1. Introduction
1.1. Pancreas
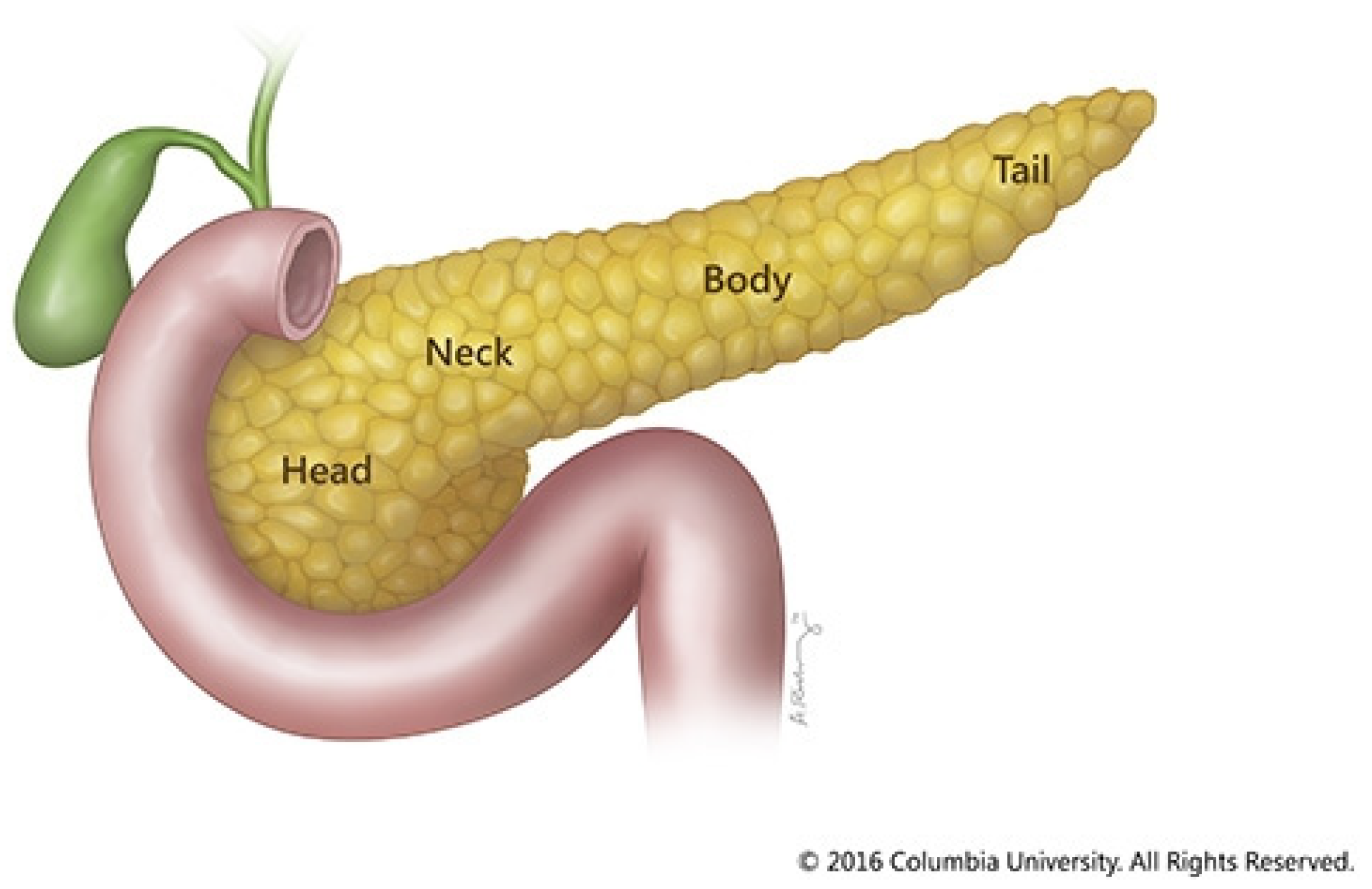
Pancreatic cancer remains one of the deadliest cancers in the world, spotlighting the need for improved therapeutics to combat this disease. The pancreas is an organ in the upper-left quadrant of the abdomen. It is about six to ten inches long horizontally. The wide part of the pancreas is called the head. This head is adjacent to the duodenum, the first portion of the small intestine. As the food travels from the stomach to the small intestine, the pancreas releases digestive enzymes to help break down food. The central section of the pancreas is called the neck, and the tapered end is called the tail [
8].
Figure 1.
Diagram of pancreas. Diagram courtesy of Columbia University.
Figure 1.
Diagram of pancreas. Diagram courtesy of Columbia University.
95% of the pancreatic tissue serves exocrine functions, while the remaining tissue forms the islets of Langerhans. These cell clusters produce hormones that regulate pancreatic secretions and blood sugar.
The pancreas executes exocrine and endocrine functions. The exocrine functions include the creation of vital digestive enzymes. These enzymes include lipase, which breaks down lipids; amylase, which breaks down carbohydrates; and trypsin and chymotrypsin, which break down proteins. When food enters the stomach, these pancreatic secretions are discharged into a network of ducts that convene in the main pancreatic duct. The ampulla of Vater, which is situated in the duodenum, is formed by merging the pancreatic and common bile ducts. Bile is another crucial digestive liquid secreted from the liver and gallbladder. The body breaks down fats, carbs, and proteins with the aid of the pancreatic fluids and bile secreted into the duodenum. [
8].
Pancreatic endocrine functions are mediated by islet cells (islets of Langerhans), which make vital hormones and release them into the bloodstream. The two primary pancreatic hormones are insulin, which lowers blood sugar, and glucagon, which raises it. To operate properly, blood sugar levels must be maintained for vital organs like the brain, liver, and kidneys [
8].
1.2. Cancer
Cancer is a process of uncontrolled cell proliferation and often metastasis. Genetic alterations that impact cell function, primarily functions governing division and growth, begin the process of cancer formation, known as malignant transformation. Numerous factors, such as mistakes made during cell division, exposure to dangerous environmental chemicals like tobacco smoke or UV rays, and inherited genetic abnormalities, can result in these genetic changes. These genetic alterations cause cells to resist normal growth signals and avoid designed cell death systems, which results in unchecked cell division [
9].
There are several key differences between cancer cells and normal cells. Cancer cells can evade the immune system, grow without the help of outside growth signals, reject stop signals, and encourage the formation of new blood vessels to provide tumors with nutrition, oxygenation, and waste removal. Chromosomal abnormalities and altered metabolic pathways that promote rapid development are frequently present in cancer cells. Tumors go through several growth phases, progressively developing more malignant traits and becoming less responsive to signals from the cellular environment. Though some cancers can occur swiftly, in most cancers, malignant transformation is a years-long process. Consequently, cancer incidence tends to rise with age [
9].
Metastasis, the capacity to infiltrate adjacent tissues and spread to far-off locations, is an important influencer of cancer lethality. Changes in the activity of certain genes, such as proto-oncogenes (which stimulate cell growth) and tumor suppressor genes (which regulate cell growth), are frequently associated with the onset and spread of cancer. Mutations in these genes may accelerate the disease process, catalyzing the promotion of cancer traits or deactivating cellular regulations designed to check those traits [
10].
1.3. Pancreatic Cancer
Pancreatic cancer is the 10th most common cancer in the United States, with an estimated 66,440 new cases expected in 2024 [
1]. As of right now, pancreatic cancer ranks third among cancer-related deaths in the US, but epidemiologic predictions suggest that by 2025, its mortality will rank second only to lung cancer [
11]. Pancreatic cancer has one of the poorest prognoses among cancers. The overall 5-year survival rate is only about 12%, significantly lower than many other common cancers [
1]. For comparison, the 5-year survival rates for breast cancer (90%) and prostate cancer (98%) are much higher [
12,
13].
After the pancreatic tumor establishes itself, cancer cells often travel throughout the body via the bloodstream or the lymphatic system to seed new tumors in other tissues, known as metastasis. Some common sites of metastasis are the liver, lungs, and abdominal cavity. One of pancreatic cancer’s most lethal qualities is its location, as the pancreas resides in the middle of the abdomen, close to many other vital organs. Another lethal quality is that the tumor is often asymptomatic until late-stage disease. 60% of patients with pancreatic cancer have metastasis before receiving their diagnosis. Another 20-25% of the time, though the cancer has not yet spread, it is still inoperable. Finally, even if pancreatic cancer is detected in the early stages, 70% of successful resections still ultimately result in recurrence and death [
2]. The prevalence of diagnosis in late-stage disease is due to a lack of effective screening and biomarker tools for patients with a high risk of pancreatic cancer [
14].
Four major genes contribute to pancreatic cancer:
KRAS,
CDKN2A,
TP53, and
SMAD4. The
KRAS gene codes for producing the K-Ras protein. It is a GTPase that transmits signals to the cell nucleus from outside the cell, telling the cell to proliferate or differentiate [
3]. This gene has the highest mutation frequency observed in pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer. There is a 95% mutation rate in this gene for PDAC [
15].
CDKN2A is a cell cycle regulator. Studies have found that 1.5-3.3% of patients with familial pancreatic cancer (FPC) carry a CDKN2A variant [
16]. A study analyzing the genomes of 638 PDAC patients found that 16 of 638 patients had a pathogenic germline CDKN2A variant [
17].
The
TP53 gene encodes a tumor suppressor protein. Inactivating wild-type TP53 function directly impacts cell cycle progression, apoptosis, and senescence induction [
18]. This mutation occurs in 50–75% of PDAC, usually following a
KRAS mutation. The
TP53 mutation leads to the production of the mutant p53 protein [
19].
The
SMAD4 protein belongs to the SMAD family and is the primary mediator of the transforming growth factor-beta (TGF-β) signaling pathway. When activated, the TGF-β receptor phosphorylates the proteins SMAD2/3, which can subsequently form a protein heterocomplex in the cytoplasm with SMAD4. This complex can travel to the nucleus and alter the expression of genes related to cell migration, survival, proliferation, microenvironment regulation, and immunoregulation. SMAD4 is inactivated in 50–55% of PDACs [
20].
Surgery, radiation therapy, chemotherapy, immunotherapy, cell therapy, and combinations thereof are all used to combat pancreatic cancer. Exploration of the current treatment options for pancreatic cancer can aid in understanding the treatment process for this disease [
21].
2. Surgery
Several surgical procedures can be utilized to resect a tumor and help stop the progression of pancreatic cancer. Surgical resection remains the only potential curative treatment. However, only 15-20% of patients qualify for surgery (their disease is still early enough for surgery to be effective) [
4]. For people who receive surgery, the one-year survival rate is about 73%, while the 5-year survival rate is about 20% [
22].
The location of the tumor determines the surgical procedure used for resection and reorganization of the gut as needed. About 65% of pancreatic cancers are found in the head (HD) of the pancreas, while 15% occur in the body and tail (BT). The remaining cases involve the glands. HD pancreatic cancers have a higher survivability rate than BT tumors because BT tumors tend to present clinically later in the disease course and, therefore, have a higher chance of having already metastasized [
4]. One study found BT lesions may be linked to poorer survival outcomes than HD, neck, and uncinate lesions, regardless of the stage at diagnosis and disease extent [
23]. Specifically, BT tumors had a significantly higher rate of metastatic disease (67% vs 36%), had a substantially lower rate of cancer-directed surgery (16% vs 30%), and the median survival was significantly lower (four months vs six months) [
4].
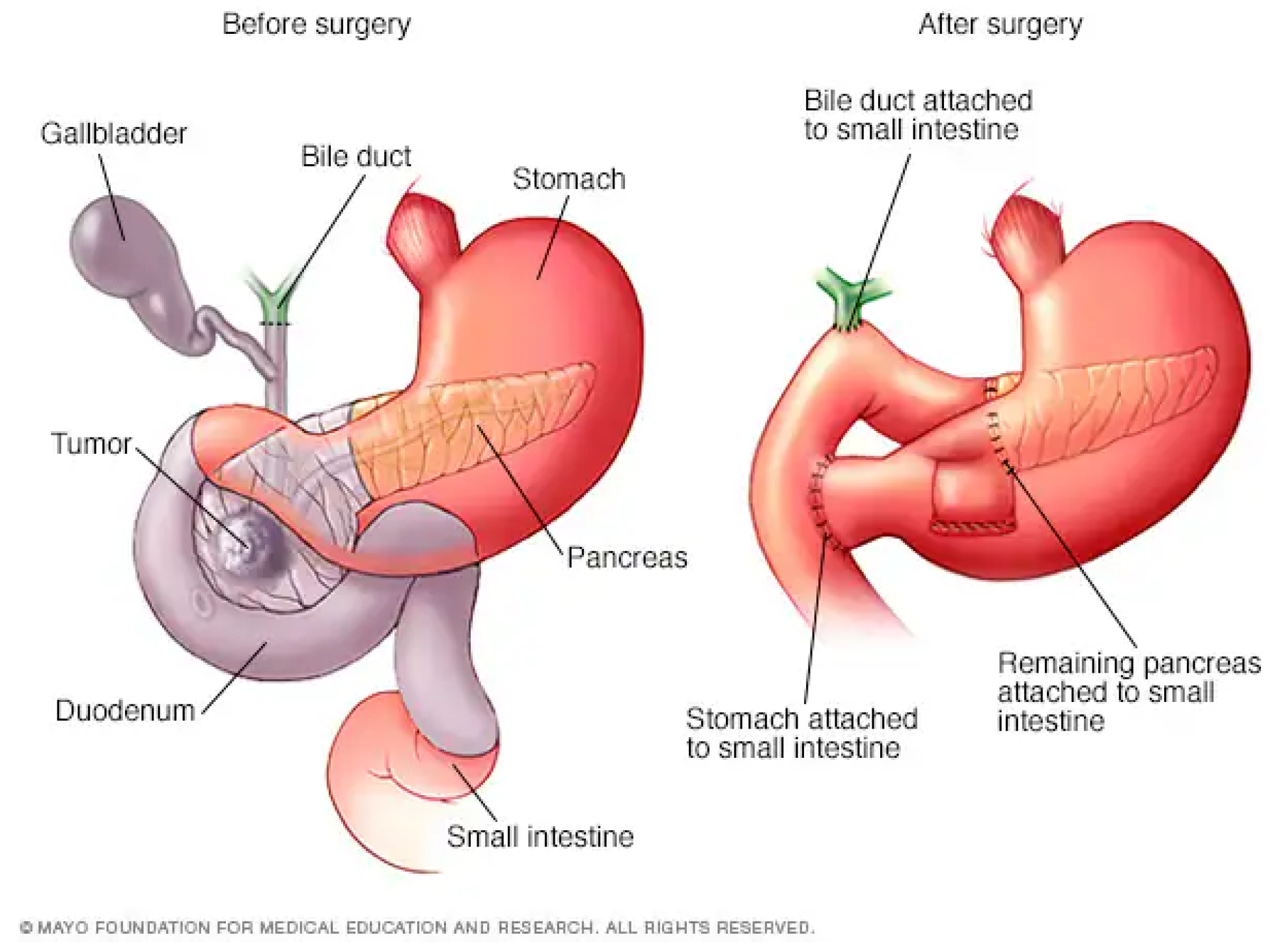
2.1. Whipple Procedure
The most common pancreatic cancer resection procedure is the Whipple procedure, also known as a pancreaticoduodenectomy. The surgeon removes the head of the pancreas, the first part of the small intestine, the gallbladder, and the bile duct. This procedure can only be performed if the cancer has not spread beyond the pancreas [
24].
Recently, a new form of Whipple procedure was introduced, called a pylorus-preserving pancreaticoduodenectomy (PPPD). In this operation, the head of the pancreas, the duodenum, the gallbladder, and part of the bile duct are removed [
24].
According to one study, there was no difference in survival between a group that underwent PPPD and another that underwent the standard Whipple procedure. Both operations, however, led to weight loss, with a median of 8 kg for the Whipple procedure and 13.5 kilograms for the PPPD procedure. Weight loss side effects are common to both procedures because both procedures involve gut remodeling. This causes delayed gastric emptying for both groups [
25]. Also known as gastroparesis, delayed gastric emptying is a condition where the passage of food from the stomach to the small intestine is delayed. This can result in nausea, vomiting, bloating, and stomach pain. Severe cases can result in difficulty absorbing nutrients from foods and medications and lead to dehydration [
26]. All these factors explain the reason behind the weight loss in both groups of patients.
The average hospital stay is about a week for the Whipple procedure. Most patients can return to normal activities four to six weeks after surgery, but full recovery can take two to six months [
27,
28]. Because the Whipple is a far more complex pancreatic procedure than the others, the mortality rate is approximately 1-3% at high-volume medical centers [
29]. This number increases up to five times in low-volume medical centers, around a 16.3% mortality rate. This number has significantly improved in recent decades, as the morality rates used to be much higher (around 25% in the 1960s and 70s) [
30].
Figure 2.
Diagram of the Whipple Procedure. Diagram courtesy of Mayo Foundation.
Figure 2.
Diagram of the Whipple Procedure. Diagram courtesy of Mayo Foundation.
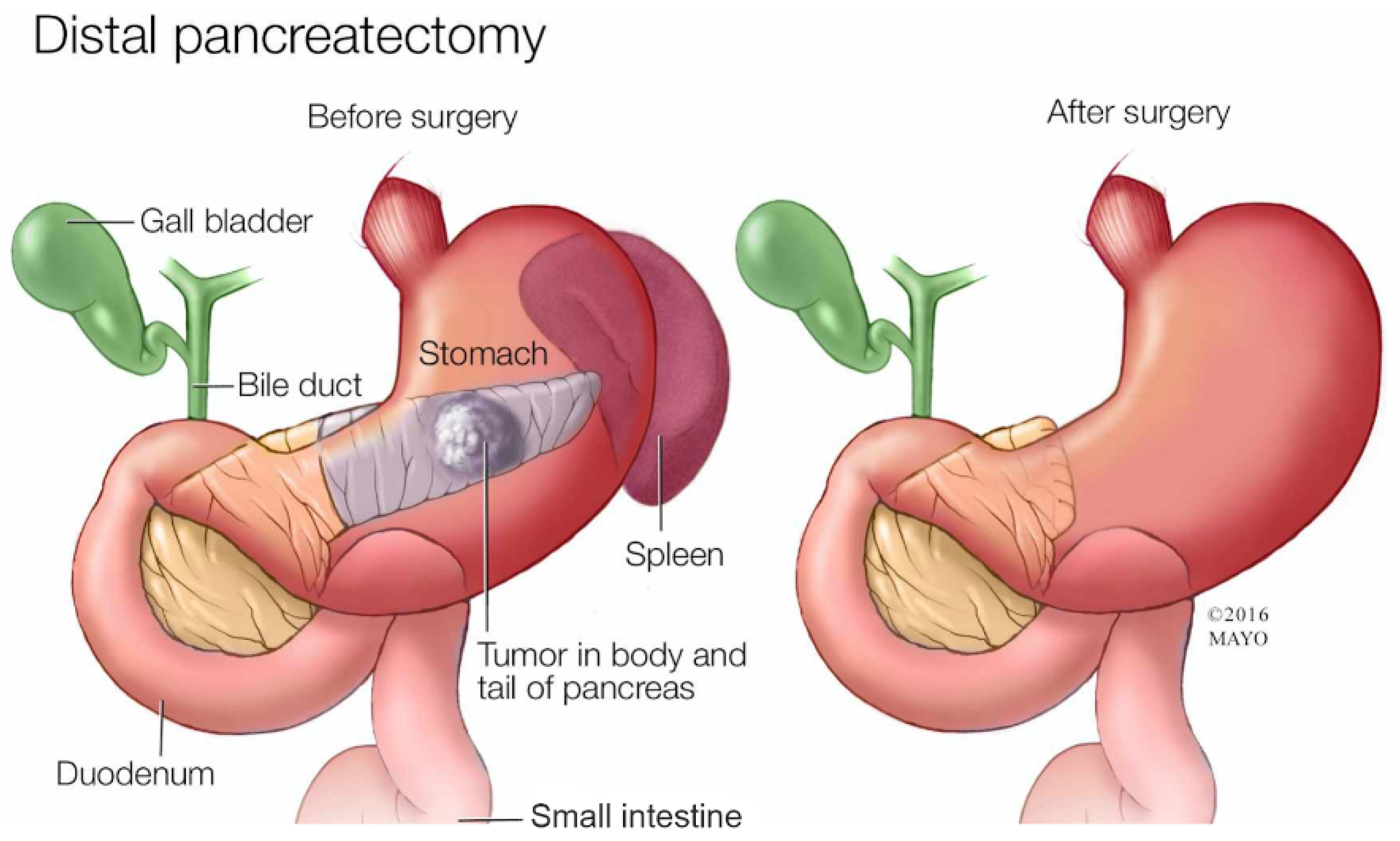
2.2. Distal Pancreatectomy
A distal pancreatectomy removes the tail of the pancreas (hence the name distal) and sometimes part of the body. Since the tail of the pancreas is close to the spleen, a distal pancreatectomy also removes the spleen [
31]. The recovery time is normally shorter than a Whipple procedure, as patients can fully recover in around a month or two [
32]. The mortality rate is typically lower than the Whipple procedure as the surgery is less complex, being 1.6% [
33].
Figure 3.
Diagram of the Distal Pancreatectomy. Diagram courtesy of Mayo Foundation.
Figure 3.
Diagram of the Distal Pancreatectomy. Diagram courtesy of Mayo Foundation.
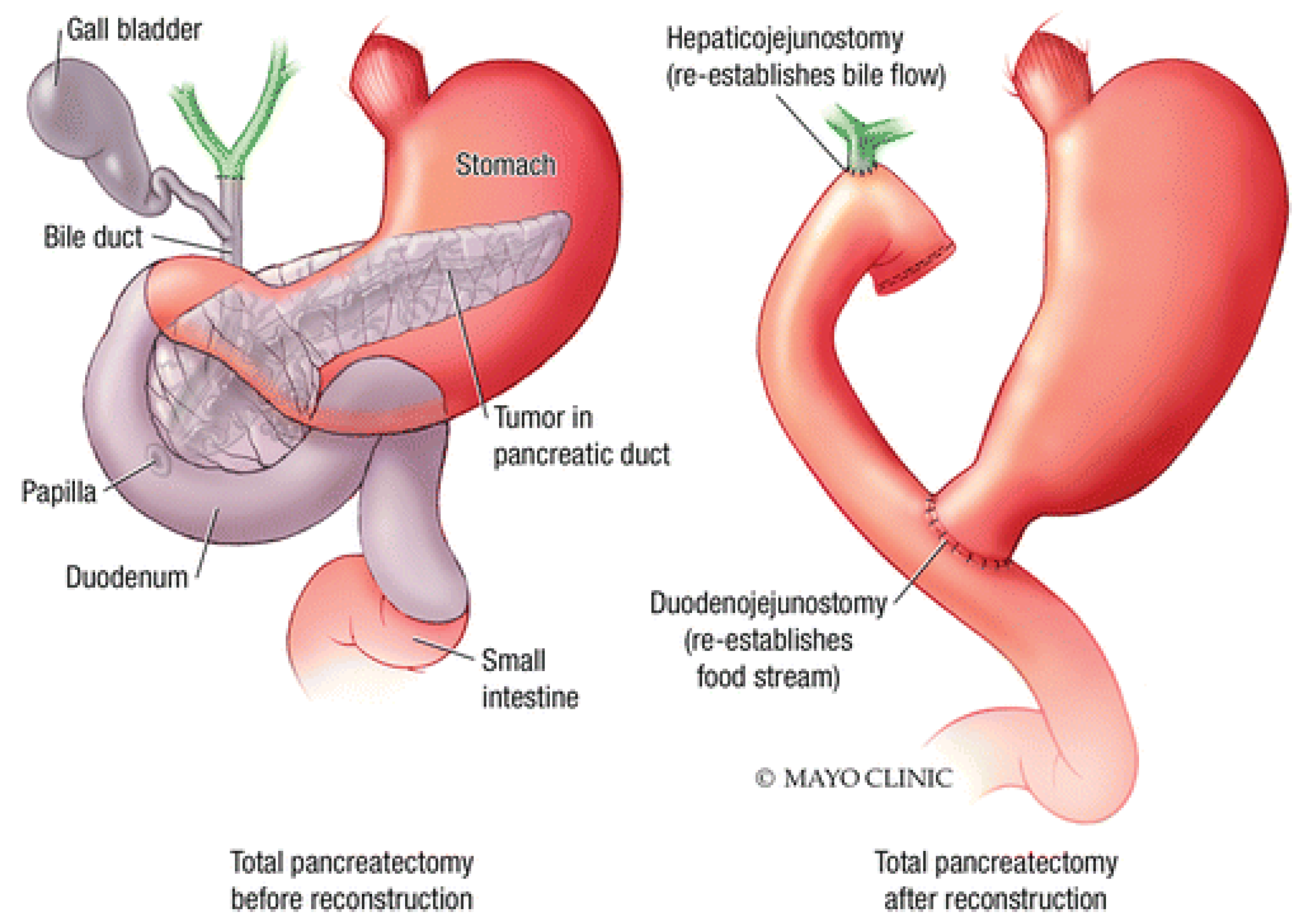
2.3. Total Pancreatectomy
A total pancreatectomy is a last resort when cancer affects the entire pancreas. In this procedure, the whole pancreas, along with adjacent lymph nodes, vessels, and other organs, are removed. Some of these organs include the spleen, gallbladder, duodenum, and the lower part of the stomach. After the procedure, the small intestine is reconnected to the stomach and bile duct [
31]. The recovery is typically slightly longer than a Whipple procedure. Since the pancreas is fully removed, the patient must take lifelong medications to replace the hormones and enzymes the pancreas would have otherwise made. Full recovery takes several months [
32].
Figure 4.
Diagram of the Total Pancreatectomy. Diagram courtesy of Mayo Foundation.
Figure 4.
Diagram of the Total Pancreatectomy. Diagram courtesy of Mayo Foundation.
One study found that out of 488 patients that underwent exploration and resection for pancreatic cancer, 28 patients (5.7%) underwent total pancreatectomy; 409 patients (83.8%) underwent pancreaticoduodenectomy, both the standard Whipple procedure and the PPPD; and 51 patients (10.5%) underwent distal pancreatectomy [
34].
3. Chemotherapy
In chemotherapy, drugs are administered intravenously to prevent the development of cancer cells by either killing them or stopping them from dividing [
21]. Chemotherapy is administered to 92.9% of patients with pancreatic cancer [
35]. Chemotherapy affects all populations of fast-dividing cells in the body, of which cancer is one. Chemotherapy can be used before surgery to help shrink a tumor and make surgical resection more feasible. It can also be used after surgery to kill any of the remaining cancer cells or portions of tumors that cannot be resected. This helps lower the chance of the cancer coming back. Finally, if a patient is not a surgical candidate (i.e., metastasis), chemo can be used to help slow down the rate of cancer. Though typically, in this situation, the disease has progressed beyond the potential for a cure [
36].
Doctors administer chemotherapy in cycles, where each treatment period is followed by a rest period to allow your body to recover from the drug’s effects. Because chemotherapy indiscriminately targets fast-dividing cells, common side effects come from other target cell populations, such as hair, gut, and skin cells. These lead to common side effects such as nausea, vomiting, and hair loss, among others. The chemotherapy cycles typically last 2 to 3 weeks, though the schedule can vary based on the specific drug regimen. For instance, some medications are administered only on the first day of the cycle, while others may be given for several consecutive days or once a week. At the end of each cycle, the schedule repeats for the next cycle. Adjuvant and neoadjuvant chemotherapies are usually given for a total of 3 to 6 months, depending on the drug regimen. For advanced pancreatic cancer, the duration of treatment is determined by the regimen effectiveness and the side effect severity [
36].
However, only 71.6% of people complete the entire regimen [
35]. This is primarily because of the cost vs benefit analysis. Many times, the cancer has spread and grown so much that chemotherapy would not help but instead just add to the side effects. Because of this, there would be no point continuing it.
The most common chemotherapy regimen consists of six months with modified folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin (mFOLFIRINOX) in patients who can tolerate this protocol; for patients who cannot tolerate this protocol, six months of gemcitabine and capecitabine will be used instead [
37]. Patients who received mFOLFIRINOX had more side effects than patients who received gemcitabine. However, according to a study, after three years, 63.5% of patients who received mFOLFIRINOX were still alive, compared to 48.6% of patients still alive after gemcitabine [
5].
4. Radiation Therapy
In radiation therapy, high-energy X-rays halt or reduce cancer cell growth [
21]. The most typical course of treatment for pancreatic cancer is a daily regimen of external beam radiation administered by three-dimensional (3D) conformal or intensity-modulated radiation therapy (IMRT) for five to six weeks. High toxicity rates are caused by the inability of these 3D approaches to avoid intestinal structures and the requirement for wide treatment fields to cover the pancreas and surrounding regional areas. Overall, it has been shown that the standard radiation dose (40 to 60 Grays) has little to no effect on patients’ overall survival. Therefore, radiation therapy is rarely used in pancreatic cancer treatment [
6].
A relatively new radiotherapy technique, stereotactic body radiation therapy (SBRT), is being researched. This technique can deliver a precise and high dose to the tumor, limiting toxicity to other gut organs. SBRT has been proven to be effective in treating several solid cancers, including prostate cancer, hepatic cancer, and non-small cell lung cancer. Recently, SBRT has been indicated as a potential treatment for pancreatic cancer. SBRT has garnered a great deal of attention from patients with pancreatic cancer because it can be finished in one to five fractions, needs less time away from full doses of chemotherapy, and uses lower target volumes than traditional radiotherapy. Recent findings on the use of SBRT for pancreatic cancer show it to delay local disease progression [
6].
5. Immunotherapy
Immunotherapy leverages the body’s immune system to help combat cancers. T cells are essential members of the human immune system and are controlled by stimulatory and inhibitory signals. T cells can identify and destroy tumor cells. Multiple immunotherapy classes have proven effective in the fight against cancer. Certain immunosuppressive entities known as immune checkpoints function normally to preserve self-tolerance and prevent the body from unintentionally and collaterally damaging healthy cells in the presence of infection or inflammation. Tumor cells can evade immune cell detection and destruction by completing these immunosuppressive checkpoints. Checkpoint inhibitors can reduce cancer immune evasion by interrupting the connection between the immune checkpoint and its corresponding ligand. Notably, the first immunological checkpoints to be identified were PD-1 (programmed cell death protein 1), PD-L1 (programmed death-ligand 1), and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4). These checkpoints have attracted much interest as potential treatment targets [
38].
Monoclonal antibodies can prevent PD-1 from binding to PD-L1 and PD-L2 and binding to the CD80 protein. This restores the immune system’s ability to target and eliminate tumor cells. Likewise, disrupting the ligand-receptor interactions of B7-1/B7-2 and CTLA-4 enables effector cells to sustain their ability to recognize and kill tumor cells. However, these combinations of immunotherapies show little impact on prognosis in patients with pancreatic cancer [
38].
5.1. mRNA Vaccines
Excitingly, mRNA vaccines have shown promising results in the context of pancreatic cancer. A segment of mRNA that matches a viral protein—typically a tiny portion of a protein on the virus’s outer membrane—must be introduced for mRNA vaccines to function. Cells can create the viral protein using this mRNA. The immune system produces specific proteins known as antibodies in response to the foreign protein as part of a typical immunological response. Through their ability to identify certain viruses or other pathogens, bind to them, and mark them for elimination, antibodies aid in the body’s defense against infection. Immunologic memory enables the immune system to respond to the same pathogen or cancer in the future. After being vaccinated against a virus using mRNA, antibodies can identify the target protein swiftly, attach to it, and label it for elimination before it can cause a serious sickness [
39].
The administration of an mRNA vaccine following surgical resection has demonstrated significantly longer recurrence-free survival (>18 months) than that of a surgical resection alone (13.4 months). Other more traditional vaccine platforms are also under investigation. The KRAS gene is a potential target. A KRAS-targeting peptide vaccine is currently under clinical testing. This vaccine has high potential, as KRAS appears in most cases of pancreatic cancer [
7].
6. Combinations
A combination of treatment types is commonly employed to combat pancreatic cancer effectively. Common regimens combine chemotherapy with drugs targetting specific pro-cancer processes. For example, gemcitabine and targeted medication combinations have significantly reduced the growth of pancreatic cancer cells. Mutated KRAS is a key target. Inhibitors of KRAS and its downstream proteins, such as RAF, MEK, and ERK, slow pancreatic cancer progression. Antibodies that bind to the activated GTP-bound form of oncogenic KRAS mutants have been developed and significantly sensitize pancreatic cancer cells to gemcitabine [
40].
Immunotherapy and vaccination are another effective combination of therapies. Many barriers prevent an effective antitumor immune response, such as low tumor mutation burden (a slow mutation rate delays immune system recognition of tumors), impaired T cell activation, and a suppressive tumor microenvironment. One strategy to overcome these issues combines a vaccine that induces and activates host effector T cells with immune-modulating agents that enhance antitumor T cell activity. One study found that neoadjuvant and adjuvant GVAX, a pancreatic cancer vaccine, used with PD-1 blockade and CD137 agonist antibody therapy, increases activation of intratumoral cytotoxic T cells and suggests the combinational therapy is an effective treatment in the battle against pancreatic adenocarcinoma [
41].
7. Conclusions
Pancreatic cancer remains one of the deadliest forms of cancer. The metabolic importance and interconnectedness of the pancreas with other organs result in metastatic cancer being nearly impossible to treat. Along with this, pancreatic cancer is hard to diagnose, only presenting clinical symptoms late in the disease course [
2]. Currently, the only way to potentially cure pancreatic cancer is through surgical resection, though surgical resection only occurs in 15-20% of cases [
4]. As the overall 5-year survival rate is only about 12%, the current treatments are profoundly insufficient to manage this disease [
1].
Broad-spectrum chemotherapy does not tend to be an efficient form of pancreatic cancer treatment either. While drugs such as mFOLFIRINOX and gemcitabine may be effective, by themselves, their rates of success aren’t high enough to significantly alter the disease course (63.5% and 48.6%, respectively) [
5]. Radiation is also a poor therapy for pancreatic cancer management, and the typical treatment has high toxicity rates due to the abdominally embedded location of the pancreas. However, with the advent of SBRT, radiation therapy may yet become a more effective and more prominent component of pancreatic cancer treatment [
6]. Immunotherapy has shown promising results in pancreatic cancer treatment. Monoclonal antibodies have enabled the immune system to target tumor cells better and eliminate them [
38]. mRNA vaccines have also shown promising results, potentially reducing the risk of cancer recurrence after surgery [
7]. Combined therapy regimens are more effective than individual therapies alone. These include both multi-drug regimens as well as combination immunotherapies.
Matching the difficulty of pancreatic cancer management is the difficulty of early pancreatic cancer diagnosis. Prophylactic immunotherapies, such as vaccines, may provide a long-awaited breakthrough in pancreatic cancer prevention and reducing recurrence. Pancreatic cancer remains a deadly disease, and additional research and development are needed to improve preventative and therapeutic management strategies and ultimately turn the tide of pancreatic cancer morbidity and mortality.
Acknowledgments
I would like to thank Chloe Cavanaugh for helping mentor me on this review paper.
References
- Cancer of the Pancreas—Cancer Stat Facts. (n.d.). SEER. Retrieved July 8, 2024. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html.
- Why Is Pancreatic Cancer So Deadly and What Are the Treatment Options. (n.d.). Retrieved July 8, 2024. Available online: https://www.medstarhealth.org/blog/pancreatic-cancer-deadly-treatment-options.
- KRAS gene: MedlinePlus Genetics. (n.d.). Retrieved July 8, 2024. Available online: https://medlineplus.gov/genetics/gene/kras/.
- Artinyan, A., Soriano, P. A., Prendergast, C., Low, T., Ellenhorn, J. D. I., & Kim, J. (2008). The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB: The Official Journal of the International Hepato Pancreato Biliary Association, 10(5), 371–376. [CrossRef]
- Chemotherapy Change Improves Pancreatic Cancer Outcomes—NCI (nciglobal, ncienterprise). (2018, June 28). [cgvBlogPost]. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2018/pancreatic-cancer-chemotherapy-regimen-change.
- Burkoň, P., Trna, J., Slávik, M., Němeček, R., Kazda, T., Pospíšil, P., Dastych, M., Eid, M., Novotný, I., Procházka, T., & Vrzal, M. (2022). Stereotactic Body Radiotherapy (SBRT) of Pancreatic Cancer—A Critical Review and Practical Consideration. Biomedicines, 10(10), 2480. [CrossRef]
- Huang, X., Zhang, G., Tang, T.-Y., Gao, X., & Liang, T.-B. (2022). Personalized pancreatic cancer therapy: From the perspective of mRNA vaccine. Military Medical Research, 9(1), 53. [CrossRef]
- Pancreas Functions, Location & Disease | Columbia Surgery. (n.d.). Retrieved July 8, 2024. Available online: https://columbiasurgery.org/pancreas/pancreas-and-its-functions.
- What Is Cancer? - NCI (nciglobal, ncienterprise). (2007, September 17). [cgvArticle]. Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer.
- How cancer can spread. (2014, October 29). Cancer Research UK. Available online: https://www.cancerresearchuk.org/about-cancer/what-is-cancer/how-cancer-can-spread.
- About Pancreatic Cancer | Penn Pancreatic Cancer Research Center | Perelman School of Medicine at the University of Pennsylvania. (n.d.). Retrieved July 8, 2024. Available online: https://www.med.upenn.edu/pcrc/aboutpancreaticcancer.html.
- Prostate Cancer Prognosis. (2023, November 6). Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/prostate-cancer/prostate-cancer-prognosis.
- Survival Rates for Breast Cancer. (n.d.). Retrieved July 8, 2024. Available online: https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html.
- Kamisawa, T., Wood, L. D., Itoi, T., & Takaori, K. (2016). Pancreatic cancer. The Lancet, 388(10039), 73–85. [CrossRef]
- Zhang, Z., Zhang, H., Liao, X., & Tsai, H. (2023). KRAS mutation: The booster of pancreatic ductal adenocarcinoma transformation and progression. Frontiers in Cell and Developmental Biology, 11. [CrossRef]
- Kimura, H., Klein, A. P., Hruban, R. H., & Roberts, N. J. (2021). The Role of Inherited Pathogenic CDKN2A Variants in Susceptibility to Pancreatic Cancer. Pancreas, 50(8), 1123–1130. [CrossRef]
- Roberts, N. J., Norris, A. L., Petersen, G. M., Bondy, M. L., Brand, R., Gallinger, S., Kurtz, R. C., Olson, S. H., Rustgi, A. K., Schwartz, A. G., Stoffel, E., Syngal, S., Zogopoulos, G., Ali, S. Z., Axilbund, J., Chaffee, K. G., Chen, Y.-C., Cote, M. L., Childs, E. J., … Klein, A. P. (2016). Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discovery, 6(2), 166–175. [CrossRef]
- McCubrey, J. A., Yang, L. V., Abrams, S. L., Steelman, L. S., Follo, M. Y., Cocco, L., Ratti, S., Martelli, A. M., Augello, G., & Cervello, M. (2022). Effects of TP53 Mutations and miRs on Immune Responses in the Tumor Microenvironment Important in Pancreatic Cancer Progression. Cells, 11(14), 2155. [CrossRef]
- Morton, J. P., Timpson, P., Karim, S. A., Ridgway, R. A., Athineos, D., Doyle, B., Jamieson, N. B., Oien, K. A., Lowy, A. M., Brunton, V. G., Frame, M. C., Evans, T. R. J., & Sansom, O. J. (2010). Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proceedings of the National Academy of Sciences, 107(1), 246–251. [CrossRef]
- Racu, M.-L., Bernardi, D., Chaouche, A., Zindy, E., Navez, J., Loi, P., Maris, C., Closset, J., Van Laethem, J.-L., Decaestecker, C., Salmon, I., & D’Haene, N. (2023). SMAD4 Positive Pancreatic Ductal Adenocarcinomas Are Associated with Better Outcomes in Patients Receiving FOLFIRINOX-Based Neoadjuvant Therapy. Cancers, 15(15), 3765. [CrossRef]
- Pancreatic Cancer Treatment—NCI (nciglobal, ncienterprise). (2024, April 26). [pdqCancerInfoSummary]. Available online: https://www.cancer.gov/types/pancreatic/patient/pancreatic-treatment-pdq.
- Prognosis if you can have surgery. (n.d.). Pancreatic Cancer UK. Retrieved August 5, 2024. Available online: https://www.pancreaticcancer.org.uk/information/just-diagnosed-with-pancreatic-cancer/if-you-can-have-surgery-to-remove-the-cancer-early-pancreatic-cancer/prognosis-if-you-can-have-surgery/.
- Sohn, T. A., Yeo, C. J., Cameron, J. L., Koniaris, L., Kaushal, S., Abrams, R. A., Sauter, P. K., Coleman, J., Hruban, R. H., & Lillemoe, K. D. (2000). Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract, 4(6), 567–579. [CrossRef]
- Types of surgery for pancreatic cancer. (n.d.). Retrieved July 15, 2024. Available online: https://www.cancerresearchuk.org/about-cancer/pancreatic-cancer/treatment/surgery/which-surgery.
- Tran, K. T. C., Smeenk, H. G., van Eijck, C. H. J., Kazemier, G., Hop, W. C., Greve, J. W. G., Terpstra, O. T., Zijlstra, J. A., Klinkert, P., & Jeekel, H. (2004). Pylorus Preserving Pancreaticoduodenectomy Versus Standard Whipple Procedure. Annals of Surgery, 240(5), 738–745. [CrossRef]
- Gastroparesis. (n.d.). Yale Medicine. Retrieved August 5, 2024. Available online: https://www.yalemedicine.org/conditions/gastroparesis.
- Whipple procedure—Mayo Clinic. (n.d.). Retrieved July 31, 2024. Available online: https://www.mayoclinic.org/tests-procedures/whipple-procedure/about/pac-20385054.
- DeMarco, C. (n.d.). Whipple procedure: 9 things to know. MD Anderson Cancer Center. Retrieved July 31, 2024. Available online: https://www.mdanderson.org/cancerwise/whipple-procedure-and-pancreatic-cancer-treatment-9-things-to-know.h00-159459267.html.
- Whipple Procedure: What Is It, Treatment & Recovery. (n.d.). Cleveland Clinic. Retrieved July 31, 2024. Available online: https://my.clevelandclinic.org/health/treatments/21650-whipple-procedure-pancreaticoduodenectomy.
- 10-year survival rate after whipple surgery—Seena Magowitz Foundation. (n.d.). Retrieved July 31, 2024. Available online: https://seenamagowitzfoundation.org/whipple-operation/.
- Pancreatectomy Surgery: Procedure, Types & Definition. (n.d.). Cleveland Clinic. Retrieved July 16, 2024. Available online: https://my.clevelandclinic.org/health/treatments/23134-pancreatectomy-surgery-removal-pancreas.
- Pancreatectomy (Removal of Pancreas). (n.d.). UPMC Hillman Cancer Center. Retrieved July 31, 2024. Available online: https://hillman.upmc.com/cancer-care/pancreatic/treatment/surgery/pancreatectomy.
- Loos, M., Mack, C. E., Xu, A. T. L., Hassenpflug, M., Hinz, U., Mehrabi, A., Berchtold, C., Schneider, M., Al-Saeedi, M., Roth, S., Hackert, T., & Büchler, M. W. (2024). Distal Pancreatectomy: Extent of Resection Determines Surgical Risk Categories. Annals of Surgery, 279(3), 479. [CrossRef]
- Karpoff, H. M., Klimstra, D. S., Brennan, M. F., & Conlon, K. C. (2001). Results of Total Pancreatectomy for Adenocarcinoma of the Pancreas. Archives of Surgery, 136(1), 44–47. [CrossRef]
- Maggino, L., Malleo, G., Marchegiani, G., Viviani, E., Nessi, C., Ciprani, D., Esposito, A., Landoni, L., Casetti, L., Tuveri, M., Paiella, S., Casciani, F., Sereni, E., Binco, A., Bonamini, D., Secchettin, E., Auriemma, A., Merz, V., Simionato, F., … Salvia, R. (2019). Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surgery, 154(10), 932–942. [CrossRef]
- Chemotherapy for Pancreatic Cancer. (n.d.). Retrieved July 31, 2024. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/treating/chemotherapy.html.
- Springfeld, C., Jäger, D., Büchler, M. W., Strobel, O., Hackert, T., Palmer, D. H., & Neoptolemos, J. P. (2019). Chemotherapy for pancreatic cancer. La Presse Médicale, 48(3, Part 2), e159–e174. [CrossRef]
- Ye, X., Yu, Y., Zheng, X., & Ma, H. (2024). Clinical immunotherapy in pancreatic cancer. Cancer Immunology, Immunotherapy, 73(4), 64. [CrossRef]
- What are mRNA vaccines and how do they work?: MedlinePlus Genetics. (n.d.). Retrieved August 19, 2024. Available online: https://medlineplus.gov/genetics/understanding/therapy/mrnavaccines/.
- Nishimoto, A. (2022). Effective combinations of anti-cancer and targeted drugs for pancreatic cancer treatment. World Journal of Gastroenterology, 28(28), 3637–3643. [CrossRef]
- Heumann, T., Judkins, C., Li, K., Lim, S. J., Hoare, J., Parkinson, R., Cao, H., Zhang, T., Gai, J., Celiker, B., Zhu, Q., McPhaul, T., Durham, J., Purtell, K., Klein, R., Laheru, D., De Jesus-Acosta, A., Le, D. T., Narang, A., … Zheng, L. (2023). A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma. Nature Communications, 14, 3650. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).