1. Introduction
Osteoporosis, a subclinical condition, is the most common metabolic bone disease globally, characterized by an increased risk of fractures [
1,
2]. The osteoporosis is fundamentally attributed to imbalance in bone remodeling, resulting from the bone resorption function of osteoclasts exceeding the bone formation function of osteoblasts [
3,
4,
5]. There are over 8.9 million osteoporosis-related fractures worldwide [
6]. Over 33% of women and 10% of men over 50 years will experience at least one osteoporosis-related fracture in their lifetime [
7]. With the aging of the population, osteoporosis has become a public chronic disease, with associated fractures, high disability rates, and mortality rates, thereby increasing social and economic burdens [
8]. Moreover, an estimated 80% to 90% of adults with osteoporosis do not receive adequate treatment even during the secondary prevention phase, indicating a notable deficiency in the primary prevention of osteoporosis [
9]. Therefore, elucidating the novel pathogenic mechanisms of osteoporosis may provide promising therapeutic approach for the clinical treatment.
Oxygen is an essential factor for bone growth, development, and metabolism. Hypoxia, defined as the threshold where oxygen concentration limits normal cell function [
10], results from inadequate oxygen supply or excessive consumption, hindering normal cellular metabolism [
11]. The oxygen tension in most normal tissues ranges from 2% to 9% (14-65 mmHg) [
12], while <6.6%-8.6% in bone [
13]. Hypoxia conditions, such as reduced oxygen partial pressure, poor oxygen diffusion and perfusion environment, may trigger cellular hypoxic responses. Studies have demonstrated that both osteoblasts and osteoclasts are oxygen-sensing cells [
14]. Pathological or environmental hypoxia can disrupt bone homeostasis, thereby affecting bone health [
15,
16]. Mice exposed to a simulated altitude of 5500 meters for 28 days exhibited significant reductions in bone mineral density of the fourth lumbar vertebra, bone volume fraction, trabecular thickness, and trabecular number, indicating that hypoxia may increase the risk of fractures [
17]. However, the mechanisms by which hypoxia involves in osteoclast differentiation require further clarification.
Research has shown that hypoxia can regulate the transcription of numerous deubiquitinating enzymes (DUBs) through the hypoxia-inducible-factor (HIF) signaling pathway, thereby mediating the occurrence and development of various diseases [
18,
19,
20,
21,
22]. he human genome encodes approximately 100 DUBs, which are further classified into six subfamilies [
23,
24,
25,
26]. In recent years, an increasing amount of evidence has indicated that ubiquitination plays a significant regulatory role in maintaining bone homeostasis [
27]. Several DUBs, including CYLD [
28,
29], USP15, USP18 (Type I IFN) [
30], USP26 [
31], USP34 [
32], A20 [
33,
34], MYSM1 [
35], and POH1 [
36]), have been implicated in modulating osteoclast function. It should be noted that pioneering researches rarely focused on whether and how hypoxia regulates the expression DUBs to modulate osteoporosis progression. Additionally, mounting studies gradually revealed that targeting ubiquitin-proteasome system (UPS) might be a promising strategy for clinical applications of osteoporosis [
37,
38,
39,
40,
41,
42]. Therefore, a thorough understanding of hypoxia-DUBs axis in osteoporosis could not only improve the therapeutic efficacy but also minimize side effects for specificity of targeted treatment.
In this study, we found that hypoxia significantly promoted osteoclast differentiation, whereas induced obvious reduction of USP18 in RANKL-induced differentiation of RAW264.7 cells to osteoclasts. Further functional results revealed that USP18 exhibited as a negative regulator in RANKL-induced osteoclast differentiation. In addition, overexpressing USP18 remarkably rescued hypoxia-enhanced osteoclast differentiation. Mechanically, the USP18-mediated suppression on NF-κB signaling pathway was responsible for its inhibition in osteoclastogenesis. Thus, hypoxia might weaken the USP18-mediated suppression on NF-κB signaling pathway to significantly promote osteoclast differentiation, consequently participating in osteoporosis development. Our research provided novel insights into molecular basis of osteoporosis pathogenesis for developing potential therapeutic strategies.
2. Materials and Methods
2.1. Cell Culture
RAW264.7 cell were cultured in dulbecco’s modified eagle medium (DMEM) (Gibco, cat. C11995500BT) supplemented with 10% fetal bovine serum (FBS) (Gibco, cat. 10099141C), 0.1 mg/ml penicillin-streptomycin (Gibco) at 37° C under 5% CO2 (Thermo Fisher Scientific). Murine bone-marrow-derived monocytes (BMDM) flushed from the long bones of C57BL/6 mice aged 6–8 weeks were maintained in α-minimal essential medium (α-MEM, Gibco, cat. C12571500BT) containing 10% heat-inactivated FBS, penicillin(50 U/ml), and streptomycin sulfate (50 µg/ml). BMDM were cultured as follows: first, marrow cells flushed from the long bones of mice were cultured in α-MEM containing 10% FBS in the presence of M-CSF (10 ng/ ml) at 37 °C in 5% CO2 for 3 days. Non-adherent cells were removed by washing the culture dishes with phosphate-buffered saline followed by incubation in the presence of M-CSF (10 ng/ml) and 75ng/ml RANKL (RD, cat. 462-TEC-010) for 7 days for osteoclast detection. Cells were incubated in a hypoxic chamber (HERACELL 150i, Thermo Fisher Scientific, Waltham, MA, USA) at 5% CO2 and 1% O2, balanced with N2 as indicated or in a normal incubator containing 5% CO2 and approximately 20% O2 (Forma Series II, Thermo Fisher Scientific). Hypoxic conditions were achieved in a hypoxia chamber (memmert) flushed with a preanalyzed gas mixture of 1% O2, 5%CO2, and 94% N2 or 2% O2, 5%CO2, and 93% N2. For induction of osteoclast differentiation, RAW264.7 macrophages were seeded at a density of 1×104/ well in 24-well plates and cultured overnight in complete DMEM. The cells were then replaced with α-MEM/DMEM containing 75ng/ml RANKL (RD, cat. 462-TEC-010), 10% FBS, and 0.1 mg/ml penicillin-streptomycin. The time point of RANKL addition was designated as Day 0, and the medium was changed every other day. The corresponding days were induced according to the requirements of subsequent experiments.
2.2. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from preosteoclasts using TRIzol reagent ((Invitrogen, cat. 15596018) and subjected to cDNA synthesis using HiScript II Q RT SuperMix for quantitative polymerase chain reaction (+gDNA wiper; Vazyme, cat. R223). qRT-PCR was performed on QuantStudio 5 flex (Thermo Fisher) using 2×RealStar green power mixture (GenStar, cat. A311). Relative quantification calculations were performed using the 2−ΔCT method. The gene-specific primer sets (all for murine genes) were as follows:
cFos, 5′- cgggtttcaacgccgacta-3′
5′-ttggcactagagacggacaga-3′;
Acp5, 5′-cactcccaccctgagatttgt-3′
5′-catcgtctgcacggttctg-3′;
Nfatc1, 5′-cagtgtgaccgaagatacctgg-3′
5′-tcgagacttgatagggacccc-3′;
Trap, 5′-tggtccaggagcttaactgc-3′
5′- gtcaggagtgggagccatatg-3′;
Usp18, 5′-caggagtccctgatttgcgt-3′
5′-caaggcatcctccagggttt-3′;
β-actin, 5′- tctgctggaaggtggacagt -3′
5′- cctctatgccaacacagtgc -3′.
2.3. Immunoblot and Antibodies
For immunoblot, whole cell lysates were obtained using low-salt lysis buffer supplemented with protease and phosphatase inhibitors on ice, followed by centrifugation at 12,000 g for 5 min at 4 ℃. Then the protein samples were heated at 99 ℃ for 10 min with SDS loading buffer (Solarbio, cat. P1040) and resolved on SDS-PAGE gels. Proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad, cat. 1620177). The membranes were blocked with 5% (w/v) reagent-grade nonfat milk and further incubated with the appropriate antibodies. Antibodies used in immunoblot are listed as follows: Rabbit anti-USP18 (1:1,000, #5348; Cell Signaling Technology), Rabbit anti-Phospho-TAK1 (1:1,000, #S412; Cell Signaling Technology), Rabbit anti-TAK1 (1:1,000, #D94D7; Cell Signaling Technology), Rabbit anti-NF-kappa B p65 (1:1,000, #D14E12; Cell Signaling Technology), Rabbit anti-Phospho-NF-kappa B pp65 (1:1,000, #5536; Cell Signaling Technology), and anti-β-actin mouse monoclonal antibody (1:8000, #66009; Proteintech). The immune complexes were then incubated with horseradish peroxidase-conjugated anti-mouse IgG (1:5000, #SA0001; Proteintech) or anti-rabbit IgG (1:5000, #RGAR001, Proteintech) and visualized with Immobilon reagents (Millipore, Billerica, MA, USA).
2.4. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
After the RAW264.7 cells were induced and cultured for a certain number of days according to the experimental requirements, the medium was aspirated, and the cells were washed twice with 0.5ml of pre-warmed PBS added along the side wall of the well. Then, 0.25ml of pre-cooled TRAP fixative solution at 2-8 °C was added to the 24-well plate for 50 s in the dark. The cells were washed 3 times with PBS added along the side wall of the well to remove residual fixative solution. The TRAP incubation solution was prepared by mixing AS-BI Buffer, GBC staining solution, and ACP Buffer in a ratio of 10:1:90, protected from light. The TRAP incubation solution was added dropwise to cover the cells, incubated in a 37 °C incubator in the dark for 60 minutes, then the staining solution was discarded and washed three times with distilled water. The cells were counterstained with hematoxylin in the dark for 5 minutes, then washed three times with tap water, and photographed under a microscope after air drying.
2.5. Cell Transfection
For Small infected RNA, RAW264.6 cells were plated at a density of 1×105 per well and cultured overnight at 37 ºC with 5% CO2 for transfection. A total amount of 5 pmol siRNA per well was transfected into the cells with Lipofectamine RNAiMAX (Invitrogen, USA) according to the manufacturer’s protocol. The cells were then cultured for 24 h under normoxia conditions (supplemented with 20% O2, 5% CO2 and 75% N2before proceeding with subsequent experiments. The target and control sequences were provided by Ribobio, including:
siUsp18-1 GGACGCAAAGCCTCTGAAA,
siUsp18-2 CAATCTGGAACCTGACTAA,
siUsp18-3 CCTATGGGAACCACAGATA.
For overexpression, RAW264.6 cells were plated at the same density and 400ng/ml plasmid were transfected into the cells with Lipofectamine2000 according to the manufacturer’s protocol for 24 h, before proceeding with subsequent experiments.
2.6. Statistical Methods
All data were analyzed using GraphPad Prism 8.0 (GraphPad Software) and presented as “mean ± standard deviation (SD)”. Comparisons between two groups were performed using the t-test, while multiple comparisons between groups were analyzed using Two-way ANOVA. Statistical significance was defined as P < 0.05.
3. Results
3.1. Hypoxia Significantly Enhances the Osteoclasts Differentiation of both RAW264.7 and BMDM Cells
To identify optimal osteoclasts induction conditions, RAW264.7 cells were cultured in either complete α-MEM or DMEM medium, with the addition of 75 ng/mL RANKL (Fig. S1A-C). RAW264.7 cells, known for their smaller size and rapid growth, showed noticeable proliferation and clustering after one day of induction, while no TRAP-positive cells were observed (Fig. S1A). On day 2, TRAP-positive monocytes began to appear, whereas showed no diffidence between α-MEM and DMEM group (Fig. S1A-C). By day 3, numerous TRAP-positive multinucleated giant cells with irregular cell shapes and varying numbers of nuclei were observed (Fig. S1A). Some of these giant cells contained dozens of nuclei, clustered either centrally or peripherally within the cells. The cytoplasm of these cells appeared hollow. Remarkably, compared to DMEM, the number of osteoclasts in α-MEM group was dramatically higher, and the difference was pronounced at Day 3, with p-value < 0.05 (Fig. S1A-C). On day 4, a large number of mature osteoclasts disintegrated, leading to decrease in number of osteoclasts (Fig. S1A). In conclusion, the α-MEM medium was found to be more effective than DMEM in promoting RANKL-induced differentiation of RAW264.7 cells into osteoclasts. Therefore, the subsequent experiments utilized the α-MEM medium as the preferred culture condition.
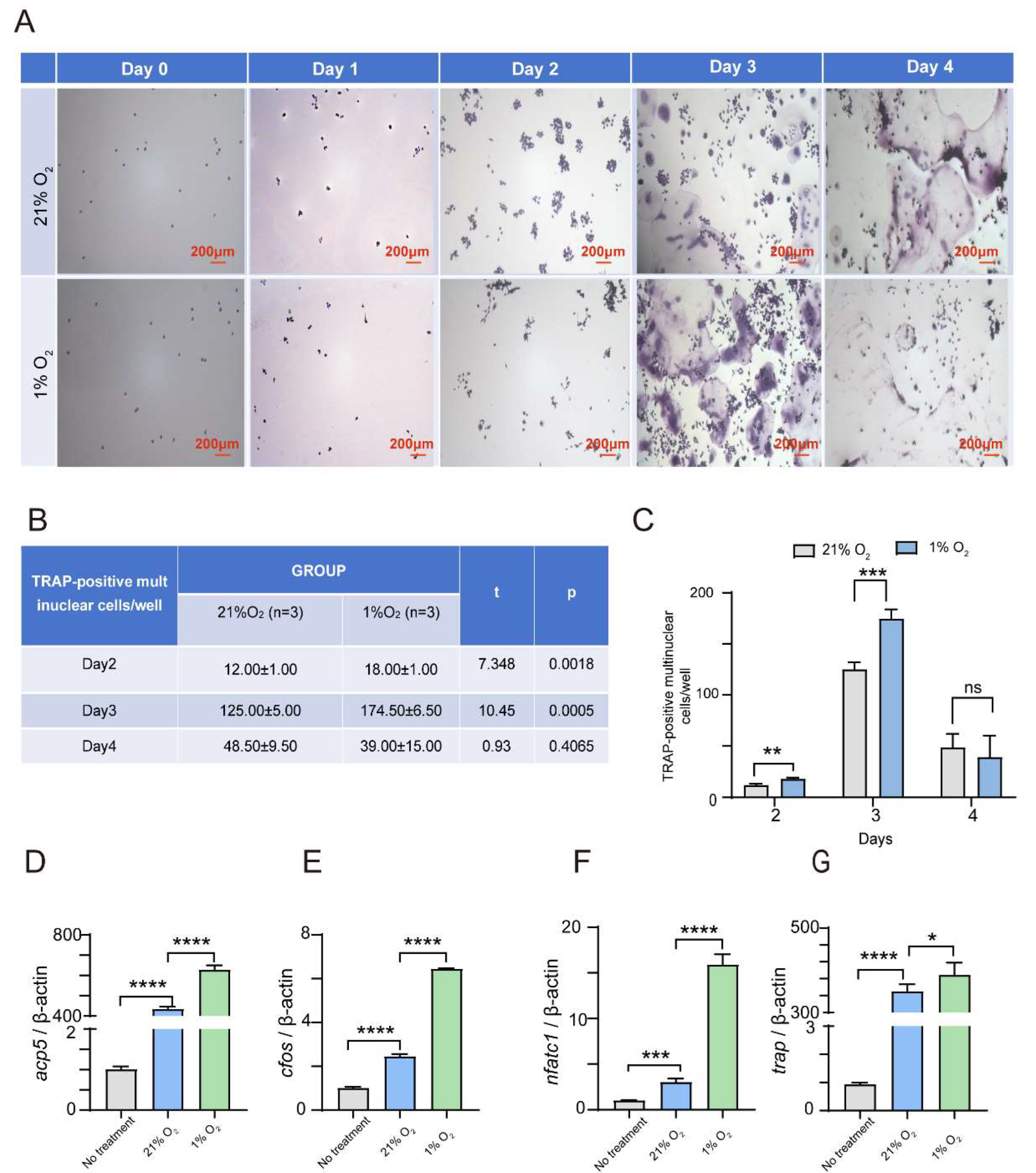
Importantly, under sustained 1% O
2 treatment, the number of TRAP-positive multinuclear cells was greater than normoxic group, particularly from Day 2 to Day 3 (
Figure 1A–C). Additionally, sustained hypoxia treatment led to a robust increase in the expression levels of osteoclast marker genes, Acp5, cFos, Nfatc1, and Trap (
Figure 1D–G). These findings indicated that sustained hypoxia significantly enhanced the differentiation of RAW264.7 cells into osteoclasts. We further examined the morphological changes in osteoclasts formation under short-term hypoxia treatment (24-hour exposure to 1% O
2). Similar to sustained hypoxia, there was a notable elevation in the number of TRAP-positive multinuclear cells in short-term hypoxia group, especially on Day 3 with the most pronounced increase and difference, compared to the normoxia (Fig. S2A-C). In addition, We also examined the morphological changes of BMDM in osteoclasts formation under short-term hypoxia treatment (24-hour exposure to 1% O
2). Similar to RAW264.7 cells, there was a notable elevation in the number of TRAP-positive multinuclear cells in short-term hypoxia group, especially on Day 5 with the most pronounced increase and difference, compared to the normoxia (Fig. S3A-C). Thus, these results showed that either sustained or short-term hypoxic exposure could induce a significant increase in osteoclast differentiation, highlighting the sensitivity of RAW264.7 cells to hypoxic environments for osteoclastogenesis.
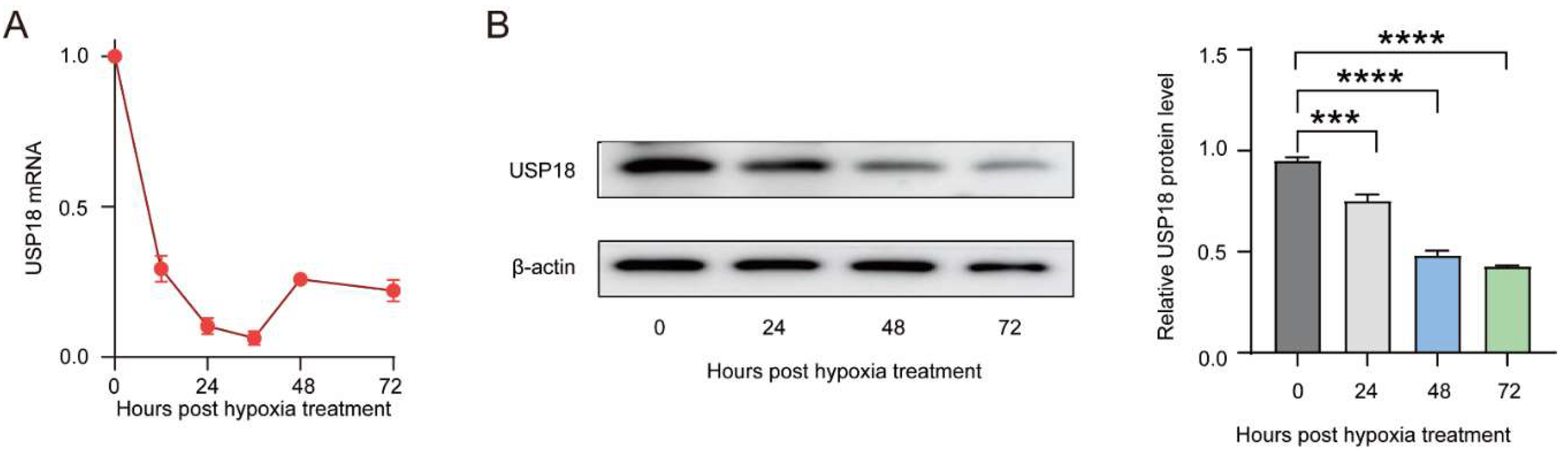
3.2. Hypoxia Dramatically Suppresses the USP18 Expression in Osteoclasts Differentiation
We also found that both USP18 mRNA and protein levels were obviously downregulated over time in response to sustained hypoxia (1% O
2) during the differentiation of RAW264.7 cells into osteoclasts (
Figure 2A–B). At the 12th hour of hypoxic stimulation, the Usp18 mRNA was significantly decreased and remained at a low level through 24, 36, 48, and 72 hours (
Figure 2A). Furthermore, at the 24th hour of hypoxic stimulation, the USP18 protein was remarkably reduced and continued to be downregulated at 48 and 72 hours (
Figure 2B). The relative fold change of USP18 protein at 24 hours dropped from 0.90 at 0 hours to approximately 0.70, and at 72 hours, it further decreased to about 0.40. Given that DUBs are involved in numerous intracellular events via regulating the availability and activity of functional proteins, we hypothesized that the USP18 might be a key factor in osteoclastogenesis under hypoxic conditions [
43,
44].
Additionally, we conducted short-term hypoxic treatment (0, 6, 12, and 24 hours) on the second day for osteoclast differentiation derived from RAW264.7 (Fig. S4A, S4B, and S4D) and BMDM (Fig. S4C) cells. Short-term hypoxic exposure also resulted in a notable decrease in USP18 mRNA and protein levels in osteoclast differentiation from both RAW264.7 and BMDM cells. During the differentiation of RAW264.7 cells into osteoclasts, both the mRNA and protein levels of USP18 were significantly reduced at the 6th hour of hypoxic stimulation (1% O2) and remained at low levels for up to 24 hours (Fig. S4A and S4D). The relative fold change of Usp18 mRNA was reduced from 1.0 (0 hour) to 0.1 at 6 hours, and to 0.09 at 24 hours (Fig. S4A). Moreover, gray scale analysis showed that the relative fold change of USP18 protein was decreased from 1.14 (0 hour) to 1.03 at 6 hours, to 0.70 at 24 hours (Fig. S4D). These results indicated that hypoxia could significantly downregulated the USP18 expression during osteoclastogenesis, highlighting the potential importance of USP 18 in osteoclast differentiation under hypoxic conditions.
3.3. USP18 Negatively Modulates Osteoclast Differentiation
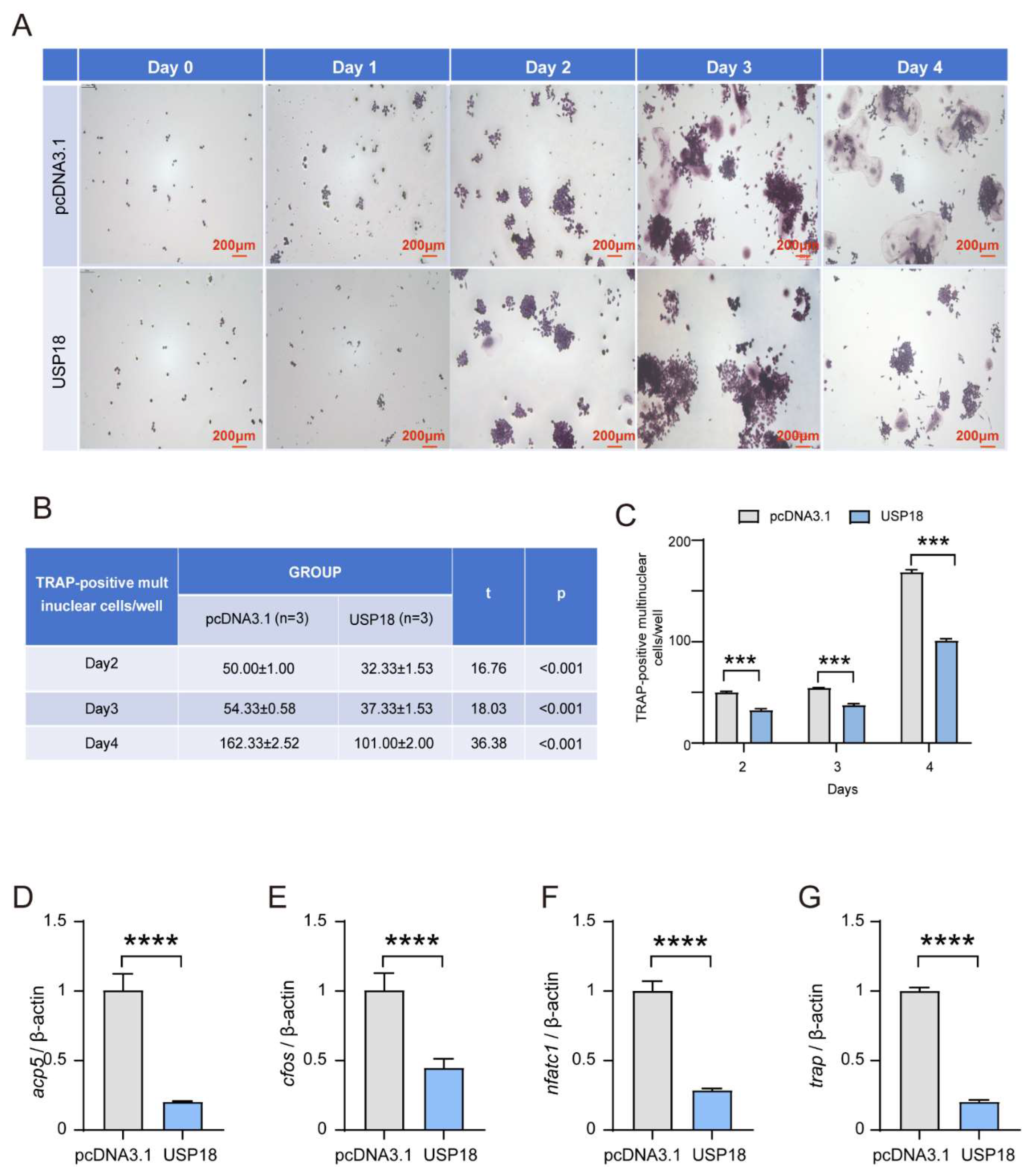
To gain insight into the function of USP18 in osteoclast differentiation, we transiently transfected Usp18 plasmid to achieve its overexpression in RAW264.7 cells. Overexpressing USP18 notably inhibited the osteoclast differentiation through TRAP staining across the observed days, with a clear reduction in TRAP-positive multinuclear cells and quantified counts (
Figure 3A–C). Consistently, overexpression of USP18 significantly downregulated the expression of osteoclast marker genes Acp5, cFos, Nfatc1, and Trap, further supporting its role in inhibiting osteoclastogenesis (
Figure 3D–G). These results concluded that overexpression of USP18 inhibits led to a marked decrease in osteoclast differentiation and related marker gene expression, indicating its critical role in suppressing osteoclastogenesis.
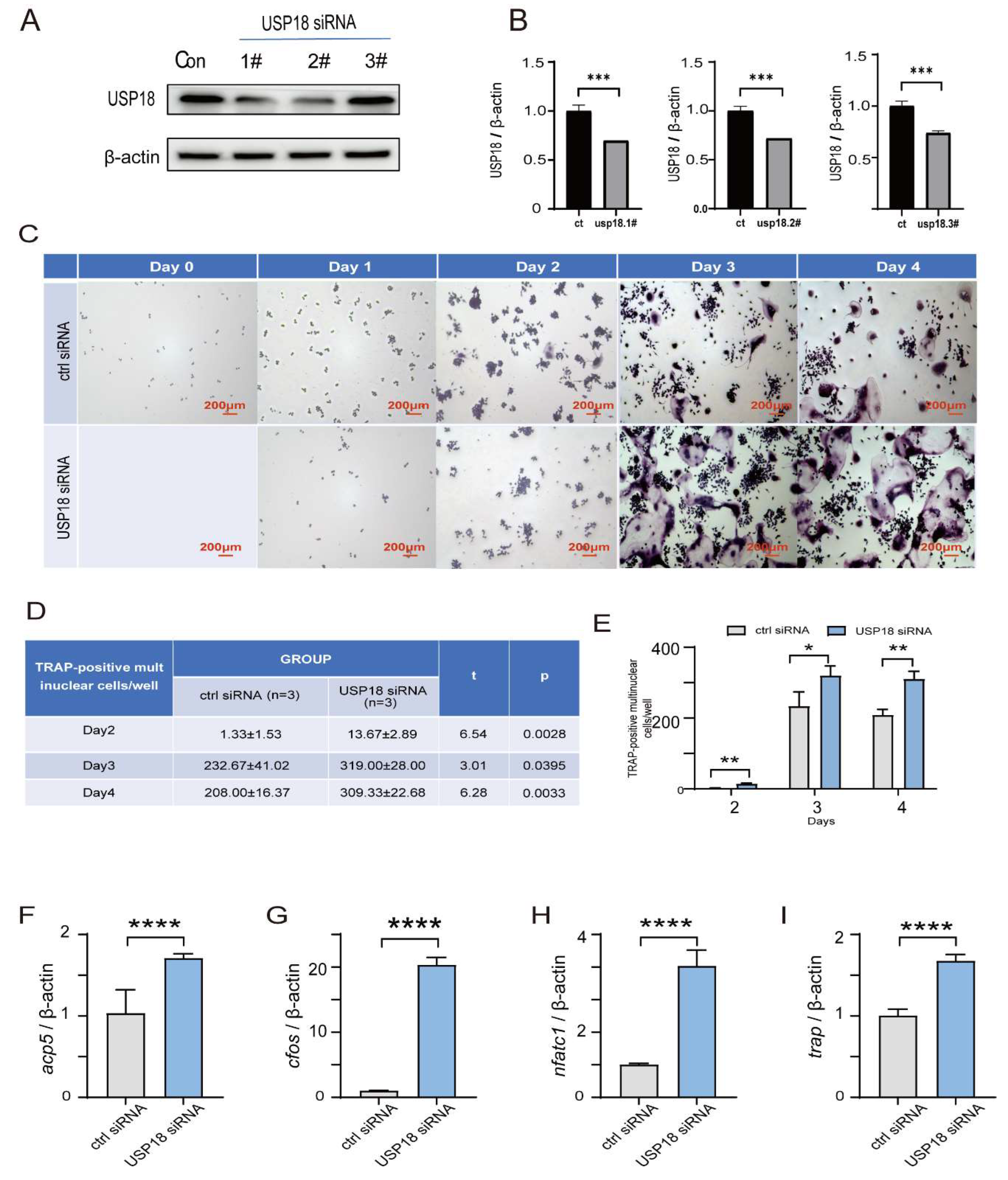
To further confirm the role of USP18 in osteoclastogenesis, we performed siRNA-mediated knockdown of USP18 in RAW264.7 cells. After transfecting the RAW264.7 cells with three siRNAs targeting USP18, we verified the knockdown efficiency by measuring the protein and mRNA levels with immunoblot and qRT-PCR, respectively (
Figure 4A,B). We found that the Usp18 mRNA level was downregulated after interference with all three siRNAs, whereas only the siUsp18-2 significantly reduced the protein level of USP18, leading us to select it for the subsequent experiments (
Figure 4A,B). TRAP-staining showed that compared to the control, knockdown of USP18 remarkably enhanced the osteoclast differentiation across the observed days, with a significant increase in TRAP-positive multinuclear cells and quantified counts (
Figure 4C–E). Consistently, RT-qPCR analysis revealed that silencing USP18 obviously facilitated the expression of osteoclast marker genes Acp5, cFos, Nfatc1, and Trap (
Figure 4F–I). Our findings implicated that USP18 was an inhibitor for osteoclastogenesis, which was consistent with above overexpression experiments.
3.4. Overexpressing USP18 Rescues Hypoxia-Enhanced Osteoclast Differentiation
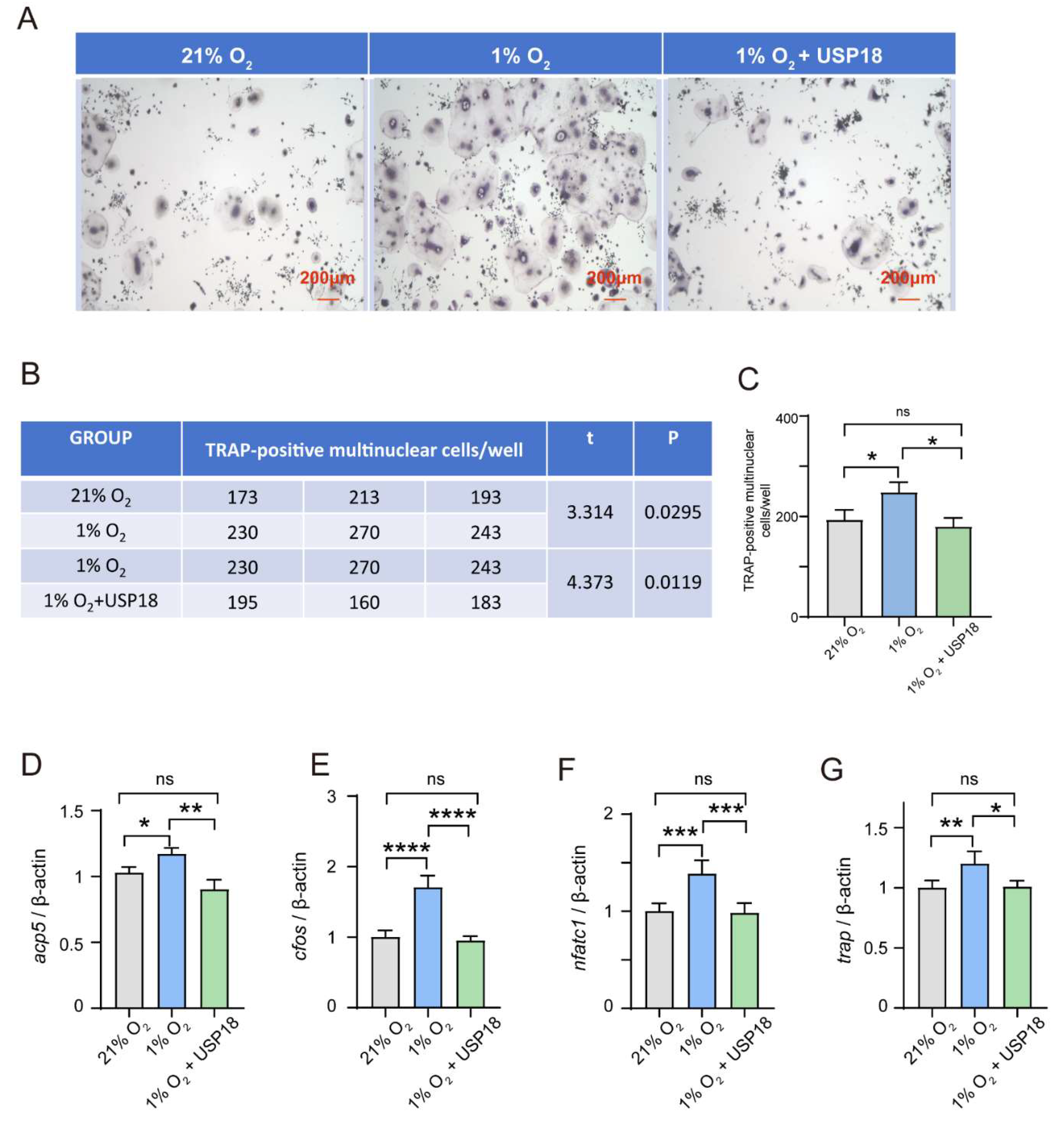
Given that the hypoxia facilitates osteoclastogenesis by suppressing the expression of USP18, an inhibitory factor of osteoclast differentiation, we sought to explore whether overexpressing USP18 is sufficient to rescue the pro-osteoclastogenic effect of hypoxia. As shown in
Figure 1, the counts of osteoclasts reached its peak on day 3, and the promoting effect of hypoxia on osteoclastogenesis was also most pronounced on this day. Consequently, we investigated the impact of USP18 overexpression on hypoxia-promoted osteoclastogenesis by performing TRAP staining on day 3 and assessing the expression of osteoclast markers on day 2. Our study revealed that overexpression of USP18 under hypoxic conditions resulted in a significant reduction in the number of TRAP-positive cells compared to the control group (
Figure 5A–C). Strikingly, no significant difference was observed between the hypoxia group with USP18 overexpression and the normoxia group. These findings indicated that overexpression of USP18 significantly reversed the hypoxia-induced increase in osteoclastogenesis. In consistent, when USP18 was overexpressed under hypoxic treatment, the expression levels of osteoclast markers, including genes Acp5, cFos, Nfatc1, and Trap, were remarkably reduced compared to control hypoxic group (
Figure 5D–G). Collectively, these results delineated that USP18 played a critical role in counteracting the effects of hypoxia on osteoclast differentiation, highlighting that the hypoxia-mediated enhancement of osteoclastogenesis was achieved through downregulating USP18 expression.
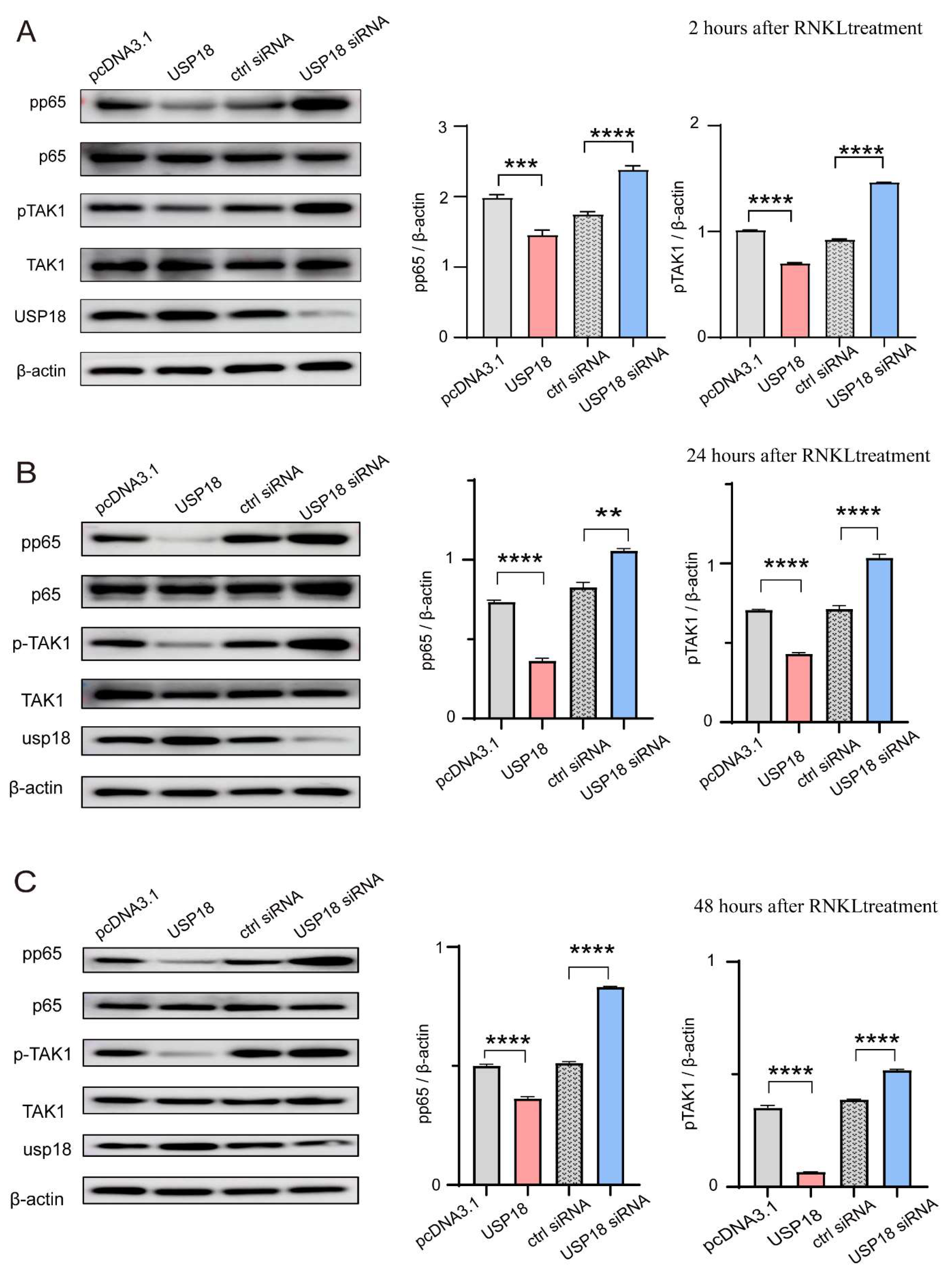
3.5. USP18 Inhibits Osteoclast Differentiation by Suppressing the NF-κB Signaling Pathway
The NF-κB pathway plays a crucial role in osteoclast differentiation [
45]. To determine whether USP18-mediated inhibition on osteoclast differentiation is NF-κB pathway dependent, we transfected RAW264.7 cells with Usp18 plasmid or siRNA-Usp18. Immunoblot analysis showed that overexpressing USP18 resulted in reduced activation of NF-κB pathway, as evidenced by decreased pp65 levels following 2/24/48 hours of RANKL treatment (
Figure 6A–C). Conversely, increased pp65 levels were observed in the USP18 knockdown group after 2/24/48 hours of RANKL treatment, leading to augmented activation of the NF-κB pathway (
Figure 6A–C). These findings indicated that the USP18 protein level was inversely correlated with the activation of NF-κB pathway, suggesting that the USP18 exhibited as a negative regulator of NF-κB pathway. Additionally, pTAK1 also showed a negative correlation with the USP18protein level (
Figure 6A–C), revealing that the potential molecular targets by which USP18 inhibited the activation of NF-κB pathway might target on TAK1 or its upstream molecules, such as MyD88, TRIF, IRAK1, TRAF2, TRAF6. In conclusion, the inhibitor effect of USP18 on osteoclast differentiation was dependent on the NF-κB signaling pathway potentially by targeting TAK1 or its upstream adaptors.
4. Discussion
Our study revealed that both sustained and transient hypoxia significantly promotes osteoclast differentiation and contributes to the pathogenesis of osteoporosis. Prior research has indicated that hypoxia could promote the differentiation BMDMs into osteoclast, with the peak effect observed at 2% O
2, resulting in a fourfold increase in the number of mature multinucleated cells and a 21-fold increase in resorptive activity [
14,
46]. Similar results were found for the effect of hypoxia on cat osteoclast activity [
46]. However, the specific impact of hypoxia on osteoclast differentiation has been inconsistent across studies, with some reporting enhanced osteoclast differentiation and activity while others showing reduced osteoclastogenesis. Knowles HJ et al. found that hypoxia (1% O
2) reduced the formation and activity of osteoclasts in vitro [
47]. Gorissen et al further reported that hypoxia inhibited cellular multinucleation during osteoclast differentiation [
48]. Conversely, Muzylak et al. observed that despite a reduction in osteoclast numbers, bone resorption was augmented due to the enlargement of osteoclasts induced by hypoxia [
49]. These discrepancies might be attributed to variations in the methods used to identify osteoclasts, the oxygen concentrations applied, and the duration of hypoxic treatment. Nonetheless, these findings from our group and others suggested that hypoxia may exert complex effects on osteoclast differentiation and bone resorption, which warrant further in vivo investigation.
Meanwhile, hypoxia downregulates USP18 expression during RANKL-induced osteoclast differentiation in both BMDMs and RAW264.7 cells. Prior to our study, the role of USP18 in osteoclasts has not been previously reported. We propose that the downregulated USP18 expression may be involved in hypoxia-induced augmented osteoclastogenesis. Further experiments confirmed that USP18 exhibits as a negative regulator of osteoclast differentiation, implicating that the hypoxia-mediated enhancement of osteoclast differentiation is achieved through downregulating USP18 expression. However, the precise mechanisms underlying USP18-mediated suppression on osteoclastogenesis require further elucidation. Originally identified as a type I interferon response gene, USP18 is rapidly upregulated via the JAK/STAT kinase pathway following IFN-β stimulation in osteoclast differentiation [
50]. Moreover, USP18 exerts as an inhibitor of type I IFN signaling pathway both ISG15-dependent [
51] and -independent manners [
52,
53]. Additionally, USP18 also acts as a novel negative regulator in toll-like receptor (TLR)-mediated NF-κB signaling via attenuating the ubiquitination of the TAK1/TAB1 complex and IKKα/β-NEMO complex in both isopeptidase-dependent and -independent ways [
54]. In mammals, the NF-κB/Rel family comprises five members: NF-kB1 (p50), NF-kB2 (p52), RelA (p65), Rel-B and c-Rel, which form homo- or heterodimers and remain as an inactive complex with IκB inhibitory proteins to block their nuclear import [
55]. The most abundant form of NF-κB activated by the canonical pathway is the p65:p50 heterodimer [
56]. Considering the pivotal role of NF-κB signaling pathway in the development of various endocrine system disorders, particularly osteoporosis [
57,
58,
59], we conducted immunoblotting to detect the activation/phosphorylation of p65 under varied USP18 expression conditions. Our findings unveiled that the USP18 serves as a negative regulator of NF-κB signaling pathway potentially by targeting TAK1 or its upstream adaptors. The exact molecular targets of USP18 in NF-κB-related osteoclastogenesis warrant further exploration.
In conclusion, our study reveals a novel mechanism by which hypoxia promotes osteoclast differentiation through the downregulation of USP18, which subsequently relieves the suppression on the activation of NF-κB pathway. The findings from this study provide novel insights into the molecular mechanisms by which hypoxia modulated bone homeostasis and suggest potential therapeutic targets for the treatment of osteoporosis. Future studies should focus on validating these findings in vivo and exploring the potential of USP18 as a therapeutic target for the treatment of bone diseases associated with altered oxygen levels.
Figure 1.
Hypoxia significantly enhances the osteoclasts differentiation of RAW264.7. RAW264.7 cells were treated with RANKL for 0- 4 days. (a-c) Osteoclastogenesis was investigated by tartrate-resistant acid phosphatase (TRAP) staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (d-g) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. The relative mRNA level of individual genes was expressed as fold induction compared with NT. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n = 3). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Figure 1.
Hypoxia significantly enhances the osteoclasts differentiation of RAW264.7. RAW264.7 cells were treated with RANKL for 0- 4 days. (a-c) Osteoclastogenesis was investigated by tartrate-resistant acid phosphatase (TRAP) staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (d-g) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. The relative mRNA level of individual genes was expressed as fold induction compared with NT. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n = 3). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Figure 2.
Hypoxia dramatically suppresses the USP18 expression in osteoclasts differentiation. RAW264.7 cells were treated with RANKL for 0h, 12h, 24h, 48h and 72h. (a) qRT-PCR analysis was performed for USP18. (b) The protein expression of USP18 was observed by Western blot.Data are presented as mean ± SD (***P < .001, ****P < .0001).
Figure 2.
Hypoxia dramatically suppresses the USP18 expression in osteoclasts differentiation. RAW264.7 cells were treated with RANKL for 0h, 12h, 24h, 48h and 72h. (a) qRT-PCR analysis was performed for USP18. (b) The protein expression of USP18 was observed by Western blot.Data are presented as mean ± SD (***P < .001, ****P < .0001).
Figure 3.
Overexpression USP18 inhibited RANKL-induced osteoclast differentiation. RAW264.7 Cells were transfected with USP18 plasmid for overexpression, followed by treatment with RANKL. (a-c) Osteoclastogenesis was investigated by TRAP staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (d-g) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n=3). (***P < .001, ****P < .0001).
Figure 3.
Overexpression USP18 inhibited RANKL-induced osteoclast differentiation. RAW264.7 Cells were transfected with USP18 plasmid for overexpression, followed by treatment with RANKL. (a-c) Osteoclastogenesis was investigated by TRAP staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (d-g) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n=3). (***P < .001, ****P < .0001).
Figure 4.
Si-USP18 inhibits RANKL-induced osteoclast differentiation. RAW264.7 Cells were transfected with USP18 for silencing, (a) Immunoblot of whole-cell lysates using USP18 antibodies. β-actin served as a loading control. (b) The relative mRNA level of USP18 was expressed as fold induction compared with NT. (c-e) Osteoclastogenesis was investigated by tartrate-resistant acid phosphatase (TRAP) staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (f-i) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n=3). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Figure 4.
Si-USP18 inhibits RANKL-induced osteoclast differentiation. RAW264.7 Cells were transfected with USP18 for silencing, (a) Immunoblot of whole-cell lysates using USP18 antibodies. β-actin served as a loading control. (b) The relative mRNA level of USP18 was expressed as fold induction compared with NT. (c-e) Osteoclastogenesis was investigated by tartrate-resistant acid phosphatase (TRAP) staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (f-i) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n=3). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Figure 5.
USP18 rescues RANKL-induced osteoclast differentiation under hypoxia. RAW264.7 Cells were transfected with USP18 for silencing, and then treated in 1% O₂ or 21% O₂ for 3days with RANKL. (a-c) Osteoclastogenesis was investigated by TRAP staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (d-g) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n=3). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Figure 5.
USP18 rescues RANKL-induced osteoclast differentiation under hypoxia. RAW264.7 Cells were transfected with USP18 for silencing, and then treated in 1% O₂ or 21% O₂ for 3days with RANKL. (a-c) Osteoclastogenesis was investigated by TRAP staining, and TRAP-positive multi-nucleated cells were considered mature osteoclasts. Scale bar, 200 μm. (d-g) qRT-PCR was performed using RNA isolated from RAW264.7 cells cultured in the presence of RANKL for 2days. Data represent mean values of 3 independent experiments. Data are presented as mean ± SD (n=3). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Figure 6.
USP18 inhibits osteoclast differentiation by suppressing the NF-κB signaling pathway. RAW264.7 Cells were transfected with USP18 for overexpression and silencing. (a-c) The protein activation of pp65, p65, pTAK1, TAK1, and β-actin were measured by immunoblotting with the indicated antibodies at different time points. Data are presented as mean ± SD, (n=3). (**P < .01, ***P < .001, ****P < .0001).
Figure 6.
USP18 inhibits osteoclast differentiation by suppressing the NF-κB signaling pathway. RAW264.7 Cells were transfected with USP18 for overexpression and silencing. (a-c) The protein activation of pp65, p65, pTAK1, TAK1, and β-actin were measured by immunoblotting with the indicated antibodies at different time points. Data are presented as mean ± SD, (n=3). (**P < .01, ***P < .001, ****P < .0001).