Submitted:
20 October 2024
Posted:
21 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
| Korelacja porządku rang Spearmana | ||||
|---|---|---|---|---|
| N Ważnych |
R Spearman |
t(N-2) | p-Value | |
| Apgar score & time of diagnosis of CP | 133 | 0,273912 | 3,259739 | 0,001421 |
| Test U Manna-Whitneya (z poprawką na ciągłość) Względem zmiennej: skolioza neurogenna. Zaznaczone wyniki są istotne z p <,05000 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum.rang Grupa 1 |
Sum.rang Grupa 2 |
U | Z | p | Z popraw. |
p-Value | N ważn. Grupa 1 |
N ważn. Grupa 2 |
2*1str. dokł. p |
||
| GMFCS | 7064,500 | 1846,500 | 1069,500 | -1,39250 | 0,163771 | -1,45842 | 0,144726 | 109 | 24 | 0,163869 | |
| Kolumna1 | Kolumna2 | ||
|---|---|---|---|
| Chi-kwadrat (df=1) | 5,27 | p = ,0217 |
| Test U Manna-Whitneya (z poprawką na ciągłość) Względem zmiennej: podwichnięcie bioder. Zaznaczone wyniki są istotne z p <,05000 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum.rang Grupa 1 |
Sum.rang Grupa 2 |
U | Z | p | Z popraw. |
p-Value | N ważn. Grupa 1 |
N ważn. Grupa 2 |
2*1str. dokł. p |
||
| BMI | 7867,000 | 1044,000 | 744,0000 | 3,296957 | 0,000977 | 3,297007 | 0,000977 | 109 | 24 | 0,000782 | |
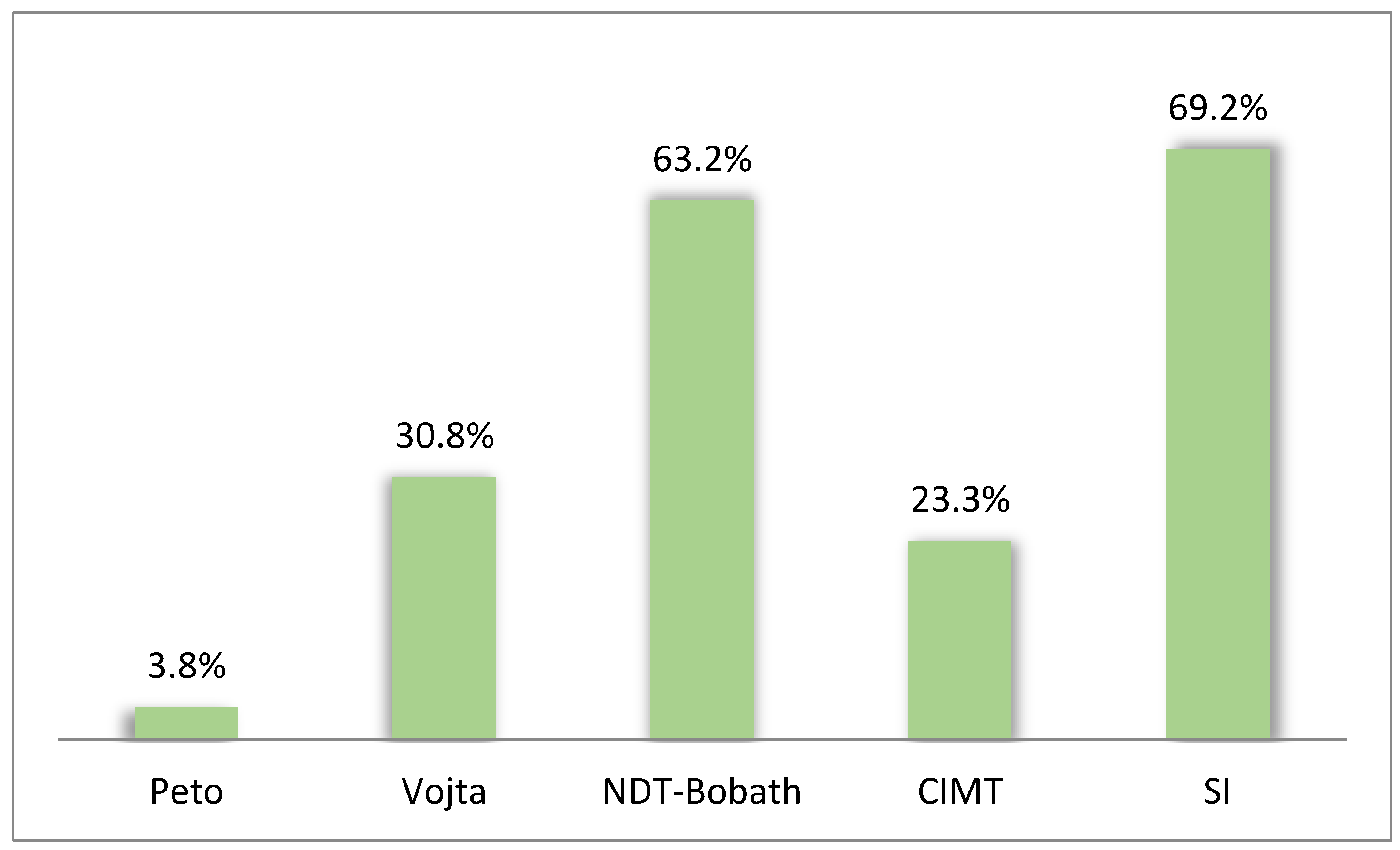
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Włodzisław Kuliński, Krzysztof Zeman (red.) przy współpracy Teresy Orlik. Fizjoterapia w Pediatrii, PZWL; Warszawa: 2014.
- Vitrikas K, Dalton H, Breish D. Cerebral Palsy: An Overview. Am Fam Physician. 2020 Feb 15;101(4):213-220. [PubMed]
- Milewska, Agnieszka, et al. “Analiza czynników ryzyka mózgowego porażenia dziecięcego.” Nowa Pediatria 4 (2011): 79-84.
- Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers. 2016 Jan 7;2:15082. [CrossRef] [PubMed] [PubMed Central]
- Krigger, KW. Cerebral palsy: an overview. Am Fam Physician. 2006 Jan 1;73(1):91-100. [PubMed]
- Sadowska M, Sarecka-Hujar B, Kopyta I. Analysis of Selected Risk Factors Depending on the Type of Cerebral Palsy. Brain Sci. 2021 Oct 30;11(11):1448. [CrossRef] [PubMed] [PubMed Central]
- Madigan RR, Wallace SL. Scoliosis in the institutionalized cerebral palsy population. Spine (Phila Pa 1976). 1981 Nov-Dec;6(6):583-90. [CrossRef] [PubMed]
- Sawin PD, Menezes AH. Neuromuscular scoliosis: diagnostic and therapeutic considerations. Semin Pediatr Neurol. 1997 Sep;4(3):224-42. [CrossRef] [PubMed]
- Persson-Bunke M, Hägglund G, Lauge-Pedersen H, Wagner P, Westbom L. Scoliosis in a total population of children with cerebral palsy. Spine (Phila Pa 1976). 20 May 2012 ;37(12):E708-13. [CrossRef] [PubMed]
- Koop, SE. Scoliosis in cerebral palsy. Dev Med Child Neurol. 2009 Oct;51 Suppl 4:92-8. [CrossRef] [PubMed]
- Hägglund, Gunnar, et al. “Incidence of scoliosis in cerebral palsy: a population-based study of 962 young individuals.” Acta Orthopaedica 89.4 (2018): 443-447.
- Kasperczyk, T. Wady postawy ciała, diagnostyka i leczenie, Wyd. FHU Kasper, Kraków, 2002.
- Vialle R, Thévenin-Lemoine C, Mary P. Neuromuscular scoliosis. Orthop Traumatol Surg Res. 2013 Feb;99(1 Suppl):S124-39. Epub 2013 Jan 19. [CrossRef] [PubMed]
- Małgorzata Domagalska-Szopa, Andrzej Szopa. Postępowanie usprawniające w mózgowym porażeniu dziecięcym, Edra Urban & Partner; Wrocław: 2023.
- Cloake T, Gardner A. The management of scoliosis in children with cerebral palsy: a review. J Spine Surg. 2016 Dec;2(4):299-309. [CrossRef] [PubMed] [PubMed Central]
- McAviney J, Roberts C, Sullivan B,. The prevalence of adult de novo scoliosis: A systematic review and meta-analysis. Eur Spine J. 2020 Dec;29(12):2960-2969. Epub 2020 May 22. [CrossRef] [PubMed]
- Koop SE, Lonstein JE, Winter RB, Denis F. The Natural History of Spine Deformity in Cerebral Palsy. Scoliosis Research Society Annual Meeting, September 1991.
- Dariusz, Białoszewski; et al. (red). Fizjoterapia w Ortopedii, PZWL; Warszawa: 2015.
- Kobylińska M, Malak R, Majewska K, Kędzia A, Samborski W. Assessment of anterior-posterior spinal curvatures in children suffering from hypopituitarism. BMC Endocr Disord. 2019 Dec 11;19(1):137. [CrossRef] [PubMed] [PubMed Central]
- Kobylińska M, Malak R, Majewska K, Fechner B, Samborski W, Kędzia A. Angle of trunk rotation in children suffering from growth hormone deficiency. Pediatria Polska - Polish Journal of Paediatrics. 2023;98(1):30-35. [CrossRef]
- Suken, A. Shah, MD Pediatric Orthopaedic and Scoliosis Surgery Department of Orthopaedics 1600 Rockland Road, PO Box 269 Wilmington, DE 19899 302-651-5904.
- Vinje, Svend, Terje Terjesen, and Thomas Kibsgård. “Scoliosis in children with severe cerebral palsy: a population-based study of 206 children at GMFCS levels III–V.” European Spine Journal 32.11 (2023): 4030-4036.
- Garg, Sumeet, et al. “The relationship of gross motor functional classification scale level and hip dysplasia on the pattern and progression of scoliosis in children with cerebral palsy.” Spine Deformity 1.4 (2013): 266-271.
- 24. Willoughby KL, Ang SG, Thomason P, Rutz E, Shore B, Buckland AJ, Johnson MB, Graham HK. Epidemiology of scoliosis in cerebral palsy: A population-based study at skeletal maturity. J Paediatr Child Health. 2022 Feb;58(2):295-301. Epub 2021 Aug 28. Erratum in: J Paediatr Child Health. 2022 Apr;58(4):743. doi: 10.1111/jpc.15943. PMID: 34453468; PMCID: PMC9291795. [CrossRef] [PubMed] [PubMed Central]
- Manikowska, Faustyna, Marek Jóźwiak, and Maciej Idzior. “Wpływ nasilenia spastyczności na możliwości funkcjonalne dziecka z mózgowym porażeniem.” Neurologia Dziecięca 18.36 (2009): 31-35.
- Hägglund, G. Association between pelvic obliquity and scoliosis, hip displacement and asymmetric hip abduction in children with cerebral palsy: a cross-sectional registry study. BMC Musculoskelet Disord. 2020 Jul 14;21(1):464. [CrossRef] [PubMed] [PubMed Central]
- Radcliff, Kristen E., et al. “Is pelvic obliquity related to degenerative scoliosis?.” Orthopaedic surgery 5.3 (2013): 171-176.
- Dalén, Ylva, et al. “Effects of standing on bone density and hip dislocation in children with severe cerebral palsy.” Advances in Physiotherapy 12.4 (2010): 187-193.
- Kraus, Manuel Johannes, Reinald Brunner, and Erich Rutz. Risk profile of bony intervention for hip displacement in our youngest cohort of children with cerebral palsy. Diss. Medizinische Fakultät der Universität Basel, 2019.
- Houdek MT, Watts CD, Wyles CC, Trousdale RT, Milbrandt TA, Taunton MJ. Total Hip Arthroplasty in Patients with Cerebral Palsy: A Cohort Study Matched to Patients with Osteoarthritis. J Bone Joint Surg Am. 2017 Mar 15;99(6):488-493. [CrossRef] [PubMed]
- Saito, Naoto, et al. “Natural history of scoliosis in spastic cerebral palsy.” The Lancet 351.9117 (1998): 1687-1692.
- Bertoncelli, Carlo M., et al. “Risk factors for developing scoliosis in cerebral palsy: a cross-sectional descriptive study.” Journal of child neurology 32.7 (2017): 657-662.
- Lin, Ching-Yueh, et al. “Long-term effect of botulinum toxin A on the hip and spine in cerebral palsy: A national retrospective cohort study in Taiwan.” PLoS One 16.7 (2021): e0255143.
- Lie, Kari Kveim, Else-Karin Grøholt, and Anne Eskild. “Association of cerebral palsy with Apgar score in low and normal birthweight infants: population based cohort study.” Bmj 341 (2010).
- Moster, Dag, et al. “The association of Apgar score with subsequent death and cerebral palsy: a population-based study in term infants.” The Journal of pediatrics 138.6 (2001): 798-803.
- Khalaji, Masoud, et al. “The effect of hydrotherapy on health of cerebral palsy patients: An integrative review.” Iranian Rehabilitation Journal 15.2 (2017): 173-180.
- Bairaktaridou, Aikaterini, et al. “The effect of hydrotherapy on the functioning and quality of life of children and young adults with cerebral palsy.” Int J Adv ResMed 3 (2021): 21-4.
- Warutkar VB, Krishna Kovela R. Review of Sensory Integration Therapy for Children With Cerebral Palsy. Cureus. 2022 Oct 26;14(10):e30714. [CrossRef] [PubMed] [PubMed Central]
- Tekin, Fatih, et al. “Effectiveness of Neuro-Developmental Treatment (Bobath Concept) on postural control and balance in Cerebral Palsied children.” Journal of back and musculoskeletal rehabilitation 31.2 (2018): 397-403.
- Senthilkumar, S., and P. Swarnakumari. “A study on Vojta therapy approach to improve the motor development of cerebral palsy children.” Int J Med Sci 4.1&2 (2011): 39-45.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





