0.006 0.841 |
| Low pressure nitrogen adsorption, 77K | Symbol | Biochar before sorption | Biochar after sorption |
|---|---|---|---|
| Langmuir total sorption capacity, cm³/g STP | amL | 34.27 | 25.63 |
| Langmuir coefficient, 1/kPa | K | 0.85 | 0.48 |
| Langmuir specific surface area, m²/g | SSAL | 149.15 | 111.55 |
| BET total sorption capacity, cm³/g STP | amBET | 22.61 | 16.28 |
| BET specific surface area, m²/g | SSABET | 98.42 | 70.88 |
| NLDFT total pore volume, cm3/g | VNLDFT | 0.06 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





