1. Introduction and Review of TLS Protocols
Venetoclax is a potent, orally bioavailable, and selective BCL-2 inhibitor [
1]. A phase I trial evaluated venetoclax monotherapy for multiple myeloma in 66 patients [
2]. TLS prophylaxis involved 1-2L of oral hydration >72 h before the first dose and at each dose increase. Prophylaxis with uric acid-reducing agents was considered for patients with elevated uric acid levels. Doses were escalated over a two-week lead-in to reach the final doses of 300, 600, 900, or 1200 mg. The maximum tolerated dose (MTD) was not reached, no TLS events were reported, and there were no differences in safety events between doses.
In phase I and II trials of venetoclax plus dexamethasone (VenDex) for t(11;14) RRMM, patients were advised to drink 1-2L per day before the first dose of venetoclax and to take venetoclax after breakfast [
3]. Labs were drawn more frequently for patients at higher risk of TLS (t(11;14) and > 50% marrow plasma cell infiltration, or CrCl < 50mL/min). There was no venetoclax lead-in period for the phase II cohorts of this trial. TLS was observed in 3/51 patients treated with 800 mg daily venetoclax plus dexamethasone. All three cases were grade 3 or 4. TLS was managed with IV fluids (> 1 mL/kg/hr), medications for hyperkalemia and hyperphosphatemia, and/or dialysis. Similarly, 66 patients were enrolled in a phase I trial of venetoclax plus bortezomib and dexamethasone (VenBd) with venetoclax doses ranging from 100-1200mg [
4]. Allopurinol was given >72 hours before the first dose of venetoclax for TLS prophylaxis, along with oral hydration. No TLS events occurred in the study, and the MTD of VenBd was not reached.
The phase III BELLINI trial compared 800 mg venetoclax plus bortezomib and dexamethasone (VenBd, n = 193) vs placebo plus bortezomib and dexamethasone (Bd, n = 96) [
5]. TLS prophylaxis was at investigator discretion. Recommendations were for oral hydration >72 h prior to the first dose of venetoclax for all patients and uric acid-reducing agents for patients with high uric acid levels. High-risk patients were monitored more intensely with frequent labs and IV hydration at the investigator's discretion. TLS was reported in 1/193 patients in the VenBd group and 0/96 patients in the placebo + Bd group. Protocols for TLS management were not described.
Venetoclax plus carfilzomib and dexamethasone for RRMM was investigated in a phase II trial of 43 patients and showed favorable safety and efficacy [
6]. This trial provided the rationale for treating this patient with VenKd. TLS prophylaxis was oral and IV hydration >72 hours before the first dose. 1/43 patients developed TLS on cycle 1 day 2 of 800mg venetoclax plus 20mg/m
2 carfilzomib. The patient who developed TLS in this trial had 60% bone marrow infiltration prior to treatment and t(11;14). The patient was treated with hydration and allopurinol, and treatment resumed after 4 days when labs normalized.
Taken together, TLS is a rare occurrence in patients treated with venetoclax mono- or combination therapy, and clear protocols guiding TLS prophylaxis are lacking. Our case report demonstrates the importance of a uniform TLS prophylaxis protocol in treating t(11;14) positive RRMM with venetoclax.
2. Case Presentation
We present a 53-year-old gentleman with a past medical history of steroid-induced diabetes mellitus with triple-class-refractory multiple myeloma (MM) treated with venetoclax, carfilzomib and dexamethasone leading to severe tumor lysis syndrome complicated by acute kidney injury, requiring multiple rounds of hemodialysis (HD). A detailed hematologic history is presented in
Supplemental Table S1. Serum creatinine was within normal limits prior to initiation of therapy.
For TLS prophylaxis, he was given allopurinol, 300 mg PO daily, was encouraged to orally hydrate himself well for 3 days and was given 500mL of IV normal saline 1h before and after his treatment. He received a single dose of venetoclax 800 mg PO, carfilzomib 20 mg/m2 IV, and dexamethasone 40 mg PO. He was put on levofloxacin, trimethoprim-sulfamethoxazole, and acyclovir for infection prevention.
One day later, the patient presented to his local hospital for fluid overload and acute kidney injury, for which he received one round of HD, as well as treatment with allopurinol and rasburicase. Kidney failure was attributed to MM, and the patient was discharged to hospice. Five days after the first and thus far only dose of VenKd, the patient presented to our hospital for a second opinion. His chief concerns were shortness of breath, cough, weight gain (37 lbs), and anuria for 24 hours before presentation. Vital signs were stable with BP 121/79, T 98.2 °F, HR 83, RR 16, and SpO
2 100% on room air. Physical exam was concerning for abdominal distention and bilateral lower extremity edema extending to his thighs. The sudden rise in creatinine, electrolyte profile, and lactate dehydrogenase of >4,500 units/dL made acute renal failure (ARF) secondary to TLS the most likely diagnosis, and the patient was hospitalized for aggressive management. ARF was defined as a rapid fall in glomerular filtration rate, manifesting with disrupted salt and water homeostasis and associated increases in serum urea and creatinine. Tumor lysis syndrome was diagnosed using the Cairo-Bishop criteria [
7]. Labs on presentation and throughout the admission are summarized in
Table 1. The patient was started on diuretics, sevelamer and other supportive care for his ARF along with HD. The patient received five treatments of hemodialysis, through day 9 of this 13-day hospitalization. Urine output gradually increased to ~2 liters per day. The patient was discharged on 100 mg daily allopurinol and 800 mg sevelamer 3x daily. Serum creatinine and urine output continued to improve post-hospital discharge, heralding gradual restoration of kidney function.
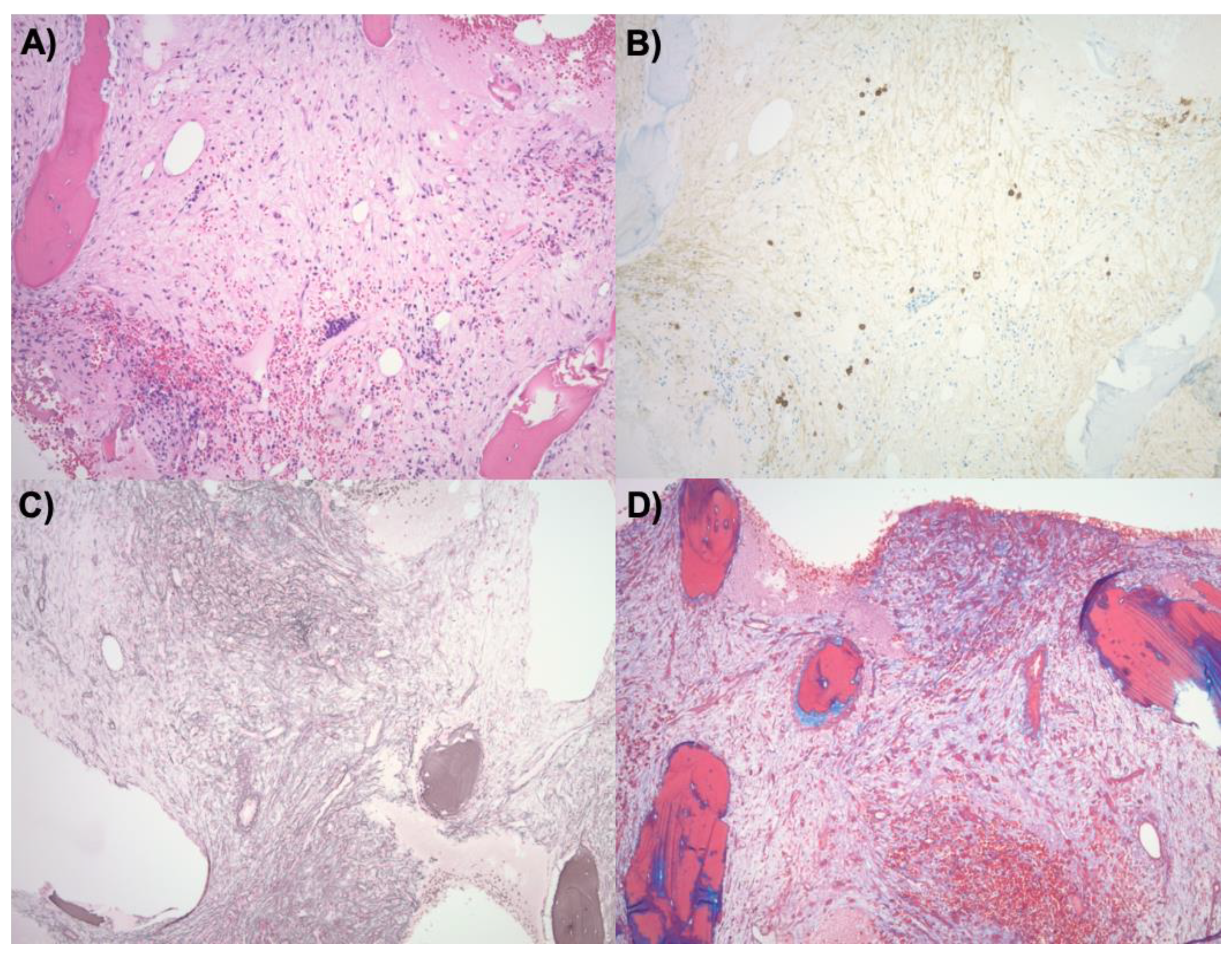
Our patient was pancytopenic and required 4 units of packed red blood cells and 10 units of platelets during this hospitalization. A repeat bone marrow biopsy taken 2 weeks after treatment while the patient was in the hospital was negative for plasma cell neoplasm. It demonstrated a markedly hypocellular marrow with extensive fibrosis (
Figure 1). Flow cytometry showed no significant population of plasma cells.
Follow-up and Outcomes:
The patient’s health and urine output improved after discharge, with resolution of pancytopenia. Two months later, a repeat bone marrow biopsy showed a normocellular (50% cellular) bone marrow with trilineage hematopoiesis, extensively involved by plasma cell neoplasm at 90%. Flow cytometry revealed a clonal plasma cell population. Reticulin and trichrome stains demonstrated WHO grade 1 fibrosis, suggesting partial resolution of the marrow fibrosis.
The patient restarted venetoclax at 200 mg daily in a controlled hospital setting, aiming for disease control to enable consolidation with high-dose chemotherapy/autologous stem cell transplant or autologous chimeric antigen receptor T-cell therapy. Phosphorus and uric acid levels rose slightly on day three but improved with sevelamer. After 1 week, venetoclax was increased to 400 mg daily with good tolerance. Carfilzomib (20 mg/m2 IV/week followed by 27 mg/m2 IV twice weekly) and dexamethasone (at 20 mg PO twice weekly) were added outpatient. The patient continued VenKd treatment with good disease response and performance status.
3. Discussion
In summary, after one dose of VenKd (800mg Ven, 20 mg/m2 carfilzomib, and 40 mg dex), our patient developed ARF due to TLS, requiring a two-week hospital stay and six rounds of HD. To our knowledge, this is the first case of a MM patient’s bone marrow transitioning from a hypercellular, severely diseased marrow to a hypocellular, fibrotic marrow without monoclonal plasma cells after a single VenKd dose.
TLS is rare in RRMM. Although there are scarcely reported cases of spontaneous TLS in RRMM [
8,
9], most TLS is associated with myeloma-directed therapy, especially high-dose chemotherapy [
10] and proteaosome inhibitors (PIs) [
11]. Of the commercially available PIs, twice weekly carfilzomib plus dexamethasone, was associated with higher rates of TLS in a single-center study from Japan. In this study, 50% of patients treated with carfilzomib plus dex developed TLS with a median onset of 5.5 days after carfilzomib [
11]. Venetoclax represents a new group of potent drugs used in RRMM with t(11;14), with the potential to cause TLS. The rising use of these drugs, as monotherapy or combination, necessitates improved TLS risk assessment tools and risk-adapted prophylaxis measures.
TLS associated with venetoclax use is well described in chronic lymphocytic leukemia (CLL). TLS risk assessment tools using absolute lymphocyte count and lymph node size to guide prophylactic measures are implemented in clinical trials utilizing venetoclax [
12]. A slow, 5-week dose escalation of venetoclax leads to lower TLS rates compared to high initial doses and rapid escalation [
13]. Retrospective data in RRMM shows higher disease burden, doses, and drug potency increase TLS rates in RRMM, as do increased LDH, hepatosplenomegaly, and chromosome 13 aberrations [
8,
10,
11]. Studies have also shown worse survival outcomes for patients who develop TLS compared to those who don’t, highlighting the disease’s inherently aggressive nature. As such, risk assessment tools that consider disease burden, cytogenetics, and serum LDH should be evaluated and validated with future clinical trials to guide TLS prophylaxis measures, including IV versus oral hydration, the frequency of TLS lab monitoring, the need for slow dose escalation, and the timing and dosing of uric acid lowering therapies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Timeline of Multiple Myeloma Treatment History.
Author Contributions
RF: AK, and EB performed study concept and design, writing, review, illustration, and revision of the paper. AL performed expert histopathological analysis of tissues. All authors read and approved the final paper.
Funding
This work supported by the Vanderbilt Medical Scientist Training Program (MSTP) T32 training grant, NIGMS of the National Institutes of Health, award number T32GM007347. We also acknowledge the American Society of Hematology Minority Hematology Fellow Award, the Robert A. Winn CDA Diversity in Clinical Trials Career Development Award, and the Multiple Myeloma Research Foundation Research Scholars Award.
Informed Consent Statement
Verbal informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data is contained within the article and supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the patient for agreeing to participate in the writing of this case report.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seymour, J. ABT-199 for Chronic Lymphocytic Leukemia. Clin. Adv. Hematol. Oncol. HO 2014, 12, 698–700. [Google Scholar]
- Kumar, S.; Kaufman, J. L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; Punnoose, E. A.; Alzate, S.; Dunbar, M.; Xu, T.; Agarwal, S. K.; Enschede, S. H.; Leverson, J. D.; Ross, J. A.; Maciag, P. C.; Verdugo, M.; Touzeau, C. Efficacy of Venetoclax as Targeted Therapy for Relapsed/Refractory t(11;14) Multiple Myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J. L.; Gasparetto, C.; Schjesvold, F. H.; Moreau, P.; Touzeau, C.; Facon, T.; Boise, L. H.; Jiang, Y.; Yang, X.; Dunbar, F.; Vishwamitra, D.; Unger, S.; Macartney, T.; Pesko, J.; Yu, Y.; Salem, A. H.; Ross, J. A.; Hong, W.; Maciag, P. C.; Pauff, J. M.; Kumar, S. Targeting BCL-2 with Venetoclax and Dexamethasone in Patients with Relapsed/Refractory t(11;14) Multiple Myeloma. Am. J. Hematol. 2021, 96, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Chanan-Khan, A.; Roberts, A. W.; Agarwal, A. B.; Facon, T.; Kumar, S.; Touzeau, C.; Punnoose, E. A.; Cordero, J.; Munasinghe, W.; Jia, J.; Salem, A. H.; Freise, K. J.; Leverson, J. D.; Enschede, S. H.; Ross, J. A.; Maciag, P. C.; Verdugo, M.; Harrison, S. J. Promising Efficacy and Acceptable Safety of Venetoclax plus Bortezomib and Dexamethasone in Relapsed/Refractory MM. Blood 2017, 130, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. K.; Harrison, S. J.; Cavo, M.; Rubia, J. de la; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; Punnoose, E. A.; Hong, W.-J.; Freise, K. J.; Yang, X.; Sood, A.; Jalaluddin, M.; Ross, J. A.; Ward, J. E.; Maciag, P. C.; Moreau, P. Venetoclax or Placebo in Combination with Bortezomib and Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma (BELLINI): A Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Costa, L. J.; Davies, F. E.; Monohan, G. P.; Kovacsovics, T.; Burwick, N.; Jakubowiak, A.; Kaufman, J. L.; Hong, W.-J.; Dail, M.; Salem, A. H.; Yang, X.; Masud, A. A.; Munasinghe, W.; Ross, J. A.; Bueno, O. F.; Kumar, S. K.; Stadtmauer, E. A. Phase 2 Study of Venetoclax plus Carfilzomib and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma. Blood Adv. 2021, 5, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M. S.; Bishop, M. Tumour Lysis Syndrome: New Therapeutic Strategies and Classification. Br. J. Haematol. 2004, 127, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, S.; Yim, B.; Thekkekara, R. Tumor Lysis Syndrome in Multiple Myeloma: An Increasingly Recognized Risk—A Report of Seven Cases. Indian J. Hematol. Blood Transfus. 2017, 33, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Saravu, K.; Kumar, S.; Shastry, A. B.; Kurien, A.; Prabhu, R.; Kumar, R. Spontaneous Tumour Lysis Syndrome in a Case of Multiple Myeloma – A Rare Occurrence. Australas. Med. J. 2013, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Fassas, A. B.-T.; Desikan, K. R.; Siegel, D.; Golper, T. A.; Munshi, N. C.; Barlogie, B.; Tricot, G. Tumour Lysis Syndrome Complicating High-Dose Treatment in Patients with Multiple Myeloma. Br. J. Haematol. 1999, 105, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nishiwaki, K.; Gunji, T.; Katori, M.; Hosoba, R.; Hirano, K.; Masuoka, H.; Yano, S. Tumor-Lysis Syndrome in Relapsed or Refractory Multiple Myeloma Patients Treated with Proteasome Inhibitors. Blood 2018, 132, 5631. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.-M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; Liberati, A. M.; Pinilla-Ibarz, J.; Opat, S.; Sivcheva, L.; Dû, K. L.; Fogliatto, L. M.; Niemann, C. U.; Weinkove, R.; Robinson, S.; Kipps, T. J.; Boettcher, S.; Tausch, E.; Humerickhouse, R.; Eichhorst, B.; Wendtner, C.-M.; Langerak, A. W.; Kreuzer, K.-A.; Ritgen, M.; Goede, V.; Stilgenbauer, S.; Mobasher, M.; Hallek, M. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K. L.; Huang, Y.; Dotson, E. K.; Sheredy, S.; Bhat, S. A.; Byrd, J. C.; Desmond, E.; Ford, J.; Iarocci, S.; Jones, J. A.; Lucas, M. S.; Moran, M. E.; Wiczer, T. E.; Woyach, J. A.; Awan, F. T.; Rogers, K. A. Safety of Venetoclax Rapid Dose Escalation in CLL Patients Previously Treated with B-Cell Receptor Signaling Antagonists. Blood Adv. 2020, 4, 4860–4863. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).