Submitted:
17 October 2024
Posted:
18 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
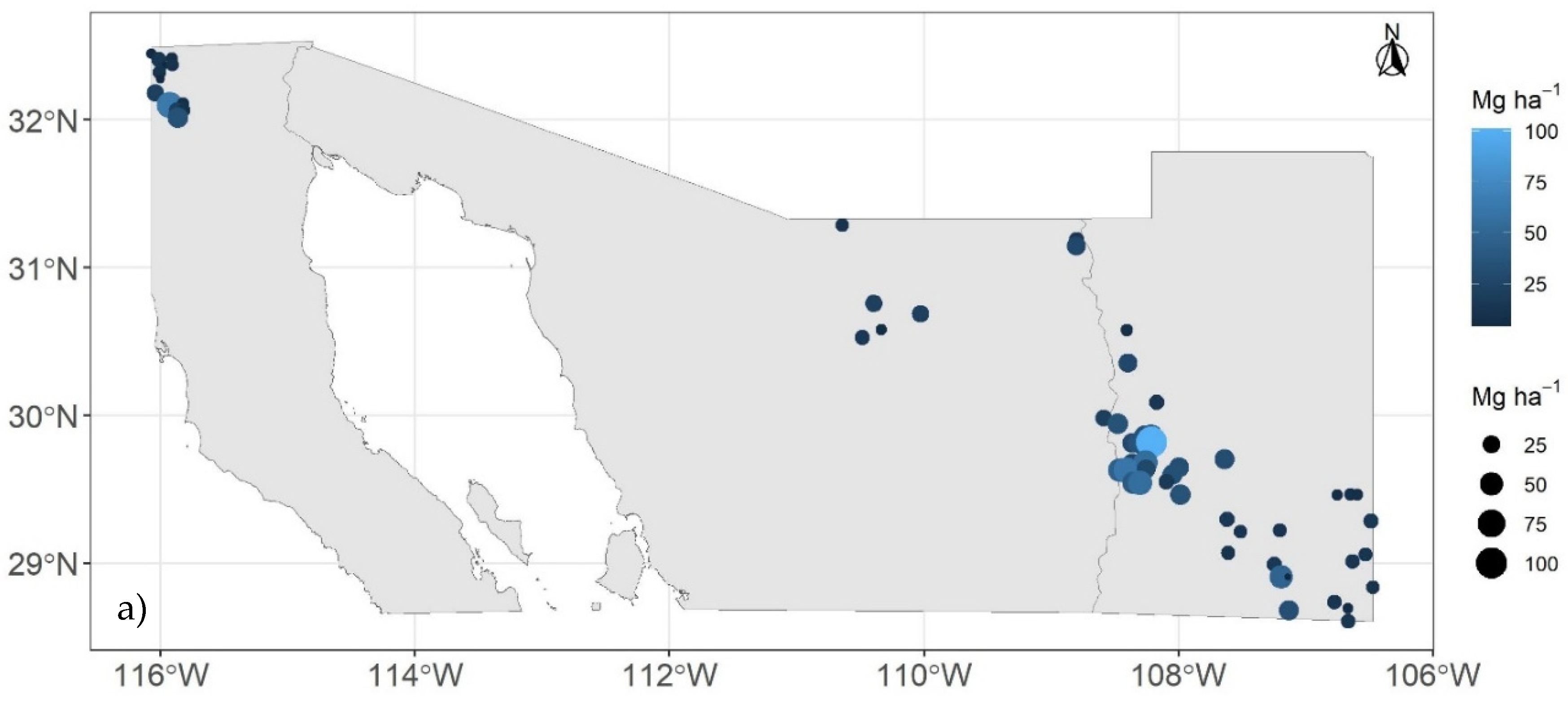
2.1. Study Area Description
2.2. Data Acquisition and Cleaning Process
2.3. Predictive Modeling of cdAGB
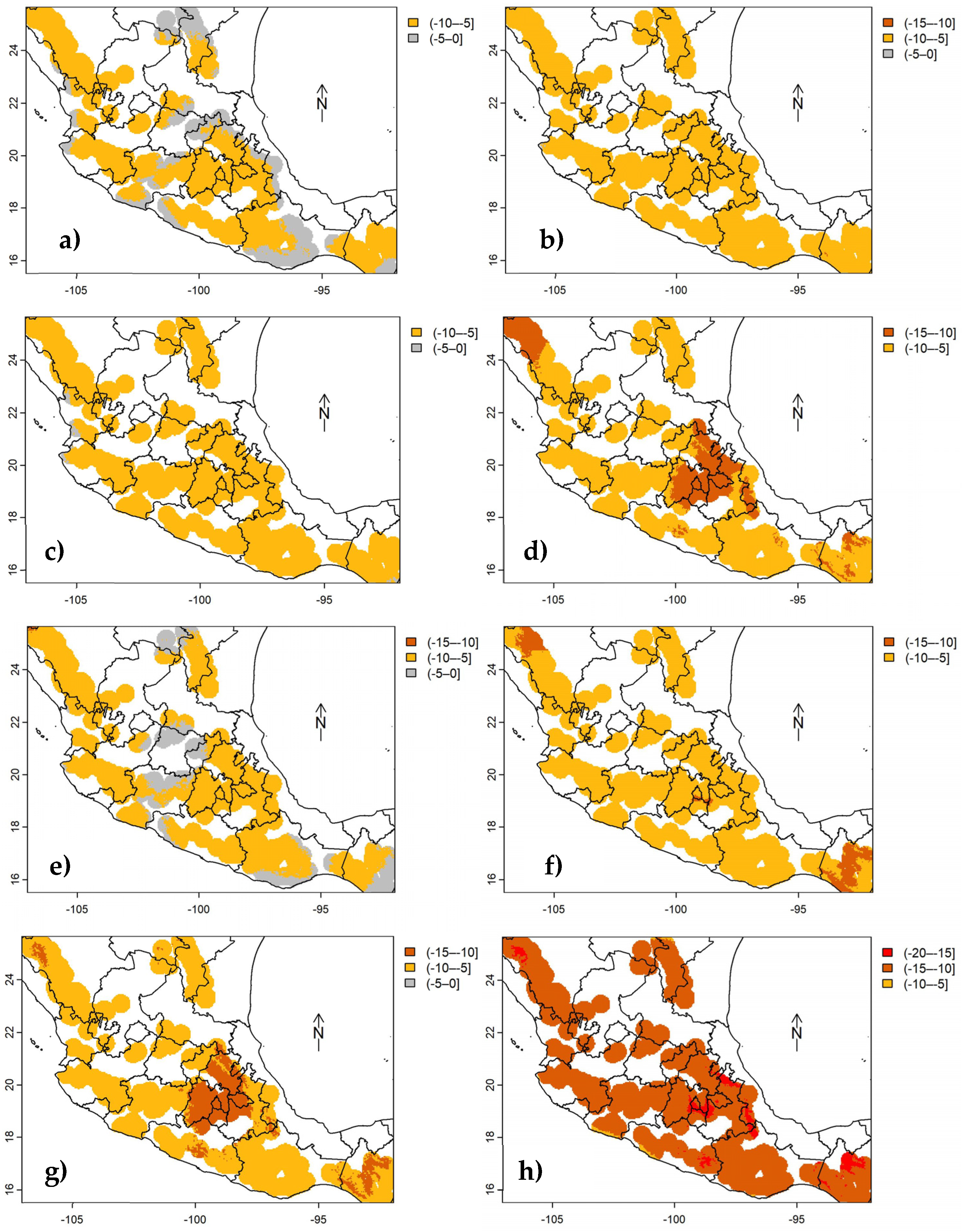
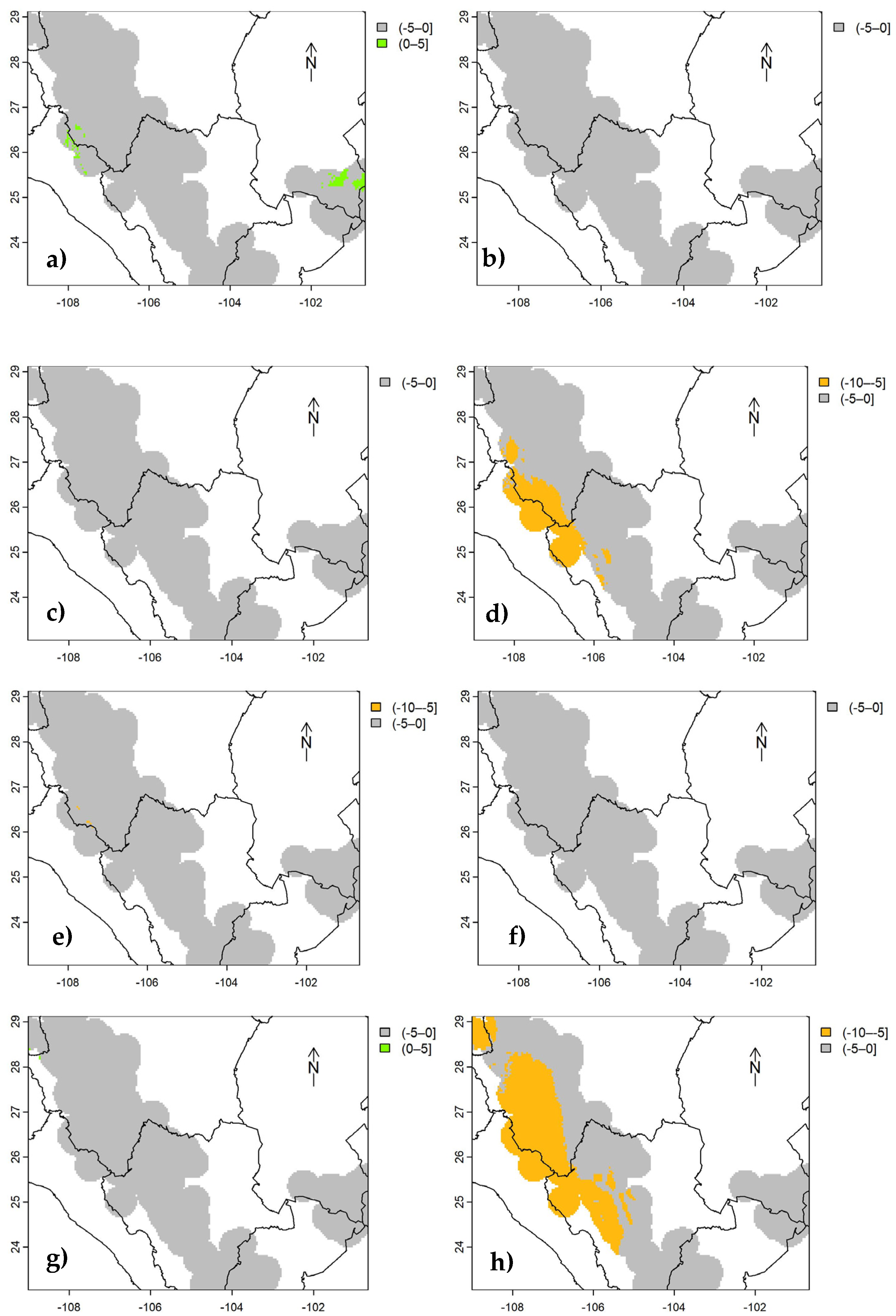
2.4. Current and Future Prediction and Rate of Change of cdAGB
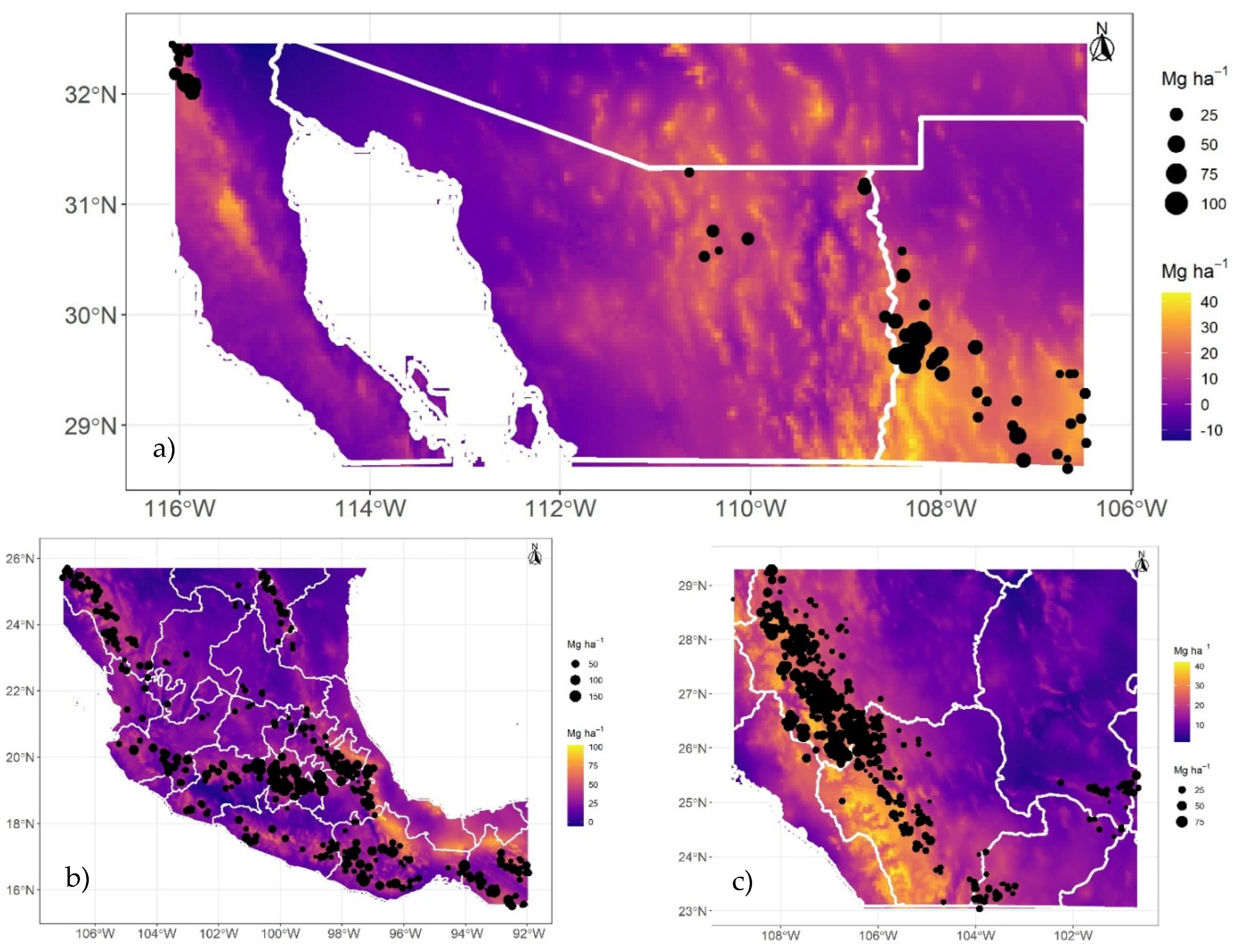
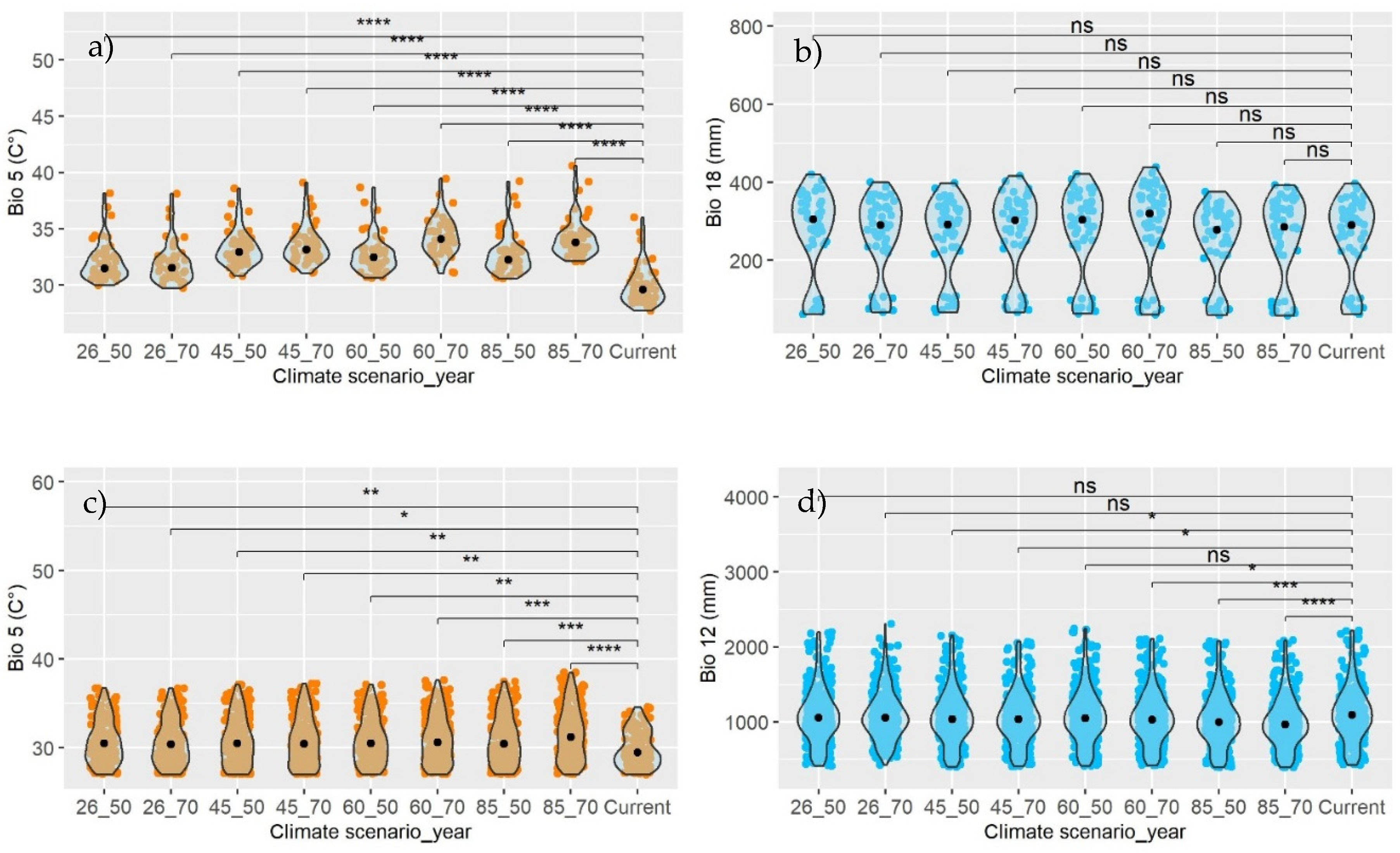
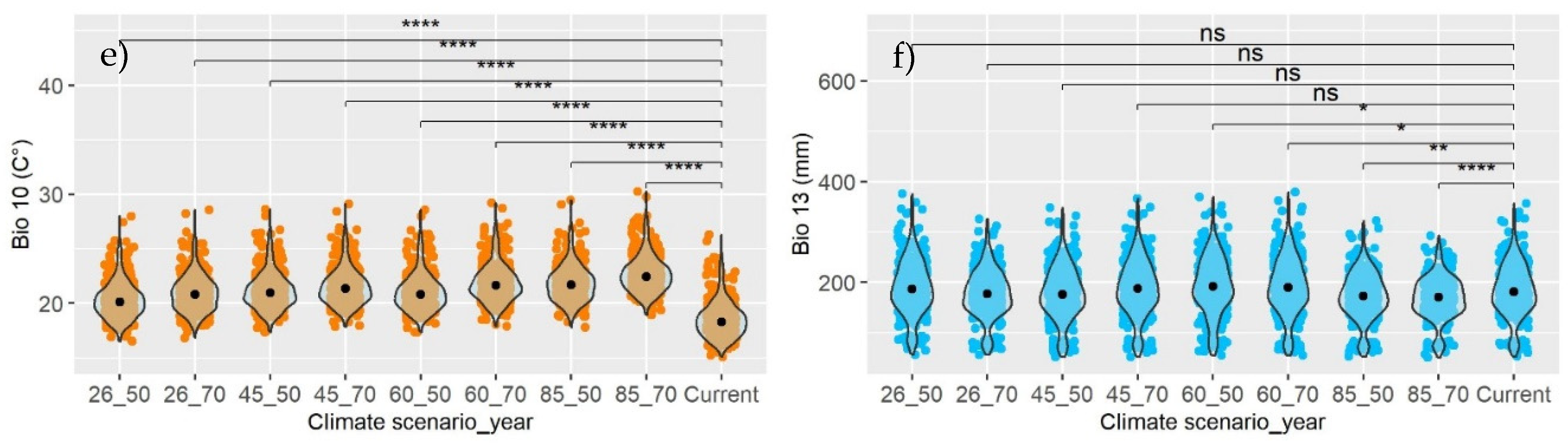
2.5. Bioclimatic Predictors: Current Analysis and Future Projections
3. Results
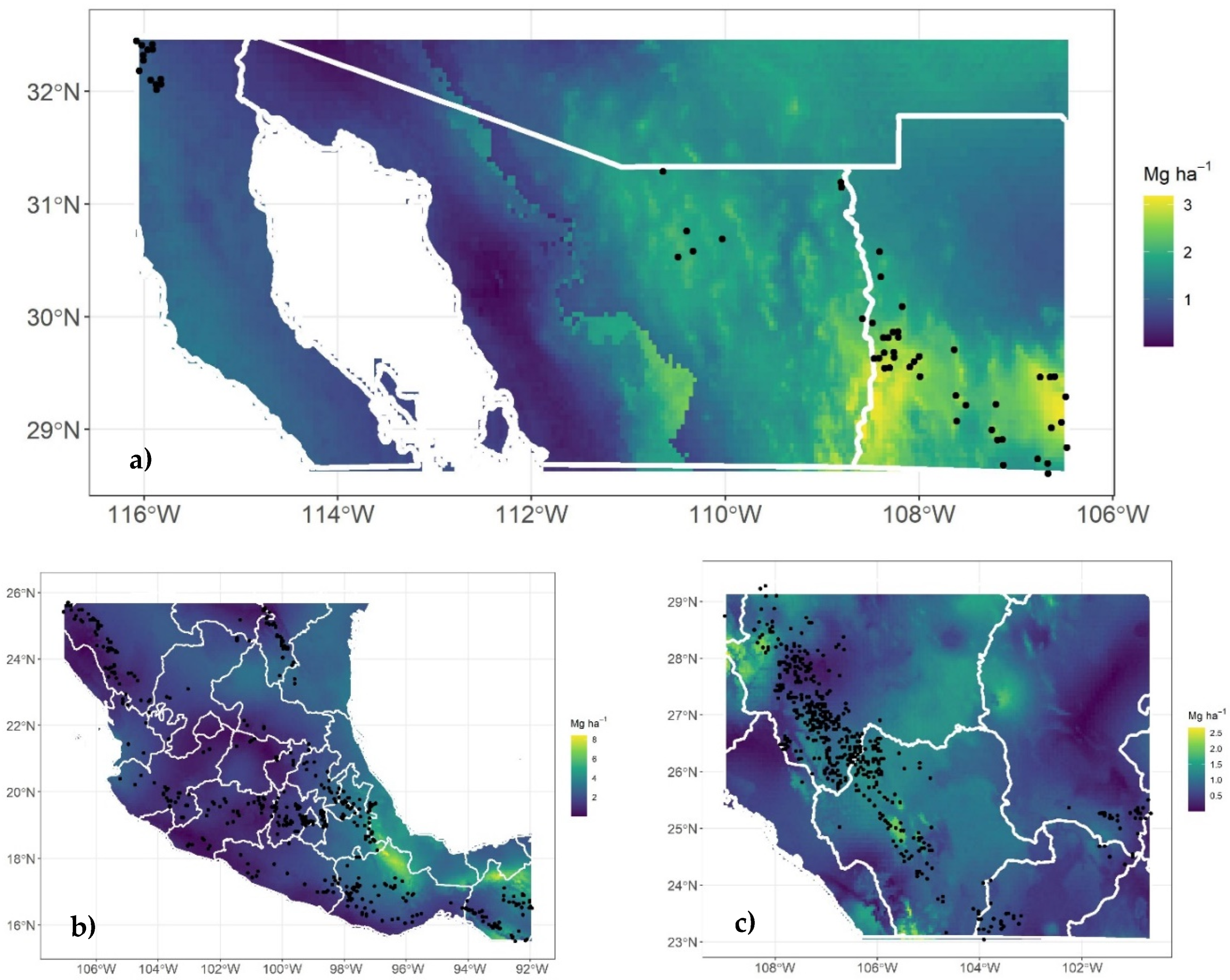
3.1. Distribution Carbon Density Of Aboveground Live Biomass
3.2. Models For Predicting Carbon Density in the Aboveground Live Biomass
3.3. Validation of Predictive Models for cdAGB
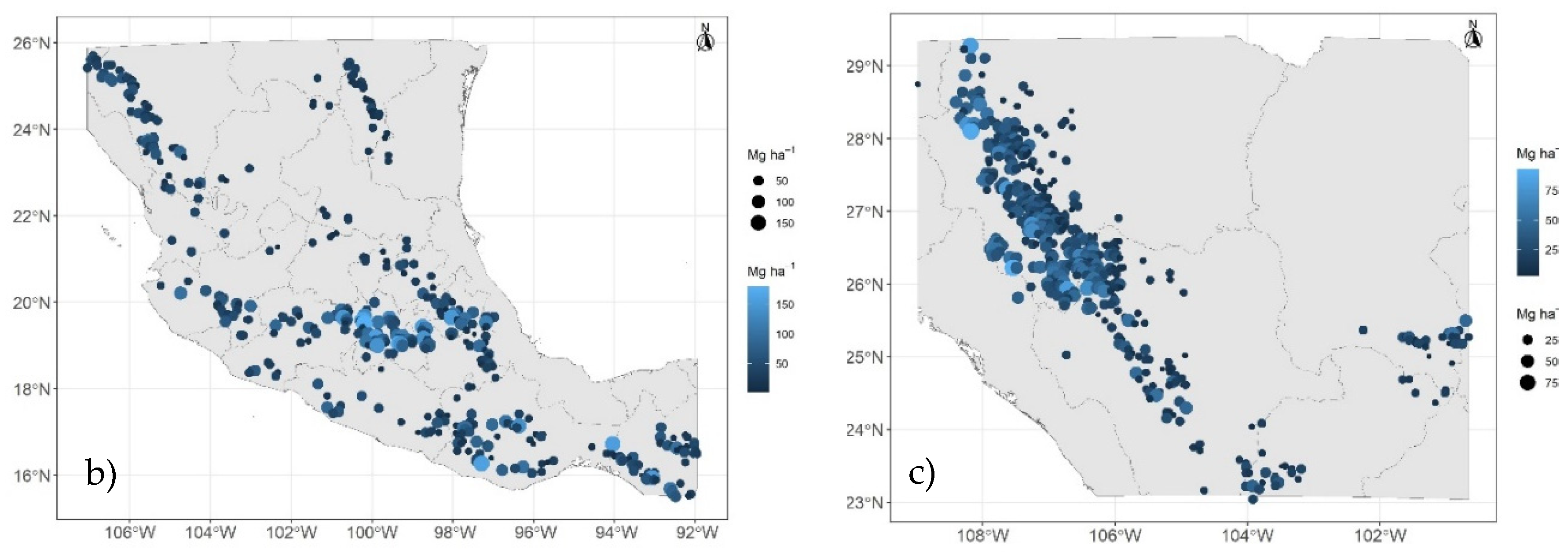
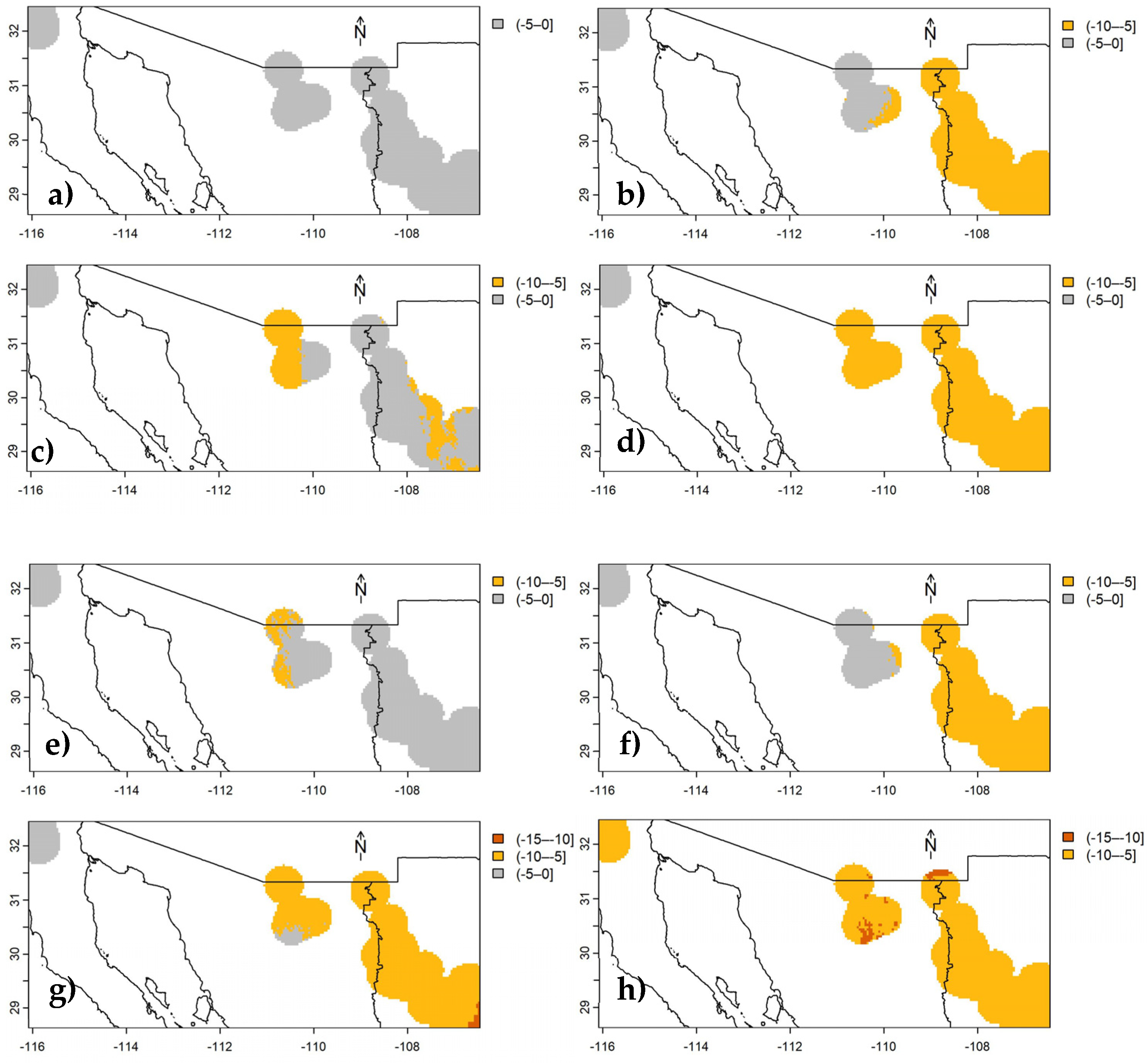
3.4. Current And Future Prediction of Carbon Density of Aboveground Live Biomass
| Stratum | Changes in cdAGB |
RCP 26 | RCP 45 | RCP 60 | RCP 85 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2050 | 2070 | 2050 | 2070 | 2050 | 2070 | 2050 | 2070 | ||
| I | (-20 – -15 |  ) ) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
(-15 – -10 |  ) ) |
0 | 0 | 0 | 0 | 0 | 35 | 0 | 107 | |
(-10 – -5 |  ) ) |
0 | 240 | 2361 | 2280 | 906 | 2885 | 3011 | 3180 | |
(-5 – 0 |  ) ) |
3287 | 3047 | 926 | 1007 | 2381 | 367 | 276 | 0 | |
(0 – 5 |  ) ) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| II | (-20 – -15 |  ) ) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 1148 |
(-15 – -10 |  ) ) |
0 | 30 | 6 | 1526 | 0 | 3928 | 4796 | 22466 | |
(-10 – -5 |  ) ) |
17750 | 19473 | 23821 | 22317 | 23571 | 19912 | 19047 | 229 | |
(-5 – 0 |  ) ) |
6093 | 4340 | 16 | 0 | 272 | 3 | 0 | 0 | |
(0 – 5 |  ) ) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| III | (-20 – -15 |  ) ) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
(-15 – -10 |  ) ) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
(-10 – -5 |  ) ) |
0 | 6 | 0 | 0 | 0 | 0 | 1290 | 3281 | |
(-5 – 0 |  ) ) |
9680 | 9819 | 9825 | 9825 | 9825 | 9822 | 8535 | 6544 | |
(0 – 5 |  ) ) |
145 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | |
3.5. Evaluation of Climate Projections on Variables Predicting cdAGB
4. Discussion
4.1. Predictor Variables of Aboveground Biomass
4.2. Correlation Between Bioclimatic Variables and Carbon Density
4.3. Predictive Capacity of Bioclimatic Models
4.4. Current and Future Projection of cdAGB
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Bioclimatic Variables
| Variable | Description | Units | Scale |
| Bio 1 | Annual Mean Temperature | °C | Annual |
| Bio 2 | Mean Diurnal Range (max temp - min temp) | °C | Monthly |
| Bio 3 | Isothermality (Bio02/Bio07) (×100) | % | Annual |
| Bio 4 | Temperature Seasonality (standard deviation × 100) | % | Annual |
| Bio 05 | Max Temperature of Warmest Month | °C | Monthly |
| Bio 06 | Min Temperature of Coldest Month | °C | Monthly |
| Bio 07 | Temperature Annual Range (Bio5–Bio6) | °C | Annual |
| Bio 08 | Mean Temperature of Wettest Quarter | °C | Quarterly |
| Bio 09 | Mean Temperature of Driest Quarter | °C | Quarterly |
| Bio 10 | Mean Temperature of Warmest Quarter | °C | Quarterly |
| Bio 11 | Mean Temperature of Coldest Quarter | °C | Quarterly |
| Bio 12 | Annual Precipitation | mm | Annual |
| Bio 13 | Precipitation of Wettest Month | mm | Monthly |
| Bio 14 | Precipitation of Driest Month | mm | Monthly |
| Bio 15 | Precipitation Seasonality (Coefficient of variation) | % | Quarterly |
| Bio 16 | Precipitation of Wettest Quarter | mm | Quarterly |
| Bio 17 | Precipitation of Driest Quarter | mm | Quarterly |
| Bio 18 | Precipitation of Warmest Quarter | mm | Quarterly |
| Bio 19 | Precipitation of Coldest Quarter | mm | Quarterly |
References
- FAO, 2022. El Estado de Los Bosques Del Mundo 2022; FAO, 2022; ISBN 978-92-5-136479-6.
- Arasa-Gisbert, R.; Vayreda, J.; Román-Cuesta, R.M.; Villela, S.A.; Mayorga, R.; Retana, J. Forest Diversity Plays a Key Role in Determining the Stand Carbon Stocks of Mexican Forests. For Ecol Manage 2018, 415–416, 160–171. [CrossRef]
- Rodríguez-Veiga, P.; Saatchi, S.; Tansey, K.; Balzter, H. Magnitude, Spatial Distribution and Uncertainty of Forest Biomass Stocks in Mexico. Remote Sens Environ 2016, 183, 265–281. [CrossRef]
- Guo, Y.; Peng, C.; Trancoso, R.; Zhu, Q.; Zhou, X. Stand Carbon Density Drivers and Changes under Future Climate Scenarios across Global Forests. For Ecol Manage 2019, 449. [CrossRef]
- Ma, Y.; Eziz, A.; Halik, Ü.; Abliz, A.; Kurban, A. Precipitation and Temperature Influence the Relationship between Stand Structural Characteristics and Aboveground Biomass of Forests—A Meta-Analysis. Forests 2023, 14.
- IPCC, 2021. In Climate Change 2021 – The Physical Science Basis; Cambridge University Press, 2021; pp. 3–32.
- 2024; 7. MEXICO CLIMATE RISK COUNTRY PROFILE; 2024;
- Bennett, A.C.; Penman, T.D.; Arndt, S.K.; Roxburgh, S.H.; Bennett, L.T. Climate More Important than Soils for Predicting Forest Biomass at the Continental Scale. Ecography 2020, 43, 1692–1705. [CrossRef]
- Cysneiros, V.C.; Coelho de Souza, F.; Gaui, T.D.; Pelissari, A.L.; Orso, G.A.; Machado, S. do A.; de Carvalho, D.C.; Silveira-Filho, T.B. Integrating Climate, Soil and Stand Structure into Allometric Models: An Approach of Site-Effects on Tree Allometry in Atlantic Forest. Ecol Indic 2021, 127. [CrossRef]
- Gao, J.; Zhao, P.; Shen, W.; Rao, X.; Hu, Y. Physiological Homeostasis and Morphological Plasticity of Two Tree Species Subjected to Precipitation Seasonal Distribution Changes. Perspect Plant Ecol Evol Syst 2017, 25, 1–19. [CrossRef]
- Yuan, J.; Yan, Q.; Wang, J.; Xie, J.; Li, R. Different Responses of Growth and Physiology to Warming and Reduced Precipitation of Two Co-Existing Seedlings in a Temperate Secondary Forest. Front Plant Sci 2022, 13. [CrossRef]
- Xin, S.; Wang, J.; Mahardika, S.B.; Jiang, L. Sensitivity of Stand-Level Biomass to Climate for Three Conifer Plantations in Northeast China. Forests 2022, 13. [CrossRef]
- Eamus, D. How Does Ecosystem Water Balance Affect Net Primary Productivity of Woody Ecosystems? Functional Plant Biology 2003, 30, 187–205. [CrossRef]
- Liu, Y.; Yu, G.; Wang, Q.; Zhang, Y. How Temperature, Precipitation and Stand Age Control the Biomass Carbon Density of Global Mature Forests. Global Ecology and Biogeography 2014, 23, 323–333. [CrossRef]
- Sim-Hee Han 2012.
- Devi, V.; Kaur, A.; Sethi, M.; Avinash, G. Perspective Chapter: Effect of Low-Temperature Stress on Plant Performance and Adaptation to Temperature Change;
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, Distribution, and Biomass of the World’s Forests. Annu Rev Ecol Evol Syst 2013, 44, 593–622.
- Keith, H.; Mackey, B.G.; Lindenmayer, D.B. Re-Evaluation of Forest Biomass Carbon Stocks and Lessons from the World’s Most Carbon-Dense Forests. Proc Natl Acad Sci U S A 2009, 106, 11635–11640. [CrossRef]
- He, X.; Lei, X.; Zeng, W.; Feng, L.; Zhou, C.; Wu, B. Quantifying the Effects of Stand and Climate Variables on Biomass of Larch Plantations Using Random Forests and National Forest Inventory Data in North and Northeast China. Sustainability (Switzerland) 2022, 14. [CrossRef]
- Chen, X.; Luo, M.; Larjavaara, M. Effects of Climate and Plant Functional Types on Forest Above-Ground Biomass Accumulation. Carbon Balance Manag 2023, 18. [CrossRef]
- Dai, L.; Ke, X.; Guo, X.; Du, Y.; Zhang, F.; Li, Y.; Li, Q.; Lin, L.; Peng, C.; Shu, K.; et al. Responses of Biomass Allocation across Two Vegetation Types to Climate Fluctuations in the Northern Qinghai–Tibet Plateau. Ecol Evol 2019, 9, 6105–6115. [CrossRef]
- Khan, D.; Muneer, M.A.; Nisa, Z.U.; Shah, S.; Amir, M.; Saeed, S.; Uddin, S.; Munir, M.Z.; Lushuang, G.; Huang, H. Effect of Climatic Factors on Stem Biomass and Carbon Stock of Larix Gmelinii and Betula Platyphylla in Daxing’anling Mountain of Inner Mongolia, China. Advances in Meteorology 2019, 2019. [CrossRef]
- David, H.C.; de Araújo, E.J.G.; Morais, V.A.; Scolforo, J.R.S.; Marques, J.M.; Péllico Netto, S.; MacFarlane, D.W. Carbon Stock Classification for Tropical Forests in Brazil: Understanding the Effect of Stand and Climate Variables. For Ecol Manage 2017, 404, 241–250. [CrossRef]
- López-Serrano, P.M.; Domínguez, J.L.C.; Corral-Rivas, J.J.; Jiménez, E.; López-Sánchez, C.A.; Vega-Nieva, D.J. Modeling of Aboveground Biomass with Landsat 8 Oli and Machine Learning in Temperate Forests. Forests 2020, 11. [CrossRef]
- Rezashobairi, S.; Lin, H.; Usoltsev, V.; Osmirko, A.; Tsepordey, I.; Ye, Z.; Anees, S. A Comparative Pattern for Populus Spp. and Betula Spp. Stand Biomass in Eurasian Climate Gradients. Croatian Journal of Forest Engineering 2022, 43, 457–467. [CrossRef]
- Stegen, J.C.; Swenson, N.G.; Enquist, B.J.; White, E.P.; Phillips, O.L.; Jørgensen, P.M.; Weiser, M.D.; Monteagudo Mendoza, A.; Núñez Vargas, P. Variation in Above-Ground Forest Biomass across Broad Climatic Gradients. Global Ecology and Biogeography 2011, 20, 744–754. [CrossRef]
- Girón-Gutiérrez, D.; Méndez-González, J.; Osorno-Sánchez, T.G.; Cerano-Paredes, J.; Soto-Correa, J.C.; Cambrón-Sandoval, V.H. Climate as a Driver of Aboveground Biomass Density Variation: A Study of Ten Pine Species in Mexico. Forests 2024, 15, 1160. [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature Drives Global Patterns in Forest Biomass Distribution in Leaves, Stems, and Roots. Proc Natl Acad Sci U S A 2014, 111, 13721–13726. [CrossRef]
- Khan, D.; Muneer, M.A.; Nisa, Z.U.; Shah, S.; Amir, M.; Saeed, S.; Uddin, S.; Munir, M.Z.; Lushuang, G.; Huang, H. Effect of Climatic Factors on Stem Biomass and Carbon Stock of Larix Gmelinii and Betula Platyphylla in Daxing’anling Mountain of Inner Mongolia, China. Advances in Meteorology 2019, 2019. [CrossRef]
- Gernandt, D.S.; Pérez-De La Rosa, J.A. Biodiversity of Pinophyta (Conifers) in Mexico. Rev Mex Biodivers 2014, 85. [CrossRef]
- Rzedowski, J.; Huerta, L. Vegetación de México; 2006;
- Granados-Sánchez, D.; López-Ríos, G.F.; Hernández-García, M.A. Sistema de Información Científica;
- Challenger, A. Utilización y Conservación de Los Ecosistemas Terrestres de México: Pasado, Presente y Futuro. 1998.
- Penman, J.; Carruthers, I.; López, C. Capítulo 1: Panorama General AUTORES Y EDITORES REVISORES Autores Principales Coordinadores Editores Revisores;
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. International Journal of Climatology 2017, 37, 4302–4315. [CrossRef]
- Li, Y.; Li, M.; Wang, Y. Forest Aboveground Biomass Estimation and Response to Climate Change Based on Remote Sensing Data. Sustainability (Switzerland) 2022, 14. [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling [Data Set]. En CRAN: Contributed Pa-Ckages. The R Foundation. 2010.
- Lê, S.; Josse, J.; Rennes, A.; Husson, F. FactoMineR: An R Package for Multivariate Analysis; 2008; Vol. 25;.
- Bivand, R., G.-R.V., & P.E. GeoStratR: Tools for Simple Stratification of Spatial Patterns. 2022.
- Kuhn, M. Journal of Statistical Software Building Predictive Models in R Using the Caret Package; 2008;
- Grömping, U. Relative Importance for Linear Regression in R: The Package Relaimpo; 2006; Vol. 17;.
- IPCC, 2013 Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (Eds.)]. 2013.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. 2024.
- Luo, M.; Wang, Y.; Xie, Y.; Zhou, L.; Qiao, J.; Qiu, S.; Sun, Y. Combination of Feature Selection and Catboost for Prediction: The First Application to the Estimation of Aboveground Biomass. Forests 2021, 12, 1–22. [CrossRef]
- Bjork, S.; Anfinsen, S.N.; Naesset, E.; Gobakken, T.; Zahabu, E. On the Potential of Sequential and Nonsequential Regression Models for Sentinel-1-Based Biomass Prediction in Tanzanian Miombo Forests. IEEE J Sel Top Appl Earth Obs Remote Sens 2022, 15, 4612–4639. [CrossRef]
- Ortiz-Reyes, A.D.; Valdez-Lazalde, J.R.; Ángeles-Pérez, G.; de los Santos-Posadas, H.M.; Schneider, L.; Aguirre-Salado, C.A.; Peduzzi, A. Synergy of Landsat, Climate and LiDAR Data for Aboveground Biomass Mapping in Medium-Stature Tropical Forests of the Yucatan Peninsula, Mexico. Revista Chapingo, Serie Ciencias Forestales y del Ambiente 2021, 27, 383–400. [CrossRef]
- Zhang, H.; Feng, Z.; Shen, C.; Li, Y.; Feng, Z.; Zeng, W.; Huang, G. Relationship between the Geographical Environment and the Forest Carbon Sink Capacity in China Based on an Individual-Tree Growth-Rate Model. Ecol Indic 2022, 138. [CrossRef]
- Cartus, O.; Kellndorfer, J.; Walker, W.; Franco, C.; Bishop, J.; Santos, L.; Fuentes, J.M.M. A National, Detailed Map of Forest Aboveground Carbon Stocks in Mexico. Remote Sens (Basel) 2014, 6, 5559–5588. [CrossRef]
- Yan, M.; Xia, Y.; Yang, X.; Wu, X.; Yang, M.; Wang, C.; Hou, Y.; Wang, D. Biomass Estimation of Subtropical Arboreal Forest at Single Tree Scale Based on Feature Fusion of Airborne LiDAR Data and Aerial Images. Sustainability (Switzerland) 2023, 15. [CrossRef]
- Chen, L.; Ren, C.; Zhang, B.; Wang, Z.; Xi, Y. Estimation of Forest Above-Ground Biomass by Geographically Weighted Regression and Machine Learning with Sentinel Imagery. Forests 2018, 9. [CrossRef]
- Adhikari, H.; Heiskanen, J.; Siljander, M.; Maeda, E.; Heikinheimo, V.; Pellikka, P.K.E. Determinants of Aboveground Biomass across an Afromontane Landscape Mosaic in Kenya. Remote Sens (Basel) 2017, 9.
- Opelele, O.M.; Yu, Y.; Fan, W.; Chen, C.; Kachaka, S.K. Biomass Estimation Based on Multilinear Regression and Machine Learning Algorithms in the Mayombe Tropical Forest, in the Democratic Republic of Congo. Appl Ecol Environ Res 2021, 19, 359–377. [CrossRef]
- Wang, Z.; Yi, L.; Xu, W.; Zheng, X.; Xiong, S.; Bao, A. Integration of UAV and GF-2 Optical Data for Estimating Aboveground Biomass in Spruce Plantations in Qinghai, China. Sustainability (Switzerland) 2023, 15. [CrossRef]
- Zhou, R.; Zhang, Y.; Peng, M.; Jin, Y.; Song, Q. Effects of Climate Change on the Carbon Sequestration Potential of Forest Vegetation in Yunnan Province, Southwest China. Forests 2022, 13. [CrossRef]
- Villers-Ruiz, L.; Trejo-Vazquez, I. CLIMATE RESEARCH Clim Res Assessment of the Vulnerability of Forest Ecosystems to Climate Change in Mexico; 1997; Vol. 9;.
- Ferreira, I.J.M.; Campanharo, W.A.; Fonseca, M.G.; Escada, M.I.S.; Nascimento, M.T.; Villela, D.M.; Brancalion, P.; Magnago, L.F.S.; Anderson, L.O.; Nagy, L.; et al. Potential Aboveground Biomass Increase in Brazilian Atlantic Forest Fragments with Climate Change. Glob Chang Biol 2023, 29, 3098–3113. [CrossRef]
| Stratum | Coefficient | Estimate | 2.5 | 97.5 | Std. | T | Pr | Residual | VIF | Imp.(%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Err | value | (>|t|) | deviance | |||||||
| I | β0 (intercept) | 64.8332 | 22.7606 | 97.3943 | 23.2221 | 2.79 | 0.00772 ** | 13.246 | ||
| (n=48) | β1 (Bio 5) | -0.1897 | -0.2932 | -0.0544 | 0.0737 | -2.57 | 0.01355 * | 1.02 | 10.33 | |
| β2 (Bio 18) | 0.0586 | 0.0337 | 0.0829 | 0.0123 | 4.77 | 2.02e-05 *** | 1.02 | 8.74 | ||
| II | β0 (intercept) | 87.6362 | 66.3661 | 108.9076 | 10.4558 | 8.38 | 1.22e-15 *** | 172.17 | ||
| (n=360) | β1 (Bio 5) | -0.2572 | -0.3296 | -0.1841 | 0.0376 | -6.84 | 3.37e-11 *** | 1.03 | 8.04 | |
| β2 (Bio 12) | 0.0190 | 0.0106 | 0.0272 | 0.0037 | 5.12 | 4.91e-07 *** | 1.03 | 3.35 | ||
| III | β0 (intercept) | 26.6379 | 12.3682 | 40.4383 | 8.4994 | 3.13 | 0.00186 ** | 132.39 | ||
| (n=370) | β1 (Bio 10) | -0.0959 | -0.1571 | -0.0293 | 0.0391 | -2.45 | 0.01461 * | 1.16 | 3.28 | |
| β2 (Bio 13) | 0.0933 | 0.0708 | 0.1160 | 0.0135 | 6.93 | 1.8e-11 *** | 1.16 | 14.95 |
| Stratum | Parameter | n | Min | P25 | Mean | Median | P75 | Max | SD | CV | Shapiro | Anderson |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-value | p-value | |||||||||||
| I | cdAGB | 48 | 4.23 | 11.54 | 23.14 | 16.23 | 33.35 | 62.05 | 15.04 | 65 | 0.0001 | 0.0001 |
| Bio 5 | 27.7 | 28.7 | 29.97 | 29.6 | 30.75 | 36 | 1.76 | 5.89 | 0.0001 | 0.0001 | ||
| Bio 18 | 62 | 223 | 256.89 | 290 | 330.5 | 397 | 101.66 | 39.57 | 0.0001 | 0.0001 | ||
| II | cdAGB | 360 | 4.46 | 19.87 | 42.57 | 34.35 | 54.75 | 179.69 | 33.01 | 77.54 | 0.0001 | 0.0001 |
| Bio 5 | 17.5 | 22.78 | 25.72 | 25.3 | 28.7 | 34.6 | 3.93 | 15.28 | 0.0001 | 0.0001 | ||
| Bio 12 | 426 | 895.25 | 1109.42 | 1092 | 1314.25 | 2216 | 367.25 | 33.1 | 0.0001 | 0.0001 | ||
| III | cdAGB | 370 | 3.15 | 12.76 | 26.26 | 23.24 | 35.98 | 92.92 | 16.56 | 63.05 | 0.0001 | 0.0001 |
| Bio 10 | 14.7 | 17.4 | 18.55 | 18.3 | 19.4 | 26.3 | 1.74 | 9.39 | 0.0001 | 0.0001 | ||
| Bio13 | 53 | 154 | 184.03 | 181 | 219 | 357 | 52.54 | 28.55 | 0.0001 | 0.0001 |
| Stratum | Method | Set | n | Pseudo R2 | RMSE | MAE |
|---|---|---|---|---|---|---|
| Training | 48 | |||||
| I | LOOCV | Validation | 12 | 0.031 | 38.319 | 30.806 |
| CV | Validation | 12 | 0.177 | 29.908 | 29.379 | |
| RCV | Validation | 12 | 0.177 | 31.949 | 31.272 | |
| II | Bootstrap | Validation | 12 | 0.316 | 42.426 | 34.457 |
| Training | 360 | |||||
| LOOCV | Validation | 90 | 0.128 | 29.938 | 21.789 | |
| CV | Validation | 90 | 0.249 | 28.493 | 21.720 | |
| RCV | Validation | 90 | 0.246 | 28.620 | 21.685 | |
| Bootstrap | Validation | 90 | 0.150 | 30.107 | 22.302 | |
| III | Training | 370 | ||||
| LOOCV | Validation | 92 | 0.153 | 13.887 | 10.699 | |
| CV | Validation | 92 | 0.231 | 13.181 | 10.543 | |
| RCV | Validation | 92 | 0.238 | 13.330 | 10.600 | |
| Bootstrap | Validation | 92 | 0.192 | 14.175 | 10.986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





