1. Introduction
CXC chemokine receptor 1 (CXCR1) is a G protein-coupled receptor that plays an essential regulator for migration and activation of neutrophil granulocytes [
1]. CXCR1 serves as a receptor for interleukin-8 (IL-8, also known as C-X-C motif chemokine ligand 8), a central mediator of immune and inflammatory responses involved in many disorders including cancer [
2]. Upon binding to IL-8, CXCR1 triggers a rapid and transient increment of free calcium in neutrophil granulocytes through a GTP-binding protein [
2], which results in the migration to the site of tissue damage or infection [
3]. The attracted neutrophil granulocytes kill and phagocytose bacteria at the sites of inflammation. Furthermore, the IL-8-CXCR1 axis plays an essential role in the tumor microenvironment to promote inflammation and resistance to immunotherapy [
4]. Therefore, the blockade of the IL-8-CXCR1 axis is a promising strategy to improve antitumor efficacy in combination with other immunotherapy [
5].
The activation of CXCR1 involves both N-terminal residues and extracellular loops [
3,
6]. The structure of human CXCR1 in a lipid bilayer was solved using nuclear magnetic resonance spectroscopy, which facilitated molecular modeling and the understanding of interactions with small molecule inhibitors [
7]. The structure of CXCR1 complexed with IL-8 and Gαi1 protein was solved using cryo-EM [
8]. The CXCR1 N-terminal residues fit loosely into an IL-8 groove to form the interaction surface chemokine recognition site 1 (CRS1) [
8]. Therefore, monoclonal antibodies (mAbs) that recognize the CXCR1 N-terminus are expected to neutralize the IL-8 binding.
We have developed anti-mouse chemokine receptor mAbs against CXCR1 (clone Cx
1Mab-1) [
9], CXCR3 (clone Cx
3Mab-4) [
10], CXCR4 (clone Cx
4Mab-1) [
11], CCR1 (clone C
1Mab-6) [
12], CCR3 (clones C
3Mab-2, C
3Mab-3, and C
3Mab-4) [13-15], CCR5 (clone C
5Mab-2) [
16], CCR8 (clones C
8Mab-1, C
8Mab-2, and C
8Mab-3) [17-19], using the Cell-Based Immunization and Screening (CBIS) method. The CBIS method includes immunizing antigen-overexpressed cells and high-throughput hybridoma screening using flow cytometry. Furthermore, we established anti-mouse chemokine receptor mAbs against CCR2 (clone C
2Mab-6) [
20], CCR3 (clones C
3Mab-6 and C
3Mab-7) [
21], CCR4 (clone C
4Mab-1) [
22], CCR5 (clone C
5Mab-4 and C
5Mab-8) [
23], CCR9 (clone C
9Mab-24) [
24], CXCR6 (clone Cx
6Mab-1) [
25], and ACKR4 (clone A
4Mab-1, A
4Mab-2, and A
4Mab-3) [
26] using the N-terminal peptide immunization.
In this study, a high-affinity anti-mouse CXCR1 (mCXCR1) mAb was developed by N-terminal peptide immunization.
2. Materials and Methods
2.1 Cell Lines and Plasmids
The LN229, Chinese hamster ovary (CHO)-K1, and P3X63Ag8U.1 (P3U1) cell lines were sourced from the American Type Culture Collection (ATCC, Manassas, VA).
A pCMV6neo-myc-DDK plasmid carrying mCXCR1 (Accession No.: NM_178241) was obtained from OriGene Technologies, Inc. (Rockville, MD). The mCXCR1 plasmid was introduced into CHO-K1 and LN229 cells via the Neon electroporation system (Thermo Fisher Scientific, Waltham, MA). Stable transfectants were generated through cell sorting using a SH800 cell sorter (Sony Corp., Tokyo, Japan), and selection was maintained in a medium supplemented with 0.5 mg/mL of Zeocin (InvivoGen, San Diego, CA).
CHO-K1, mCXCR1-overexpressing CHO-K1 (CHO/mCXCR1), mCXCR1-PA-overexpressing CHO-K1 (CHO/mCXCR1-PA), and P3U1 cells were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc., Kyoto, Japan), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B (Nacalai Tesque, Inc.). LN229 and mCXCR1-overexpressing LN229 (LN229/mCXCR1) were cultured in Dulbecco's Modified Eagle Medium (DMEM; Nacalai Tesque, Inc.), also supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B. All cell cultures were maintained at 37°C in a humidified incubator with 5% CO2 and 95% air.
2.2 Peptides
A partial sequence of the N-terminal extracellular domain of mCXCR1 (1-MAEAEYFIWTNPEGDFEKE-19) with an additional C-terminal cysteine was synthesized by Eurofins Genomics KK (Tokyo, Japan). The peptide was conjugated to keyhole limpet hemocyanin (KLH) at its C-terminus.
2.3. Production of Hybridomas
A five-week-old Sprague–Dawley rat was obtained from CLEA Japan (Tokyo, Japan) and housed under specific pathogen-free conditions. All animal experiments complied with relevant guidelines and regulations to minimize animal discomfort and distress. The experimental procedures were approved by the Animal Care and Use Committee of Tohoku University (Permit No.: 2022MdA-001).
The rat's health was monitored daily over the four-week experimental period. A humane endpoint was established as a reduction in body weight exceeding 25%. The rat was euthanized by cervical dislocation, with death confirmed by observing respiratory and cardiac arrest.
To generate monoclonal antibodies (mAbs) against mCXCR1, a rat was immunized intraperitoneally with 100 µg of KLH-conjugated mCXCR1 peptide (mCXCR1-KLH) mixed with Alhydrogel adjuvant 2% (InvivoGen). The immunization protocol included three additional weekly injections (100 µg per rat) and a final booster injection (100 µg per rat) two days before spleen cell collection. The spleen cells were harvested and fused with P3U1 cells using PEG1500 (Roche Diagnostics, Indianapolis, IN). The resulting hybridomas were cultured in RPMI-1640 medium containing 10% FBS, 5% Briclone (NICB, Dublin, Ireland), 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B. Hypoxanthine, aminopterin, and thymidine (HAT; Thermo Fisher Scientific Inc.) were added to the medium to select for hybridomas. Supernatants were then screened by enzyme-linked immunosorbent assay (ELISA) using the mCXCR1 peptide, followed by flow cytometry with CHO/mCXCR1 and CHO-K1 cells.
2.4. Aantibodies
Alexa Fluor 488-conjugated anti-rat IgG and peroxidase-conjugated anti-rat IgG were obtained from Cell Signaling Technology, Inc. (Danvers, MA) and Sigma-Aldrich Corp. (St. Louis, MO), respectively.
The culture supernatants from Cx1Mab-8 hybridomas were processed using a 1 mL Ab-Capcher column (ProteNova, Kagawa, Japan). After washing with phosphate-buffered saline (PBS), the monoclonal antibodies (mAbs) were eluted using an IgG elution buffer (Thermo Fisher Scientific Inc.). The eluates were then concentrated, and the buffer was exchanged for PBS using Amicon Ultra filters (Merck KGaA, Darmstadt, Germany).
2.5. ELISA
The mCXCR1 peptide (MAEAEYFIWTNPEGDFEKEC) was immobilized onto Nunc Maxisorp 96-well plates (Thermo Fisher Scientific Inc.) at a concentration of 1 µg/mL for 30 minutes at 37°C. After thorough washing with phosphate-buffered saline containing 0.05% Tween 20 (PBST; Nacalai Tesque, Inc.), the wells were blocked for 30 minutes at 37°C with PBST supplemented with 1% bovine serum albumin (BSA). Following this, the plates were incubated with supernatants from hybridoma cultures, followed by adding peroxidase-conjugated anti-rat IgG at a dilution of 1:20,000. The enzymatic reactions were subsequently carried out using the ELISA POD Substrate TMB Kit (Nacalai Tesque, Inc.), and the optical density was measured at 655 nm using an iMark microplate reader (Bio-Rad Laboratories, Inc., Berkeley, CA).
2.6. Flow Cytometric Analysis
Cells were collected following brief treatment with 0.25% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). Afterward, the cells were rinsed with a blocking buffer composed of 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and incubated with varying concentrations (0.01, 0.1, 1, and 10 µg/mL) of Cx1Mab-8 for 30 minutes at 4°C. Subsequently, the cells were exposed to Alexa Fluor 488-conjugated anti-rat IgG diluted to 1:2,000. Fluorescence measurements were then obtained using the SA3800 Cell Analyzer (Sony Corp.).
2.7. Determination of Dissociation Constant (KD) by Flow Cytometry
CHO/mCXCR1 cells were incubated in a series of diluted solutions of Cx1Mab-8 for 30 minutes at a temperature of 4°C. Following this, the cells were treated with Alexa Fluor 488-conjugated anti-rat IgG at a dilution of 1:200. Fluorescence measurements were then obtained using the SA3800 Cell Analyzer. The dissociation constant (KD) was determined by fitting the saturation binding curves to the one-site binding models provided in GraphPad PRISM 6 software (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Development of Anti-mCXCR1 mAbs Using N-Terminal Peptide Immunization
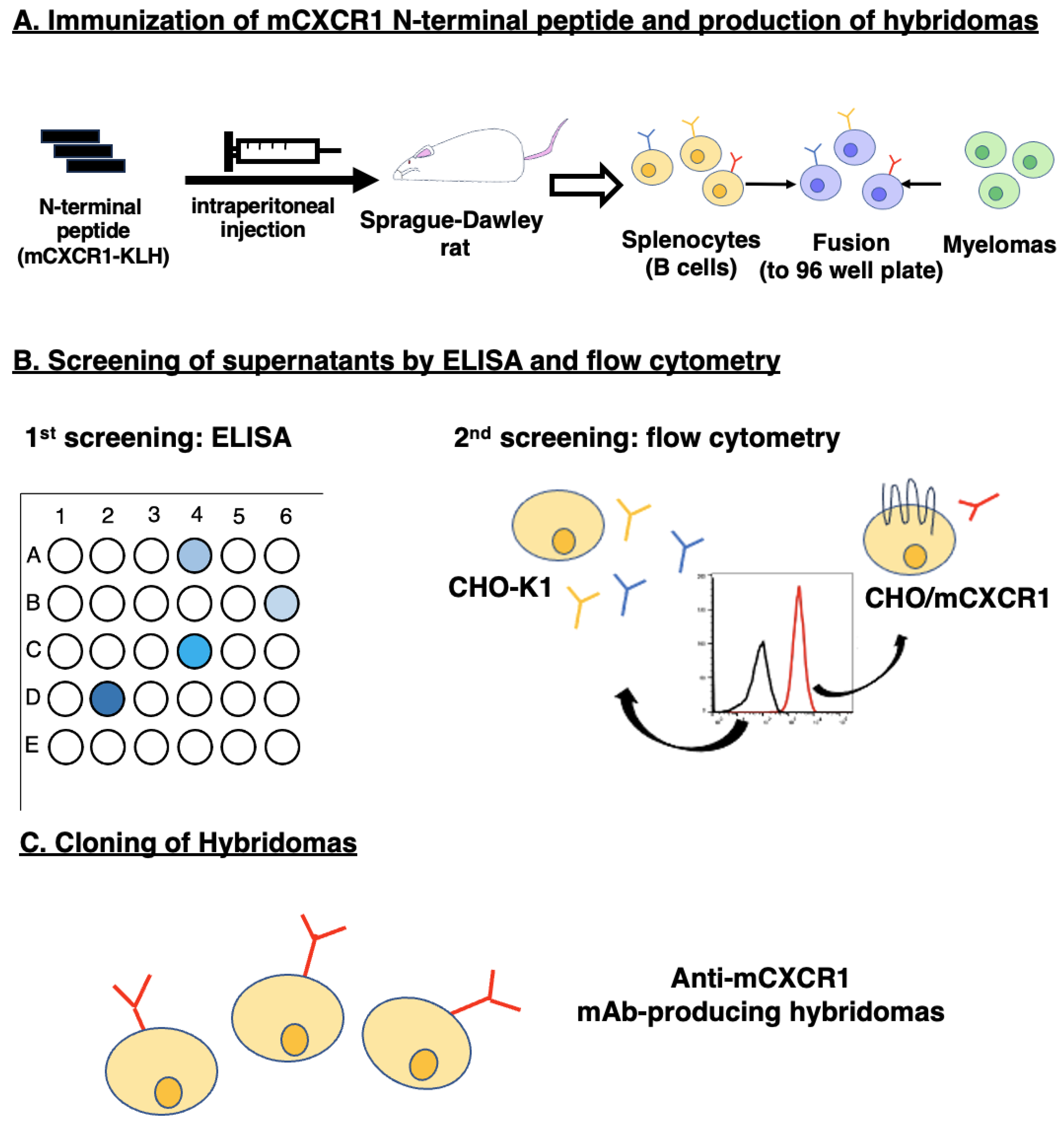
To develop anti-mCXCR1 mAbs, one rat was immunized with mCXCR1-KLH (
Figure 1A). Spleen was then excised from the rat, after which splenocytes were fused with myeloma P3U1 cells. Developed hybridomas were seeded into ten 96-well plates and cultivated for six days. Then, positive wells for the naked mCXCR1 peptide were selected using ELISA, followed by the selection of CHO/mCXCR1-reactive and CHO-K1-non-reactive supernatants using flow cytometry (
Figure 1B). The ELISA screening identified 102 out of 958 wells (10.6%), which strongly reacted with the naked mCXCR1 peptide. The flow cytometric screening identified 8 out of the 102 wells (7.8%) exhibiting strong signals to CHO/mCXCR1 cells but not CHO-K1 cells. After the limiting dilution and several additional screenings, Cx
1Mab-8 (rat IgG
2b, kappa) was finally established (
Figure 1C).
3.2. Flow Cytometric Analysis using Cx1Mab-8
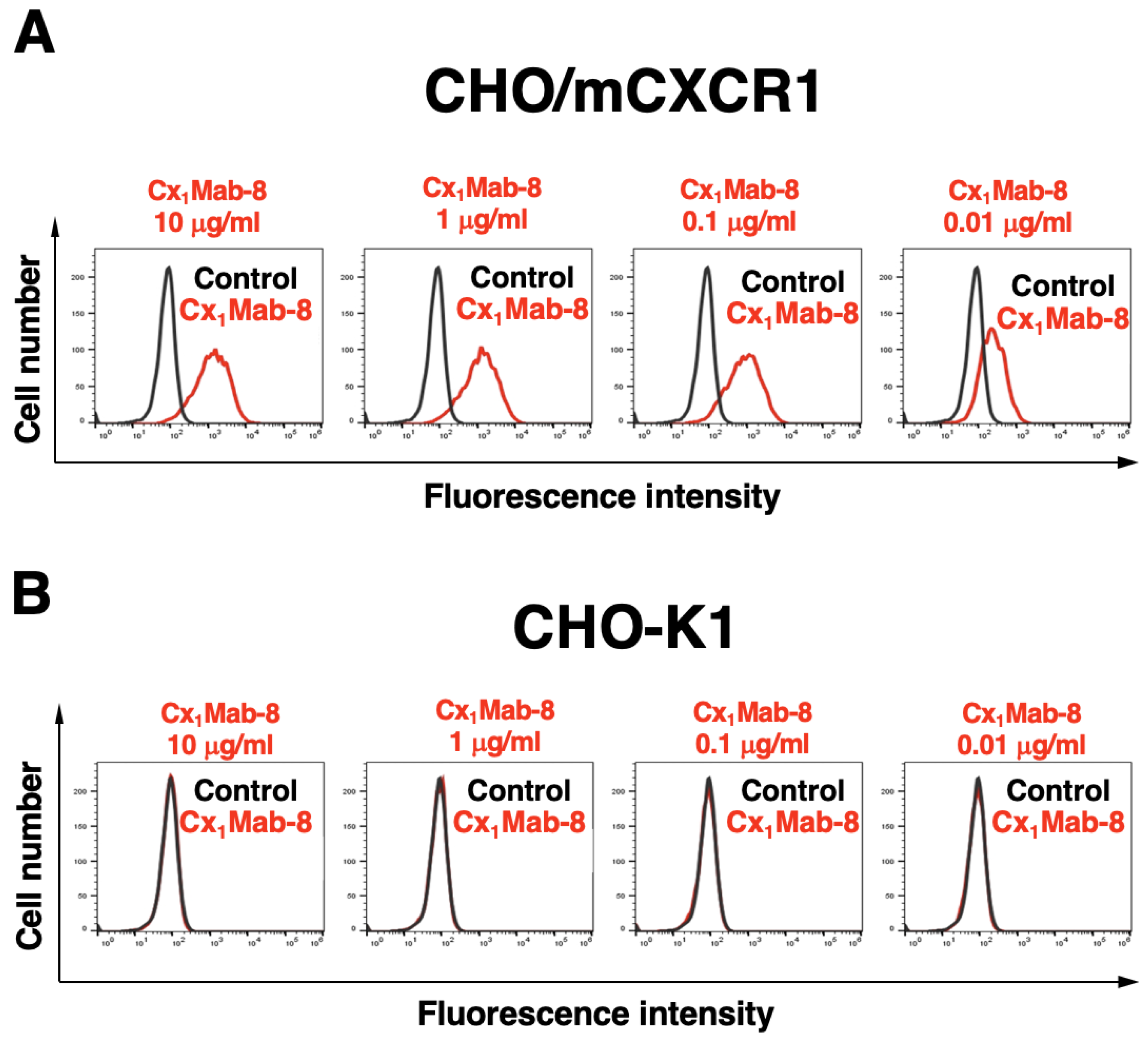
We conducted flow cytometry using Cx
1Mab-8 against CHO/mCXCR1 and CHO-K1 cells. Cx
1Mab-8 recognized CHO/mCXCR1 cells dose-dependently at 10, 1, 0.1, and 0.01 μg/mL (
Figure 2A). Parental CHO-K1 cells were not recognized by Cx
1Mab-8 even at 10 μg/mL (
Figure 2B). The similar reactivity of Cx
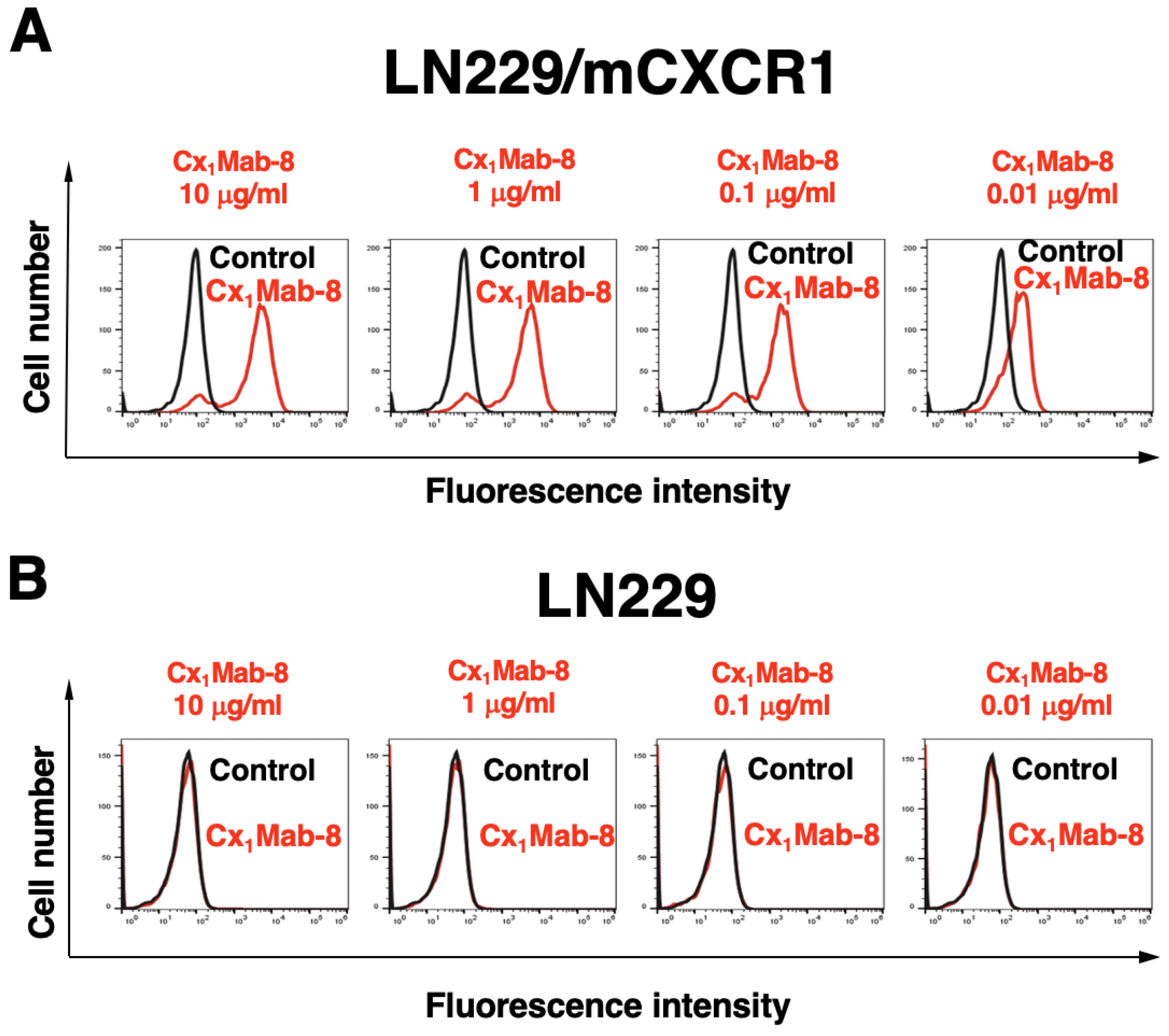
1Mab-8 was also observed in LN229/mCXCR1 cells (
Figure 3).
3.3. Determination of the Binding Affinity of Cx1Mab-8 Using Flow Cytometry
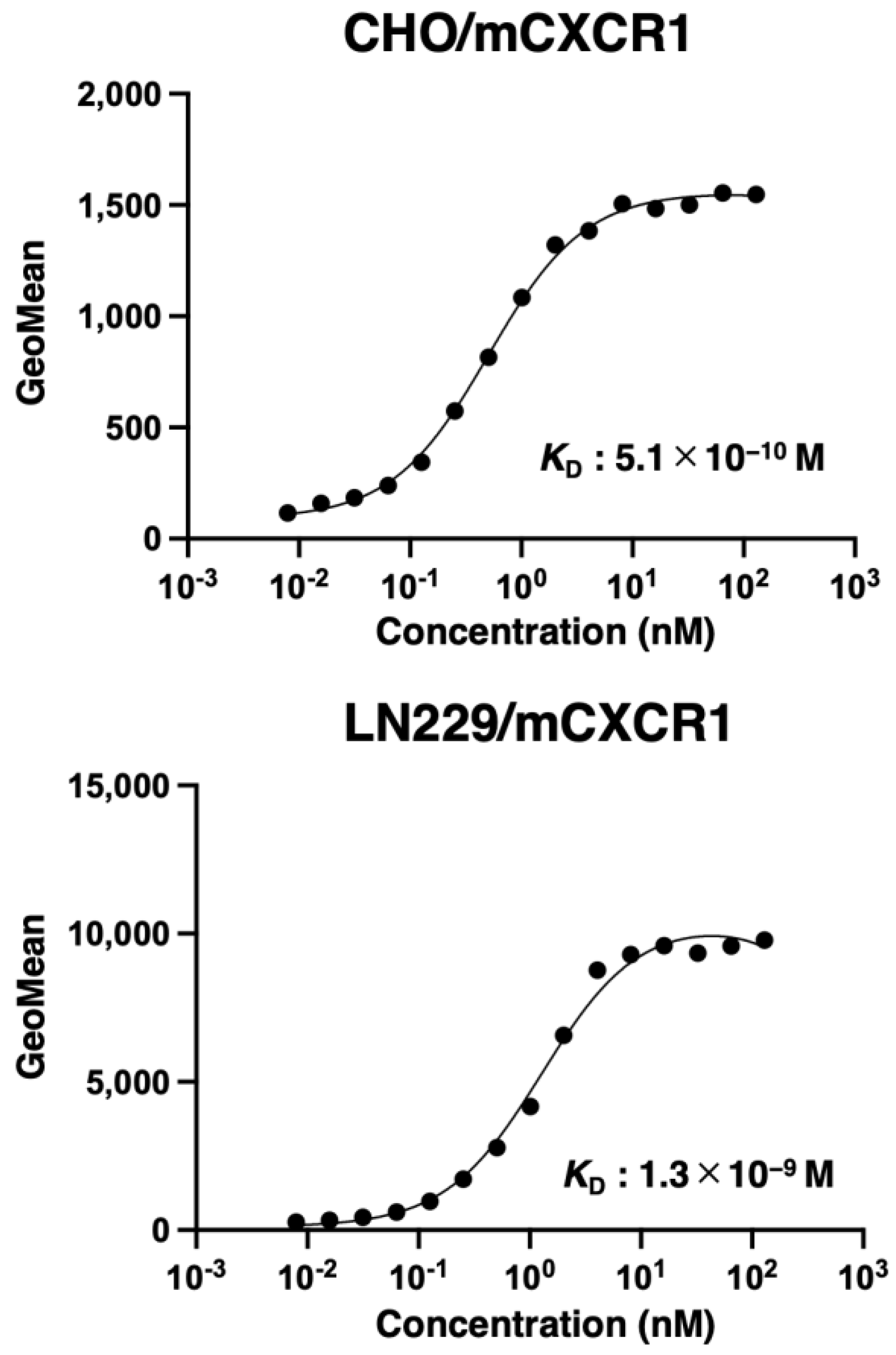
To determine the
KD values of Cx
1Mab-8 against CHO/mCXCR1 and LN229/mCXCR1, we conducted flow cytometry, and the geometric mean of the fluorescence intensity was plotted versus the concentration. The
KD values of Cx
1Mab-8 for CHO/mCXCR1 were LN229/mCXCR1 determined as 5.1 × 10
−10 M and 1.3 × 10
−9 M, respectively (
Figure 4).
4. Discussion
In this study, we developed a novel anti-mCXCR1 mAb, Cx
1Mab-8 using the N-terminal peptide immunization and showed the usefulness of flow cytometry (
Figure 2 and
Figure 3) to detect mCXCR1. Cx
1Mab-8 possess superior affinities: 5.1 × 10
−10 M (CHO/mCXCR1) and 1.3 × 10
−9 M (LN229/mCXCR1), respectively (
Figure 4) compared to that of a previously established anti-mCXCR1 mAb, Cx
1Mab-1: 2.6 × 10
−9 M (CHO/mCXCR1) and 2.1 × 10
−8 M (LN229/mCXCR1) [
9].
As described in the result section, less than 10% of ELISA-positive supernatants recognized CHO/mCXCR1 in flow cytometry. One possible explanation is a disulfate bond connecting the CXCR1 N-terminus (Cys30 in humans) to the extracellular start of transmembrane 7 (Cys277) [
7,
8]. The Cys pair is highly conserved in the chemokine receptors and is essential for ligand binding. Furthermore, it plays a critical role in shaping the extracellular structure of the chemokine receptors and provides a restriction for the structure formation. Therefore, determining the Cx
1Mab-8 epitope is essential to understand the recognition. We previously identified the Cx
6Mab-1 epitope using 1× and 2× alanine scanning methods [
27]. Future studies should focus on determining the epitope of Cx
1Mab-8.
The N-terminus of chemokine receptors plays an essential role in chemokine specificity. Structural studies have shown that the receptor N-terminus binds to the chemokine core at an interface of CRS1. In contrast, the chemokine N-terminus fits within a pocket of the receptor’s TM helical domain (called CRS2) [
28,
29]. HuMax-IL8 (BMS-986253) is a fully human monoclonal antibody against IL-8. HuMax-IL8 inhibits tumor progression by suppressing IL-8-mediated epithelial-mesenchymal transition, immune escape, and recruitment of myeloid-derived suppressor cells [
30]. A clinical trial is currently underway in hormone-sensitive prostate cancer, examining its combination with nivolumab in patients with rising prostate-specific antigen [
31]. Although CXCR1 antagonists such as navarixin and reparixin have been developed for asthma, pneumonia, and solid tumors [
5], mAb therapy using anti-CXCR1 has not been explored. Further studies are needed to investigate the neutralizing activity of Cx
1Mab-8 against mouse IL-8 orthologues, including KC, MIP-2, and LIX [
32]. Furthermore, class-switched mAbs of Cx
1Mab-8 to mouse immunoglobulins could facilitate preclinical studies for inhibiting mCXCR1 or depletion of mCXCR1-positive cells.
Author Contributions
Guanjie Li: Investigation, Writing – original draft. Hiroyuki Suzuki: Investigation, Funding acquisition, Writing – original draft. Tomohiro Tanaka: Investigation, Funding acquisition. Hiroyuki Satofuka: Investigation, Funding acquisition. Mika K. Kaneko: Conceptualization, Funding acquisition. Yukinari Kato: Conceptualization, Funding acquisition, Project administration, Writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP24am0521010 (to Y.K.), JP24ama121008 (to Y.K.), JP24ama221339 (to Y.K.), JP24bm1123027 (to Y.K.), and JP24ck0106730 (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 22K06995 (to H.Suzuki), 21K20789 (to T.T.), 24K11652 (to H.Satofuka), 21K07168 (to M.K.K.), and 22K07224 (to Y.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest involving this article.
References
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol 2014;10(5): 593-619.
- Thelen, M.; Peveri, P.; Kernen, P.; et al. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. Faseb j 1988;2(11): 2702-2706.
- Sallusto, F.; Baggiolini, M. Chemokines and leukocyte traffic. Nat Immunol 2008;9(9): 949-952.
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017;7(6): 1543-1588.
- McClelland, S.; Maxwell, P.J.; Branco, C.; et al. Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance. Cancers (Basel) 2024;16(16).
- Rajagopalan, L.; Rajarathnam, K. Ligand selectivity and affinity of chemokine receptor CXCR1. Role of N-terminal domain. J Biol Chem 2004;279(29): 30000-30008.
- Park, S.H.; Das, B.B.; Casagrande, F.; et al. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 2012;491(7426): 779-783.
- Ishimoto, N.; Park, J.H.; Kawakami, K.; et al. Structural basis of CXC chemokine receptor 1 ligand binding and activation. Nat Commun 2023;14(1): 4107.
- Li, G.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Cx1Mab-1: A Novel Anti-mouse CXCR1 Monoclonal Antibody for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2024;in press.
- Ouchida, T.; Isoda, Y.; Tanaka, T.; et al. Cx(3)Mab-4: A Novel Anti-Mouse CXCR3 Monoclonal Antibody for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2024.
- Ouchida, T.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Cx(4)Mab-1: A Novel Anti-Mouse CXCR4 Monoclonal Antibody for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2023.
- Ouchida, T.; Isoda, Y.; Nakamura, T.; et al. Establishment of a Novel Anti-Mouse CCR1 Monoclonal Antibody C(1)Mab-6. Monoclon Antib Immunodiagn Immunother 2024.
- Tateyama, N.; Asano, T.; Suzuki, H.; et al. Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies Using Flow Cytometry. Antibodies (Basel) 2022;11(4).
- Saito, M.; Harigae, Y.; Li, G.; et al. C(3)Mab-2: An Anti-Mouse CCR3 Monoclonal Antibody for Immunocytochemistry. Monoclon Antib Immunodiagn Immunother 2022;41(1): 45-49.
- sano, T.; Suzuki, H.; Tanaka, T.; et al. C(3)Mab-3: A Monoclonal Antibody for Mouse CC Chemokine Receptor 3 for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2022;41(2): 74-79.
- Suzuki, H.; Tanaka, T.; Li, G.; et al. Development of a Sensitive Anti-Mouse CCR5 Monoclonal Antibody for Flow Cytometry Monoclon Antib Immunodiagn.Immunother 2024;in press.
- Suzuki, H.; Saito, M.; Asano, T.; et al. C(8)Mab-3: An Anti-Mouse CCR8 Monoclonal Antibody for Immunocytochemistry. Monoclon Antib Immunodiagn Immunother 2022;41(2): 110-114.
- Saito, M.; Tanaka, T.; Asano, T.; et al. C(8)Mab-2: An Anti-Mouse C-C Motif Chemokine Receptor 8 Monoclonal Antibody for Immunocytochemistry. Monoclon Antib Immunodiagn Immunother 2022;41(2): 115-119.
- Saito, M.; Suzuki, H.; Tanaka, T.; et al. Development of an Anti-Mouse CCR8 Monoclonal Antibody (C(8)Mab-1) for Flow Cytometry and Immunocytochemistry. Monoclon Antib Immunodiagn Immunother 2022;41(6): 333-338.
- Tanaka, T.; Li, G.; Asano, T.; et al. Development of a Novel Anti-Mouse CCR2 Monoclonal Antibody (C(2)Mab-6) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022;41(2): 80-86.
- Asano, T.; Suzuki, H.; Goto, N.; et al. Establishment of Novel Anti-Mouse CCR3 Monoclonal Antibodies (C(3)Mab-6 and C(3)Mab-7) by N-terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022;41(2): 94-100.
- Takei, J.; Suzuki, H.; Asano, T.; et al. Development of a Novel Anti-Mouse CCR4 Monoclonal Antibody (C(4)Mab-1) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022;41(2): 87-93.
- Ubukata, R.; Suzuki, H.; Tanaka, T.; et al. Development of Sensitive Anti-Mouse CCR5 Monoclonal Antibodies Using the N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2024;43(4): 112-118.
- Kobayashi, H.; Asano, T.; Suzuki, H.; et al. Establishment of a Sensitive Monoclonal Antibody Against Mouse CCR9 (C(9)Mab-24) for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2023;42(1): 15-21.
- Kitamura, K.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Cx(6)Mab-1: A Novel Anti-Mouse CXCR6 Monoclonal Antibody Established by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022;41(3): 133-141.
- Hirose, M.; Suzuki, H.; Ubukata, R.; et al. Development of specific anti-mouse atypical chemokine receptor 4 monoclonal antibodies. Biochem Biophys Rep 2024;40: 101824.
- Isoda, Y.; Tanaka, T.; Suzuki, H.; et al. Epitope Mapping of an Anti-Mouse CXCR6 Monoclonal Antibody (Cx(6)Mab-1) Using the 2 × Alanine Scanning Method. Monoclon Antib Immunodiagn Immunother 2022;41(5): 275-278.
- Prado, G.N.; Suetomi, K.; Shumate, D.; et al. Chemokine signaling specificity: essential role for the N-terminal domain of chemokine receptors. Biochemistry 2007;46(31): 8961-8968.
- Catusse, J.; Liotard, A.; Loillier, B.; Pruneau, D.; Paquet, J.L. Characterization of the molecular interactions of interleukin-8 (CXCL8), growth related oncogen alpha (CXCL1) and a non-peptide antagonist (SB 225002) with the human CXCR2. Biochem Pharmacol 2003;65(5): 813-821.
- Dominguez, C.; McCampbell, K.K.; David, J.M.; Palena, C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2017;2(21).
- Bilusic, M.; Heery, C.R.; Collins, J.M.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer 2019;7(1): 240.
- Rovai, L.E.; Herschman, H.R.; Smith, J.B. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol 1998;64(4): 494-502.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).