1. Introduction
According to the 2022-23 European Centre for Disease Prevention and Control (ECDC) Point Prevalent Study, bloodstream infections (BSI) represent almost 18.0% of all healthcare-associated infections (HAI) in Intensive Care Units (ICU) across Europe [
1]. It is therefore important that BSIs be diagnosed accurately.
Although novel, non-culture, methods for the detection of bacteraemia are evolving, blood cultures (BC) remain the gold standard against which all new methods are compared [
2,
3]. Timely and accurate reporting of microbiologic data from positive BCs improves clinical outcomes and reduces healthcare costs [
4]. In fact, obtaining BCs before antibiotic administration is one of five elements of the “Hour-1 Bundle for initial resuscitation for sepsis and septic shock” [
5]. Thus, BCs are among the most frequently performed and clinically important tests in microbiology. Unfortunately, contamination of BCs is a frequent problem, with reported rates ranging from 0.8% to 30% depending on the setting and other factors [
6,
7]. Blood culture contamination has multiple unwanted effects, such as additional unnecessary testing (i.e. to confirm the presence and to investigate the origin of the spurious BSI), increased antimicrobial exposure and inappropriate hospital admissions, leading to increased laboratory workload and healthcare cost [
8,
9]. Several interventions to reduce BCs contamination are supported by evidence, e.g. appropriate selection and preparation of venipuncture site, sterile technique during venipuncture and inoculation of blood in the BC vials, collection of appropriate blood volume and expedited transport to the laboratory [
8,
10]. Although there are relatively few studies on BC collection care bundles, they are suggested as a quality improvement intervention [
11,
12,
13,
14]. While the effectiveness of some care bundles, e.g. central venous catheter insertion and prevention, has been repeatedly confirmed in practice [
15], Greece ranks above the 75th percentile of the EU/EE countries with 69.4 BC sets drawn per 1000 patient-days [
16]. The success of care bundles is, at least in part, context-dependent, and requirements in terms of organizational culture and staffing are probably critical to successful care bundle implementation [
17,
18].Therefore, we designed this study to examine prospectively the effect of the implementation of a relevant care bundle on BCs contamination rates in our setting which was a high workload ICU, with high rates of BCs and limited experience on the implementation of care bundles..
2. Results
2.1. Patient characteristics
Data were collected from 419 patients in PRE and from 328 patients in POST. Demographic and clinical characteristics of patients are presented in
Table 1. Blood cultures (BCs) were drawn from 255/419 (60.9%) patients in PRE, and from 211/328 (64.3%) patients in POST (Chi squared=0.944, p= 0.33). Mortality rates were similar in both phases (37.8% in PRE and 34.1% in POST), as were Charlson, APACHE II, and SOFA scores upon admission. The only difference between the study phases was the frequency of wound drainage tubes, which was larger in the PRE phase PRE 102/419 (24.3%) vs POST 59 (18.0%), p=0.036]. The rates of infections between the two phases were similar.
2.2. Blood culture information
During the study, a total of 4236 BC vials were collected (2050 in PRE and 2186 in POST phase). Of the 1763 complete BC sets, 989/1763 (56.1%) were paired BC sets (464 in the PRE and 525 in the POST phase), while 443/4236 (10.4%) vials were solitary. Details of the BC yield are shown in
Table 2. The most common indication for obtaining BCs overall was increased CRP (1647/2206 BC sets, 74.7%), followed by any of leukocytosis, leukopenia or neutropenia (1432/2206, 64.9%). Fever was the indication for BC in only 904/2206 (41.0%) BC sets, while in 80/2206 (3.6%) BC sets there was no clear indication. Some indications were significantly more frequent in the PRE phase (CVC change and previously positive BC), while increased CRP was more frequent in the POST phase (See
supplementary Table 1 for details).
2.3. Contaminated and indeterminate blood cultures
There were 314/1763 (17.8%) positive BC sets and 61/443 (13.8%) positive solitary BC vials. Of the 114 BC sets with a common commensal (5.2% of all BC sets), 36 (1.6%) were classified as CBC and 78 (3.5%) as IBC (see
Table 2). Contamination rate decreased significantly after the intervention from 6.2% (29/464) to 1.3% (7/525), [Chi-square 16.98, p<0.0001, relative risk=0.21 (95% confidence interval 0.09-0.47)]. The proportion of indeterminate BC sets was also significantly lower in the POST phase [PRE 65/1210 (5.2%) vs 15/996 (1.5%), relative risk=0.28 (95% confidence interval 0.16-0.50), chi-square 21.93, p<0.001]. The contamination rate by pooling CBC and IBC sets, again it was lower for the POST phase [PRE 92/1210 (7.6%) vs 22/996 (2.2%), relative risk=0.28 (95% CI 0.17-0.50), chi-square 32.44 p<0.0001].
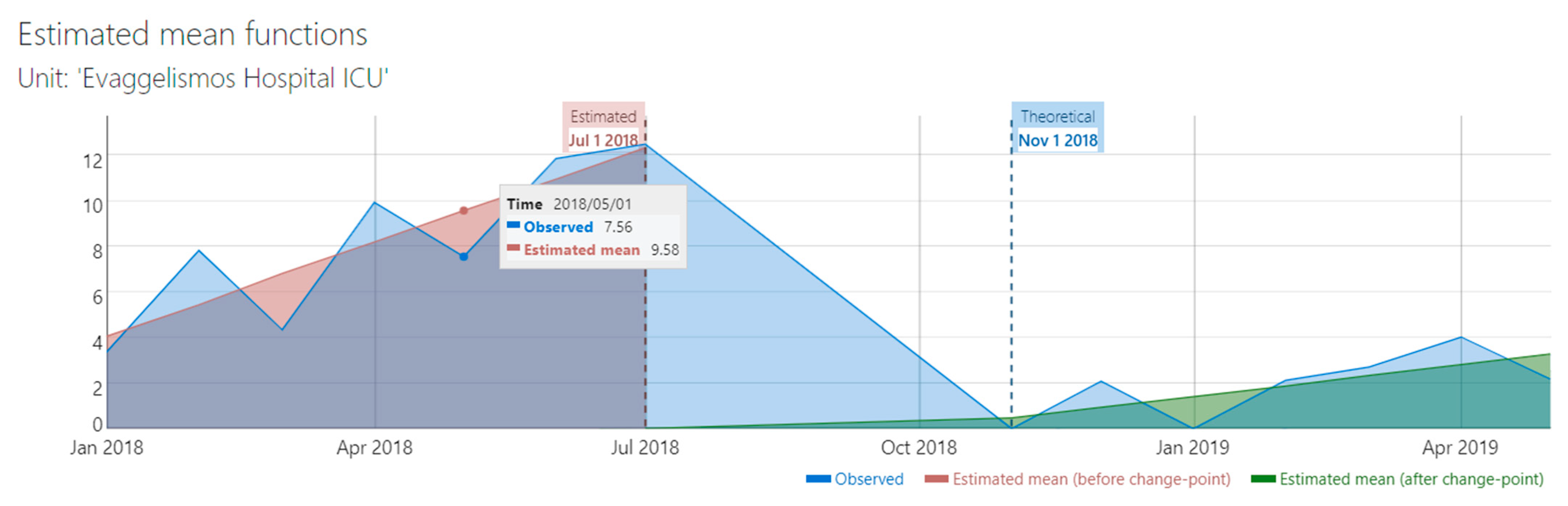
The duration of the study was relatively short for a robust interrupted time series analysis (ITS). However, a preliminary ITS analysis which included CBC and IBC sets, showed that there was a significant change in trend after the implementation of the intervention (from 1.38 to 0.47, difference -0.91, 95% CI -1.46 to -0.36,
Figure 1). Supremum Wald test confirmed the presence of a change point in the series (p=6.03 x 10-9).
The incidence rate of CBC sets was significantly lower in the POST phase: 0.461 vs 0.154 BC sets per 100 ICU bed-days (Rate difference = -0,307, 95% confidence interval = -0.527 to -0,086). Indeterminate BC incidence rate was also significantly lower in the POST phase:1.00 vs 0.330 (Rate difference = -0.671, 95% confidence interval = -0.996 to -0.347).
Overall, CBC and IBC sets represented respectively 7.4% (36/489) and 16.0% (78/489) of BC sets yielding any microorganism (pathogen or commensal). The proportion of CBC sets was significantly lower in the POST phase: 29/308 (9.4%) vs 7/181 (3.9%), Chi-square 5.14, p=0.023. Similarly, the proportion of IBC sets was also lower in the POST phase: 63/308 (20.4%) vs 15/181 (8.3%), Chi-square 12.59, p=0.0004.
2.4. Quality indicators
The proportion of paired BC sets increased significantly after the intervention from 464/882 (52.6%) to 525/881 (59.6%) [chi-square=8.7, p=0.0031, relative risk=1,13 with 95% confidence interval 1.04-1.23]. The proportion of solitary BC vials decreased significantly from 328/2050 (16.0 %) to 115/2186 (5.3%), chi-square 130.0, p<0.0001.
The appropriate blood volume per BC vial is at least 8 ml [
19,
20] however the proportion of BC vials with appropriate blood volume was only 709/2050 (34.6%) in the PRE phase. This proportion increased significantly to 2024/2186 (93.5%) in the POST phase (Chi-square 1614.0, p<0.001). Median vial blood volume also increased significantly from 6.40 ml (SD=2.61) to 8.73 ml (SD=1.04), t-test, p<0.0001. Further details on the blood volume per vial are shown in
Supplementary Table 2.
2.5. Care bundle compliance
Compliance to care bundle was assessed by direct observation of randomly selected BC collection events both in PRE and POST phases. Overall, we collected 275 observations: 145 in PRE (100 for venipuncture and 45 for CVC blood sampling) and 130 in POST phase (95 for venipuncture and 35 for CVC). The overall compliance in the PRE phase was very low at 3.4% (5/145) but it increased to 96.9% (126/130) in the POST phase (Fisher’s exact test, p<0.0001). Details of compliance by study phase and bundle element in
supplementary Tables 3 and 4.
2.6. Factors associated with BC contamination
To analyze risk factors for BC contamination we used a case-control design, in which the negative BC sets were the controls. We run two analyses, one in which cases included CBC sets only and one in which cases included CBC and IBC sets. Positive (BC sets n=375) were not included in any risk factor analysis [
21].
For the analysis of different indications for BC, we included all BC sets for each patient (n=1831). There was no association between CBC and the different indications for BC. However, the risk of CBC or IBC was lower in increased CRP [74/1363 (5.4%) vs 40/468 (8.5%) relative risk=1.40, 95% CI 1.07 – 1.80, chi-square=5.8 p=0.02). In contrast, the risk of CBC or IBC was higher in previous positive BC [34/402 (8.5%) vs 80/1428 (5.6%), relative risk=1.11, 95% CI 1.006 – 1.28, chi-square=4.4 p=0.036). The complete results are shown in
Supplementary Tables 5 and 6. Contaminated BC sets were associated with lower BC volumes [CBC, median =6.0 ml (interquartile range=3.0 ml) vs Negative BC, median=8.0 ml (interquartile range=4.0 ml), Mann-Whitney test p=0.007]. There was no association between contamination and the interval in days between hospital or ICU admission and BC day (see
Supplementary Table 7 for details).
Patient-specific variables included demographic variables, underlying diseases and comorbidities, pharmaceutical and other interventions, clinical status assessment scales (e.g. APACHE, SOFA) and laboratory parameters (e.g. albumin and C-reactive protein). For these variables we planned to analyse only the initial BC set for each patient [
22]. This dataset included 423 patients (236 from PRE phase and 187 from POST phase), of which 6/423 (1.4%) had contaminated initial BC, 16/423 (3.8%) had indeterminate initial BC result and 401/423 (94.8%) had a negative initial BC set. However, the analysis of CBC vs negative BC sets, did not reveal any associations, possibly due to the low percentage of CBCs (details in
Supplementary Table 8). We repeated the analysis for CBC or IBC vs negative BC. We only found that patients on chronic hemodialysis had significantly higher risk of CBC or IBC: 4/13 (23.5%) vs 18/388 (4.4%), Fisher’s exact test p=0.008, odds ratio=6.6 (95% CI 1.96-22.38). No other significant association was found (see
Table 3).
3. Discussion
Blood culture contamination is associated with increased laboratory workload (increased number of BCs) and healthcare cost (increased antibiotic exposure) thus it is important BC contamination rate to be reduced [
8,
10]. In the present study we have found that the introduction of the bundle in the study ICU was associated with a significant reduction in the BC contamination rate from 6.2% to 1.3%. Although the study had the limitations of the before-after design, the effect of the care bundle was confirmed by the significant reductions in the incidence rate (per 100 ICU bed-days) of both CBC and IBC sets, and by a preliminary ITS analysis (
Figure 1). Our results are very similar with those of Kai et al. who implemented a BC care bundle in the Emergency Department [
11]. Minami et al., in a retrospective study, found a similar effect after the implementation of a BC care bundle, across a large hospital [
12]. Our study was conducted exclusively in an ICU; thus, one may conclude that BC care bundles are effective across practically all hospital departments.
The intervention was associated with improvement in all other BC quality indicators such as proportion of paired and complete BC sets, proportion of solitary BC vials, proportion of BC vials with appropriate blood volume and mean vial blood volume (see RESULTS). The overall compliance with the BC care bundle increased enormously after the intervention, from 3.4% to 96.9%. This increase is too large to be real. There should probably be a strong Hawthorne effect, as the observation was overt [
27]. A recent study in an ICU found that the difference between overt and covert observations of the hand hygiene compliance could be as high as 25% [
28]. It should also be noted the POST period was relatively short and it did not allow us to assess how sustained was the impact of the intervention. Unfortunately, we could not reassess the BC care bundle compliance after the end of the study.
It is important to note that the study ICU was characterized by a baseline BC contamination rate of 6.2%, which is clearly above the acceptable range of <3% [
2,
8]. The low overall compliance with the BC bundle, the many solitary vials (328/2050, 16.0 %), and the low proportion of paired BS sets (56.1%) in the PRE phase, all point to poor BC collection practices. Another characteristic of our setting was the increased proportion of IBC sets (5.2%, 63/1210) at baseline, which may represent a not fully recognized problem. The management of a CBC set is rather straightforward, as most laboratories do not perform susceptibility testing, and the clinician can relatively safely conclude that there is no infection, at least from the isolated common commensal [
29,
30]. In contrast, the management of an indeterminate BC result requires a decision based on clinical judgement, and this might lead to full BC workup with susceptibility testing and subsequent antimicrobial use [
21]. To our knowledge, there is no literature on the significance of IBC sets. In our study the number of IBC sets was much larger than the CBC sets, but we had no detailed data regarding antimicrobial use, thus we could not investigate possible differences between patients with CBC and IBC sets. Indeterminate classification is a consequence of the BC set being single, therefore the proportion of single BC sets might be another important indicator of BC collection quality. Current CDC guidance suggests the calculation of single-set BC rate at least monthly in conjunction with the contaminated BC rate, as a quality sub-measure [
23]. A similar suggestion has been made specifically for the Emergency Department by Hills et al [
31]. In our opinion, the indeterminate BC set rate, calculated as the number of indeterminate BC sets divided by the number of single-set BC might be more useful than single-set BC rate, as it expresses more accurately the BC sets that might lead to excess laboratory workload and antimicrobial use.
Our study was not designed to study risk factors for BC contamination, and the small sample size and the low number of outcomes suggests that the power of the study was inadequate to establish associations with patient-specific factors [
25,
26]. Furthermore, we had no data regarding BC collection practices and compliance with the BC care bundle for individual BC collection events. Therefore, we could not assess these variables as risk factors for contamination. The only BC-specific risk factors that we could assess were the indication for the BC collection and the blood volume. We found that lower blood volumes were associated with CBC sets, as reported in the literature [
32].
Our study has several limitations; perhaps the more important is that the duration of the study did not allow us to have enough data time points to perform a robust ITS analysis. However, a preliminary ITS analysis suggested that the improvement in the outcomes studied was indeed associated with the implementation of the BC care bundle. Second, we had no data to associate BC bundle compliance in an individual BC collection procedure with the presence of contamination in the collected BC sets. This has been documented by Minami et al in a retrospective study [
12]. Third, the studied ICU had poor BC collection quality indicators at baseline, therefore the margin for improvement was very large. The observed effects of the intervention might not be expected across settings with e.g. better baseline BC collection quality. Finally, we were not able to document in the long term the duration and the size of the effect of the intervention.
4. Materials and Methods
4.1. Setting
The study was conducted at the intensive care unit (ICU) of Evaggelismos Hospital, a 945-bed university affiliated tertiary care hospital in Athens, Greece. The ICU has 30 beds, with 89% bed occupancy rate and 9.750 patient-days per year.
4.2. Study design
The study was quasi-experimental with a before-after design: a pre-intervention phase (PRE, 01 January 2018 to 31 July 2018), an implementation interval (01 August 2018 to 31 October 2018) and a post-intervention phase (POST, 01 November 2018 to 31 May 2019). To analyze risk factors for BC contamination we used a case-control design, where contaminated or contaminated plus indeterminate BC sets were classified as cases and the negative BC sets were the controls.
The study protocol was approved by the Institutional Review Board of the hospital (No. 302/04-12-2017).
4.3. Inclusion / exclusion criteria
All BCs obtained from patients >18 years old in the ICU during the PRE and POST phase were included in the study. If multiple BCs had been collected from one patient, they were all included. Blood cultures obtained from patients before they were transferred to the ICU were excluded.
4.4. Data collection
Clinical data were collected from the medical records and included demographic data, comorbidities, presence of various devices (such as vascular stents etc), therapeutic interventions (e.g. immunosuppressive drugs), vital signs and laboratory results at different time points. We also calculated Acute Physiology and Chronic Health Evaluation II (APACHE II) and the Sequential Organ Failure Assessment (SOFA) score on admission and on the day of blood culture.
4.5. Intervention: Care bundle implementation.
To support the implementation of the care bundle we performed various educational activities for the healthcare workers (physicians, nurses, and nursing assistants) of the ICU. The activities were in-person training sessions, focused on the correct technique of obtaining BCs and the “Care bundle to obtain BCs”. These activities were supported by written material which included a detailed protocol for obtaining BCs, a leaflet outlining the care bundle and the care bundle checklist. We adopted the “Care bundle to obtain blood cultures” of Antimicrobial Resistance and Healthcare Associated Infection Scotland, with some modifications for BCs from CVCs [
14]. The elements of care bundle are presented in
Table 1.
4.6. Definitions and outcomes
We defined as a set all BC vials drawn during the same venipuncture, including at least an aerobic vial. “Complete” was any BC set which included at least two vials. When only anaerobic or fungal BC vials were collected, they were classified as “solitary” BC vials. When a second BC set had been obtained within 48 hours of the initial BC set, the set was defined as “paired”; if not, the BC set was defined as “single”.
The BC sets which yielded a common commensal were considered contaminated following the CDC guidance; however, we extended the time frame for the repeat BC to 48 (instead of 24) hours [
23]. Pathogenic microorganisms and common commensals were classified based on the National Healthcare Safety Network Organisms List [
24].
A set was classified as positive, negative, contaminated or indeterminate. “Positive” BC set was defined as any BC set yielding a pathogenic microorganism, or all BC sets drawn within 48 hours from the same patient, yielding the same commensal microorganism. A BC set was classified as “Negative” when no vial yielded any microorganism. “Contaminated” BC set (CBC) was defined as any set with at least one BC vial (aerobic, anaerobic or fungal) of a set yielding a common commensal, provided that a) the BC set was paired, and b) the particular organism had not been isolated from another of these BC sets. As “Indeterminate” BC set (IBC) was defined any BC set yielding a common commensal when the set was single [
25].
The primary outcome was contamination rate calculated by dividing the number of CBC sets divided by the number of paired BC sets [
23]. Secondary outcomes included IBC set rate and pooled CBC or IBC sets rate (both in relation to all BC sets obtained), incidence rate of CBC and IBC sets per 100 ICU bed-days, proportion of CBC and IBC sets as % of BC sets which yielded a microorganism, and compliance with the care bundle (overall and for each element). We compared all outcomes in the PRE and POST phases.
To investigate risk factors for contamination among BC set-specific variables (e.g. indication, day of ICU hospitalization, blood volume) we have analysed BC sets, excluding positive and indeterminate BC sets. For patient-specific variables (e.g. age, sex, underlying diseases etc) we have analysed only the initial BC set of each patient (REF). Again, we excluded positive BC sets; however, as the number of patients with contaminated initial BC set was low (n=6), we performed the analysis both excluding Indeterminate initial BC sets and pooling Contaminated and Indeterminate initial BC sets.
4.7. Statistical analysis
For descriptive statistics, for continuous variables we calculated means and standard deviations (SD) or medians and interquartile ranges (IQR) as appropriate. For categorical variable we calculated counts and percentages. We used Pearson’s chi-square test or Fisher's exact test for nominal variables and 2-sample independent t tests or the non-parametric Mann-Whitney test for continuous variables, to investigate associations between the outcome and the different variables, as appropriate. Incidence rates of events per patient-days were calculated and compared using Poisson’s rates. Interrupted time series analysis was performed using the “Robust Interrupted Time Series” (RITS) toolbox [
26]. Significance levels were set at a two-tailed p-value of 0.05. Statistical analysis was performed using IBM SPSS Statistics version 26.0 and StatsDirect version 4.04.
5. Conclusions
The implementation of a BC care bundle was associated with a significant improvement in the BC contamination indicators as well as with improvement in almost all other BC collection quality indicators. It is noted that the poor baseline BC collection practices in the study ICU, suggests that this effect should not be expected across all settings. An important finding was the large number of indeterminate BC sets, which is associated with the respective proportion of single-set BC. Indeterminate BC sets may have an equal or a larger impact than CBC sets, and we suggest that they should be included in the BC collection quality indicators
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, M.S., F.V. and A.K.; methodology, F.V., M.S.; software, M.S.; validation, M.S., and F.V.; formal analysis, M.S; investigation, F.V., X.X., P-M.V.; data curation, F.V., and P-M.V.; writing—original draft preparation, F.V.; writing—review and editing, M.S.; supervision, A.K.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Evaggelismos Hospital (protocol code 302/04-12-2017).
Informed Consent Statement
Patient consent was waived because the study was part of a quality improvement initiative and only routinely generated data were collected, anonymously.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to legal restrictions.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest..
References
- Suetens, C.K., T.; Plachouras, D. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2022-23.; European Centre for Disease Prevention and Control.: Stockholm, 2024.
- Wilson, M.L. Principles and Procedures for Blood Cultures ( CLSI guideline M47), 2nd ed.; Clinical and Laboratory Standards Institute: 2022.
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gonzalez, M.D.; Harrington, A.; Jerris, R.C.; Kehl, S.C.; Leal, S.M., Jr.; et al. Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2024 Update by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clinical infectious diseases 2024. [CrossRef]
- Kirn, T.J.; Weinstein, M.P. Update on blood cultures: how to obtain, process, report, and interpret. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2013, 19, 513-520. [CrossRef]
- Ko, B.S.; Choi, S.H.; Shin, T.G.; Kim, K.; Jo, Y.H.; Ryoo, S.M.; Park, Y.S.; Kwon, W.Y.; Choi, H.S.; Chung, S.P.; et al. Impact of 1-Hour Bundle Achievement in Septic Shock. J Clin Med 2021, 10, 527. [CrossRef]
- Story-Roller, E.; Weinstein, M.P. Chlorhexidine versus Tincture of Iodine for Reduction of Blood Culture Contamination Rates: a Prospective Randomized Crossover Study. Journal of clinical microbiology 2016, 54, 3007-3009. [CrossRef]
- Washer, L.L.; Chenoweth, C.; Kim, H.W.; Rogers, M.A.; Malani, A.N.; Riddell, J.t.; Kuhn, L.; Noeyack, B., Jr.; Neusius, H.; Newton, D.W.; et al. Blood culture contamination: a randomized trial evaluating the comparative effectiveness of 3 skin antiseptic interventions. Infection control and hospital epidemiology 2013, 34, 15-21. [CrossRef]
- Doern, G.V.; Carroll, K.C.; Diekema, D.J.; Garey, K.W.; Rupp, M.E.; Weinstein, M.P.; Sexton, D.J. Practical Guidance for Clinical Microbiology Laboratories: A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem. Clinical microbiology reviews 2019, 33, e00009-00019. [CrossRef]
- Schinkel, M.; Boerman, A.; Carroll, K.; Cosgrove, S.E.; Hsu, Y.J.; Klein, E.; Nanayakkara, P.; Schade, R.; Wiersinga, W.J.; Fabre, V. Impact of Blood Culture Contamination on Antibiotic Use, Resource Utilization, and Clinical Outcomes: A Retrospective Cohort Study in Dutch and US Hospitals. Open forum infectious diseases 2024, 11, ofad644. [CrossRef]
- Hughes, J.A.C., C.J.; Williams, J.; Ray, M.; Coyer, F. Interventions to reduce peripheral blood culture contamination in acute care settings: A systematic review and meta-analysis. MedRxiv 2023. [CrossRef]
- Kai, M.; Miyamoto, K.; Akamatsu, K.; Tsujita, A.; Nishio, M. Effect of a bundle-approach intervention against contamination of blood culture in the emergency department. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy 2020, 26, 785-789. [CrossRef]
- Minami, K.; Yamada, T.; Yoshioka, K.; Kawanishi, F.; Ogawa, T.; Ukimura, A. Effect of the introduction of a management bundle for blood culture collection. American journal of infection control 2022, 50, 772-776. [CrossRef]
- Murphy, T.; Maile, D.; Barsch, T.; Jerdan, F. Investigating the impact of blood culture bundles on the incidence of blood culture contamination rates. J Infus Nurs 2014, 37, 205-210. [CrossRef]
- What are the key infection prevention and control recommendations to inform a prevention of blood culture contamination. Antimicrobial Resistance and Healthcare Associated Infection Scotland. Available online: https://www.nss.nhs.scot/media/2276/3_blood-culture-review-v3.pdf (accessed on 02/09/2024).
- Ista, E.; van der Hoven, B.; Kornelisse, R.F.; van der Starre, C.; Vos, M.C.; Boersma, E.; Helder, O.K. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis 2016, 16, 724-734. [CrossRef]
- Country factsheet Greece: Key indicators. Point prevalence survey of healthcare-associated infections and antimicrobial use in acute care hospitals, 2022-2023. Available online: https://www.ecdc.europa.eu/en/publications-data/country-factsheet-greece (accessed on 02/09/2024).
- Gilhooly, D.; Green, S.A.; McCann, C.; Black, N.; Moonesinghe, S.R. Barriers and facilitators to the successful development, implementation and evaluation of care bundles in acute care in hospital: a scoping review. Implement Sci 2019, 14, 47. [CrossRef]
- Karapanou, A.; Vieru, A.M.; Sampanis, M.A.; Pantazatou, A.; Deliolanis, I.; Daikos, G.L.; Samarkos, M. Failure of central venous catheter insertion and care bundles in a high central line-associated bloodstream infection rate, high bed occupancy hospital. American journal of infection control 2020, 48, 770-776. [CrossRef]
- Preventing Adult Blood Culture Contamination: A Quality Tool for Clinical Laboratory Professionals. Available online: https://www.cdc.gov/labquality/blood-culture-contamination-prevention.html (accessed on 14-09-2024).
- BACTEC™ Standard/10 Aerobic/F Culture Vials Instructions for use. Available online: http://static.bd.com/documents/eifu/500017524_ZMG_E_RL_500017524.pdf (accessed on 02-09-2024).
- Klucher, J.M.; Davis, K.; Lakkad, M.; Painter, J.T.; Dare, R.K. Risk factors and clinical outcomes associated with blood culture contamination. Infection control and hospital epidemiology 2022, 43, 291-297. [CrossRef]
- Liaquat, S.; Baccaglini, L.; Haynatzki, G.; Medcalf, S.J.; Rupp, M.E. Patient-specific risk factors contributing to blood culture contamination. Antimicrob Steward Healthc Epidemiol 2022, 2, e46. [CrossRef]
- Blood Culture Contamination - An Overview for Infection Control and Antibiotic Stewardship Programs Working with the Clinical Laboratory. Available online: https://www.cdc.gov/antibiotic-use/core-elements/pdfs/fs-bloodculture-508.pdf (accessed on 02-09-2024).
- Common commensals - National Health Safety Network Organism List. Available online: https://www.cdc.gov/nhsn/xls/master-organism-com-commensals-lists.xlsx (accessed on 02-09-2024).
- Weinstein, M.P. Blood culture contamination: persisting problems and partial progress. Journal of clinical microbiology 2003, 41, 2275-2278. [CrossRef]
- Cruz, M.; Pinto-Orellana, M.A.; Gillen, D.L.; Ombao, H.C. RITS: a toolbox for assessing complex interventions via interrupted time series models. BMC Med Res Methodol 2021, 21, 143. [CrossRef]
- Chen, L.F.; Vander Weg, M.W.; Hofmann, D.A.; Reisinger, H.S. The Hawthorne Effect in Infection Prevention and Epidemiology. Infection control and hospital epidemiology 2015, 36, 1444-1450. [CrossRef]
- Bruchez, S.A.; Duarte, G.C.; Sadowski, R.A.; Custodio da Silva Filho, A.; Fahning, W.E.; Belini Nishiyama, S.A.; Bronharo Tognim, M.C.; Cardoso, C.L. Assessing the Hawthorne effect on hand hygiene compliance in an intensive care unit. Infect Prev Pract 2020, 2, 100049. [CrossRef]
- Richter, S.S.; Beekmann, S.E.; Croco, J.L.; Diekema, D.J.; Koontz, F.P.; Pfaller, M.A.; Doern, G.V. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory-based algorithm. Journal of clinical microbiology 2002, 40, 2437-2444. [CrossRef]
- Weinstein, M.P.; Towns, M.L.; Quartey, S.M.; Mirrett, S.; Reimer, L.G.; Parmigiani, G.; Reller, L.B. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clinical infectious diseases 1997, 24, 584-602. [CrossRef]
- Hills, A.Z.; Ray, M.; Williams, J.; Greenslade, J. Benchmarking blood culture quality in the emergency department: Contamination, single sets and positivity. Emergency medicine Australasia : EMA 2024, 36, 206-212. [CrossRef]
- Gonsalves, W.I.; Cornish, N.; Moore, M.; Chen, A.; Varman, M. Effects of volume and site of blood draw on blood culture results. Journal of clinical microbiology 2009, 47, 3482-3485. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).