1. Introduction
Kiwifruit (
Actinidia chinensis) is a popular and highly nutritious fruit with a unique flavor and aroma compounds [
1]. It is known for its high vitamin C content and a range of other health benefits [
2]. The main kiwifruit cultivar marketed in the world is ‘Hayward’ (
Actinidia chinensis var.
deliciosa). The introduction of new cultivars in the kiwifruit industry is confronted with significant challenges pertaining to the successful commercialization, ensuring that the product meets consumer preferences in terms of good taste and “ready to eat”, as well as being attractive appearance to the consumer [
3]. In Chile, kiwifruit exports are primarily concentrated in the ‘Hayward’ cultivar which constitutes 98% of the total exports. The remaining 2% are comprised of yellow-fleshed cultivars, including ‘Dori’, ‘Jintao’ (Jing Gold), ‘Soreli’, A19 (‘Enza Gold’) and ‘Kiss’ [
4]. Despite the strong dominance of the ‘Hayward’ cultivar in Chilean plantations, there is an increasing intertest in growing new cultivars, particularly those with yellow and red flesh [
5].
From the point of view of the consumer, one of the main characteristics of the commercial cultivars is the vibrant color of their flesh due to the accumulation of pigments including chlorophylls (green flesh), carotenoids (yellow flesh) and anthocyanins (red flesh). Adequate color development is therefore of commercial relevance, particularly for
A. chinensis var.
chinensis cultivars, which requires the breakdown of chlorophyll pigments (green) present in the flesh during fruit development to reveal the pigments associated with yellow or red flesh color, a process known as flesh degreening [
6]. In Hayward Kiwifruit (
Actinidia chinensis var.
deliciosa) flesh color remains green throughout fruit development and ripening. However, in the yellow-fleshed cultivars (
A. chinensis var.
chinensis) the color changes from green to yellow due to activation of the chlorophyll breakdown pathway and the transformation of plastid structure from chloroplast to chromoplast along with the unmasking the carotenoid pigments already present [
7]. The main increase in carotenoids, once chlorophyll degradation has been initiated, is attributed to beta-carotene concentration, under control of
LCY-β (lycopene beta cyclase) genes [
8]. Carotenoid pigment concentrations in yellow fleshed kiwifruit (
Actinidia chinensis var.
chinensis) and green fleshed kiwifruit (
Actinidia chinensis var.
deliciosa) can be similar even in ripe fruit [
9].
Changes in flesh color can occur on and off the vine [
10]. To achieve the commercially required color, it is necessary to harvest the fruit already degreened, or at least with a sufficient level of degreening to allow the process during postharvest [
11]. Initial studies performed in the yellow flesh kiwifruit ‘Hort16A’ showed an influence of harvest maturity and temperature regimens on the flesh degreening potential [
12,
13]. These studies showed that ‘ Hort16A’, when picked at the usual harvest maturity, achieved complete degreening postharvest if stored at storage temperatures between 10 °C and 15 °C. Flesh degreening was complete if fruit were harvested earlier than commercial harvest with an initial color of 107.9 ° hue and stored for 42 days at 15 °C. Subsequent studies on ‘Zesy003’ (Gold9) indicated complete degreening after 42 days storage at between 10 °C and 15 °C and with the same sigmoidal decrease in hue angle [
14]. Similar results were found with an early cultivar, ‘Dori’, harvested seven days before commercial maturity [
15]. Conversely, the late cultivar ‘Kiss’ (initially described as Y-374) exhibited a more complex color evolution during its development on the plant, indicating a slower progression in color change compared to other maturity parameters [
16]. Therefore, temperature and exposure time are key factors for achieving complete flesh degreening off the vine. While high degreening temperatures can lead to increased softening in yellow flesh cultivars, they also demonstrated a beneficial effect by reducing symptoms of chilling injury [
15,
17].
Studies of degreening in species other than kiwifruit showed complete inhibition of degreening at high temperatures. Peel degreening in Cavendish bananas was reduced at temperatures between 30 and 34 °C, compared to at 20 °C when degreening was complete [
18]. In a later study with bananas (
Musa acuminata cv. Cavendish,
AAA group) peel degreening was analyzed for treatments in which temperatures of 20 or 30 °C were alternated or continuous [
19]. Peel degreening was inhibited when the fruit was treated with continuous temperatures at 30 °C, although the changes triggered by the maturation process continued normally and degreening was completed at 20 °C. The optimal temperature range for banana degreening lies between 18 and 24 °C [
19]. The inhibitory effect of 30°C on degreening flesh has not been previously tested in kiwifruit, and it may serve as a means of elucidating the role of enzymes in the degreening process, particularly with consideration of the specific functions of chlorophyllase and magnesium dechelatase enzymes, closely associated with early chlorophyll degradation.
The genes involved in the enzymes of the biosynthesis and degradation pathway of chlorophyll are expressed in the green and yellow fleshed cultivars of kiwifruit [
20]. Chlorophyll degradation involves a series of enzymatic reactions that lead to the degradation of the chlorophyll molecule and the formation of colorless breakdown products. This process chlorophyll breakdown can be divided in an early phase occurring within the thylakoid membrane with the degradation of chlorophylls to simpler products, known as PAO/Phyllobilin pathway. Then a later phase in which these colorless chlorophyll catabolites are further modified and translocated from the chloroplast to the vacuole [
21]. Views differ on the specific enzymes responsible for triggering chlorophyll breakdown and on the role played by chlorophyllase in removing the phytol chain of the chlorophyll molecule through the formation of chlorophyllide as an intermediate compound [
21]. Additionally, studies indicate that magnesium dechelatase plays a pivotal role in initiating chlorophyll degradation by catalyzing the removal of the central magnesium atom. This step converts chlorophyll
a to pheophytin
a. leading to the formation of pheophytin which is considered a fundamental part of the breakdown process. In the PAO/ Phyllobilin pathway Chlorophyll
a is converted to Pheophytin
a and the production of the magnesium-free porphyrin ring, colorless Pheophorbide
a by Pheophorbide oxidase [
22,
23]. On the other hand, PAO enzyme (Pheophorbide
a oxygenase) participates in the conversion of pheophorbide
a to red chlorophyll colorless catabolite (RCCs), which are primarily associated with the cellular processes of senescing leaves [
23,
24]. Additionally, STAY GREEN (SGR) protein is involved in regulating the chlorophyll degradation process. Although it is not an enzyme itself, SGR plays a crucial role in modulating the activity of chlorophyll degradation enzymes and determining the timing of chlorophyll breakdown [
22].
The study of genes involved in kiwifruit flesh degreening in ‘Zesy003’ (yellow-fleshed) and Hayward (green fleshed) suggest that the expression of
SGR2 and
PAO1 genes account for the main differences in degreening among the yellow and green-fleshed kiwifruit cultivars [
14]. Both genes are described as components of the chlorophyll degradation pathway;
SGR, in particular, is proposed to encode magnesium dechelatase enzyme, responsible for the conversion chlorophyll
a to pheophytin
a by removing the central magnesium atom [
25]. In particular, SGR2 was found to be slightly upregulated in yellow fleshed kiwifruit [
20]. However, the information regarding these genes has yet to be fully elucidated at the protein level.
Our research aims to further clarify the degreening behaviors and to assess the enzymatic response of chlorophyllase and magnesium dechelatase considering different storage temperature regimes, including high temperature, in two contrasting kiwifruit cultivars: the yellow-fleshed cv. ‘Kiss’ and the green-fleshed cv. ‘Hayward’.
4. Discussion
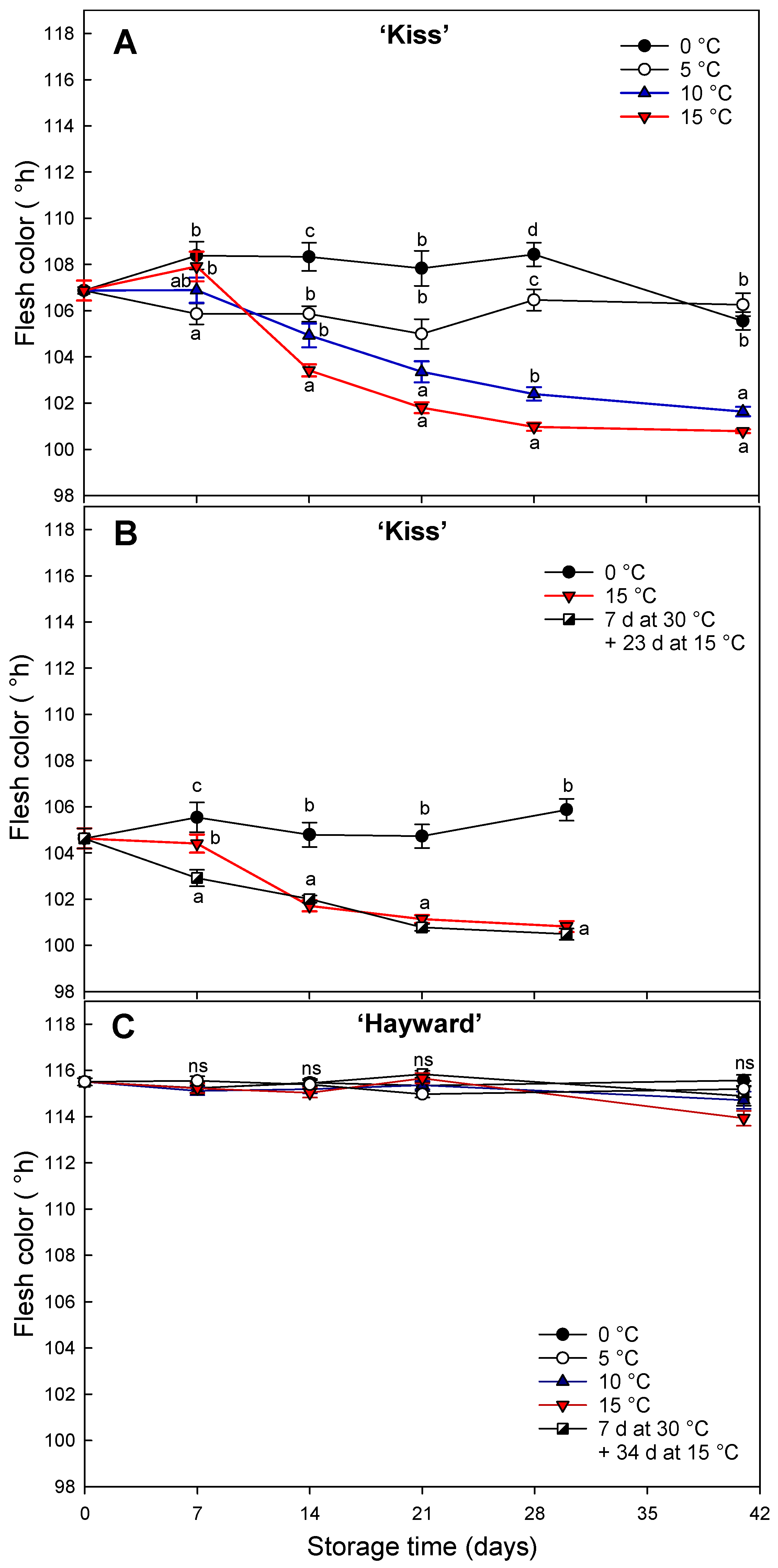
The kiwifruit cultivars ‘Kiss’ and ‘Hayward’ showed different degreening behaviors when exposed to 0, 5, 10 or 15 °C and a short exposure of seven days at 30 °C. Degreening of ‘Kiss’ kiwifruit was greater and faster at higher temperatures. The decrease in hue angle was sigmoidal, being most rapid in the first two weeks; a finding similar to that of Gambi et al. [
14] in cv. ’Zesy003’ that degreened almost completely after 30 d at 10 °C or 15 °C. The slightly later harvest resulted in a shortening of the sigmoidal pattern of hue angle decline while the slightly earlier harvest accentuated it [
13]. This suggests a need to determine the optimal harvest time, to achieve complete degreening off-vine as discussed for the yellow-flesh cv. ’Dori’ [
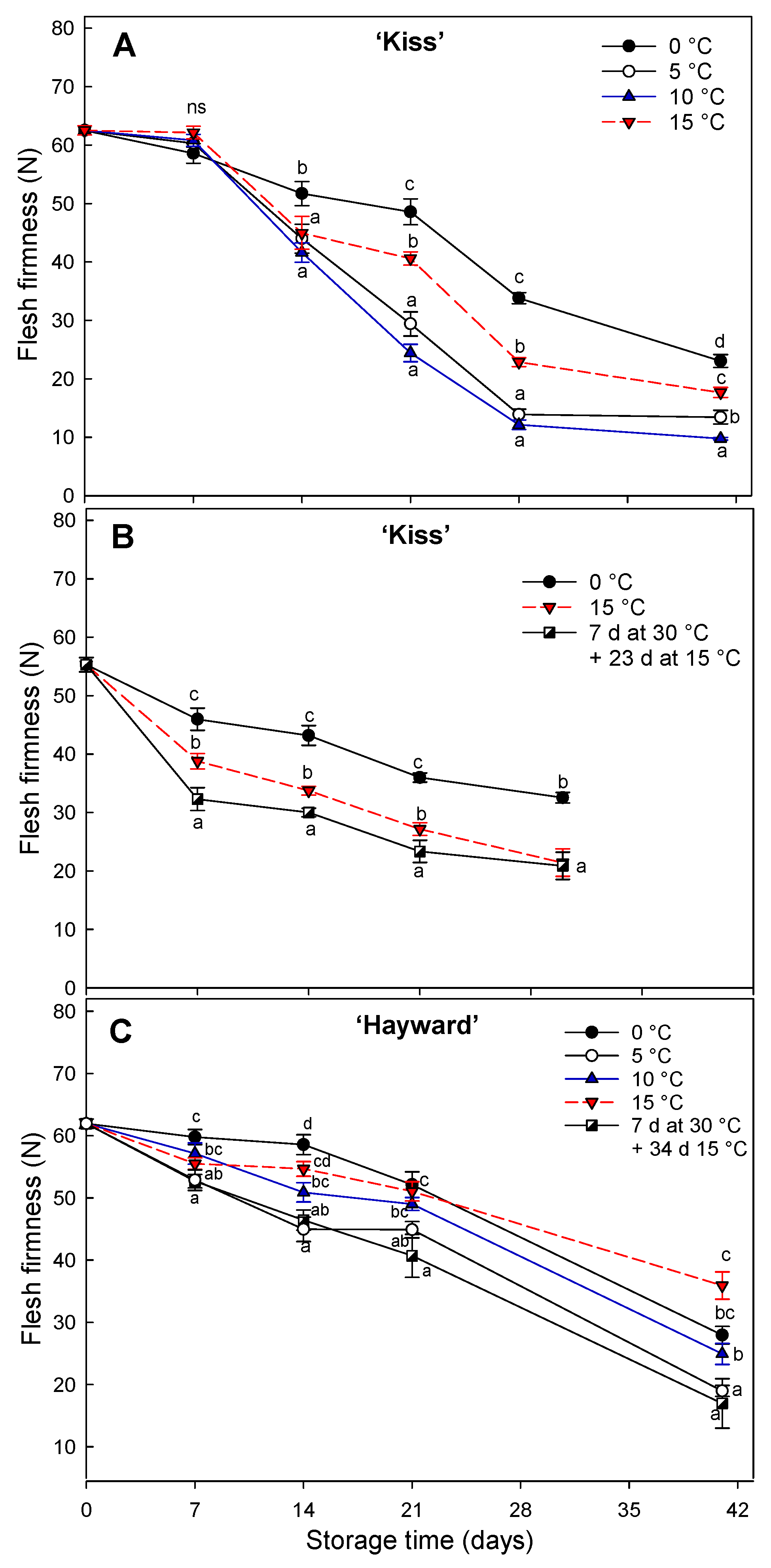
15]. Unlike other fruit types, fruit firmness in these two kiwifruit cultivars was not positively related to increases in storage temperature. This behavior has been noted previously [
33] suggesting that softening is regulated by low temperatures, independently of ethylene and where some chilling injury occurs at 0 °C. With fruit degreened at 15 °C softening more slowly than fruit degreened at 5 or 10 °C. Similar results have been reported for the kiwifruit cvs. ‘Hayward’ and ’Zesh004’ where fruit held at 16°C softened less than fruit held at temperatures lower than 10 °C [
34].
The high-temperature degreening responses studied here (7 d at 30 °C) have previously been reported in other species too including in bananas and lemons that require degreening of the peel. In bananas
Musa acuminata group AAA, use of high temperatures (above 25 °C) for more than 48 hours is associated with inhibition of chlorophyll breakdown, resulting in a retention of their green color that persists even when fruit temperature is subsequently dropped to 20°C [
18,
19,
27]. Similarly, with lemons at temperatures above 25 °C that inhibit chlorophyll breakdown resulting in incomplete degreening [
35]. In our study, high temperatures (7 d at 30 °C) did not affect negatively the degreening of the flesh but instead favored an initial rapid decline in the hue angle compared to the high values maintained when the fruit was stored continuously at 0 °C. However, this rapid decrease in hue angle was not persistent, resulting in a similar result as when stored continuously at 15 °C. This is probably because it was not supported by the production of internal ethylene.
Most cultivars of kiwifruit have green flesh early in development. In
A. deliciosa the green color persists to maturity. However,
A. chinensis the chlorophylls are degraded to reveal the underlying carotenoids and/or anthocyanins [
9,
36,
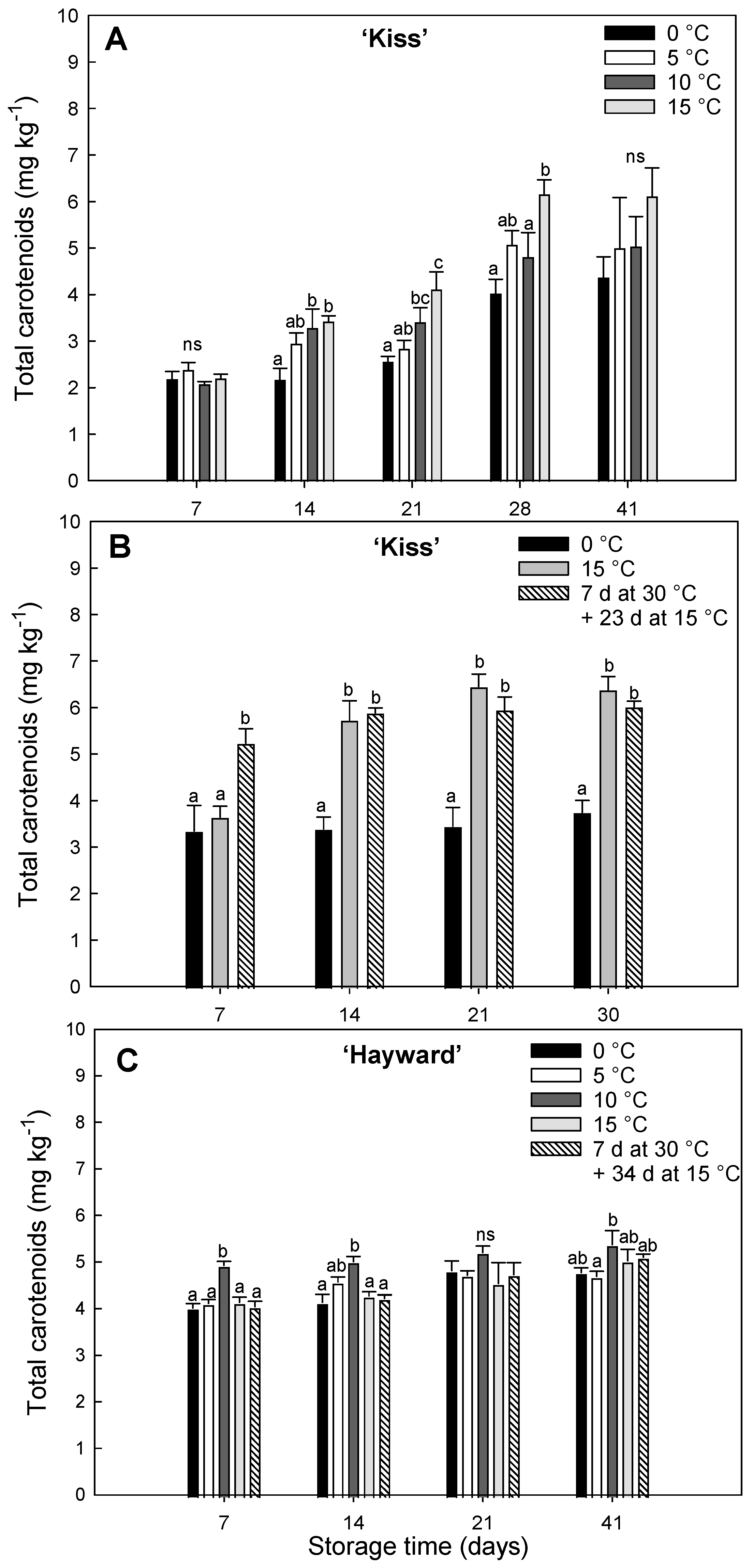
37]. The total carotenoid concentration in ‘Kiss’ was higher when fruit were degreened at higher temperature, with an increase during the first weeks of storage. According to Xia et al. [
29] in the yellow-fleshed cultivars ‘Jinshi 1’ and ‘Jinyan’, an increase in carotenoid concentration was detected after storage for 32 d at 20 °C with a rise in the expressions of genes associated with carotenoid metabolism, upregulating the synthetic gene
PSY (Phytoene synthase) and downregulating of the genes
CCD1 (carotenoid cleavage dioxygenase) and
NCED1 (9-cisepoxycarotenoid dioxygenase). Increases in total carotenoids have also been reported in other species, such as in satsuma mandarins (
Citrus unshiu Marc.) that show increases after prolonged exposure to 20 °C under ethylene-free conditions [
38]. This suggests the yellow flesh color in kiwifruit, in addition to being related to chlorophyll degradation under high temperature conditions, is also linked to increases in the synthesis of carotenoids [
29].
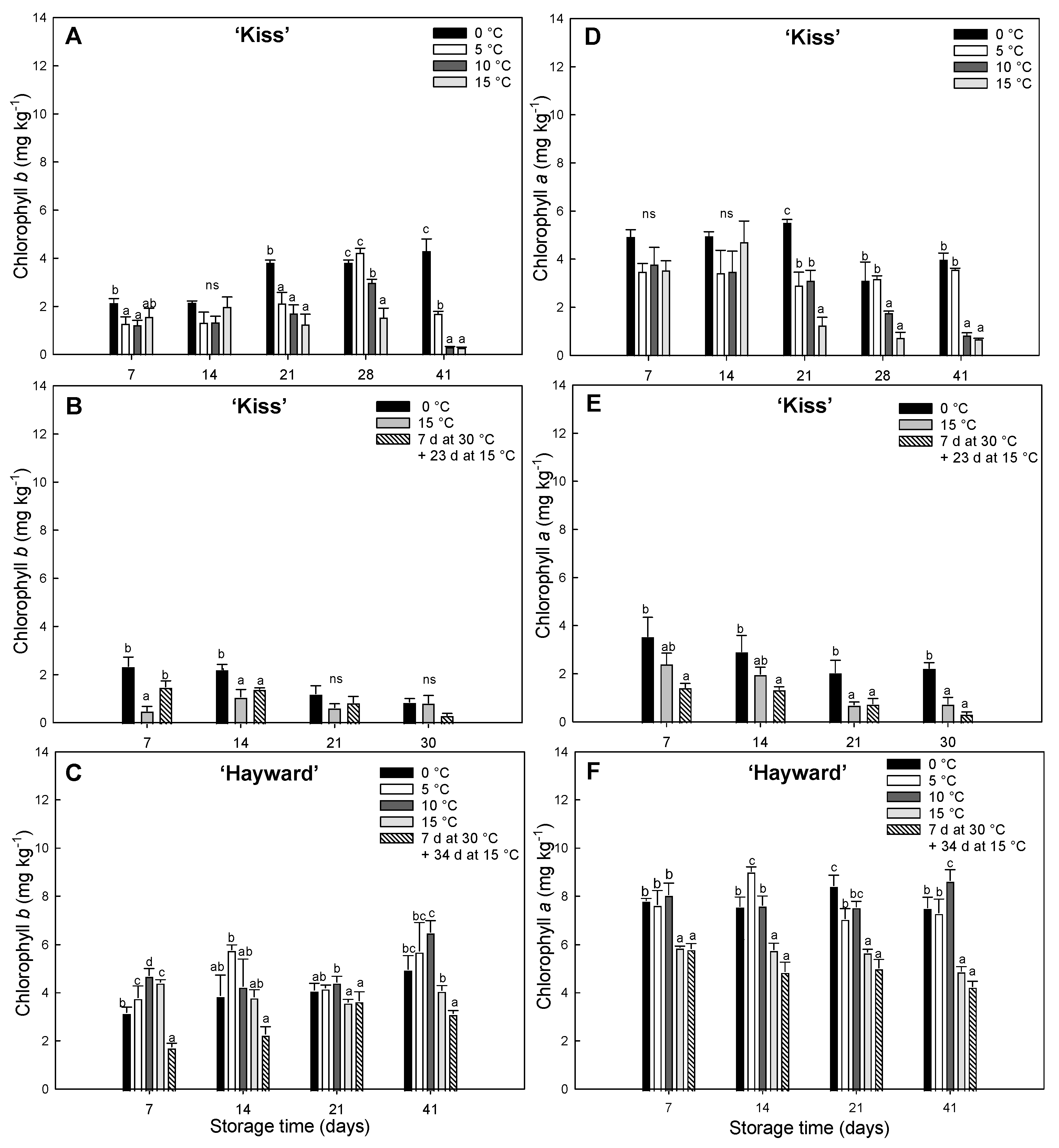
The degreening of ‘Kiss’ kiwifruit at high temperatures enhanced carotenoid synthesis and this was accompanied by decreases in chlorophyll. High temperature treatments at 15 °C (either continuously or with an initial 7 d at 30 °C) achieved the fastest decreases in chlorophyll, indicating an activation of the chlorophyll degradation pathway at these temperatures. At lower temperatures (0 and 5 °C) the high chlorophyll levels persisted until the end of the storage period. These were also accompanied by decreases in the chlorophyll
a/
b ratio, with higher concentrations of chlorophyll
b (Data not shown). There are also reports of chlorophyll retention in other species, such as
Arabidopsis thaliana and Broccoli (
Brassica oleracea L. var. italica) under specific conditions of light and temperature. The reaction of chlorophyll
b to chlorophyll
a is carried out by chlorophyllide reductase enzymes
a and
b; this is an early phase of chlorophyll degradation [
39].
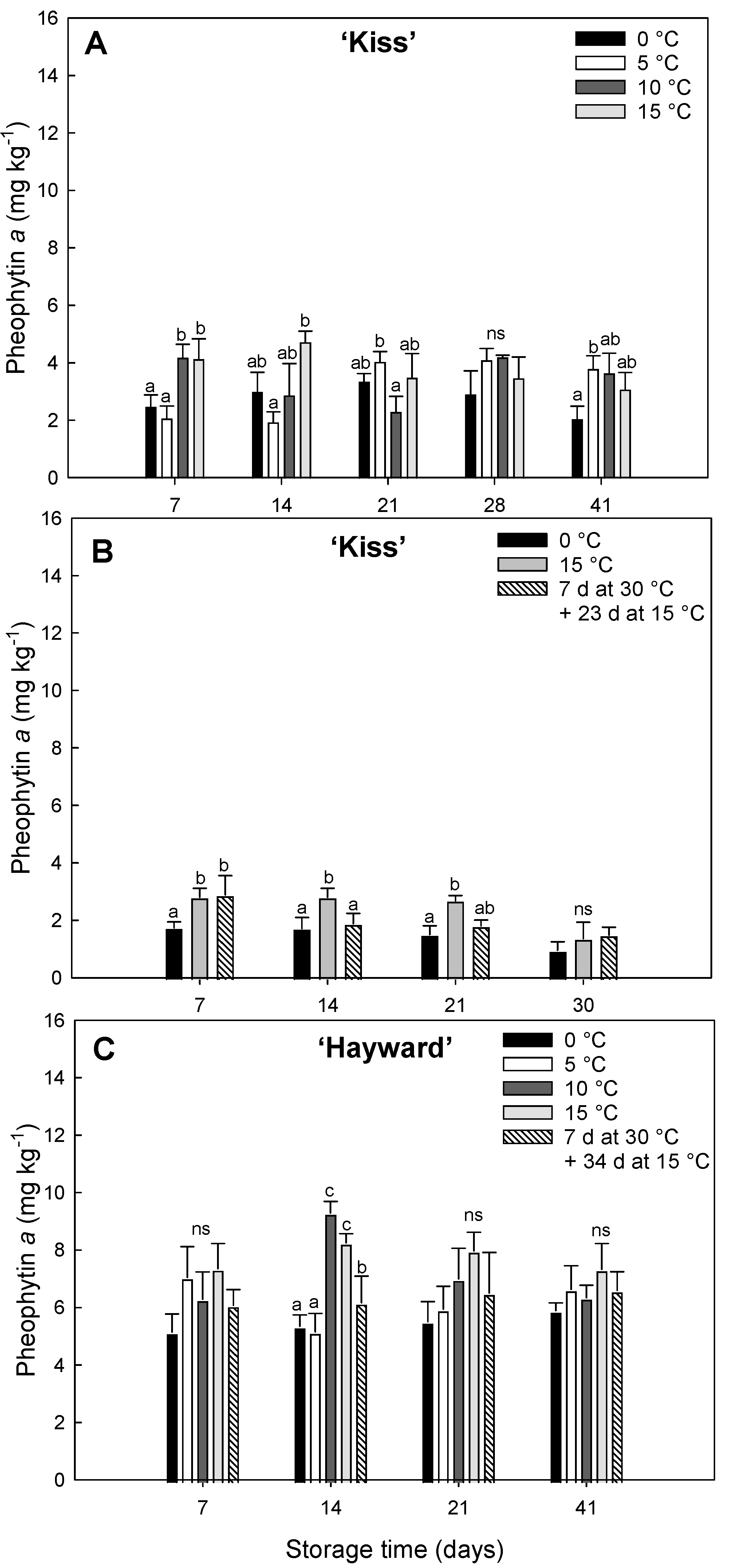
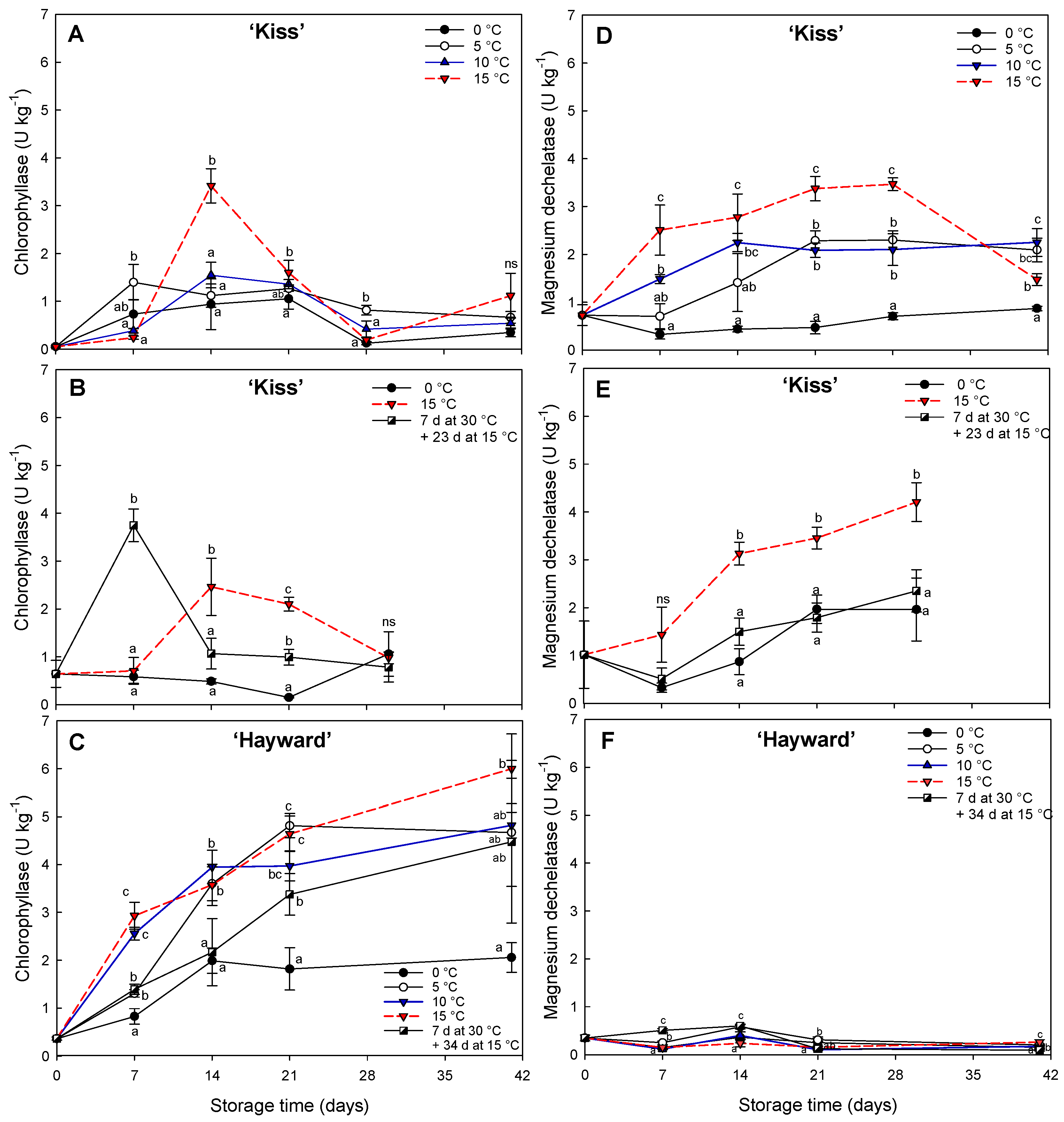
The relationships between the concentrations of different pigments and the activities of the enzymes associated with chlorophyll breakdown, provide information on the behavior of flesh degreening. Traditionally, the participation of chlorophyllase enzymes in the initial chlorophyll degradation process was described in the reaction of chlorophyll
a to pheophorbide
a through the generation of chlorophyllide intermediates [
21], in addition to enzymes with a magnesium dechelatase activity apparently involved in the direct generation of the chlorophyllide intermediates [
21]. The kiwifruit cv. ‘Kiss’ showed a transient increase in chlorophyllase activity at 15 °C at both 14 and 21 d at 15 °C. In contrast, magnesium dechelatase activity remained high during the period at temperatures higher than 0 °C but was diminished at low temperatures (0 °C) or when degreening at 15 °C was preceded by seven days at 30 °C. Interestingly, during the initial period of 7 d at 30 °C, a storage condition that stimulates the degreening process of the yellow-flesh kiwifruits, the change was associated mainly with chlorophyllase activity in conjunction with a low activity of magnesium dechelatase. This indicates there is a differential activity of enzymes related to chlorophyll degradation according to exposure to different temperatures. For example, in tropical fruit such as bananas (
Musa acuminata group AAA) and plantains (
Musa acuminata group ABB) the activity of enzymes associated with the degreening of the skin differ at different temperature ranges. In the case of bananas, the full peel degreening (hue less than 95 °) was achieved with temperatures between 15 and 20 °C and characterized by low chlorophyll content and accompanied by greater activity of magnesium dechelatase enzymes and chlorophyllase.
The opposite occurs at 30 °C where the green color persists and the concentration of chlorophylls and chlorophyllase activity are high but magnesium dechelatase remains low [
27]. In plantains the situation is reversed, presenting greater chlorophyllase activity at 20 °C and high magnesium dechelatase activity at 30 °C with fully degreening [
27]. This suggests both enzymes work together to stimulate chlorophyll degradation, with chlorophyllase playing a more prominent role during this particular stage. In ‘Hayward’, chlorophyllase activity was very high, in contrast with remarkable low activity of magnesium dechelatase enzyme despite maintaining the green color with high levels of chlorophylls present with a high chlorophyll
a/
b ratio. Similar results have been observed by Ghasemnezhad et al. [
40] in cv. ‘Hayward’ and cv. ’Tomua’, both green-fleshed kiwifruits.
Previous senescence studies in
Arabidopsis thaliana suggest that the chlorophyllase enzymes are not the only ones involved in the initial degradation of the chlorophyll
a molecule [
41,
42] postulating the existence of enzymes with magnesium dechelatase activity. The expression of
SGR (
STAY GREEN) genes has been described in regulate enzymes with magnesium dechelatase activity. Gamby et al. [
14] demonstrated that SGR gene expression was high in yellow-fleshed kiwifruit degreened at storage temperatures higher than conventional, and specifically with high expression at 15 °C and low expression in green flesh kiwifruit. Similarly in our work at enzyme level, the comparison with cv. ‘Hayward’, which retains its green flesh, with high concentrations of chlorophylls, high chlorophyllase activity and practically no magnesium dechelatase activity, suggests a central role for magnesium dechelatase enzymes in the degreening of the yellow-fleshed kiwifruit cultivars
5. Conclusions
Postharvest degreening at 15 °C of fruit harvested 14 or 21 d before the usual commercial harvest date, improves the development of flesh color in ‘Kiss’ kiwifruit, a process dependent on exposure time and temperature. This is different in ‘Hayward’ where it is not possible to induce the same process. This suggests the use of storage temperatures above 0 °C in yellow-fleshed kiwifruit offers the potential for enhanced degreening, with the possibility of complete degreening at continuous temperatures of 15 °C. The application of high temperatures (30°C) over a brief period time did not impede the flesh degreening process in the ‘Kiss’ kiwifruit. Nevertheless, the decline in the hue angle was faster, with the activity of the chlorophyllase enzyme prevailing over that the enzyme magnesium dechelatase (inhibited) then 7 d at 30°C. The findings substantiate the pivotal role of temperature in the degreening process.
According to the enzyme study at different temperature treatments, in the yellow-flesh kiwifruit, an increase in degreening temperature is associated with a higher activity of magnesium dechelatase, indicating its involvement in the breakdown of chlorophyll, mainly at 15 °C. In contrast, the chlorophyllase enzymes showed increased activities associated with exposure to very high temperatures (30 °C), thus triggering a much faster flesh degreening and associated with a drop in firmness. The coexistence of a high chlorophyll concentration, a high chlorophyllase activity and a low magnesium dechelatase activity observed in ‘Hayward’ in response to high storage temperatures supports the hypothesis that chlorophyllase enzymes are not the only enzymes involved in the process of chlorophyll breakdown and the development of the yellow flesh in the Actinidia chinensis cultivars.
Elucidating the enzymatic processes involved in chlorophyll degradation will improve our understanding of differences in degreening both on and off the vine among the different cultivars. This knowledge will contribute to the development of more effective degreening strategies to achieve the desired flesh color in a wide range of yellow-fleshed kiwifruit cultivars.
Figure 1.
Change of flesh color (hue angle, °h) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher’s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 1.
Change of flesh color (hue angle, °h) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher’s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 2.
Evolution of total carotenoid concentration (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 2.
Evolution of total carotenoid concentration (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 3.
Evolution of pigment concentrations of chlorophyll b (A, B, C) and chlorophyll a (D, E, F) (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B, D, E) and green-fleshed ‘Hayward’ (C, F) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 3.
Evolution of pigment concentrations of chlorophyll b (A, B, C) and chlorophyll a (D, E, F) (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B, D, E) and green-fleshed ‘Hayward’ (C, F) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 4.
Evolution of Pheophytin a concentration (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 4.
Evolution of Pheophytin a concentration (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 5.
Evolution of pigment concentrations of chlorophyll b (A, B, C) and chlorophyll a (D, E, F) (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B, D, E) and green-fleshed ‘Hayward’ (C, F) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 5.
Evolution of pigment concentrations of chlorophyll b (A, B, C) and chlorophyll a (D, E, F) (mg kg-1) in yellow-fleshed ‘Kiss’ at two harvest times (A, B, D, E) and green-fleshed ‘Hayward’ (C, F) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 6.
Change of flesh firmness (N) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Figure 6.
Change of flesh firmness (N) in yellow-fleshed ‘Kiss’ at two harvest times (A, B) and green-fleshed ‘Hayward’ (C) degreening at different range of times and temperatures. Error bars represent the standard error of the means of four replicates. Mean values with the same letter are not significantly different according to Fisher´s least significant differences (LSD) (P ≤ 0.05), ns: non-significant.
Table 1.
Maturity parameters of flesh color, flesh firmness, dry matter (DM), soluble solids concentration (SSC), and titratable acidity (TA) in yellow fleshed ‘Kiss’ and green fleshed ‘Hayward’ kiwifruit at harvest (days after full bloom, DAFB). Data presented are values of means ± standard deviation.
Table 1.
Maturity parameters of flesh color, flesh firmness, dry matter (DM), soluble solids concentration (SSC), and titratable acidity (TA) in yellow fleshed ‘Kiss’ and green fleshed ‘Hayward’ kiwifruit at harvest (days after full bloom, DAFB). Data presented are values of means ± standard deviation.
| Cultivar |
Harvest (DAFB) |
Flesh color (°h) |
Firmness (N) |
DM (%) |
SSC (%) |
TA (%) |
| Kiss |
158 |
106.9 ± 2.74 |
54.7 ± 6.80 |
17.3 ± 0.63 |
6.8 ±0.93 |
1.88 ± 0.05 |
| Kiss |
166 |
104.6 ± 2.76 |
54.6 ± 6.95 |
18.5 ± 0.75 |
7.3 ±0.65 |
1.88 ± 0.08 |
| Hayward |
161 |
115.5 ± 1.01 |
61.4 ± 4.71 |
16.6 ± 1.18 |
6.1 ±0.48 |
1.67 ±0.07 |
Table 2.
Pigment concentration of total carotenoids, chlorophyll b, chlorophyll a and pheophytin a in yellow-fleshed ‘Kiss’ and green-fleshed ‘Hayward’ at harvest (days after full bloom, DAFB). Data presented are values of means ± standard deviation.
Table 2.
Pigment concentration of total carotenoids, chlorophyll b, chlorophyll a and pheophytin a in yellow-fleshed ‘Kiss’ and green-fleshed ‘Hayward’ at harvest (days after full bloom, DAFB). Data presented are values of means ± standard deviation.
| Cultivar |
Harvest
(DAFB) |
Pigment concentration (mg kg-1) |
Total
carotenoids |
Chlorophyll b
|
Chlorophyll a
|
Pheophytin a
|
| Kiss |
158 |
2.13 ± 0.37 |
2.20 ± 0.16 |
5.34 ± 0.74 |
1.01 ± 0.84 |
| Kiss |
166 |
2.63 ±0.33 |
1.30 ± 0.28 |
3.97 ± 0.20 |
2.74 ± 1.24 |
| Hayward |
161 |
3.86 ± 0.12 |
4.39 ± 1.03 |
7.88 ± 1.08 |
6.19 ± 1.15 |