Submitted:
18 October 2024
Posted:
21 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EPPO. Rhynchophorus ferrugienus. EPPO Global Database. European and Mediterranean Plant Protection Organization. Available online: https://gd.eppo.int/taxon/RHYCFE/distribution (accessed on 23 September 2024).
- Resolucion, no. Resolucion no. 1.079/022 DGSA aprueba plan de contingencia para Rhynchophorus ferrugineus. Ministerio de Ganadería, Agricultura y Pesca, Gobierno de Uruguay. 2022, p. 2. Available online: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/institucional/normativa/resolucion-n-1079022-dgsa-aprueba-plan-contingencia-para-rhynchophorus (accessed on 23 September 2024).
- Martin, M.M.; Cabello, T. Biologia y ecologia del curculionido rojo de la palmera, Rhynchophorus ferrugineus (Olivier, 1790) (Col.: Dryophthoridae); University of Almeria: Almeria, Spain, 2005; pp. 1–202. [Google Scholar]
- Cabello, T. Population biology and dynamics of the red palm weevil. In Rhynchophorus ferrugineus (Col.: Dryophothoridae) in Spain. First International Conference on the Red Palm Weevil; Valencia Fundacion, Agroalimed, Ed.; Generalitat Valenciana, Conselleria de Agricultura y Medio Ambiente: Valencia, Spain, 2006; pp. 19–34. [Google Scholar]
- Dembilio, O.; Jaques, J.A. Biology and management of Red Palm Weevil. In Sustainable pest management in date palm: current status and emerging challenges; Waqas, W., Faleiro, J.R., Miller, T.A., Eds.; Springer International: Cham, Switzerland, 2015; pp. 13–36. [Google Scholar] [CrossRef]
- Rochat, D.; Dembilio, O; Jaques, J.A.; Suma, P.; Pergola, A.; Hamidi, R.; Kontodimas, D.; Soroker, V. Rhynchophorus ferrugineus: taxonomy, distribution, biology, and life cycle. In: Handbook of major palm pests; Audsley, N., Soroker, V., Colazza, S., Eds.; John Willey & Sons Ltd.: Oxford, UK, 2017; pp. 69–104. [Google Scholar] [CrossRef]
- Faleiro, J.R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Col.: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sc. 2006, 26, 135–154. [Google Scholar] [CrossRef]
- Cabello, T.; de la Pena, J.; Barranco, P.; Belda, J. Laboratory evaluation of imidacloprid and oxamyl against Rhynchophorus ferrugineus. Tests of Agrochemicals and Cultivar. Annals of Applied Biology 18 Supplement 130, 6–7.
- Barranco, P.; de la Peña, J.; Martin, M.M.; Cabello, T. Efficacy of chemical control of the new palm pest Rhynchophorus ferrugineus (Curculionidae: Coleoptera). Bol. San. Veg. Plagas 1998, 24, 301–306. [Google Scholar]
- Solano-Rojas, Y.; Gamez, M.; Lopez, I.; Garay, J.; Varga, Z.; Cabello, T. Conservation strategy for palm groves: Optimal chemical control model for red palm weevil Rhynchophorus ferrugineus. Agronomy 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Bracchetti, L.; Cocci, P.; Palermo, F.A. Multiple aspects of the fight against the red palm weevil in an urban area: study case, San Benedetto del Tronto (Central Italy). Insects 2023, 14, 502. [Google Scholar] [CrossRef]
- Hernandez, D.; Folk, F.; Sanchez, A.; Fernandez, R. Control of red palm weevil (Rhynchophorus ferrugineus) using trunk injections and foliar sprays. Bol. San. Veg. Plagas 2003, 29, 563–573. [Google Scholar]
- Dembilio, O.; Riba, J.M.; Gamon, M.; Jacas, J.A. Mobility and efficacy of abamectin and imidacloprid against Rhynchophorus ferrugineus in Phoenix canariensis by different application methods. Pest Manag. Sci. 2015, 71, 1091–1098. [Google Scholar] [CrossRef]
- FAO. Red Palm Weevil: Guidelines on management practices; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 1–86. [Google Scholar] [CrossRef]
- See, A.S; Salleh, A.B.; Bakar, F. A, Yusof, N.A.; Abdulamir, A.S.; Heng, L.Y. Risk and health effect of boric acid. Am. J. Appl. Sci. 2010, 7, 620–627. [Google Scholar] [CrossRef]
- Boone, C.; Bond, C.; Stone, D. Boric Acid General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. Available online: http://npic.orst.edu/factsheets/boricgen.pdf] (accessed on 29 June 2024).
- Alarcon, F.J.; Martinez, T.F.; Barranco, P.; Cabello, T.; Diaz, M.; Moyano, F.J. Digestive proteases during development of larvae of red palm weevil, Rhynchophorus ferrugineus (Col.: Curculionidae). Insect Biochem. Molec. 2002, 32, 265–274. [Google Scholar] [CrossRef]
- Martin, M.M.; Cabello, T. Rearing management of red palm weevil, Rhynchophorus ferrugineus (Col.: Dryophthoridae), in artificial diet and effects on biology and adult biometry. Bol. San. Veg. Plagas 2006, 32, 631–641. [Google Scholar]
- Andujar, A.; Barranco, P.; Belda, J.E.; Cabello, T.; Carreño, R. Analisis de eficacia de productos fitosanitarios. Phytoma España 1997, 92, 32–42. [Google Scholar]
- Merghem, A.; Mohamed, A.A. Impact of neem extracts, Azadirachta indica induced against red palm weevil, Rhynchophorus ferrugineus attacking date palm orchards in Egypt. Egypt. Acad. J. Biolog. Sci. 2017, 9, 109–117. [Google Scholar] [CrossRef]
- Dhra, G.; Ahmad, M.; Kumar, J.; Patanjali, P.K. Mode of action of azadirachtin: a natural insecticide. Int. Res. J. Biol. Sci. 2018, 7, 41–46. [Google Scholar]
- Kilani-Morakchi, S.; Morakchi-Goudjil, H.; Sifi, K. Azadirachtin-based insecticide: overview, risk assessments, and future directions. Front. Agron. 2021, 3. [Google Scholar] [CrossRef]
- Morgan, E.D. Azadirachtin, a scientific goldmine. Bioorgan. Med. Chem. 2009, 17, 4096–4105. [Google Scholar] [CrossRef]
- Naveed, H.; Andoh, V.; Islam, W.; Chen, L.; Chen, K. Sustainable pest management in date palm ecosystems: unveiling the ecological dynamics of red palm weevil (Col.: Curculionidae) Infestations. Insects 2023, 14, 859. [Google Scholar] [CrossRef]
- Gabr, B.; Lemmons, J.M.; El-Bokl, M.M. Potential of neem oil extract against palmetto weevil larvae, Rhynchophorus cruentatus (Col.: Curculionidae) and its impact on some detoxification enzymes. J. Entomol. Acarol. Res. 2022, 54, 10470. [Google Scholar] [CrossRef]
- EPA. R.E.D. Fact Boric acid. United States Environmental Protection Agency, EPA-738-F-93-006. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/2000E7FQ.PDF?Dockey=2000E7FQ.PDF] (accessed on 2 October 2024).
- Woods, W. G. An introduction to boron: history, sources, uses and chemistry. Environ. Health Perspect. 1994, 102, 5–11. [Google Scholar]
- Cox, C. Boric acid and borates. J. Pesticide Reform 2004, 24, 10–15. [Google Scholar]
- Bernard, C.E.; Harrass, M.C.; Manning, M.J. Boric acid and inorganic borate pesticides. In Hayes’ handbook of pesticide toxicology, 3rd ed.; Krieger, R., Ed.; Elsevier: Amsterdam, NL, 2010; pp. 2033–2053. [Google Scholar] [CrossRef]
- Cochran, D.G. Toxic effects of boric acid on the german cockroach. Experientia 1995, 51, 561–63. [Google Scholar] [CrossRef]
- Habes, D.; Morakchi, S.; Aribi, N.; Farine, J.P.; Soltani, N. Boric acid toxicity to the german cockroach, blattella germanica: alterations in midgut structure, and acetylcholinesterase and glutathione s-transferase activity. Pestic. Biochem. Phys. 2006, 84, 17–24. [Google Scholar] [CrossRef]
- Klotz, J.; Amrhein, C.; McDaniel, S.; Rust, M.; Reierson, D. Assimilation and toxicity of boron in the Argentine ant (Hym.: Formicidae). J. Entomol. Sci. 2002, 37, 193–99. [Google Scholar] [CrossRef]
- Sharawi, S.E. Mortality and malformation effects of boric acid against larval stage of Aedes aegypti (Culicidae: Diptera). bioRxiv 5562. [Google Scholar] [CrossRef]
- Thompson, S.N.N.; Simpson, S.J.J. Nutrition. In Encyclopedia of Insects; Resh, V.H., Carde, R.T., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 807–813. [Google Scholar]
- Kashkouli, M.; Mehrabadi, M.; Fathipour, Y. The symbionts. In Microbial approaches for insect pest management; Omkar, *!!! REPLACE !!!*, Ed.; Springer Nature: Singapore, 2021; pp. 219–269. [Google Scholar]
- Gupta, A.; Nair, S. Dynamics of insect–microbiome interaction influence host and microbial symbiont. Front. Microbiol. 2020, 11, 1357. [Google Scholar] [CrossRef]
- Drishnan, N.; Csiszar, V.; Mori, T.F.; Garay, J. Genesis of ectosymbiotic features based on commensalistic syntrophy. Sci. Rep. 2024, 14, 1366. [Google Scholar] [CrossRef]
- Lefevre, C; Charles, H.; Vallier, A.; Delobel, B.; Farrell, B.; Heddi, A. Endosymbionte phylogenesis in the Dryophthoridae weevils: evidence for bacterial replacement. Mol. Biol. Evol. 2004, 21, 965–973. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Antony, B.; El-Shafie, H.A.F.; Chamorro, M.L.; Milosavljevic, I.; Lohr, B.; Faleiro, J.R. Taxonomy, biology, symbionts, omics, and management of Rhynchophorus palm weevils (Col.: Curculionidae: Dryophthorinae). Annu. Rev. Entomol. 2024, 69, 455–479. [Google Scholar] [CrossRef]
- Tagliavia, M.; Messina, E.; Manachini, B.; Cappello, S.; Quatrini, P. The gut microbiota of larvae of Rhynchophorus ferrugineus (Col.: Curculionidae). BMC Microbiol. 2014, 14, 136. [Google Scholar] [CrossRef]
- Habineza, P.; Muhammad, A.; Ji, T.; Xiao, R.; Yin, X.; Hou, Y.; Shi, Z. The promoting effect of gut microbiota on growth and development of red palm weevil, Rhynchophorus ferrugineus (Col.: Dryophthoridae) by modulating its nutritional metabolism. Front. Microbiol. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Butera, G.; Ferraro, C.; Colazza, S.; Alonzo, G.; Quatrini, P. The culturable bacterial community of frass produced by larvae of Rhynchophorus ferrugineus (Col.: Curculionidae) in the Canary Island date palm. Lett. Appl. Microbiol. 2012, 54, 530–536. [Google Scholar] [CrossRef]
- Habineza, P.; Muhammad, A.; Ji, T.; Xiao, R.; Yin, X.; Hou, Y.; Shi, Z. The promoting effect of gut microbiota on growth and development of red palm weevil, Rhynchophorus ferrugineus (Col.: Dryophthoridae) by modulating its nutritional metabolism. Front. Microbiol. 2019, 10, 1212. [Google Scholar] [CrossRef]
- McDonnell, G.E. Antisepsis, disinfection, and sterilization: type, action, and resistance, 2nd ed.ASM Press: Washington, DC, USA, 2017; pp. 1–432. [Google Scholar]
- Celikezen, F.C.; Sahin, I.H. Investigation of antimicrobial effects of some boron compounds. Bitlis Eren Univ. J. Sci. 2023, 12, 591–595. [Google Scholar] [CrossRef]
- Boric acid and precursor to boric acid: environmentier II assessment. CAS Registry number: 10043-35-3, 11113-50-1, 13460-51-0, 12008-41-2, 26038-87-9, 1303-96-4. Government of Australia. Available online: https://www.industrialchemicals.gov.au/sites/default/files/Boric%20acid%20and%20precursors%20to%20boric%20acid_%20Environment%20tier%20II%20assessment.pdf (accessed on 5 October 2014).
- Boric acid. Government of Canada, Canada. Available online: https://www.canada.ca/en/health-canada/services/chemicals-product-safety/boric-acid.html (accessed on 5 October 2014).
- Boucard, P.; Denize, C. Potential for substitution of substances used in wood preservative (PT8). Ineris-207016-2757679-v1.0. Available online: https://www.ineris.fr/en/potential-substitution-substances-used-wood-preservatives-pt8-issues-related-future-approval (accessed on 5 October 2014).
- Jiang, M.; Dong, F.Y.; Pan, X.Y.; Zhang, Y.N.; Zhang, F. Boric acid was orally toxic to different instars of Blattella germanica (Blattodea: Blattellidae) and caused dysbiosis of the gut microbiota. Pestic. Biochem. Physiol. 2021, 172, 104756. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, A.R.; Schwarz, M.; Schal, C.; Mikaelyan, A. Lethal disruption of the symbiotic gut community in Eastern subterranean termite caused by boric acid. BioRxiv. 2024, 2024.06.26.600876. [Google Scholar] [CrossRef]
- Govindarajan, R.; Jayaraj, S.; Narayanan, K. Mortality of the tobacco caterpillar, Spodoptera litura, when treated with Bacillus thuringiensis combinations with boric acid and insecticides. Phytoparasitica 1976, 4, 193–1966. [Google Scholar] [CrossRef]
- Cisneros, J.; Perez, J.A.; Penagos, D.I.; Ruiz, J.V.; Goulson, D.; Caballero, P.; Cave, R.D.; Williams, T. Formulation of nucleopolyhedrovirus with boric acid for control of Spodoptera frugiperda (Lep.: Noctuidae) in maize. Biol. Control 2002, 23, 87–95. [Google Scholar] [CrossRef]
- Biswal, G.; Singh, D.; Dhal, N.K. Synergistic effect of Bacillus subtilis and boric acid on management of bacterial with disease of potato caused by Rlastonia solanacearum in coastal plans of Odisha under field condition. Indian Phytopathology 2018, 71, 431–434. [Google Scholar] [CrossRef]
- Chao, Y.; Wang, M.; Dai, W.; Dong, F.; Wang, K.; Zhang, F. (Synergism between hydramethylnon and Metarhizium anisopliae and their influence on the gut microbiome of Blattella germanica. Insects 2020, 11, 538. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, M.; Schal, C.; Jiang, M.; Cai, T.; Zhang, F. Boric acid enhances Metarhizium anisopliae virulence in Blattella germanica by disrupting the gut and altering its microbial community. Biol. Control. 2021, 152, 104430. [Google Scholar] [CrossRef]
- Thakur, S.; Sinha, A.; Bag, A.G. Boron – a critical element for fruit nutrition. Commun. Soil Sci. Plan. 2023, 54, 2899–2914. [Google Scholar] [CrossRef]
- Pillay, A.E.; Williams, J.R.; El-Mardi, M.O.; Hassan, S.M.; Al-Hamdi, A. Boron and the alternate-bearing phenomenon in the date palm (Phoenix dactylifera). J. Arid Environ. 2005, 62, 199–207. [Google Scholar] [CrossRef]
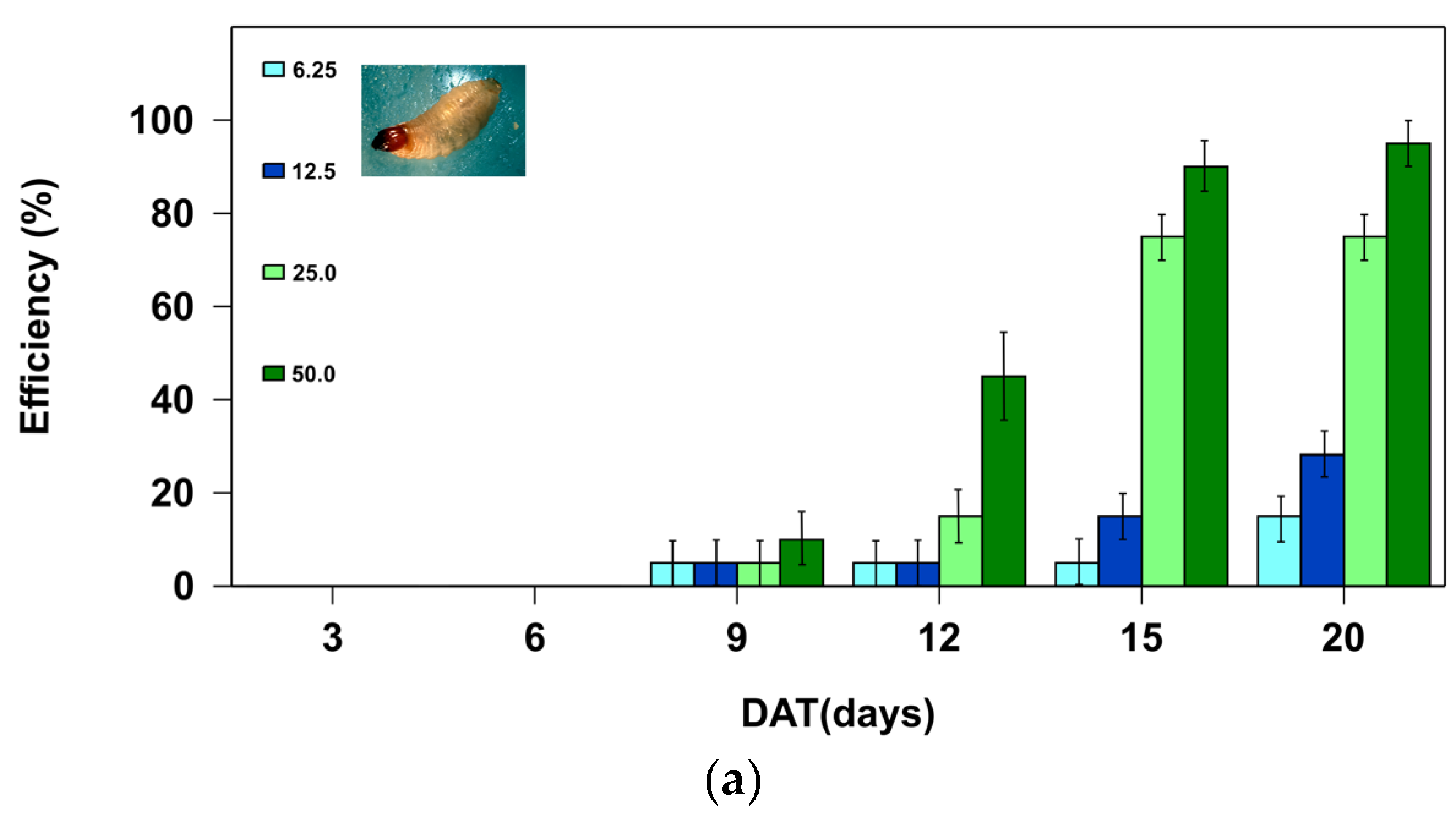
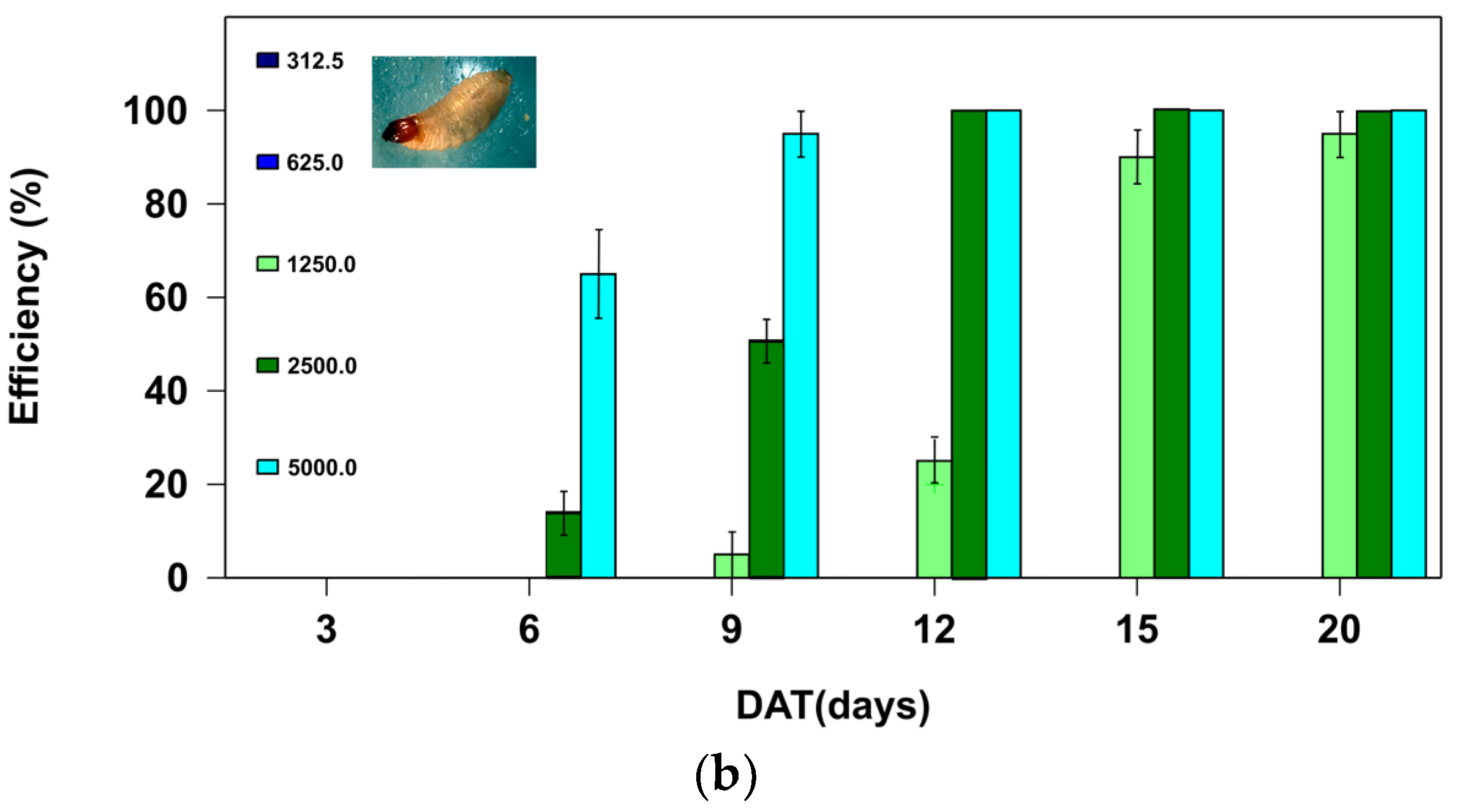
| Active Ingredient | Dose (ppm) | Day after treatments (DAT) | |||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 20 | ||
| Azadirachtin | 0 (control) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
| 6.3 | 5.0 (0.0) |
5.0 (0.0) |
4.8 (0.3) |
4.8 (0.3) |
4.8 (0.3) |
4.3 (0.3) |
|
| 12.5 | 5.0 (0.0) |
5.0 (0.0) |
4.8 (0.3) |
4.8 (0.3) |
4.8 (0.3) |
4.3 (0.3) |
|
| 25.0 | 5.0 (0.0) |
5.0 (0.0) |
4.8 (0.3) |
4.8 (0.3) |
4.3 (0.3) |
4.0 (0.0) |
|
| 50.0 | 5.0 (0.0) |
5.0 (0.0) |
4.5 (0.3) |
2.8 (0.5) |
0.5 (0.3) |
0.3 (0.3) |
|
| Boric Acid | 0 (control) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
| 312.5 | 5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
|
| 625.0 | 5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
5.0 (0.0) |
|
| 1250.0 | 5.0 (0.0) |
5.0 (0.0) |
4.8 (0.3) |
3.8 (0.3) |
0.5 (0.3) |
0.3 (0.3) |
|
| 2500.0 | 5.0 (0.0) |
4.3 (0.3) |
2.3 (0.5) |
0 | 0 | 0 | |
| 5000.0 | 5.0 (0.0) |
1.8 (0.5) |
0.3 (0.3) |
0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





