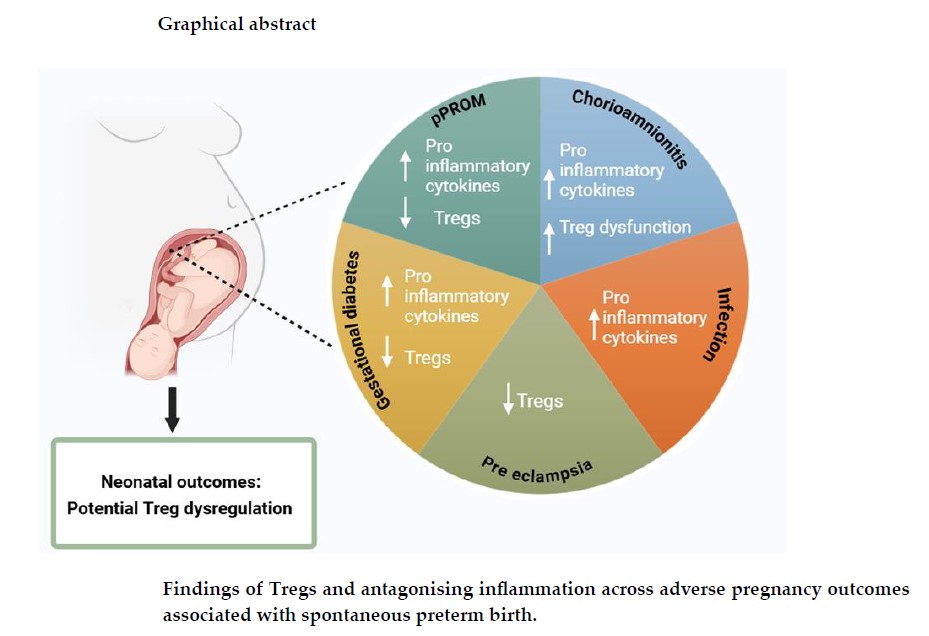
1. Introduction
Spontaneous preterm birth (sPTB) is an unplanned, non-iatrogenic, live birth occurring before 37 weeks of gestation, where the newborn is not yet considered to be term and fully developed [
1]. The negative impacts on both the health and growth of the baby have gained infamy as the leading cause of mortality and morbidity in neonates, responsible for the global death of nearly one million children under five [
2]. sPTB can be classified into four gestational outcome categories: with 85%, considered to be either ‘moderate’ with delivery occurring between 32 and 33 weeks or ‘late’ between 34 and 36 weeks. Furthermore, 10% are classified as ‘very’ preterm at 28 to 31 weeks, and the rarest cases, at 5% are ‘extremely’ preterm, where delivery occurs 28 weeks or prior [
3] with the poorest fetal outcomes associated with these earlier births. Prediction of sPTB and neonatal consequences remains difficult with most cases of sPTB devoid of a known cause, rendering them as idiopathic [
4].
Several findings point to a link between the integrity of the maternal immune response and pregnancy outcomes [
5,
6,
7], suggesting this could potentially hold the key to understanding which pregnancies make it to term, while others deliver prematurely. With reduced regulatory T cell (Treg) presence and functionality reported in adverse pregnancy conditions, including sPTB [
8], profiling, and targeting this cell type in pregnancy holds promise to furthering our understanding and ability to predict idiopathic sPTBs. This review aims to explore the role of Tregs and a variety of conditions associated with sPTB, to understand their role in sPTB pathophysiology and improve neonatal outcomes.
2. Tregs in Term Birth
Tregs are T cells broadly identified by the CD4 surface marker and occasionally CD8 [
9]. Tregs are largely heterogeneous with several subpopulations characterised by specific surface/internal markers. Each subpopulation of Tregs contributes a different role to the body’s immune response, but in general their function involves using immunosuppressive mechanisms to illicit an anti-inflammatory state [
10]. Two common subpopulations of Tregs are the thymus-derived (tTregs) and the peripherally-derived (pTregs) [
11,
12]. Both subpopulations share common Treg markers, including CD25, GITR, CTLA4, and FOXP3, contributing to their role against both foreign and self-antigens [
13]. Deficiencies in these expected markers can signify a disruption in immune response, with decreased FOXP3 showing increased rates of autoimmune and inflammatory diseases [
14,
15]. FOXP3, also known as the master transcription factor of regulatory T cells, is a forkhead box P3 protein that when internally expressed in naive T cells is key for their development into their regulatory form, allowing the identification of many Treg subsets. Although some subsets of regulatory T cells can be deficient in this marker the majority of Tregs rely on FOXP3 as a key protein for their maturation and therefore, effective function through the promotion of anti-inflammatory states [
16].
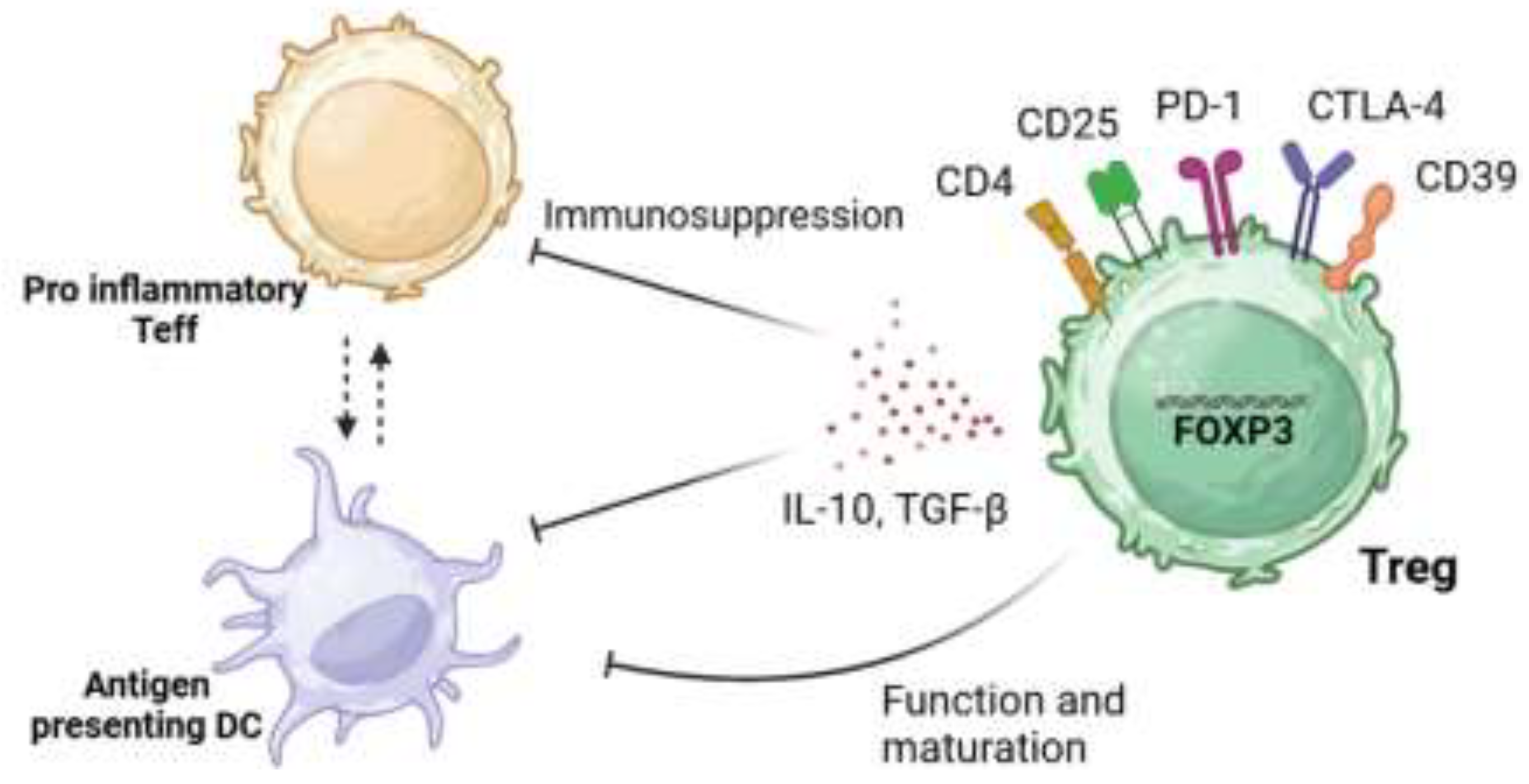
As demonstrated in
Figure 1, Tregs can carry out these anti-inflammatory states through mechanisms which lead to either the suppression of pro-inflammatory T effector cells (Teffs) (CD4
+ CD25
-), or the inhibition of antigen-presenting dendritic cells (DCs). These mechanisms can be regulated through the release of anti-inflammatory cytokines (i.e. interleukin-10 (IL-10) and transforming growth factor- β (TGF- β)), consumption of cytokines needed by Teffs, or the manipulation of biological pathways that can either trigger apoptosis or regulate T cell differentiation [
17]. Alternatively Tregs can suppress their target cells by cell:cell contact. For example, the co-inhibitory molecule PD-1 elicits its immunosuppressive functions through interactions with its ligand PDL-1 on effector T cells resulting in exhaustion, neutralization, dysfunction and increased IL-10 production by the Treg themselves [
18]. Additionally, the expression of CTLA-4 can also have immunosuppressive functions with its ability to cause trogocytosis of its ligands CD80/CD86 expressed on antigen presenting cells, leading to their immunosupresion [
19]
Figure 1.
Suppressive mechanisms elicited by Tregs.
Figure 1.
Suppressive mechanisms elicited by Tregs.
Tregs can elicit their immunosuppressive functions through various mechanisms. These include the expression of co-inhibitory external markers such as PD-1 or CTLA-4 and the production of anti-inflammatory cytokines, IL-10 and TGF-β. This allows for the inhibition of the function and maturation of DC and overall immunosuppression of pro-inflammatory effector T cells (Teffs). This image was created in BioRender, adapted from Kempkes, R. W. M., Joosten, I., Koenen, H. J. P. M. & He, X., 2019 [
20].
These anti-inflammatory mechanisms allow for Tregs to induce antigen-specific tolerance, making them invaluable in pregnancy, which requires maternal immune tolerance of fetal antigens for successful placentation and development [
21]. Maternal CD4+ CD25+ Tregs are present in both peripheral blood and the decidua basalis layer at the maternal-fetal interface, [
22,
23] interestingly, approximately 95% of the Tregs present in the placenta in first trimester are classified as nTregs. Given that nTregs work against self-antigens and would theoretically be less effective in promoting fetal tolerance, it was suggested that the sheer number of nTregs and their ability to supress inflammatory T effector cells may play a bigger role than the presence of fetal-derived antigen-specific Tregs in the first trimester, but more research is needed to support this concept [
24].
In healthy term pregnancies, the numbers of Tregs have been shown to change throughout gestation with a pro-inflammatory condition (less Tregs) being favoured for implantation. As antigen presenting cells such as, dendritic cells, present small doses of fetal antigen to maternal Tregs, an anti-inflammatory is stimulated at the maternal-fetal interface which is favourable for fetal tolerance and Treg expansion [
24]. A dramatic expansion of Tregs, shortly after implantation continues to increase until 20-30 weeks of gestation, which allows time for fetal growth and development. Towards the end of the third trimester the number of activated Tregs decrease and promote another pro-inflammatory state, this time aiding in activation of parturition for a successful delivery [
21]. It is suggested that these gestational changes are driven by fluctuations in hormones such as human chorionic gonadotropin (hCG), progesterone and oestrogen which can alter the expression of several immune cells present at the maternal-fetal interface [
25,
26,
27].
2.1. Tregs in Spontaneous Preterm Birth
sPTB is a syndrome of multiple known and unknown causes, and this has resulted in difficulties in the early identification of pregnancies at risk and development of sPTB as well as the targeting of effective treatments. Most studies include participants with causes and risks of sPTB that are heterogenous in nature which further complicates the picture [
28]. This review includes several adverse pregnancy conditions and factors that are associated with an increased risk of sPTB.
A key review, which holistically addressed the role of pregnancy immunology, highlighted an overall collaborative effort between cells of the innate (e.g., uterine natural killer (uNKs) and DCs) and adaptive (e.g., Tregs) immune response that is crucial to avoid a premature trigger of sPTB. It was observed that reduced Treg suppressive functions in the third trimester could trigger labour of both term and preterm pregnancies, reaffirming the importance of the cell type beyond the first trimester due to its participation in the triggering of labour [
29]. While Tregs present in labour of both spontaneous preterm and term births appear to be from the same subpopulations, it remains to be understood whether they occur as a result of birth or if their appearance is the mechanism driving the onset of labour, be that premature or term [
30,
31]. The number of Tregs and their suppressive function has been explored to identify characteristics of Tregs in sPTB, with some studies identifying decreased levels and impaired function of Tregs in sPTB compared to term births [
32]. These findings are supported by mouse models which have shown that depletion of Tregs in late pregnancy can result in preterm delivery [
26] while Treg therapeutics support tolerance and a healthy term pregnancy through an improved maternal immune system [
33]. This was demonstrated by Siddiq et al [
33] when lipopolysaccharide was administered to induce inflammation in the perinatal liver of mice was shown to be negatively regulated by Tregs.
In FOXP3+ Tregs specifically, a decrease in numbers were identified in comparison to their term birth counterparts. This is also supported by mouse models which saw a link in the depletion of FOXP3+ Tregs and an increase in pregnancy failure [
29]. Rowe et al, identified that pre-existing FOXP3+ Tregs retained from primary mice pregnancies drove Treg expansion in secondary pregnancies, indicating the importance of FOXP3+ Treg cells in immunological memory. Additionally, it was found that secondary pregnancies were more resilient to fetal reabsorption compared to primary pregnancies where FOXP3+ Treg cells were ablated. This highlights the ability of FOXP3+ Tregs to create lasting modifications to the maternal immune system which contribute to successful pregnancies through a unique form of transferrable or sustained fetal tolerance [
34].
3. Inflammatory and Immunocompromising Pregnancy Outcomes Associated with sPTB
With the complexity in cause of idiopathic sPTBs [
35] it is essential to evaluate the commonalities between conditions that increase the risk of sPTB. Although these conditions range in their individual causes they are tied by their association to premature labour and symptoms of inflammation. Better understanding of their inflammatory mechanisms and the role of Tregs in response to these different outcomes is key to discovering potential links between these mechanisms and why they may increase incidence of sPTB.
3.1. Preterm Premature Rupture of Membranes (pPROM)
Preterm premature rupture of membranes (pPROM) is a pregnancy condition in which the fetal membranes rupture prior to 37 weeks' gestation. It can be identified through the premature release of amniotic fluid [
36]. Interestingly, pPROM has been found to precede about 40-50% of sPTB, yet the causes of pPROM and its association to other causes of sPTB remains unclear [
37]. However, what is understood is that this rupture of membranes is strongly associated with infection and an inflammatory environment, showing that sPTB cases with pPROM have increased rates of infection/inflammation in comparison to sPTB without pPROM [
38]. One study found that 70% of pPROM cases had an additional diagnosis of intraamniotic infection, indicating the crucial role of these inflammatory states in pPROM and therefore sPTB [
39].
The immunological implications of pPROM have been explored over the years with several studies reporting cases of increased levels of pro-inflammatory cytokines present in the amniotic fluid, such as IL-8, IL-6, IL-1β and tumour necrosis factor-α (TNF-α) [
40]. This is supported by a recent study comparing the transcriptomic profiles of placental decidua and amniotic tissue from pregnancies with both pPROM and sPTB, versus term pregnancies without pPROM. Tissues form the pPROM/sPTB group exhibited higher expression of pro-inflammatory Th1 and Th17 cells, while FOXP3, a characteristic internal marker of Tregs, was decreased in both chorionic and amniotic tissue [
38,
41]. Another study, performed on whole blood samples from pPROM mothers and their newborns saw no significant difference in Treg levels when compared to non-pPROM pregnancies, however the study was limited in that they did not use the intracellular staining of FOXP3 during characterisation. However, the study did see a higher presence of memory CD4+ T cells (CD45RA+, CD27-) and lower levels of naïve CD4+ T cells (CD45RA+) in the newborn whole blood. This led them to conclude that pPROM may trigger an early maturation of T cells during pregnancy, playing a role in premature labour pathophysiology [
42].
3.2. Chorioamnionitis
An example of a pro-inflammatory condition strongly affiliated with both pPROM and sPTB is chorioamnionitis, which is either caused by ascending infection via the cervix and/or vaginal tract into the uterus where it spreads to the chorioamnion of the placenta or through a cascade of proinflammatory signals that attract neutrophils [
43,
44]. This transmission can occur either with or without the presence of chorioamniotic membrane ruptures depending on the source of infection (bacterial, viral, fungal), with infective agents being present throughout the placenta and amniotic fluid [
45,
46]. Chorioamnionitis can cause inflammation and lesions in the placental membranes, along with maternal symptoms of infection including fever and increased white blood cell count. In the presence of chorioamniotic infection, pro-inflammatory chemokines and cytokines e.g., TNF-α, IL-6 etc, endotoxins, exotoxins and granulocyte colony stimulating factors are released. This increase of inflammation within the uterus induces the influx of both maternal and fetal neutrophils to the maternal-fetal interface where lesions may be present [
47,
48]. Activated neutrophils at the membrane infection site release prostaglandins which can cause cervical ripening and uterine contractility that promote preterm labour [
49,
50,
51]. This is perpetuated by the presence of prostaglandins recruiting more neutrophils and causing production of matrix metalloproteinases and subsequent degradation of fetal membranes that can lead to a preterm labour and sPTB [
47,
51]. Studies have observed a diagnosis of acute chorioamnionitis in around 94% of preterm births occurring between 21-24 weeks gestation, compared to only 3-5% in term births [
48,
52].
Interestingly, a study exploring Treg levels and functionality in ‘moderate’ preterm birth and ‘late’ preterm birth with chorioamnionitis compared to term neonates without chorioamnionitis found that while the numbers of Tregs were not vastly different between the groups, Tregs from sPTB with severe chorioamnionitis neonates had low levels of functionality, which was assessed by the Tregs ability to suppress proinflammatory response from conventional T cells [
53]. Their reduced suppressive function may provide an explanation for the manifestations of chorioamnionitis, given the Tregs would be unlikely to reduce inflammation caused by active pro-inflammatory lymphocytes at the maternal-fetal interface, such as Teffs and uNK cells, which would be responding to infective agents [
54].
3.3. Bacterial and Viral Infections during Pregnancy
A maternal immune system debilitated by an ongoing infection can also put the pregnancy at risk of premature delivery, with it being identified that 25-40% of preterm births may be attributed to intrauterine infections [
55].
Bacteria are frequently identified in the amniotic fluid in cases of sPTB [
56,
51], specifically, mycoplasmas, with the rate of sPTB being 14.3% higher when compared to mothers that had amniotic fluid negative for mycoplasma [
57]. One current hypothesis linking bacterial infection to sPTB, states that bacterial presence is identified and triggers stimulation of the developing fetal immune response, leading to a cascade of effects
in utero. These events include the activation of toll like receptors (TLRs) that bind to specific epitopes on microorganism's surfaces, specifically TLR-2 and TLR-4 which are highly expressed in the amniotic epithelium [
58]. Premature uterine contractions may then occur after the release of pro-inflammatory cytokines such as IL-1β and TNF-α, resulting in the activation of neutrophiles and prostaglandin production [
50].
Viral infections during pregnancy are also linked to a higher risk of sPTB, which is why understanding the cell mediated responses of T cells in these cases become even more crucial [
59]. One study assessing placental T cells isolated from mothers diagnosed with human immunodeficiency virus (HIV), identified an inverse CD4:CD8 T cell ratio in villous tissue between the positive and HIV negative placental controls. This was attributed to a higher number of CD8+ T cells in the villi rather than lower levels of CD4+ T cells, in cases of HIV positive pregnancies. This CD4:CD8 T cell ratio was further stratified by gestational age at delivery to observe any differences between the median gestational age for HIV positive cases (39 weeks’ gestation) and HIV negative cases (40 weeks’ gestation). Interestingly, they found no association between placental CD4:CD8 ratio between the gestational ages [
60].
In the context of pregnancies where congenital human cytomegalovirus (HCMV) is diagnosed, the risk of having a sPTB was 15 times more likely than in pregnancies that were HCMV negative [
61]. Outside of pregnancy, Tregs isolated from PBMCs of HCMV positive individuals have been found to decrease pro-inflammatory cells such as CD8+ T cells while increasing apoptosis of both CD8+ and CD4+ T cells [
62]. However, when the relationship of Tregs and HCMV was explored in pregnancy results varied. One study infected placental extravillous trophoblasts (EVTs) with HCMV
in vitro and observed their ability to increase the proportions of CD25HIFOXP3+ and PD1HI Tregs in comparison to non-infected EVTs. Overall, there was no differences between the Treg stimulation between both groups, but the authors hypothesised that this could be due to absent methods of localised induction of Tregs by EVTs or of antigen-presenting immune cells that is expected to be present at the maternal-fetal interface
in vivo [
63].
3.4. Dysbiosis of the Cervico-Vaginal Microbiome
The cervical, vaginal, and endometrial microbiome function as a line of defence against several potentially dangerous bacteria that could enter vaginally during pregnancy and lead to several of the aforementioned conditions associated with sPTB. This protection is possible through the presence of anaerobic bacteria such as
Lactobacillus spp. which produce lactic acid and create a highly acidic environment which aids in evasion of pathogens [
64,
65]. When studied in the context of non-pregnant mouse models with sexually transmitted diseases (STDs), vaginal
Lactobacillus spp have been shown to decrease inflammatory response through the promotion of M2 macrophages and Tregs, which aided in wound repair at both the cervical and vaginal sites [
66,
67].
In another model, where non-pregnant mice were depleted of Tregs and infected with herpes simplex virus-2 (HSV-2), a delay in recruiting HSV-2 targeted CD4+ T cells was observed, when compared to Treg sufficient mice. The authors suggested that this could be because Tregs in the vaginal mucosa, which are meant to be interacting at the site of the microbiome to recruit dendritic cells for CD4+ T cell activation, are absent [
66]. Whether or not it can be concluded that Tregs have an important function within the cervico-vaginal microbiome remains unclear, however, a recent study looking at Tregs in non-pregnant women following clinical diagnosis with human papilloma virus (HPV), found inflammation caused by HPV dysregulating the cervico-vaginal microbiome was associated with higher levels of circulating Tregs (CD4+CD25+FOXP3+) within the peripheral blood [
68].
The maintenance of a regulated cervico-vaginal microbiome and interacting cells is crucial to the prevention of sPTB, with several studies showing instability of the microbiome to be a leading risk factor for premature labour [
65,
66,
69]. Many of the findings focus on the importance of
Lactobacillus species, with women whose microbiome showed
Lactobacillus was not the predominant bacterial species, being at an increased risk of sPTB, compared to healthy pregnant microbiomes where
Lactobacillus was abundant with diverse specie strains [
60,
70,
71]. However, it should be noted that several of these studies did not represent a diverse sample population or account for potential confounding variables that influence the cervico-vaginal microbiome, including ethnicity, body mass index (BMI) or menstrual cycle phase.
3.5. Preeclampsia (PE)
Preeclampsia (PE) is a condition occurring in pregnancy, typically diagnosed around 20 weeks' gestation, characterised by the development of hypertension
(>140/90 mmHg) and evidence of maternal organ dysfunction (ie. renal, hepatic, and haematological dysfunction). Proteinuria, which is often a sign of kidney damage, used to be involved in classifying PE, but it is no longer regarded, given that the condition can manifest in organ issues beyond kidney dysfunction [
72]. Maternal obesity, older maternal age and having hypertensive related diseases in previous pregnancies are a few of the many risk factors that can result in PE [
4]. It is a condition with grave consequences which include maternal mortality and fetal growth restriction, which is why medically induced preterm births are usually initiated [
73].
Decreased Treg cell numbers are associated with preeclampsia, with several studies exploring their role in the maternal immune response in hypertensive pregnancies [74,4, 21, 75, 76]. In Sasaki et al, [
75] the number of Treg cells was compared between healthy pregnant women and non-pregnant women to those with pre-eclamptic pregnancies a disparity was found. Those with pre-eclampsia were found to have fewer Tregs, specifically CD25 bright T cells, whereas CD8+ T cells in the placental bed biopsies were found to be higher in the PE patients than the healthy pregnant women. FOXP3 expression was located on most CD4+ CD25 bright Treg cells in all groups, however, in the placental bed biopsies FOXP3+ Treg cells were also reduced in the pre-eclampsia group [
75].
Lower numbers of FOXP3+ CD4+ CD25 bright Tregs was most commonly found in the maternal peripheral blood and placental bed biopsies of PE patients as opposed to the non-pregnant or healthy pregnant women. This suggests that there is a correlation between low Treg numbers and preeclampsia that can eventually require a medically induced to preterm birth [
75], this was confirmed in results by Prins et al, [
76] which identified reduced levels of CD4+ FOXP3+ Tregs from isolated peripheral blood from pregnant women with PE when compared to healthy pregnant controls.
Moreover, the meta-analysis published by Green et al, [
4] further highlights this correlation between PE and Treg levels during pregnancy. Out of the 32 studies that compared the number of Tregs in women with preeclampsia and healthy pregnancies, 30 found significantly higher Treg levels in the peripheral blood of healthy pregnant individuals. An association between Tregs levels and PE is strongly supported in the literature, but further characterisation of what Tregs manifest in different PE phenotypes is crucial to improving outcomes [
4] and understanding associations between preeclampsia and preterm birth.
3.6. Gestational Diabetes (GDM)
Gestational diabetes mellitus (GDM) is one of the most common endocrinopathies that occurs during pregnancy, characterised by hyperglycaemia and glucose intolerance. Associated maternal complications that can arise from this condition include pre-eclampsia and type 2 diabetes which manifests post-natally. For the fetus, GDM can result in intrauterine death and fetal malformation [
77]. Additionally, newborns can also experience hypoglycaemia and macrosomia, with the additional risk of type 2 diabetes during their adult life [
78]. It is currently thought that the inability of the mother’s immune system to adapt to pregnancy may lead to immune dysregulation, which is a key proponent in the development of insulin resistance and chronic inflammation seen in GDM [
79,
80].
As previously mentioned Treg involvement varies throughout a healthy gestation with a pro-inflammatory phenotype characterising early implantation [
81] which is then followed by Treg expansion, followed by a steep decline in the number of Tregs prior to the onset of labour [
82]. It is currently thought that if the maternal immune system does not follow these expected patterns, there may be underlying immune dysregulation. This dysregulation is a key proponent in the development of insulin resistance and chronic inflammation seen in GDM, characterised by factors such as, increased levels of pro-inflammatory markers TNF-α and IL-6 [
83,
84].
To understand the specific potential pathogenic role that Tregs have in GDM, a study by Schober et al, [
78] investigated disparities in Treg subsets and their suppressive capabilities across healthy pregnant women, pregnant women with insulin dependent GDM and dietary adjusted GDM. With focus on assessing the CD4+ CD127low+/- CD25+ FOXP3+ Tregs which specifically in this study was further divided into naïve CD45RA+ Tregs, HLADR-CD45RA- memory Tregs (DR-Tregs), HLADRlow+CD45RA-memory Tregs (DRlow+Tregs) and HLADRhigh+CD45RA- memory Tregs (DRhigh+Tregs) [
78].
To analyse these cells, peripheral blood samples were taken from pregnant women of each group, on which flow cytometry was then performed. Similar to findings in preeclampsia, decreased Treg levels were characteristic in the GDM pregnant women when compared to non GDM cohorts, particularly naïve CD45RA+ Tregs subpopulations. This study supports various others which found reduced Tregs linked to adverse pregnancy outcomes [85,86, 87].
A metanalysis by Arain et al, [
88] studied the Treg levels in the mother’s peripheral blood and its correspondence to developing GDM. Women with GDM were looked at alongside pregnant women without GDM. In several of the studies analysed Treg levels were lower in the GDM cohort. It can be inferred that GDM has immune implications which manifest in ways in which cause low Treg cell counts in the maternal peripheral blood [
88].
4. Influence of Tregs on Preterm Neonatal Outcomes
In parallel with interrogating the role of Tregs in pregnancy and at the maternal-fetal interface there is also a need to determine how these
in utero exposures influence immune development and morbidity risk in preterm newborns. It is known that maternal response to infection and inflammation alters the functionality of the neonatal immune system [
89], with lots of research focusing on the impact this has on newborn immune function and health outcomes [
90,
91,
92].
According to Kamdar et al, both term and preterm newborns follow a similar trajectory in postnatal immune system development, yet sPTB infants were still at a higher risk for developing sepsis to their term counterparts [
93]. This may be due to decreased function of T cells, given findings show a decreased ability of preterm T cells to produce IL-8, which is important for attracting neutrophils to combat infection [
93]. This study also used flow cytometry to compare longitudinal PBMCs from preterm newborns with either no suspicion of infection following antibiotics, suspicion of infection/sepsis and given further course of antibiotic treatment or if confirmed with chorioamnionitis. Results saw that the infants with suspected sepsis or who had chorioamnionitis had a lowerFOXP3 expression on their Tregs compared to those without infection, suggesting preterm newborns may see lower levels of this cell than their preterm counterparts without suspected/confirmed infections [
93].
The involvement of Tregs in two major neonatal conditions associated with preterm birth: necrotising enterocolitis and neonatal encephalopathies in premature infants, highlight the importance of further research as understanding of Tregs mechanisms at play are still misunderstood.
4.1. Necrotising Enterocolitis (NEC)
Necrotising enterocolitis (NEC) is an inflammatory condition commonly developed by preterm newborns that affects the intestine to a degree which can be life-threatening and require emergency treatment [
94]. NEC morbidity and mortality rates are the highest in preterm newborns since they are quite fragile and have greater difficulty recovering despite surgical advancements and other intervention strategies [
95,
96] With NEC the neonatal immune system is believed to have issues regulating present inflammation, especially in cases of sPTB with babies born prematurely found to have decreased number of Tregs and are unable to maintain the integrity of the intestinal epithelium [
97] To explore this concept one study took peripheral blood samples from newborns diagnosed with NEC to compare with those without NEC, using flow cytometry to identify their respective presence of circulating Tregs. Findings identified lower numbers of Tregs within the peripheral blood of those with NEC compared to controls. This study recognised that Treg proliferation may be defective in cases where NEC is present, seeing a reduction in FOXP3 positive Treg expression. Like many other conditions associated with preterm birth, additional research is required to explore a solid causation for this pathological association [
97].
These findings are interesting when considered in parallel to studies that found neonates play an active role in immune defence through observation of T cells in the amniotic fluid which express surface markers (ie. CD103 and CD161) indicating a potential mucosal origin site for the cell. It would be beneficial to explore if preterm babies develop NEC due to insufficient Tregs unable to repair the intestinal barrier or if there is a lack of Tregs due to NEC damaging fetal intestine, where fetal T cells may find their origin [
41].
4.2. Encephalopathies of Premature Newborns
Encephalopathies in premature infants is a commonly reported neurological condition which impacts several parts of the developing newborns brain including the cerebellum, white and grey matter. Encephalopathies following sPTB often lead to long term health consequences which can impair vision, decrease motor coordination, delay cognitive development and much more. Encephalopathy of prematurity can be characterised using magnetic resonance imaging (MRI) by observing myelin sheath reduction, immature oligodendrocytes, lesions of the axon, and neuroinflammation [
98]. Gestational age has been explored in the context of neonatal encephalopathy (NE), noting that neonates born between 35-38 weeks’ or after 40 weeks' gestation saw an increased risk of NE compared to those born from 39-40 weeks, indicating potential significance for late sPTBs occurring from 35-37 weeks GA developing encephalopathy. The risk was further increased if the mother had an infection or reported autoimmune disease, specifically rheumatoid arthritis [
99].
The presence of neuroinflammatory mechanisms being involved in a condition commonly reported in premature infants has prompted the exploration of lymphocytes and their role in neonates developing NE/EOP. One review noted two different studies conducted on murine models with induced hypoxic ischemia, where in one case treatment with FTY720 (a T cell migration blocking agonist receptor) depleted Th17 T helper cells saw a decrease in pro-inflammatory cytokines and preservation of white cerebral matter [
100]. However, the other study where FTY720 depletion treatment was performed on Tregs, CD4+ T cells and CD8+ T cells saw a loss of both white and grey cerebral matter in newborn pups [
101]. The comparison between these studies is limited since they depleted different T cell types, however, it is an interesting foundation for exploring T cell mechanisms in NE.
A more recent study compared the presence and functionality of lymphocytes in the peripheral blood of school-aged children, who experienced NE as neonates with that of children with cerebral palsy and age matched healthy controls. Results showed that NE neonates and their healthy counterparts had similar values of circulating T cells, but levels of effector T cells, which produce pro-inflammatory cytokines, were significantly higher in the NE infants. They stated that further research specifically exploring Treg numbers, and several T helper subpopulations would be needed to determine their respective roles in NE, however they suspect their findings to be evidence of the role lymphocytes such as various T cells in the disease mechanism [
102].
5. Potential Immunotherapies for Adverse Pregnancy Outcomes Associated with sPTB
The involvement Tregs have shown in adverse pregnancy outcomes associated with sPTB and poor neonatal health consequences have pushed for studies to explore their role in the prevention of premature labour. Treg therapies have been used in other inflammatory conditions, but whether these therapies have a place in the context of sPTB remain unclear yet possible.
5.1. Treg Cell Infusion Therapy
Treg cell therapy is proving useful for several immune mediated diseases and beyond, looking to address the issue of a dysfunctional immune response, that can lead to excessive inflammation in several conditions. There are a variety of approaches currently being assessed in both preclinical and clinical trials which have different benefits which should be considered based on the type of condition the Tregs are being used for. Each of these approaches begin with Tregs isolated from peripheral blood and expanded in the presence of IL-2. One of the most common approaches includes polyclonal expansion of Tregs using anti-CD3/CD28 beads, allowing for a larger cell yield and potency at the expense of specificity. Another method, used primarily for cases in solid organ transplantation involves presenting the IL-2 activated Tregs with antigen presenting cells from the donor to stimulate an antigen-specific Treg response. While these are more specific than the polyclonal Treg method they usually do not have a high enough cell yield even following cellular expansion. The third most common Treg product used as a cell therapeutic involves the expansion of polyclonal Tregs which are then transduced with either a chimeric antigen receptor (CAR) or an artificial T cell receptor (TCR), the point behind each to target a specific antigen, the latter of which involves specific major histocompatibility complex (MHC)-peptide complexes. The benefit to this method is that it allows for a high cell yield, specificity, and potency [
103,
104].
A study by Gomez-Lopez et al, tested the potential of Treg infusion therapy for preterm birth using a mouse model with either partial or total depletion of Tregs at 3 weeks gestation [
24], which is comparative to the late third trimester in human pregnancies [
106]. Depletion was performed by injecting FOXP3DTR mice with various amounts of diphtheria toxin to deplete FOXP3+ Tregs prior to mating with BALB/c mice. As a result, 15% of the mice produced premature pups, which were reported as smaller and leaner than the mice without Treg depletion. An injection of Tregs was then applied to the mice with complete FOXP3+ Treg depletion, to see if this prevented preterm birth in pregnancy. This therapy was able to significantly decline the rate of preterm births from 15% to 0% and the pups born after Treg infusion had a greater survival rate compared to the group withheld [
26].
This evolving therapy shows potential clinical benefits for pregnant women, in increasing their Treg levels and preventing sPTB. To further test that this form of treatment is transferrable to humans, Dudreuilh et al, [
107] experimented with transferring Tregs into human leukocyte antigen (HLA) sensitised patients in an ongoing trial. It is possible for some kidney failure patients to become sensitised to HLA, preventing their body from accepting a kidney transplant so the intention behind this study is to test the ability of Tregs in these patients is to determine whether memory B and T cells can suppress HLA, to facilitate their kidney transplant. If these findings show improvements in tolerance in instances of organ transplant, that opens the door to Treg cell therapy for tolerance of fetal antigen by the maternal immune system.
5.2. Probiotics for the Prevention of sPTB
Probiotics have been used throughout history as a treatment for several ailments, particularly those related to intestinal disorders, given they illicit microbial and immune interactions which combat inflammation [
108]. Studies looking at the mechanisms used by probiotics to incite these anti-inflammatory processes show that different strains of probiotics can lead to the production of cytokines such as IL-12 which can stimulate Th1 and NK cells for a pro-inflammatory immune response, however, immunomodulating probiotic strains have been shown to stimulate Tregs and anti-inflammatory cytokine IL-10. These findings indicate the importance of understanding exactly which strains of probiotics should be used, with
Lactobacillus spp stimulating either pro- or anti-inflammatory mechanisms [
109,
110]
In the context of human pregnancy, probiotics have primarily been explored to prevent neonatal symptoms associated with a preterm birth, such as cases of NEC and low birth weight. When studies explored the effects, probiotics administered in pregnancy would have on breast milk, an increase in anti-inflammatory responses from IgA and TGF-β1 in breast milk and IFN-γ in cord blood were elicited [
110]. More studies are needed to confirm the use of probiotics as a preventative measure for preterm births as most of the studies in humans have restricted sample size and differ in the bacterial strains used as well as duration of probiotic administration. These limitations make it difficult to draw substantial conclusions regardless of the studies showing benefits in reducing inflammation and aiding in sPTB neonatal outcomes [
111,
112].
5.3. Tocilizumab Use for Inflammatory Rheumatoid Arthritis
Rheumatoid arthritis (RA) in adults and juvenile idiopathic arthritis (JIA) are chronic autoimmune inflammatory joint diseases from which pain and stiffness are encountered. Therefore, due to the nature of these diseases, immune dysregulation is heavily implicated in their pathogenesis. This has been demonstrated in a meta-analysis by Falcon et al, where pregnant women with RA were identified to have reduced functionality of CD4+ CD25high regulatory T cells and increased pro-inflammatory cytokines IL-6, IFN-(gamma) and TNF-α in maternal blood collected during pregnancy in the third trimester [
113].
It has been found that pregnant women with these types of inflammatory arthritis are at a greater risk of having preterm deliveries than their counterparts without arthritis [
114]. Further tested by Smith et al, who assessed the risk of sPTB for pregnant women who had either RA or JIA and compared them to pregnant women without arthritis. In both groups of arthritis women combined, they had a much higher risk of going into preterm labour, specifically a moderate sPTB at around 32 weeks of gestation [
115]. For individuals who were diagnosed specifically with RA, they had an identified increase in extreme sPTBs compared to the control and even JIA group [
115].
Immunomodulatory drugs are emerging to enhance Treg levels in patients with different autoimmune diseases, including RA. Tocilizumab is an example of one such pharmaceutical, which acts as an anti-interleukin-6 receptor antibody, to limit the inflammation seen in RA patients [
116]. Kikuchi et al, carried out a study where non-pregnant RA patients were given Tocilizumab then assessed their peripheral blood cells by flow cytometry. Based on the clinical disease activity index (CDAI) it was determined that 53.8% of RA patients went into remission as their CD4+CD25+CD127low Tregs and HLA-DR+ activated Tregs increased, resulting in a decrease in clinical symptoms of inflammation [
116]. The use of Tocilizumab increased Tregs, improving clinical symptoms of inflammation in RA patients. Even though the mechanism by which low Tregs and RA operate has yet to be fully understood, Tocilizumab is still able to raise Treg levels via inhibiting the IL-6 pathway and increase immunosuppression in these patients [
116,
117].
Interestingly, a mouse model using Tocilizumab as a preventative intervention for preterm delivery found that the drug was able to reduce LPS-induced inflammation and prolong gestation [
117]. Whether this is translational to human pregnancies remain to be explored but from the studies that have been conducted there has been found to at least have no reportable increase in spontaneous abortion or congenital issues [
118,
119]. It is believed that Tocilizumab does not cross the placenta in high amounts during the first trimester when most congenital defects may begin to form. However, in later trimesters there have been increased incidence of sPTB, but if this is due to the drug or the individual having RA is unclear [
120]. More research is needed looking at not only the safety of Tocilizumab in pregnancy but also its ability to increase Tregs and how this affects rates of sPTB, to identify if it would make a viable treatment for at risk pregnancies.
6. Discussion
As a leading cause of neonatal morbidity and mortality, preterm birth remains a pervasive issue in the context of perinatal health. sPTB is often accompanied by immune dysregulation and heightened inflammatory responses, indicating an effected immune response within conditions affiliated with increased risk of premature labour. This review set out to explore the relationship between Tregs and various pregnancy conditions that may influence sPTB occurrence, examining how Treg levels and functionality impact sPTB phenotypes and associated neonatal outcomes.
The literature consistently points to a variation in Treg levels and functionality in the context of these inflammatory pregnancy conditions. Some studies indicate a decrease in Treg levels, while others suggest Treg dysfunctionality rather than a numerical decrease. In sPTB cases specifically, findings show increased presence of pro-inflammatory cytokines, with some subpopulations of Tregs, such as memory Tregs, being elevated. Tregs also make frequent occurrences in the literature, with their decreased levels/functionality being suggested as an important marker of premature labour. The variations seen amongst Treg findings underline the complexity of the immune landscape in sPTB, highlighting the need for standardised research to fully understand the impact of Tregs on different sPTB phenotypes.
Tregs have been implicated in conditions affiliated with sPTB such as necrotising enterocolitis and neonatal encephalopathy, where their level of functionality have proposed contributions to dysregulated immune response and long-term effects on neonatal health. There is still much to be understood in these areas due to limitations, including small sample sizes and varying methodologies, which necessitate further research to confirm these findings. Additional studies regarding T cell pathways should be explored for better understanding of how Tregs can modulate inflammation in these cases and protect against long term health consequences in premature newborns.
Given these insights, Tregs may prove fruitful in guiding in utero therapeutics to decrease inflammation and potentially prevent sPTB. Treatments aimed at enhancing Treg functionality or levels, such as Tocilizumab in RA patients, have shown promise in reducing inflammation and improving clinical outcomes. Tocilizumab, specifically, increases Treg levels and reduces inflammatory markers, potentially mitigating the risk of preterm birth in RA patients. Other therapeutic strategies, such as Treg therapy, have shown success in animal models, where Treg depletion increased sPTB risk, and Treg administration reduced risk, improving neonatal outcomes. Probiotic administration has also demonstrated potential, increasing Treg levels and reducing pro-inflammatory Th17 cells in both preterm and full-term mice. Whether these treatments would benefit pregnant humans at risk for sPTB remains to be tested but there is great potential in their use.
In conclusion, while the relationship between Tregs and sPTB is complex and multifaceted, the findings suggest that enhancing Treg functionality and levels could improve neonatal outcomes and reduce sPTB risks by decreasing inflammatory mechanisms brought on by several adverse pregnancy conditions. Future research should focus on elucidating the specific mechanisms by which Tregs influence sPTB and developing targeted therapies to modulate Treg activity in utero, potentially transforming the management and prevention of sPTB associated complications.
Funding
This research was funded by the UK Medical Research Council (MRC), grant number (2022- MR/W006820/1).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the writing of the manuscript.
References
- R. P. Tedesco et al., “The role of maternal infection in preterm birth: Evidence from the brazilian multicentre study on preterm birth (EMIP),” Clinics, vol. 75, 2020, doi: 10.6061/clinics/2020/e1508.
- S. R. Walani, “Global burden of preterm birth,” International Journal of Gynecology & Obstetrics, vol. 150, no. 1, pp. 31–33, Jul. 2020, doi: 10.1002/ijgo.13195.
- C. Phillips, Z. Velji, C. Hanly, and A. Metcalfe, “Risk of recurrent spontaneous preterm birth: A systematic review and meta-analysis,” 2017. doi: 10.1136/bmjopen-2016-015402.
- S. Green et al., “Regulatory T Cells in Pregnancy Adverse Outcomes: A Systematic Review and Meta-Analysis.,” Front Immunol, vol. 12, p. 737862, 2021, doi: 10.3389/fimmu.2021.737862.
- Y. M. Hwang et al., “Maternal-fetal outcomes in patients with immune mediated inflammatory diseases, with consideration of comorbidities: a retrospective cohort study in a large U.S. healthcare system.,” medRxiv, Aug. 2023, doi: 10.1101/2023.08.07.23293726.
- R. M. Burwick, A. I. Lokki, S. D. Fleming, and J. F. Regal, “Editorial: Innate Immunity in Normal and Adverse Pregnancy,” 2021. doi: 10.3389/fimmu.2021.646596.
- K. Racicot, J. Y. Kwon, P. Aldo, M. Silasi, and G. Mor, “Understanding the complexity of the immune system during pregnancy,” 2014. doi: 10.1111/aji.12289.
- R. P. Tedesco et al., “The role of maternal infection in preterm birth: evidence from the Brazilian Multicentre Study on Preterm Birth (EMIP).,” Clinics (Sao Paulo), vol. 75, p. e1508, 2020, doi: 10.6061/clinics/2020/e1508.
- S. Bolivar-Wagers, J. H. Larson, S. Jin, and B. R. Blazar, “Cytolytic CD4+ and CD8+ Regulatory T-Cells and Implications for Developing Immunotherapies to Combat Graft-Versus-Host Disease.,” Front Immunol, vol. 13, p. 864748, 2022, doi: 10.3389/fimmu.2022.864748.
- Z. Jiang et al., “Different subpopulations of regulatory T cells in human autoimmune disease, transplantation, and tumor immunity.,” MedComm (Beijing), vol. 3, no. 2, p. e137, Jun. 2022, doi: 10.1002/mco2.137.
- A.A. Luciano et al., “Alterations in Regulatory T Cell Subpopulations Seen in Preterm Infants,” PLoS One, vol. 9, no. 5, p. e95867, May 2014, doi: 10.1371/journal.pone.0095867.
- Cleveland clinic, “ Regulatory T-cells: Purpose, Function & Development,” 07/13/2022.
- X. Lin et al., “Advances in distinguishing natural from induced Foxp3(+) regulatory T cells.,” Int J Clin Exp Pathol, vol. 6, no. 2, pp. 116–23, 2013.
- T. Magg, J. Mannert, J. W. Ellwart, I. Schmid, and M. H. Albert, “Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells,” Eur J Immunol, vol. 42, no. 6, 2012, doi: 10.1002/eji.201141838.
- F. J. Alroqi and T. A. Chatila, “T Regulatory Cell Biology in Health and Disease,” Curr Allergy Asthma Rep, vol. 16, no. 4, p. 27, Apr. 2016, doi: 10.1007/s11882-016-0606-9.
- M. Salvany-Celades et al., “Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface,” Cell Rep, vol. 27, no. 9, 2019, doi: 10.1016/j.celrep.2019.04.109.
- S. Bhaumik et al., “Investigation of the Molecular Evolution of Treg Suppression Mechanisms Indicates a Convergent Origin.,” Curr Issues Mol Biol, vol. 45, no. 1, pp. 628–648, Jan. 2023, doi: 10.3390/cimb45010042.
- H. O. Alsaab et al., “PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome,” 2017. doi: 10.3389/fphar.2017.00561.
- M. Tekguc, J. B. Wing, M. Osaki, J. Long, and S. Sakaguchi, “Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells.,” Proc Natl Acad Sci U S A, vol. 118, no. 30, Jul. 2021, doi: 10.1073/pnas.2023739118.
- R. W. M. Kempkes, I. Joosten, H. J. P. M. Koenen, and X. He, “Metabolic Pathways Involved in Regulatory T Cell Functionality.,” Front Immunol, vol. 10, p. 2839, 2019, doi: 10.3389/fimmu.2019.02839.
- S. A. Robertson et al., “Therapeutic Potential of Regulatory T Cells in Preeclampsia—Opportunities and Challenges,” Front Immunol, vol. 10, Mar. 2019, doi: 10.3389/fimmu.2019.00478.
- J. Mjösberg, G. Berg, M. C. Jenmalm, and J. Ernerudh, “FOXP3+ Regulatory T Cells and T Helper 1, T Helper 2, and T Helper 17 Cells in Human Early Pregnancy Decidua1,” Biol Reprod, vol. 82, no. 4, pp. 698–705, Apr. 2010, doi: 10.1095/biolreprod.109.081208.
- H. Xiong, C. Zhou, and G. Qi, “Proportional changes of CD4+CD25+Foxp3+ Regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm,” Clinical & Investigative Medicine, vol. 33, no. 6, p. 422, Dec. 2010, doi: 10.25011/cim.v33i6.14594.
- S. Tsuda, A. Nakashima, T. Shima, and S. Saito, “New Paradigm in the Role of Regulatory T Cells During Pregnancy,” Front Immunol, vol. 10, Mar. 2019, doi: 10.3389/fimmu.2019.00573.
- N. Huang, H. Chi, and J. Qiao, “Role of Regulatory T Cells in Regulating Fetal-Maternal Immune Tolerance in Healthy Pregnancies and Reproductive Diseases,” Front Immunol, vol. 11, Jun. 2020, doi: 10.3389/fimmu.2020.01023.
- N. Gomez-Lopez et al., “Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes.,” Cell Rep, vol. 32, no. 1, p. 107874, Jul. 2020, doi: 10.1016/j.celrep.2020.107874.
- M. K. Collins, C. R. McCutcheon, and M. G. Petroff, “Impact of Estrogen and Progesterone on Immune Cells and Host–Pathogen Interactions in the Lower Female Reproductive Tract,” The Journal of Immunology, vol. 209, no. 8, pp. 1437–1449, Oct. 2022, doi: 10.4049/jimmunol.2200454.
- A. Ferreira, J. Bernardes, and H. Gonçalves, “Risk Scoring Systems for Preterm Birth and Their Performance: A Systematic Review,” 2023. doi: 10.3390/jcm12134360.
- N. Gomez-Lopez, D. StLouis, M. A. Lehr, E. N. Sanchez-Rodriguez, and M. Arenas-Hernandez, “Immune cells in term and preterm labor,” Cell Mol Immunol, vol. 11, no. 6, 2014, doi: 10.1038/cmi.2014.46.
- N. M. Shah, L. F. Edey, N. Imami, and M. R. Johnson, “Human labour is associated with altered regulatory T cell function and maternal immune activation,” Clin Exp Immunol, vol. 199, no. 2, pp. 182–200, Jan. 2020, doi: 10.1111/cei.13384.
- N. Gomez-Lopez and E. Laresgoiti-Servitje, “T regulatory cells: regulating both term and preterm labor?,” Immunol Cell Biol, vol. 90, no. 10, pp. 919–920, Nov. 2012, doi: 10.1038/icb.2012.48.
- L. Schober, D. Radnai, E. Schmitt, K. Mahnke, C. Sohn, and A. Steinborn, “Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool,” Immunol Cell Biol, vol. 90, no. 10, pp. 935–944, Nov. 2012, doi: 10.1038/icb.2012.33.
- M. Siddiq et al., “Inhibition of lipopolysaccharide-induced inflammation via the protective role of T regulatory cells in the fetal liver in a late-pregnancy preterm mouse model.,” Clinics (Sao Paulo), vol. 75, p. e1665, 2020, doi: 10.6061/clinics/2020/e1665.
- J. H. Rowe, J. M. Ertelt, L. Xin, and S. S. Way, “Pregnancy imprints regulatory memory that sustains anergy to fetal antigen,” Nature, vol. 490, no. 7418, 2012, doi: 10.1038/nature11462.
- S. Pereyra, C. Sosa, B. Bertoni, and R. Sapiro, “Transcriptomic analysis of fetal membranes reveals pathways involved in preterm birth,” BMC Med Genomics, vol. 12, no. 1, 2019, doi: 10.1186/s12920-019-0498-3.
- D. Bouvier et al., “Risk factors and outcomes of preterm premature rupture of membranes in a cohort of 6968 pregnant women prospectively recruited,” J Clin Med, vol. 8, no. 11, 2019, doi: 10.3390/jcm8111987.
- B. H. Jena, G. A. Biks, Y. K. Gete, and K. A. Gelaye, “Incidence of preterm premature rupture of membranes and its association with inter-pregnancy interval: a prospective cohort study,” Sci Rep, vol. 12, no. 1, 2022, doi: 10.1038/s41598-022-09743-3.
- P. R. Bennett, R. G. Brown, and D. A. MacIntyre, “Vaginal Microbiome in Preterm Rupture of Membranes,” 2020. doi: 10.1016/j.ogc.2020.08.001.
- R. Menon and L. S. Richardson, “Preterm prelabor rupture of the membranes: A disease of the fetal membranes,” Semin Perinatol, vol. 41, no. 7, 2017, doi: 10.1053/j.semperi.2017.07.012.
- P. L. Grigsby, M. J. Novy, K. M. A. Waldorf, D. W. Sadowsky, and M. G. Gravett, “Choriodecidual inflammation: A harbinger of the preterm labor syndrome,” Reproductive Sciences, vol. 17, no. 1, 2010, doi: 10.1177/1933719109348025.
- D. Miller, M. Gershater, R. Slutsky, R. Romero, and N. Gomez-Lopez, “Maternal and fetal T cells in term pregnancy and preterm labor,” 2020. doi: 10.1038/s41423-020-0471-2.
- M. Amadi et al., “Neonatal lymphocyte subpopulations analysis and maternal preterm premature rupture of membranes: A pilot study,” Clin Chem Lab Med, vol. 59, no. 10, 2021, doi: 10.1515/cclm-2021-0375.
- S. W. D. Carter et al., “Chorioamnionitis: An Update on Diagnostic Evaluation,” 2023. doi: 10.3390/biomedicines11112922.
- C. J. Kim, R. Romero, P. Chaemsaithong, N. Chaiyasit, B. H. Yoon, and Y. M. Kim, “Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance,” 2015. doi: 10.1016/j.ajog.2015.08.040.
- A. T. N. Tita and W. W. Andrews, “Diagnosis and Management of Clinical Chorioamnionitis,” Clin Perinatol, vol. 37, no. 2, pp. 339–354, Jun. 2010, doi: 10.1016/j.clp.2010.02.003.
- J. R. Fowler and L. V. Simon, Chorioamnionitis. 2024.
- S. Negara Kt, R. S. M, E. W, E. Pangkahila, W. A. P, and S. Kawilarang, “Chorioamnionitis and Funisitis Increase the Risk of Preterm Labor and Early Onset Neonatal Sepsis,” Biomedical and Pharmacology Journal, vol. 10, no. 02, pp. 767–772, Jun. 2017, doi: 10.13005/bpj/1166.
- C. J. Kim, R. Romero, P. Chaemsaithong, N. Chaiyasit, B. H. Yoon, and Y. M. Kim, “Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance,” Am J Obstet Gynecol, vol. 213, no. 4, pp. S29–S52, Oct. 2015, doi: 10.1016/j.ajog.2015.08.040.
- R. J. Phillips, M. A. Fortier, and A. López Bernal, “Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation,” BMC Pregnancy Childbirth, vol. 14, no. 1, p. 241, Dec. 2014, doi: 10.1186/1471-2393-14-241.
- G. Daskalakis et al., “Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications,” Children, vol. 10, no. 5, p. 907, May 2023, doi: 10.3390/children10050907.
- L. F. Stinson and M. S. Payne, “Infection-mediated preterm birth: Bacterial origins and avenues for intervention,” Australian and New Zealand Journal of Obstetrics and Gynaecology, vol. 59, no. 6, pp. 781–790, Dec. 2019, doi: 10.1111/ajo.13078.
- S. L. Hillier, M. A. Krohn, N. B. Kiviat, D. H. Watts, and D. A. Eschenbach, “Microbiologic causes and neonatal outcomes associated with chorioamnion infection,” Am J Obstet Gynecol, vol. 165, no. 4, pp. 955–961, Oct. 1991, doi: 10.1016/0002-9378(91)90447-Y.
- C. M. Rueda, C. B. Wells, T. Gisslen, A. H. Jobe, S. G. Kallapur, and C. A. Chougnet, “Effect of chorioamnionitis on regulatory T cells in moderate/late preterm neonates,” Hum Immunol, vol. 76, no. 1, pp. 65–73, Jan. 2015, doi: 10.1016/j.humimm.2014.10.016.
- I. Pedroza-Pacheco, A. Madrigal, and A. Saudemont, “Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy,” Cell Mol Immunol, vol. 10, no. 3, pp. 222–229, May 2013, doi: 10.1038/cmi.2013.2.
- R. L. Goldenberg, J. F. Culhane, J. D. Iams, and R. Romero, “Epidemiology and causes of preterm birth,” The Lancet, vol. 371, no. 9606, pp. 75–84, Jan. 2008, doi: 10.1016/S0140-6736(08)60074-4.
- G. L. Mendz, N. O. Kaakoush, and J. A. Quinlivan, “Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women,” Front Cell Infect Microbiol, vol. 3, 2013, doi: 10.3389/fcimb.2013.00058.
- D. P. Nguyen, S. Gerber, P. Hohlfeld, G. Sandrine, and S. S. Witkin, “Mycoplasma hominis in mid-trimester amniotic fluid: relation to pregnancy outcome.,” J Perinat Med, vol. 32, no. 4, pp. 323–6, 2004, doi: 10.1515/JPM.2004.060.
- S. A. Robertson et al., “Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury,” Clin Transl Immunology, vol. 9, no. 4, Jan. 2020, doi: 10.1002/cti2.1121.
- M. Silasi, I. Cardenas, J.-Y. Kwon, K. Racicot, P. Aldo, and G. Mor, “Viral infections during pregnancy.,” Am J Reprod Immunol, vol. 73, no. 3, pp. 199–213, Mar. 2015, doi: 10.1111/aji.12355.
- N. M. Ikumi et al., “T-Cell Homeostatic Imbalance in Placentas From Women With Human Immunodeficiency Virus in the Absence of Vertical Transmission,” Journal of Infectious Diseases, vol. 224, 2021, doi: 10.1093/infdis/jiab192.
- N. Aziz, L. Jelliffe-Pawlowski, C. Chambers, R. Baer, R. Currier, and M. Norton, “1: Spontaneous preterm birth in infants with congenitally acquired cytomegalovirus infection,” Am J Obstet Gynecol, vol. 213, no. 6, p. 896, Dec. 2015, doi: 10.1016/j.ajog.2015.09.034.
- A. Tovar-Salazar and A. Weinberg, “Understanding the mechanism of action of cytomegalovirus-induced regulatory T cells.,” Virology, vol. 547, pp. 1–6, Aug. 2020, doi: 10.1016/j.virol.2020.05.001.
- M. Salvany-Celades et al., “Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface,” Cell Rep, vol. 27, no. 9, pp. 2537-2547.e5, May 2019, doi: 10.1016/j.celrep.2019.04.109.
- D. P. Nguyen, S. Gerber, P. Hohlfeld, G. Sandrine, and S. S. Witkin, “Mycoplasma hominis in mid-trimester amniotic fluid: Relation to pregnancy outcome,” J Perinat Med, vol. 32, no. 4, 2004, doi: 10.1515/JPM.2004.060.
- F. M. Lozano et al., “Characterization of the Endometrial Microbiome in Patients with Recurrent Implantation Failure,” Microorganisms, vol. 11, no. 3, 2023, doi: 10.3390/microorganisms11030741.
- P. Pandiyan, N. Bhaskaran, M. Zou, E. Schneider, S. Jayaraman, and J. Huehn, “Microbiome dependent regulation of Tregs and Th17 cells in mucosa,” 2019. doi: 10.3389/fimmu.2019.00426.
- M. Dong et al., “Interactions between microbiota and cervical epithelial, immune, and mucus barrier,” 2023. doi: 10.3389/fcimb.2023.1124591.
- B. Qingqing, Z. Jie, Q. Songben, C. Juan, Z. Lei, and X. Mu, “Cervicovaginal microbiota dysbiosis correlates with HPV persistent infection,” Microb Pathog, vol. 152, 2021, doi: 10.1016/j.micpath.2020.104617.
- A. Mohd Zaki et al., “Neutrophils Dominate the Cervical Immune Cell Population in Pregnancy and Their Transcriptome Correlates With the Microbial Vaginal Environment,” Front Microbiol, vol. 13, 2022, doi: 10.3389/fmicb.2022.904451.
- F. Flaviani et al., “Cervicovaginal microbiota and metabolome predict preterm birth risk in an ethnically diverse cohort,” JCI Insight, vol. 6, no. 16, 2021, doi: 10.1172/jci.insight.149257.
- A. Oliver et al., “Cervicovaginal microbiome composition is associated with metabolic profiles in healthy pregnancy,” mBio, vol. 11, no. 4, 2020, doi: 10.1128/mBio.01851-20.
- N. Khan, W. Andrade, H. De Castro, A. Wright, D. Wright, and K. H. Nicolaides, “Impact of new definitions of pre-eclampsia on incidence and performance of first-trimester screening.,” Ultrasound Obstet Gynecol, vol. 55, no. 1, pp. 50–57, Jan. 2020, doi: 10.1002/uog.21867.
- A. Khalil, N. Suff, A. Grande, A. David, and A. Khalil, “OS078. Fetal growth restriction: A marker of severity of early-onset pre-eclampsia?,” Pregnancy Hypertens, vol. 2, no. 3, p. 220, Jul. 2012, doi: 10.1016/j.preghy.2012.04.079.
- K. Headen et al., “The Role of Regulatory T Cells and Their Therapeutic Potential in Hypertensive Disease of Pregnancy: A Literature Review,” Int J Mol Sci, vol. 25, no. 9, 2024.
- Y. Sasaki et al., “Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia.,” Clin Exp Immunol, vol. 149, no. 1, pp. 139–45, Jul. 2007, doi: 10.1111/j.1365-2249.2007.03397.x.
- J. R. Prins et al., “Preeclampsia is Associated with Lower Percentages of Regulatory T Cells in Maternal Blood,” Hypertens Pregnancy, vol. 28, no. 3, pp. 300–311, Jan. 2009, doi: 10.1080/10641950802601237.
- T. A. Buchanan, A. H. Xiang, and K. A. Page, “Gestational diabetes mellitus: risks and management during and after pregnancy,” Nat Rev Endocrinol, vol. 8, no. 11, pp. 639–649, Nov. 2012, doi: 10.1038/nrendo.2012.96.
- L. Schober et al., “The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus,” Clin Exp Immunol, vol. 177, no. 1, pp. 76–85, Jun. 2014, doi: 10.1111/cei.12300.
- J. F. Plows, J. L. Stanley, P. N. Baker, C. M. Reynolds, and M. H. Vickers, “The pathophysiology of gestational diabetes mellitus,” 2018. doi: 10.3390/ijms19113342.
- S. Sharma, S. Banerjee, P. M. Krueger, and S. M. Blois, “Immunobiology of Gestational Diabetes Mellitus in Post-Medawar Era,” 2022. doi: 10.3389/fimmu.2021.758267.
- N. Dekel, Y. Gnainsky, I. Granot, K. Racicot, and G. Mor, “The role of inflammation for a successful implantation.,” Am J Reprod Immunol, vol. 72, no. 2, pp. 141–7, Aug. 2014, doi: 10.1111/aji.12266.
- S. A. Robertson, A. S. Care, and L. M. Moldenhauer, “Regulatory T cells in embryo implantation and the immune response to pregnancy.,” J Clin Invest, vol. 128, no. 10, pp. 4224–4235, Oct. 2018, doi: 10.1172/JCI122182.
- C. López-Tinoco et al., “Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus.,” Cytokine, vol. 58, no. 1, pp. 14–9, Apr. 2012, doi: 10.1016/j.cyto.2011.12.004.
- J.-M. Atègbo et al., “Modulation of adipokines and cytokines in gestational diabetes and macrosomia.,” J Clin Endocrinol Metab, vol. 91, no. 10, pp. 4137–43, Oct. 2006, doi: 10.1210/jc.2006-0980.
- W. Wang et al., “Dynamic changes in regulatory T cells during normal pregnancy, recurrent pregnancy loss, and gestational diabetes,” J Reprod Immunol, vol. 150, p. 103492, Mar. 2022, doi: 10.1016/j.jri.2022.103492.
- Y. Zhao et al., “Immune checkpoint molecules on T cell subsets of pregnancies with preeclampsia and gestational diabetes mellitus,” J Reprod Immunol, vol. 142, p. 103208, Nov. 2020, doi: 10.1016/j.jri.2020.103208.
- Y. Yang et al., “Functional Defects of Regulatory T Cell Through Interleukin 10 Mediated Mechanism in the Induction of Gestational Diabetes Mellitus,” DNA Cell Biol, vol. 37, no. 3, pp. 278–285, Mar. 2018, doi: 10.1089/dna.2017.4005.
- H. Arain et al., “Regulatory T cells in the peripheral blood of women with gestational diabetes: a systematic review and meta-analysis.,” Front Immunol, vol. 14, p. 1226617, 2023, doi: 10.3389/fimmu.2023.1226617.
- S. Gee et al., “The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate,” Nat Immunol, vol. 22, no. 12, 2021, doi: 10.1038/s41590-021-01049-2.
- N. Nunez, L. Réot, and E. Menu, “Neonatal immune system ontogeny: The role of maternal microbiota and associated factors. how might the non-human primate model enlighten the path?,” 2021. doi: 10.3390/vaccines9060584.
- D. L. Gibbons et al., “Neonates harbour highly active γδ T cells with selective impairments in preterm infants,” Eur J Immunol, vol. 39, no. 7, 2009, doi: 10.1002/eji.200939222.
- A. Das et al., “Identifying immune signatures of sepsis to increase diagnostic accuracy in very preterm babies,” Nat Commun, vol. 15, no. 1, 2024, doi: 10.1038/s41467-023-44387-5.
- S. Kamdar et al., “Perinatal inflammation influences but does not arrest rapid immune development in preterm babies,” Nat Commun, vol. 11, no. 1, 2020, doi: 10.1038/s41467-020-14923-8.
- Y. Su et al., “Risk factors for necrotizing enterocolitis in neonates: A meta-analysis,” 2023. doi: 10.3389/fped.2022.1079894.
- J. G. Ginglen and N. Butki, “Necrotizing Enterocolitis,” StatPearls, Aug. 2023.
- C. Zozaya et al., “Incidence, Treatment, and Outcome Trends of Necrotizing Enterocolitis in Preterm Infants: A Multicenter Cohort Study,” Front Pediatr, vol. 8, 2020, doi: 10.3389/fped.2020.00188.
- I. Pacella et al., “Reduction in regulatory T cells in preterm newborns is associated with necrotizing enterocolitis,” Pediatr Res, vol. 94, no. 5, 2023, doi: 10.1038/s41390-023-02658-3.
- G. J. Neil and J. J. Volpe, “Encephalopathy of Prematurity,” in Volpe’s Neurology of the Newborn, Elsevier, 2018, pp. 425-457.e11. doi: 10.1016/B978-0-323-42876-7.00016-8.
- G. Bandoli, D. Suttner, E. Kiernan, R. J. Baer, L. Jelliffe-Pawlowski, and C. D. Chambers, “Risk factors for neonatal encephalopathy in late preterm and term singleton births in a large California birth cohort,” Journal of Perinatology, vol. 42, no. 3, pp. 341–347, Mar. 2022, doi: 10.1038/s41372-021-01242-z.
- D. Yang et al., “Blocking lymphocyte trafficking with FTY720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns,” Journal of Neuroscience, vol. 34, no. 49, 2014, doi: 10.1523/JNEUROSCI.2582-14.2014.
- J. Herz et al., “Peripheral T cell depletion by FTY720 exacerbates hypoxic-ischemic brain injury in neonatal mice,” Front Immunol, vol. 9, no. AUG, 2018, doi: 10.3389/fimmu.2018.01696.
- N. A. B. Taher et al., “Altered distributions and functions of natural killer T cells and γδ T cells in neonates with neonatal encephalopathy, in school-age children at follow-up, and in children with cerebral palsy,” J Neuroimmunol, vol. 356, p. 577597, Jul. 2021, doi: 10.1016/j.jneuroim.2021.577597.
- C. Raffin, L. T. Vo, and J. A. Bluestone, “Treg cell-based therapies: challenges and perspectives,” 2020. doi: 10.1038/s41577-019-0232-6.
- P. J. Eggenhuizen, B. H. Ng, and J. D. Ooi, “Treg enhancing therapies to treat autoimmune diseases,” 2020. doi: 10.3390/ijms21197015.
- N. Gomez-Lopez et al., “Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes,” Cell Rep, vol. 32, no. 1, 2020, doi: 10.1016/j.celrep.2020.107874.
- K. Smith-Jackson and R. A. Harrison, “Alternative pathway activation in pregnancy, a measured amount ‘complements’ a successful pregnancy, too much results in adverse events,” 2023. doi: 10.1111/imr.13169.
- C. Dudreuilh et al., “Can regulatory T cells improve outcomes of sensitised patients after HLA-Ab incompatible renal transplantation: study protocol for the Phase IIa GAMECHANgER-1 trial.,” BMC Nephrol, vol. 24, no. 1, p. 117, Apr. 2023, doi: 10.1186/s12882-023-03157-7.
- Y. Liu, J. J. Alookaran, and J. M. Rhoads, “Probiotics in autoimmune and inflammatory disorders,” 2018. doi: 10.3390/nu10101537.
- C. Mazziotta, M. Tognon, F. Martini, E. Torreggiani, and J. C. Rotondo, “Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health,” 2023. doi: 10.3390/cells12010184.
- J. Grev, M. Berg, and R. Soll, “Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants,” 2018. doi: 10.1002/14651858.CD012519.pub2.
- L. Krauss-Silva et al., “A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: Preliminary results,” Trials, vol. 12, 2011, doi: 10.1186/1745-6215-12-239.
- S. Yang, G. Reid, J. R. G. Challis, S. O. Kim, G. B. Gloor, and A. D. Bocking, “Is there a role for probiotics in the prevention of preterm birth?,” 2015. doi: 10.3389/fimmu.2015.00062.
- R. M. G. Falcon, R. M. U. Alcazar, A. V. Mondragon, E. G. Penserga, and O. A. G. Tantengco, “Rheumatoid arthritis and the risk of preterm birth,” American Journal of Reproductive Immunology, vol. 89, no. 3, Mar. 2023, doi: 10.1111/aji.13661.
- M. Wallenius, K. Å. Salvesen, A. K. Daltveit, and J. F. Skomsvoll, “Rheumatoid arthritis and outcomes in first and subsequent births based on data from a national birth registry.,” Acta Obstet Gynecol Scand, vol. 93, no. 3, pp. 302–7, Mar. 2014, doi: 10.1111/aogs.12324.
- C. J. F. Smith, F. Förger, G. Bandoli, and C. D. Chambers, “Factors Associated With Preterm Delivery Among Women With Rheumatoid Arthritis and Women With Juvenile Idiopathic Arthritis,” Arthritis Care Res (Hoboken), vol. 71, no. 8, pp. 1019–1027, Aug. 2019, doi: 10.1002/acr.23730.
- J. Kikuchi, M. Hashizume, Y. Kaneko, K. Yoshimoto, N. Nishina, and T. Takeuchi, “Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response.,” Arthritis Res Ther, vol. 17, no. 1, p. 10, Jan. 2015, doi: 10.1186/s13075-015-0526-4.
- A. Wakabayashi et al., “Targeting interleukin-6 receptor inhibits preterm delivery induced by inflammation,” Molecular Human Reproduction, no. 11, pp. 718–726, Nov. 2013.
- K. Nakajima, O. Watanabe, M. Mochizuki, A. Nakasone, N. Ishizuka, and A. Murashima, “Pregnancy outcomes after exposure to tocilizumab: A retrospective analysis of 61 patients in Japan,” Mod Rheumatol, vol. 26, no. 5, 2016, doi: 10.3109/14397595.2016.1147405.
- N. Konagai et al., “Safe use of tocilizumab in pregnant women with Takayasu arteritis: Three case studies,” 2023. doi: 10.1136/rmdopen-2023-002996.
- Organization of Teratology Information Specialists (OTIS), “Tocilizumab (Actemra®),” Mother To Baby , Dec. 2023.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).