1. Introduction
Coumarin derivatives have been found in a variety of natural sources such as tonka beans, liquorice, and cassia cinnamon [
1]. These chemicals have been used in a broad array of consumer products including air fresheners due to the olfactory properties [
2]. Besides, derivatives of coumarin have often been synthesized to meet specific purposes. For example, 7-aminocoumarins, substituted at position 7 with an electron donating group, exhibit strong fluorescence and found a broad range of applications in various industrial and consumer products including dyes, optical brighteners, and fluorescent probes [
3]. However, 7-diethylamino-4-methylcoumarin (R-ADMC), one of the 7- aminocoumarins, has demonstrated the antiandrogenic potential in both in vitro and in vivo studies [
4,
5]. Due to the structural similarity, 7-(dimethylamino)-4-methylcoumarin (sublimation purified) (DACM) might also be a potent antiandrogenic compound.
A recent study has illustrated that wastewater treatment is continuously discharging R-ADMC and its transformers in the grams per day range into the Holtemme River, Germany, and the exposure concentrations of R-ADMC are in the low micrograms per liter range in the downstream of the river [
6]. However, little information is known regarding the occurrence, distribution and bioaccumulation of these chemicals in the aquatic ecosystem in China. In this work, we collected both abiotic (water and sediment) and biota (plants, plankton and fish) samples from Dongjiang River basin in southern China. By analyzing the two coumarins, R-ADMC and DACM, this work was purposed to: (1) determine the exposure levels of R-ADMC and DACM in the abiotic and biotic samples in the freshwater ecosystem; (2) compare bioaccumulation potentials of coumarins in the plant, plankton and fish; (3) estimate the potential dietary exposure doses of human exposure to coumarins through fish consumption.
2. Materials and Methods
2.1. Standards and Reagents
Two target analytes, 7-(dimethylamino)-4-methylcoumarin (sublimation purified) (DACM) and 7-diethylamino-4-methylcoumarin (sublimation purified) (R-ADMC), were analyzed in this study. Information regarding the physicochemical properties of the target compounds is shown in
Table S1. Internal standard linuron-d
6 was purchased from TRC (North York, Canada). DACM and R-ADMC were purchased from TCI (Shanghai, China). Purities of chemicals were greater than 97%. All the organic solvents used in the protocol were of HPLC-grade from Fisher Scientific (Massachusetts, USA). The Milli-Q water was generated using the Milli-Q system (Elga Veolia, High Wycombe, UK).
2.2. Sample Collection
Detailed information regarding sample collection has been well documented in earlier studies [
7,
8]. Briefly, a total of 208 samples, including 51 surface water samples, 32 sediment samples, and 123 biological samples (8 algae, 14 aquatic plants, 12 zooplankton, and 89 fish species), were collected during October to November 2020 from the Dongjiang (DJ) River as well as its tributaries including Xizhijiang (XZJ) River, Shima (SM) River, and Lijiang (LJ) River. All the biological samples were from the DJ River and transported to the laboratory on dry ice once collection. Prior to analysis, water samples were preserved at 4°C, while other samples were kept at -20°C.
A total of 89 fish muscle samples originated from 28 species of freshwater fish were collected, including: Coptodon zillii, Ctenogobius giurinus, Zacco platypus, Mastacembelus aculeatus, Vanmanenia pingchowensis, Hemicculter leucisculus, Channa argus, Culter alburnus, Acrossocheilus parallens, Squalidus argentatus, Oreochromis niloticus, Acrossocheilus beijiangensis, Pseudogobio vaillanti, Cyprinus carpio, Rhinogobius giurinus, Gastromyzoninae sp., Cirrhinus molitorella, Xenocypris argentea, Hypostomus plecostomus, Opsariichthys bidens, Vanmanenia stenosoma, Rhodeus sinensis, Eleotris oxycephala, Acheilognathus macropterus, Sarcocheilichthys nigripinnis, Carassius auratus, Squaliobarbus curriculus, and Puntius semifasciolatus. A total of 14 stem leaves, representing 7 species of herbaceous aquatic plants, were collected. The species of plants analyzed were Cyperus rotundus L., Water hyacinth, Miscanthus sinensis anderss, Panicum bisulcatum thunb, Wedelia trilobata, Celastrus orbiculatus, and Ludwigia octovalvis.
2.3. Sample Preparation
Surface Water. The protocol used for the surface water pretreatment was the same as those reported in earlier studies [
9,
10,
11]. Briefly, one liter of surface water was purified with the Oasis MCX
® SPE cartridges (500 mg, 6 ml). Firstly, glass fiber filter paper (1.2 μm × 55 mm, Whatman, Metstone, UK) was used to filter the particulate matter. Then 10 ng of Linuron-d
6 was added to the filtered water. Next, the SPE cartridge was cleaned with 6 ml of methanol and conditioned with 6 ml of milli-Q water. Afterwards, the filtered water passed through the SPE cartridge at a constant flow rate (5 mL/min) and dried under vacuum for 30 min. Then, the SPE extraction cartridge was eluted with 3 mL of methanol and 6 mL of acetonitrile. The extracts were combined and concentrated in a gentle stream of nitrogen. Five hundred microliter of methanol was used as reconstitution solvent and a 0.22 μm glass fiber filter (Shanghai Xinya Purification Equipment Co., Ltd., Shanghai, China) was used prior to instrumental analysis.
Sediment. Detailed information about the sediment extraction protocol has been described elsewhere [
12,
13]. Briefly, 80 mg of the freeze-dried sediment was accurately weighed and transferred to a 15 mL polypropylene (PP) tube to which 10 ng of Linuron-d
6 had been added. Subsequently, target compounds in the sample were extracted using 5 mL of methanol:water mixture (5:3, v/v). For the extraction, the sample was shaken for 60 min and then sonicated for 30 min. Then, the sample was centrifuged at 5000 rpm and the supernatant was separated and transferred to another clean 15 mL PP tube. This step was repeated twice, and the extracts were combined and concentrated to 3 ml under a gentle stream of nitrogen. Then, 10 mL of ultrapure water containing 0.2% formic acid (v/v) was added to the extracts and purified using an Oasis MCX
®SPE cartridge (500 mg, 6 mL). During the purification process, the cartridge was cleaned with 3 ml of methanol and 6 ml of acetonitrile and then conditioned with 10 ml of aqueous solution containing 0.2% formic acid. To ensure the cleanliness of the cartridge, 10 mL of ultrapure water containing 0.2% formic acid was used to clean the cartridge. After the cleaning was completed, the cartridge was dried under vacuum. Afterwards, the target compounds were eluted from the cartridge by 9 ml of methanol, and the eluate was concentrated to nearly dryness. Then, approximately 500 μL of methanol was used for reconstitution. A 0.22 μm filter paper was used to remove particles prior to instrumental analysis.
Aquatic biota. Detailed information regarding the analytical protocol of aquatic biota samples has been described elsewhere [
14,
15]. Firstly, lyophilized samples (algae, plant, zooplankton, and fish muscle samples) were pulverized and homogenized. Then, 50 mg of the sample was added to a 15 mL PP tube along with 10 μL of Linuron-d
6 (1.0 mg/L). Next, the sample was extracted with 5 mL of acetone and 2 ml of methanol:acetonitrile mixture (1:1, v/v), respectively. After centrifugation at 5000 x g for 15 min, the supernatants were combined and concentrated to nearly dryness in a mild nitrogen stream. For the reconstitution, a mixture of 500 μL of methanol:acetonitrile:water (3:3:4,v/v/v) was used and the extract was centrifuged at 5000 x g for 5 min prior to instrumental analysis.
2.4. Instrumental Analysis
Target analytes were determined in positive ion multiple reaction monitoring (MRM) mode using an ultra-high performance liquid chromatography interfaced with a tandem QTRAP 5500 (UPLC, SCIEX Technologies) triple quadrupole mass spectrometer (MS/MS, Agilent 1260). Detailed information regarding the MRM transitions and other associated mass spectrometry parameters is provided in
Table S2 in the supporting information. The ion source parameters were listed as follows: ionization voltage, 4500 V; curtain gas, 15 psi; spray gas, 55 psi; collision gas, 7 psi; auxiliary heated gas, 55 psi; ionization temperature, 500 °C. An Acquity UPLC HSS T3 column (1.8 μm, 2.1 mm × 100 mm, Waters) was used for the separation, with the column temperature at 40 °C. Mobile phases were water containing 0.1% formic acid (phase A) and methanol (phase B). Information on the gradient flow is provided in
Table S3 in the supporting information.
2.5. Quality Control and Quality Assurance
The quantification of coumarin in the samples was carried out with an internal standard method based on the response values of linuron-d
6. A 10-point calibration curve with a regression coefficient (r) > 0.99 was established for the target compounds over the concentration range of 0.01 ~ 10 ng/ml. In this work, the limit of quantification (LOQ) was calculated as 10 times the signal-to-noise ratio (S/N), which ranged from 0.003 and 0.006 ng/L for surface water, from 0.001 to 0.035 ng/g dry wt. for sediment, from 0.001 to 0.054 ng/g dry wt. for plants, from 0.002 to 0.174 ng/g dry wt for plankton, and from 0.008 to 0.041 ng/g dry wt. for fish muscle (
Table 1).
To monitor contamination arise from the glassware and reagents used in the analytical protocol, instrumental and procedural blanks were prepared within each batch of samples analyzed. In this study, the target analytes were not detected in any blank samples. The pre-extraction matrix spikes, pre-extraction matrix spike duplicates, and post-extraction matrix spikes were analyzed to determine the accuracy, recovery, and precision of the analytical protocol by fortifying 10 ng of each target analyte and passing them through the analytical method. Absolute recoveries of the two target analytes for each sample type were shown as follows: 63.0% in surface water; 50.6% to 68.3% in sediment; 94.1% to 98.0% in fish muscle; 93.5% to 95.0% in aquatic biota (plants, algae, and zooplankton).
2.6. Bioaccumulation Capacity Estimation
The bioaccumulation factor (BAF) was used to evaluate the bioaccumulation potential of analytes in aquatic organisms, which was calculated by the following equation [
16,
17]. Generally, BCFs ≥ 5000 are considered as “highly bioaccumulative”, ≥ 2000 considered as “bioaccumulative”, and ≥ 1000 considered to have significant bioaccumulation potential.
where
,
,
, and
represent the average concentration of the target analyte in fish, zooplankton, algae, and the bulk water, respectively. Notably, for the calculation of BAFs in fish muscle and other biological samples, dry weight normalized concentrations were used.
2.7. Human Exposure Risk Estimation
Freshwater fish has been an important dietary source for people living along the DJ River. To estimate the level of exposure to coumarin from fish intake by humans, the estimated dietary intake (EDI) was calculated using the following equation:
where
refers to the concentration of the target compounds in fish muscle (ng/g), DC represents the daily fish consumption rate (g/day), and BW represents the body weight (kg). For
, average and high exposure scenarios are evaluated based on mean and 95th percentile concentrations, respectively. The values of DC were obtained from the Nutrition Data Yearbook of the Chinese Centre for Disease Control and Prevention [
18]. The BW data of various age groups were obtained from the Chinese Population Exposure Parameters Manual [
19](
Table S4). In this work, an 80% moisture content value was used to convert dry weight-based concentrations to wet weight-based concentrations [
20,
21].
Further, the Hazard Quotient (HQ) was employed to assess the health risk of human beings exposed to these chemicals through fish ingestion, and the HQ was calculated with the following equation:
where ADI refers to the acceptable daily intake (ADI); HQ >1 indicates a potential health risk. Based on the data from the European Food Safety Authority, the ADI for coumarin is less than 0.07 mg/kg bw/day [
22,
23].
2.8. Statistical Analysis
Peak integration and quantification were performed using Sciex OS software version 2.2.0.5738 (Applied Biosystems, Foster City, CA). Statistical analyses were performed using Microsoft Excel 2016 and SPSS version 27.0. To facilitate the calculation of basic descriptive statistics, those values below the LOQ were replaced with LOQ/√2. The data normality was investigated using the Kolmogorov-Smirnov test. One-way ANOVA was used to assess the differences between groups of data following normal distribution; otherwise, the Mann-Whitney U test was used. Pearson correlation coefficient test was used for investigating the correlation between groups of data when the data were distributed normally; otherwise, the Spearman rank correlation coefficient was used. Statistical significance was determined at a significance level of p<0.05.
3. Results and Discussions
3.1. Coumarin and the Metabolite in Abiotic Samples
Surface water. DACM was not detected in any water samples, however, R-ADMC was detected in all samples except those from LJ River, in which the detection rate (DR) is 31.4%. The median concentration of R-ADMC was 0.105 ng/L, with the highest concentration found at 0.309 ng/L in XZJ River (
Table 1), which is around three orders of magnitude lower than that measured in the effluent of the Silstedt Wastewater Treatment Plant (0.5 μg/L) [
2]. However, in an earlier study performed in 2003, R-ADMC was not observed in the surface waters in northern Italy [
24].
Sediment. DACM was detected in 12 samples with a DR of 37.5%; R-ADMC was detected in 19 samples with a DR of 59.4% (
Table 1). The highest and median concentrations of DACM were 0.668 and 0.189 ng/g dry wt., respectively; the highest and median concentrations of R-ADMC were 0.173 and 0.012 ng/g dry wt., respectively.
3.2. Coumarin and the Metabolite in Aquatic Biota
Plant. Both DACM and R-ADMC were detected in 14.3% of plant samples (
Table 1). Median concentrations of DACM and R-ADMC in plant samples were 0.421 and 0.051 ng/g dry wt., respectively. The highest concentration of DACM was found in xx at 0.781 ng/g dry wt., and the highest concentration of R-ADMC was observed in xx at 0.096 ng/g dry wt.
Alage. DACM and R-ADMC were found in the algae samples with DRs being 75% and 62.5%, respectively (
Table 1). Median concentrations of DACM and R-ADMC in algae samples were 0.832 and 0.009 ng/g dry wt., respectively. The highest concentration of DACM was found in xx at 1.517 ng/g dry wt., and the highest concentration of R-ADMC was observed in xx at 0.031 ng/g dry wt.
Zooplankton. Both DACM and R-ADMC were detected in 58.3% of zooplankton samples (
Table 1). Median concentrations of DACM and R-ADMC were 0.798 and 0.008 ng/g dry wt., respectively. The highest concentration of DACM was found in xx at 1.712 ng/g dry wt., and the highest concentration of R-ADMC was observed in xx at 0.118 ng/g dry wt.
Fish. DACM and R-ADMC were found in fish muscle with DRs being 30.3% and 50.6%, respectively (
Table 1). Median concentrations of DACM and R-ADMC were 0.335 and 0.181 ng/g dry wt., respectively. The highest concentration of DACM was found in
Zacco platypus at 1.574 ng/g dry wt., and the highest concentration of R-ADMC was observed in
Coptodon zillii at 0.842 ng/g dry wt.
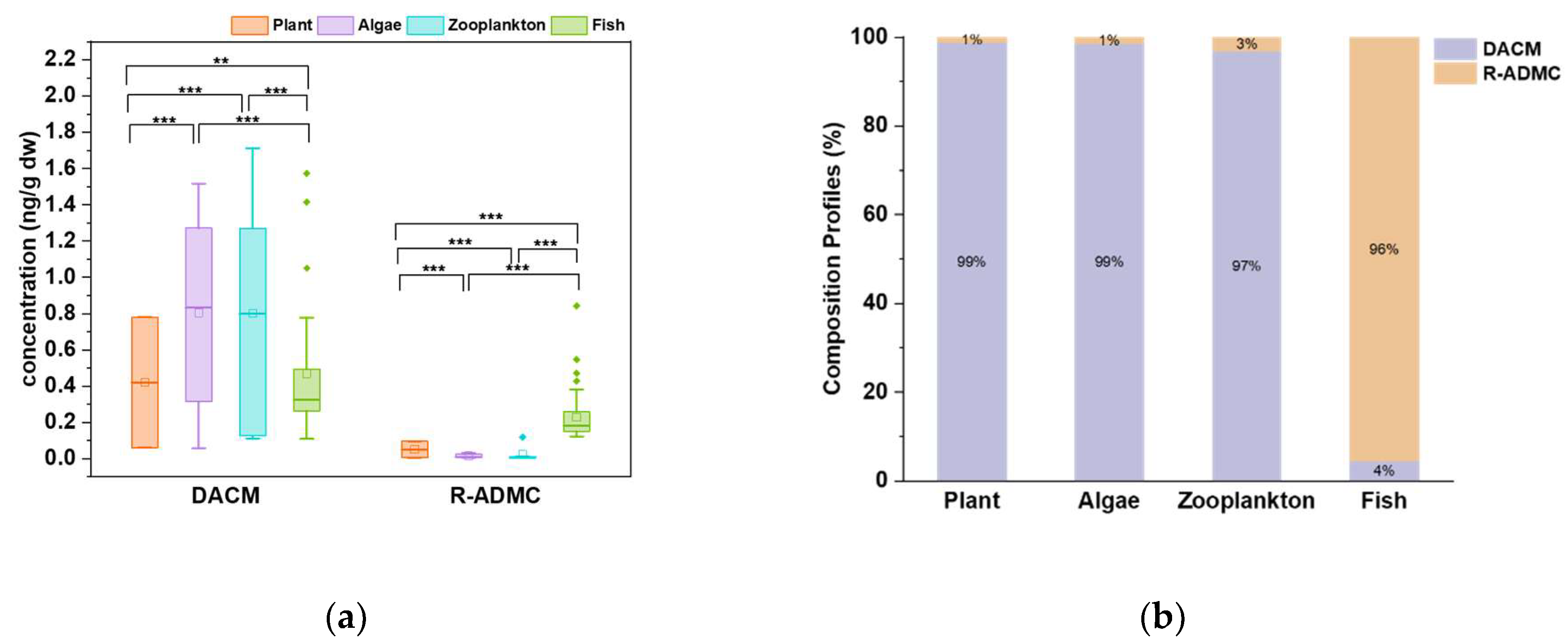
Overall, notable concentrations of DACM and its isomer R-ADMC were observed in the aquatic biotic samples analyzed in this study. Specifically, as shown in
Figure 1A, DACM is the predominant chemical found in the aquatic biota, contributing to over 97% total concentrations observed in the plant, algae, and zooplankton samples, while in fish muscle, the contributing percent ratio was 65%. We further compared the concentrations of the two chemicals in different types of samples, and the results were shown in
Figure 1B. For DACM, the concentrations in the algae, zooplankton and fish muscle samples were significantly higher than those observed in the plant samples. And the concentrations of DACM in the fish muscle were significantly lower than those found in the algae and zooplankton samples. However, for R-ADMC, the concentrations in the fish muscle were significantly higher than those detected in the plant, algae, and zooplankton samples.
3.3. Bioaccumulation of Coumarins in Aquatic Biota
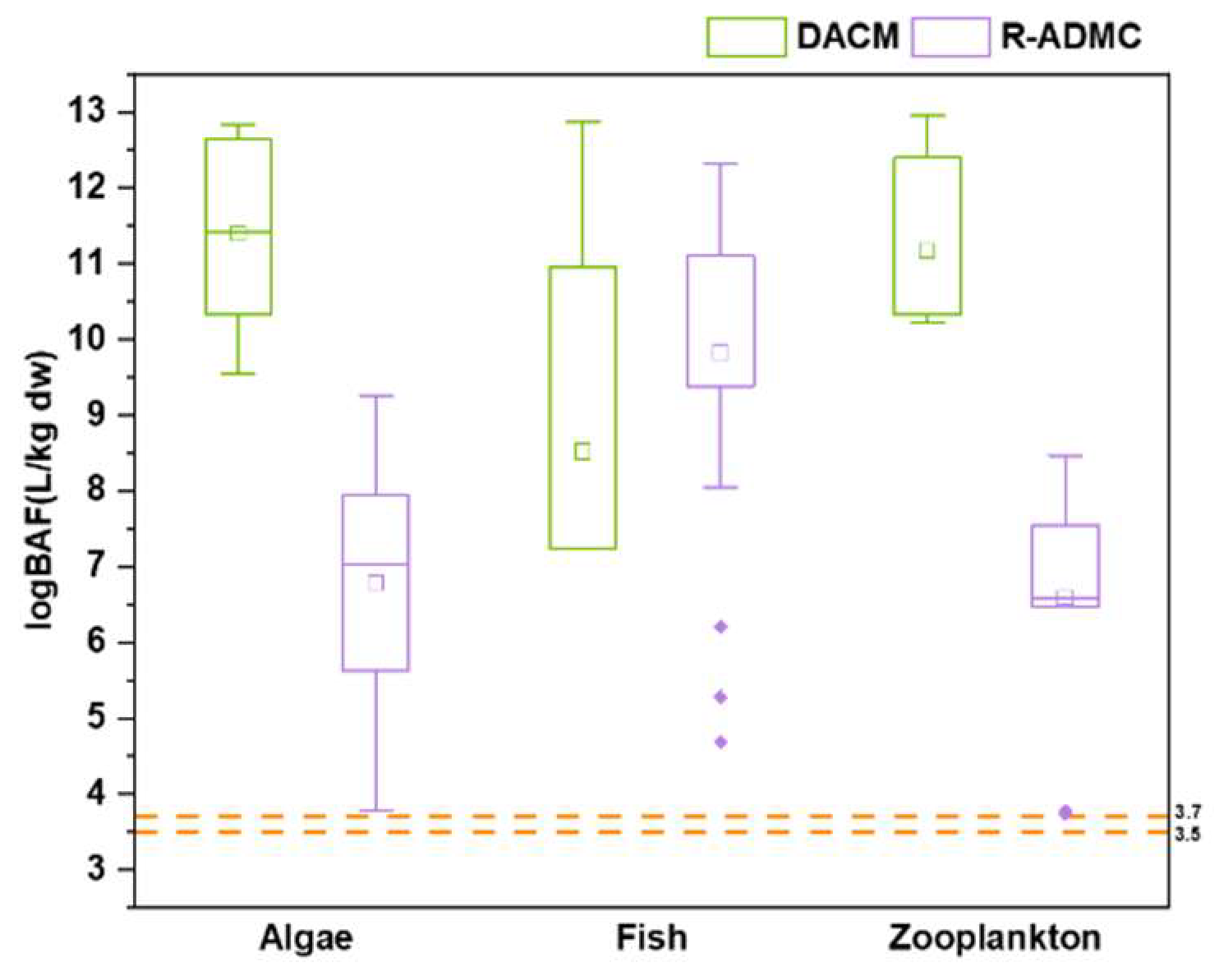
To further explore the bioconcentration capacity of coumarins in aquatic organisms, logBAF (L/kg) was calculated for algae, zooplankton, and fish based on the mean concentrations of these substances. Notably, the dry weight normalized concentrations were used in the calculation, which might lead to an overestimate of the actual values, while fish muscle-based concentrations may underestimate the real values. Thus, BAF values calculated in this study can be considered as rough estimates. BAF values between 2000 and 5000 (3.3<logBAF<3.7) were considered “bioaccumulative”, while BAF values above 5000 (logBAF>3.7) were considered “very bioaccumulative”. In both algae and zooplankton, the logBAFs of DACM (9.41and 11.2 L/kg, respectively) were higher than those calculated for R-ADMC (5.04 and 6.59 L/kg, respectively). In fish, the logBAF of DACM (8.52 L/kg) was lower than that of R-ADMC (9.83 L/kg) (
Figure 2). Overall, the logBAF values of both coumarins in all types of aquatic organisms were higher than 3.7, indicating that both DACM and R-ADMC are readily bio accumulative in aquatic biota.
3.4. Estimated Daily Intake of Coumarins through Fish Ingestion
To calculate the EDI more precisely, we divided the population into two broad categories, urban and rural, and on this basis further divided each group into three subgroups: young children (2-5 years), children and teenagers (6-17 years), and adults (≥18 years). Daily fish intake amount was obtained from the Chinese Center for Disease Control and Prevention (CDC) Nutrition Data Yearbook. It needs to note that wet weight-based concentrations were estimated from dry weight-based concentrations when performing EDI calculations. The estimated daily intakes of coumarins for different groups in China are presented in
Table 2. It was observed that urban toddlers had the highest mean and 95th percentile of daily intake of DACM (0.14 and 0.78 ng/kg bw/day, respectively), followed by urban children and teenagers (0.08 and 0.44, respectively), urban adults (0.07 and 0.36, respectively), rural young children (0.05 and 0.26, respectively), and rural adults (0.04 and 0.19, respectively). The daily intake of R-ADMC had the same distribution pattern as that of DACM, with the highest mean and 95th percentile daily intake of 0.13 and 0.41 ng/kg bw/day, respectively, among urban toddlers, followed by urban children and adolescents (0.07 and 0.23, respectively), and urban adults (0.06 and 0.19, respectively), rural young children (0.04 and 0.13, respectively) and rural adults (0.03 and 0.10, respectively). The mean and 95th percentiles of the EDI were higher for urban residents than for rural residents in all age groups. No significant difference between the exposure doses of DACM and R-ADMC was observed at average exposure levels. However, at high exposure levels, exposure dose of DACM was about twice higher than that of R-ADMC in all age groups.
To further assess the risk of human exposure to coumarins from fish intake, HQ values were calculated. The results showed that the HQs for both DACM and R-ADMC were less than 1, ranging from 4.33E-08 to 1.11E-06 and 3.86E-08 to 5.79E-07, respectively. Therefore, oral intake of freshwater fish from the Dongjiang River by residents does not pose a significant risk for coumarin exposure.
4. Conclusion
In this paper, concentrations of two coumarins were measured, for the first time, in both abiotic (water and sediment) and biotic (plankton, plant and fish) samples collected from a freshwater ecosystem. Among the matrices analyzed, different distribution patterns were observed for the two coumarins. DACM was not detected in the surface water, however, it was detected in around 40% sediment samples with the highest concentration being 0.668 ng/g dry wt. Higher concentration of R-ADMC was observed in surface water than sediment samples, suggesting that R-ADMC are readily distributed in water. Coumarins were also detected in biological samples, with algae and zooplankton samples showing higher concentrations than fish for DACM, and for R-ADMC, the highest concentration was observed in the fish muscle samples. We calculated bioconcentration factors (BAFs) for the target compounds, and the results indicate potential bioaccumulation properties for aquatic organisms. Externally, we assessed the potential human exposure risk of coumarins through consumption of freshwater fish, both posing negligible risk.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1: The physicochemical properties of the target analytes measured in this study (predicted using the US Environmental Protection Agency’s EPI Suite™); Table S2: Retention time, MRM transition and mass spectrometric parameters of coumarins; Table S3: Major parameters for the gradient flow used in the separation of coumarins; Table S4: Quality assurance/quality control (QA/QC) information of coumarins in each matrix.
Author Contributions
Conceptualization, Y.L.; methodology, Y.L. and Y.L.; software, Y.H.; validation, Y.H., R.C. and R.P.; formal analysis, S.Z.; investigation, Y.L. and G.Y.; resources, Y.L.; data curation, Y.L.; writing—original draft preparation, Y.L. and Y.L.; writing—review and editing, Y.L. and G.Y.; visualization, Y.H.; supervision, Y.L.; project administration, R.C.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52200188.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Muschket, M.; Brack, W.; Inostroza, P.A.; Beckers, L.; Schulze, T.; Krauss, M. Sources and Fate of the Antiandrogenic Fluorescent Dye 4-Methyl-7-Diethylaminocoumarin in Small River Systems. Environ. Toxicol. Chem. 2021, 40, 3078–3091. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Fukagawa, T.; Kohtani, S.; Kitoh, S.; Kunimoto, K.-K.; Nakagaki, R. Synthesis, Absorption, and Fluorescence Properties and Crystal Structures of 7-Aminocoumarin Derivatives. J. Photochem. Photobiol. Chem. 2007, 188, 378–386. [Google Scholar] [CrossRef]
- Di Paolo, C.; Kirchner, K.; Balk, F.G.P.; Muschket, M.; Brack, W.; Hollert, H.; Seiler, T.-B. Downscaling Procedures Reduce Chemical Use in Androgen Receptor Reporter Gene Assay. Sci. Total Environ. 2016, 571, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Sébillot, A.; Damdimopoulou, P.; Ogino, Y.; Spirhanzlova, P.; Miyagawa, S.; Du Pasquier, D.; Mouatassim, N.; Iguchi, T.; Lemkine, G.F.; Demeneix, B.A.; et al. Rapid Fluorescent Detection of (Anti)Androgens with Spiggin-Gfp Medaka. Environ. Sci. Technol. 2014, 48, 10919–10928. [Google Scholar] [CrossRef] [PubMed]
- Muschket, M.; Di Paolo, C.; Tindall, A.J.; Touak, G.; Phan, A.; Krauss, M.; Kirchner, K.; Seiler, T.-B.; Hollert, H.; Brack, W. Identification of Unknown Antiandrogenic Compounds in Surface Waters by Effect-Directed Analysis (EDA) Using a Parallel Fractionation Approach. Environ. Sci. Technol. 2018, 52, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, D.; Chen, H.; Cai, Y.; Liu, Y.; Guo, F.; Li, F.; Zhang, Y.; Xu, Z.; Xue, J.; et al. Distribution, Bioaccumulation and Human Exposure Risk of Bisphenol Analogues, Bisphenol A Diglycidyl Ether and Its Derivatives in the Dongjiang River Basin, South China. Sci. Total Environ. 2024, 952, 175969. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.; Liu, Y.; Lan, Y.; Zhu, J.; Cai, Y.; Guo, F.; Li, F.; Zhang, Y.; Zhang, T.; et al. Evidence of Strobilurin Fungicides and Their Metabolites in Dongjiang River Ecosystem, Southern China: Bioaccumulation and Ecological Risks. Sci. Total Environ. 2024, 908, 168427. [Google Scholar] [CrossRef] [PubMed]
- Smalling, K.L.; Orlando, J.L.; Calhoun, D.; Battaglin, W.A.; Kuivila, K. Occurrence of Pesticides in Water and Sediment Collected from Amphibian Habitats Located throughout the United States, 2009-10; U.S. Geological Survey, 2012.
- Montagner, C.C.; Vidal, C.; Acayaba, R.D.; Jardim, W.F.; Jardim, I.C.S.F.; Umbuzeiro, G.A. Trace Analysis of Pesticides and an Assessment of Their Occurrence in Surface and Drinking Waters from the State of São Paulo (Brazil). Anal Methods 2014, 6, 6668–6677. [Google Scholar] [CrossRef]
- Liu, J.; Xia, W.; Wan, Y.; Xu, S. Azole and Strobilurin Fungicides in Source, Treated, and Tap Water from Wuhan, Central China: Assessment of Human Exposure Potential. Sci. Total Environ. 2021, 801, 149733. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Lee, S.; Moon, H.-B.; Yamashita, N.; Kannan, K. Parabens in Sediment and Sewage Sludge from the United States, Japan, and Korea: Spatial Distribution and Temporal Trends. Environ. Sci. Technol. 2013, 47, 10895–10902. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, Y.; Jiang, Y.; Xia, W.; He, Z.; Xu, S. Occurrence of Azole and Strobilurin Fungicides in Indoor Dust from Three Cities of China. Environ. Pollut. 2022, 304, 119168. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Xue, J.; Liu, W.; Adams, D.H.; Kannan, K. Trophic Magnification of Parabens and Their Metabolites in a Subtropical Marine Food Web. Environ. Sci. Technol. 2017, 51, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.; Li, T.; Li, X.; Huang, X.; Gao, Y.; Li, B.; Lin, J.; Mu, W. Determination of Pyraclostrobin Dynamic Residual Distribution in Tilapia Tissues by UPLC-MS/MS under Acute Toxicity Conditions. Ecotoxicol. Environ. Saf. 2020, 206, 111182. [Google Scholar] [CrossRef] [PubMed]
- Liu,, D.; Wu, S.; Xu, H.; Zhang, Q.; Zhang, S.; Shi, L.; Yao, C.; Liu, Y.; Cheng, J. Distribution and Bioaccumulation of Endocrine Disrupting Chemicals in Water, Sediment and Fishes in a Shallow Chinese Freshwater Lake: Implications for Ecological and Human Health Risks. Ecotoxicol. Environ. Saf. 2017, 140, 222–229. [Google Scholar] [CrossRef]
- Miller, M.E.; Motti, C.A.; Hamann, M.; Kroon, F.J. Assessment of Microplastic Bioconcentration, Bioaccumulation and Biomagnification in a Simple Coral Reef Food Web. Sci. Total Environ. 2023, 858, 159615. [Google Scholar] [CrossRef] [PubMed]
- CDC Nutrition Data Yearbook. Available online: https://www.chinanutri.cn/sjnj/ (accessed on 8 October 2024).
- Zhao, X.; Duan, X.; Wang, B.; Cao, S. Environmental Exposure Related Activity Patterns Survey of Chinese Population (Children). China Environmental Science Press, Beijing. Available online: https://scholar.google.com/scholar_lookup?title=Environmental%20Exposure%20Related%20Activity%20Patterns%20Survey%20of%20Chinese%20Population%20(Children)&author=X.%20Zhao&publication_year=2016 (accessed on 8 October 2024).
- Bayen, S.; Gong, Y.; Chin, H.S.; Lee, H.K.; Leong, Y.E.; Obbard, J.P. Androgenic and Estrogenic Response of Green Mussel Extracts from Singapore’s Coastal Environment Using a Human Cell-Based Bioassay. Environ. Health Perspect. 2004, 112, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kannan, K. Temporal Trends of Parabens and Their Metabolites in Mollusks from the Chinese Bohai Sea during 2006–2015: Species-Specific Accumulation and Implications for Human Exposure. Environ. Sci. Technol. 2018, 52, 9045–9055. [Google Scholar] [CrossRef] [PubMed]
- EFSA Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Coumarin. | EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/104 (accessed on 8 October 2024).
- EFSA Coumarin in Flavourings and Other Food Ingredients with Flavouring Properties - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) | EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/793 (accessed on 8 October 2024).
- Loos, R.; Hanke, G.; Eisenreich, S.J. Multi-Component Analysis of Polar Water Pollutants Using Sequential Solid-Phase Extraction Followed by LC-ESI-MS. J. Environ. Monit. 2003, 5, 384. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).