1. Introduction
As a unique category of clay, dispersive soil is widely distributed globally. This type of soil is characterized by its susceptibility to rapid dispersion in water, low resistance to erosion, and significant stability risks[
1]. Dispersive soil exhibits distinctive properties that can trigger various hazards and pose substantial challenges during engineering construction [
2]. When exposed to water, the highly soluble particles of dispersive soil rapidly disperse and lose cohesion, leading to soil instability and a reduction in strength. These changes can result in settlement, deformation, and damage to various infrastructures [
3,
4]. In engineering construction, it is crucial to accurately identify, assess, and treat dispersive soil to minimize potential hazards and ensure project safety [
5].
Currently, dispersive soil is typically identified by the dual-density test, crumb test, pinhole test, exchangeable sodium percentage test, and pore water soluble cation test [
6]. Each of these methodologies has its distinct advantages and inherent limitations. Therefore, multiple identification tests should be conducted to comprehensively evaluate the properties of dispersive soil [
7,
8].
To prevent engineering accidents associated with dispersive soils, a variety of methods have been proposed targeting the reduction of soil dispersivity. As early as 1987, McElroy C.H. et al. suggested the use of chemical modifiers for treating dispersive soil [
9]. Conventional modification technologies for dispersive soil primarily involve the addition of lime, cement, magnesium oxide, and calcium salts [
8,
10,
11,
12,
13]. These materials undergo complex physicochemical reactions with the clay minerals in dispersive soil, including electrostatic adsorption, pozzolanic reactions, and hardening reactions, which collectively reduce soil dispersivity [
14]. When magnesium oxide is added to dispersive soils, it undergoes a series of physicochemical reactions with soil particles, thereby enhancing the attractive forces between them and reducing dispersivity [
15]. Nader Abbasi et al. [
16] discovered that the surface forces of nanoparticles, such as van der Waals and electrostatic forces, facilitate the binding and flocculation of dispersed soil particles, forming larger aggregates and thus decreasing swelling and dispersion. However, traditional chemical modifiers like cement and lime have several disadvantages, including high carbon emissions, environmental pollution, and adverse effects on groundwater and surrounding vegetation. Therefore, the development of environmentally friendly modifiers is of great significance for the application of dispersive clays. Reusing solid waste is one of the primary methods for managing solid waste in engineering projects, as it not only reduces environmental pollution but also improves the utilization efficiency of materials[
17,
18]. In recent years, numerous attempts have been made to enhance the properties of dispersive soils. Ground granulated blast furnace slag, waste coal gangue, and steel slag have been shown to effectively improve the dispersivity and mechanical properties of dispersive soil [
19,
20,
21,
22]. Additionally, polyaluminum chloride (PAC) has demonstrated significant potential in improving the dispersibility and mechanical properties of dispersive soils [
23].
Furthermore, the MICP application has demonstrated its efficacy in reducing soil dispersivity [
24]. Li Chi et al. used MICP to improve dispersive soil by depositing a large amount of calcium carbonate crystals between soil particles, thereby filling the pores and enhancing the soil's resistance to dispersion [
25]. This process effectively mitigates soil dispersivity. Moravej et al. also improved dispersive soils through microbial calcite precipitation [
24]. Tang et al. proposed that the decrease in pH during microbial activity, along with the subsequent reduction in the double layer thickness and stability of exchangeable sodium ions, are the primary mechanisms responsible for the observed decrease in soil erosion potential [
26]. V.R. Ouhadi et al. [
27] noted that the content of exchangeable sodium ions and the pH level contribute significantly to soil dispersivity. The addition of a 1.5% alum solution to dispersive soils can effectively lower the pH [
5]. Calcium lignosulfonate adsorbs soil particles, reducing the double layer thickness and providing a binding material that fills the pores between soil particles [
28,
29]. Joga J.R. et al. [
30] found that the biopolymer xanthan gum (XG) forms a thin layer on the soil surface, thereby reducing the repulsive forces in dispersive soils.
Electrokinetics (EK) is considered one of the promising methods for improving soil properties [
31,
32]. Shoghi et al. observed an average reduction of up to 50% in soil dispersivity using a rhombic electrode setup, demonstrating the efficacy of EK treatment in mitigating soil dispersivity [
2]. Vakili et al. applied a combination of lignosulfonate and electroosmosis to improve soil dispersivity. The results indicated that even a minimal lignosulfonate content of 0.5% significantly reduced the dispersivity from 88.9% to 38%. This simultaneous treatment approach yielded positive outcomes, including time savings, dispersivity reduction, and an increase in unconfined compressive strength (UCS) [
33].
Based on previous experiences, the hazards associated with dispersive soil are well recognized in engineering construction [
34]. However, most studies have focused on the individual efficacy of various improvement measures, including MICP, chemical additives, and EK remediation, with little attention to potential synergistic effects arising from the combination of two or more methods. Therefore, this study aimed to evaluate not only the individual efficacy of each method but also the synergistic effects of combining EK remediation with chemical additives to enhance dispersive soils. On the other hand, to realize the concepts of environmental protection and carbon reduction, it is an urgent problem to make resource utilization of industrial solid waste. In this study, a mixture of industrial solid waste was proposed as an additive for improving dispersive soil. The additive can be obtained by mixing raw materials such as fly ash, tailings, and slag in a specific proportion, drying until the moisture content is less than 5%, and grinding to the specified fineness. The effective use of this additive will greatly promote the resource utilization of industrial solid waste and reduce global carbon emissions. The impact of modifications on soil dispersivity was assessed through pinhole tests, double hydrometer tests, crumb tests, and exchangeable sodium percentage (ESP) tests. The modified soil was subjected to unconfined compressive strength (UCS) tests and direct shear tests to evaluate changes in strength. The microstructure of the modified soil was analyzed using energy-dispersive X-ray spectroscopy (EDS) and scanning electron microscopy (SEM) techniques. Additionally, a comparative analysis will be conducted on the improvement results of these methods to evaluate their effectiveness.
2. Materials and Methods
2.1. Materials and Apparatus
2.1.1. Dispersive Soil
The dispersive soil in this paper was sampled from Qian'an County, Jilin Province, China. The soil samples were evaluated using pinhole tests [
35], crumb tests [
36], and double hydrometer tests [
37]. The experimental results indicate that the soil sample exhibits dispersivity. The basic physical and mechanical properties of the soil samples were measured in accordance with Chinese code of GB/T 50123–2019 [
38]. The results are presented in
Table 1. According to ASTM D 2487-17 [
39], the soil is classified as lean clay (CL).
2.1.2. Additives
The additives are a composite cementitious material consisting of calcium carbide slag, iron slag powder, gypsum, and steel slag. The fundamental properties of these additives are summarized in
Table 2.
2.1.3. Bacterial Solution
Sporosarcina pasteurii[
40] (CMCC1.3687) was utilized to stabilize the soil sample. The culture medium consists of both solid and liquid forms, with the liquid medium further divided into Type A and Type B. The solid medium is used for cultivating bacterial colonies, while the liquid medium supports cell growth and reproduction to obtain a high density of microorganisms. The compositions of the bacterial culture media are listed in
Table 3.
Single colonies were extracted from the solid medium and then inoculated into test tubes containing 8 mL of Liquid Medium A. After inoculation, the medium was incubated at 32°C with shaking at 180 rpm. Subsequently, the expanded culture was conducted in Liquid Medium B at a 5% inoculation rate under the same conditions [
41]. The optical density (OD
600) of the bacterial solution reached 4.0 ± 0.5. Bacterial concentrations were determined using optical density (OD) measurement techniques [
42].
2.1.4. EK Apparatus
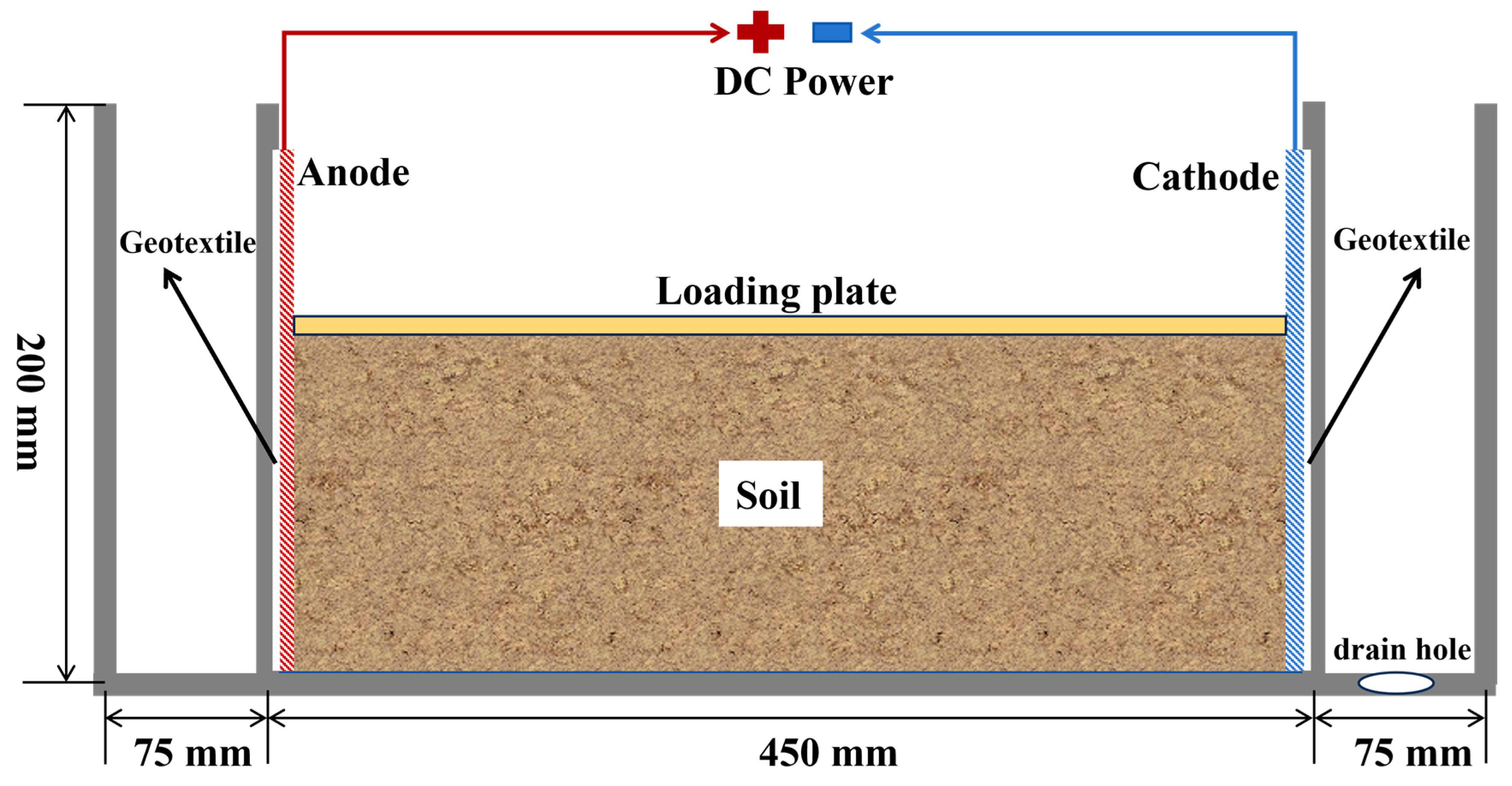
The electrokinetics (EK) experimental apparatus used in this study consists of an EK cell, two electrodes connected to a direct current (DC) power supply, and loading equipment, as shown in
Figure 1. The EK cell, constructed from 10 mm thick plexiglass, measures 600 mm in length, 100 mm in width, and 200 mm in depth. The anode and cathode were fabricated from 316 stainless steel mesh. During the tests, a loading plate was placed on the soil to apply a uniform constant load. Additionally, a geotextile was fixed between the electrode plate and the retaining plate. The geotextile allows for smooth water discharge while preventing soil particles from penetrating. The apparatus was powered by the M8811 programmable regulated DC power supply manufactured by Nanjing Merlot Company, which has a maximum power output of 150 W and a rated output of 0-30 V and 0-5 A.
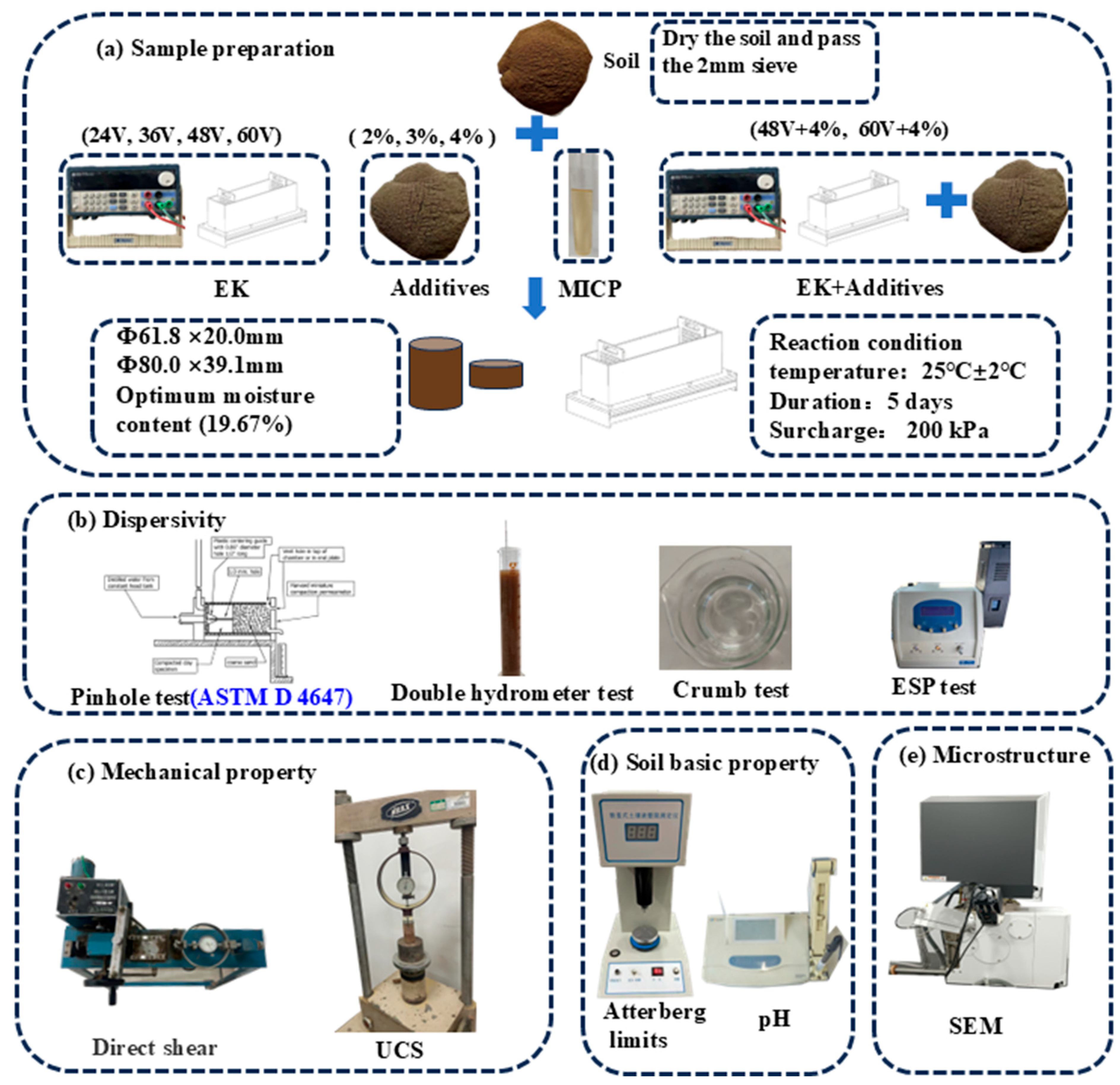
2.2. Sample Preparation
The dispersive soil was treated using various methodologies, including the addition of chemical additives at concentrations of 2%, 3%, and 4%, the use of microbial agents, electrokinetics (EK) treatments at voltages of 24V, 36V, 48V, and 60V, and combinations of EK and additives (48V + 4% and 60V + 4%). Additionally, a control test was conducted under the same loading conditions. The experimental conditions are detailed in
Table 4. The soil samples were dried and sieved through a 2 mm sieve, with an optimal water content of 19.67%. To ensure uniformity, the samples were carefully layered into the EK cell. A waterproof geotextile was then placed over the samples to prevent moisture evaporation, which can adversely affect test accuracy. During the 5-day testing period, a surcharge load of 200 kPa was applied to the soil samples until the tests were completed.
A series of post-treatment tests was conducted to evaluate the outcomes immediately after the completion of the experiments. Several tests were performed to assess the dispersivity, pH, unconfined compressive strength (UCS), and microstructure of the dispersive soil both before and after treatment. The findings are described in the subsequent sections.
2.3. Methods
In this study, pinhole tests, crumb tests, double hydrometer tests, and ESP (Exchangeable Sodium Percentage) tests were used to evaluate the dispersibility of soil samples improved by different methods. The treatment effects on the mechanical strength of the soil were assessed using unconfined compressive strength (UCS) tests and direct shear tests. The changes in the physical properties of the modified soil, including Atterberg limits and pH values, were analyzed. Energy Dispersive Spectroscopy (EDS) and Scanning Electron Microscopy (SEM) were employed to analyze the microstructure of the soil samples treated by different approaches.
Figure 2 describes the detailed test procedures of this study.
2.3.1. Dispersivity
The pinhole test [
35] is conducted using a specially designed pinhole testing device. The soil sample is compacted with a compaction instrument until the dry density reaches at least 95% of the maximum dry density. A fine axial hole with a diameter of 1.0 mm is then drilled in the center of the sample, and distilled water is used for erosion testing. The erosion of the pinhole by water is observed at water heads of 50 mm, 180 mm, 380 mm, and 1020 mm, respectively. Soil dispersivity is classified into six grades: highly dispersive (D1), dispersive (D2), transitional (ND4, ND3), and non-dispersive (ND2, ND1) [
43].
The double hydrometer test [
37] is a laboratory method used to measure soil dispersivity. In the first test, the soil sample is boiled, and the content of clay particles is determined without the addition of a dispersant. In the second test, the same soil sample is not boiled, and the content of clay particles is determined after adding a dispersant. The ratio of the clay content between the two tests is used to calculate the dispersivity of the soil sample. Soil dispersivity can be classified into three grades: dispersive (dispersivity > 50%), transitional (30% < dispersivity ≤ 50%), and non-dispersive (dispersivity ≤ 30%).
The crumb test [
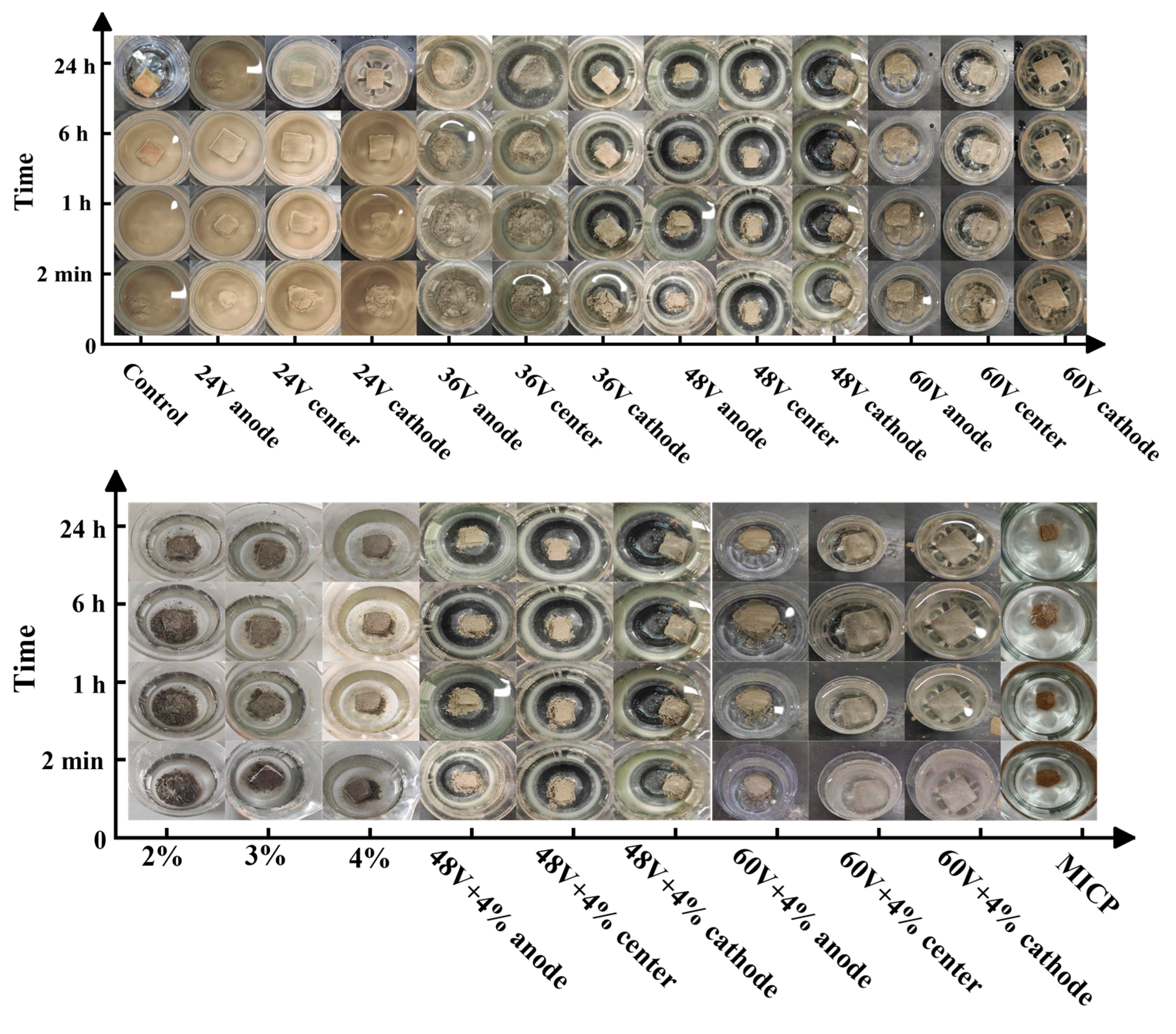
36] involves cutting the soil sample into a volume of approximately 1 cm³, filling a beaker with 200 mL of distilled water, and then placing the soil block into the beaker. Timing begins when the soil clod is placed in the beaker, and the degree of disintegration is recorded at 2 minutes, 1 hour, 6 hours, and 24 hours, respectively.
Exchangeable sodium percentage (ESP) refers to the relative content of sodium ions that can be exchanged in the soil. Soil dispersivity can be quantified by the ratio of exchangeable Na⁺ to the total exchangeable cations in the clay [
44]. Soil dispersivity can be classified into three grades: dispersive (ESP > 10%), transitional (7% < ESP ≤ 10%), and non-dispersive (ESP ≤ 7%).
2.3.2. pH
After drying, the soil sample was sieved through a 2 mm sieve and mixed with distilled water at a soil-to-water ratio of 1:5. The mixture was stirred thoroughly to ensure that the soil particles were evenly dispersed in the water, forming a uniform suspension. The pH value of the suspension was then measured using a pH meter.
2.3.3. Atterberg Limits
Plasticity reflects the sensitivity of the interaction between water and soil particles and is commonly represented by the liquid limit, plastic limit, and plasticity index. In this study, a combined liquid-plastic limit tester was used to measure the liquid limit and plastic limit of the soil samples before and after treatment, in accordance with the Chinese standard GB/T50123-2019 [
38].
2.3.4. Mechanical Tests
The strength of the soil samples was evaluated using unconfined compressive strength (UCS) tests and direct shear tests, following the Chinese standard for Geotechnical Test Procedures, GB/T50123-2019 [
38]. Cylindrical specimens measuring 80 mm in length and 39.1 mm in diameter were prepared for the UCS tests, while cylindrical specimens measuring 20 mm in length and 61.8 mm in diameter were prepared for the direct shear tests.
2.3.5. SEM
The microstructure of the dispersive soil was examined before and after improvement using an Apreoc field emission scanning electron microscope. To prepare the samples, the improved soil was cut into cubes with 100 mm edges. These cubes were then immersed in isopentane and rapidly frozen in liquid nitrogen to preserve their structural integrity. Subsequently, the samples were placed in a freeze-dryer for vacuum sublimation drying, ensuring the complete removal of moisture. After 24 hours, the fully dried soil samples were carefully broken to expose fresh, undisturbed structural planes. The samples were affixed to a circular base using conductive adhesive, with the structural plane facing upward, and then placed in a vacuum gold-coating device. The samples underwent vacuum pumping and gold coating twice to achieve optimal electrical conductivity. Finally, the soil samples were imaged and observed using the scanning electron microscope.
3. Results and Discussion
3.1. Dispersivity
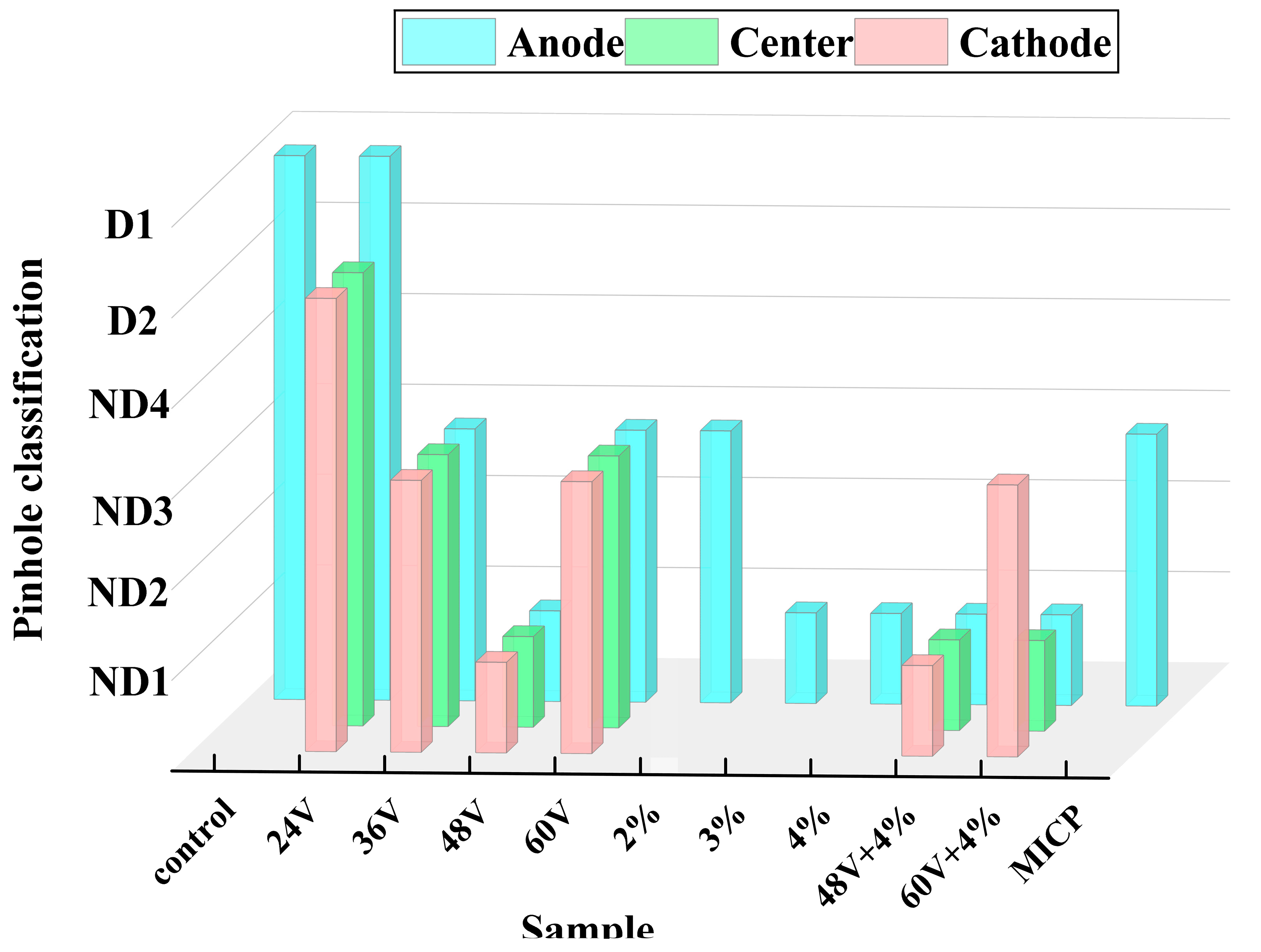
The dispersivity of the soil was analyzed using pinhole tests, crumb tests, double hydrometer tests, and exchangeable sodium percentage (ESP) tests. The treated soil exhibited significantly lower dispersivity compared to the original soil. The most pronounced enhancement effects were observed under four specific conditions: 3%, 4%, 48V (EK), and 48V + 4%, as illustrated in
Figure 3.
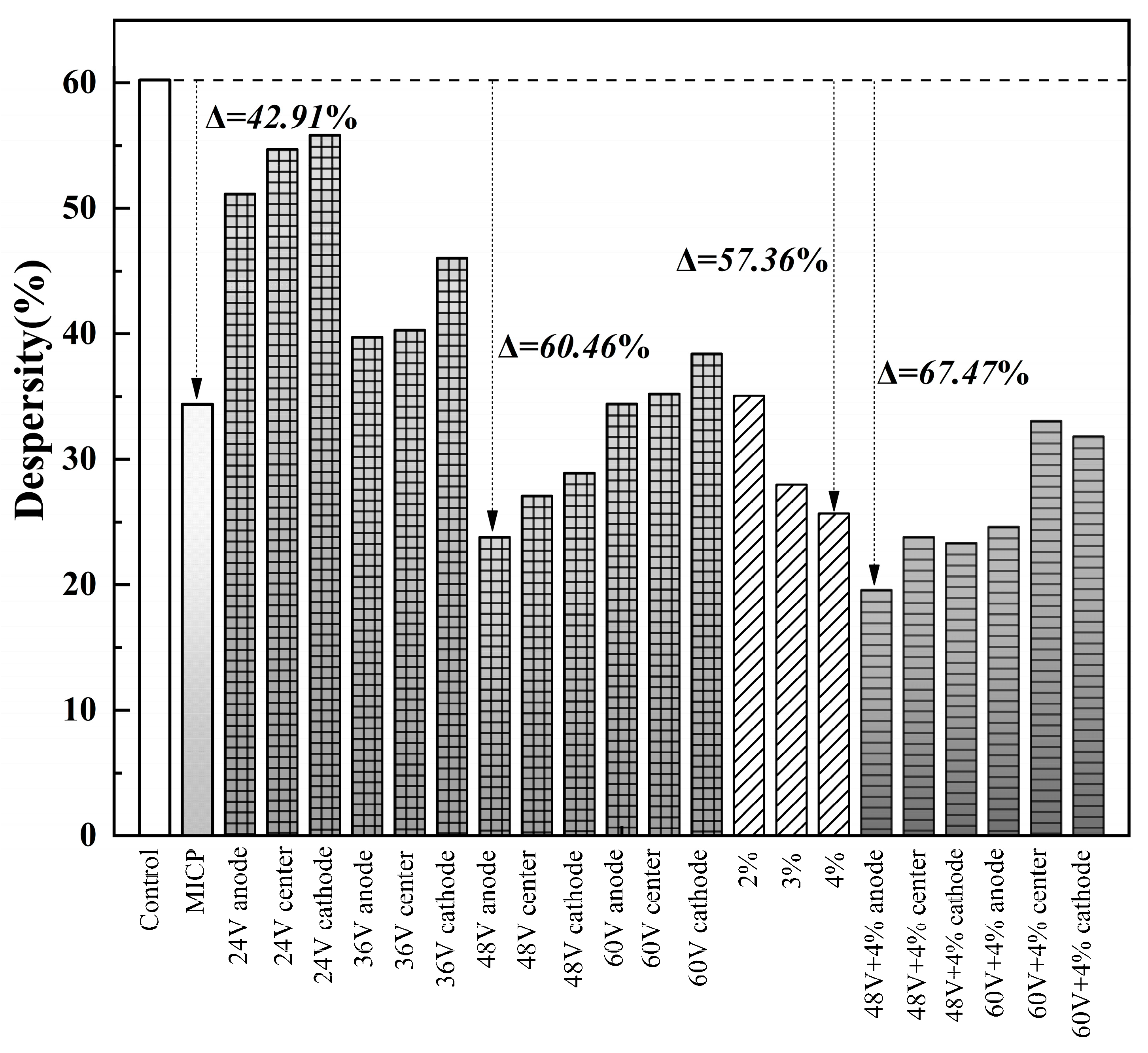
The results of double hydrometer tests are shown in
Figure 4. Among the 23 samples, 9 were classified as non-dispersive soil with dispersivity less than 30%, 10 as transitional soil with dispersivity ranging from 30% to 50%, and 3 as dispersive soil with dispersivity greater than 50%. Generally, the dispersivity of the modified soil initially decreases and then increases with voltage. The dispersivity reaches a minimum at 48 V. For the additive method, the dispersivity decreases with the concentration of the additive. The samples treated with MICP (Microbially Induced Calcite Precipitation) are classified as transitional soil.
The results of the crumb tests are presented in
Figure 5. In a beaker containing untreated dispersed soil blocks, it was observed that the soil blocks rapidly hydrolyzed, and the water became cloudy and misty. After one hour, the soil blocks had completely disintegrated, and the bottom of the container was covered with a layer of suspended matter, indicating high dispersibility in the untreated soil sample. The untreated soil was classified as dispersive due to its high dispersivity. After treatment with EK (48V), additives (4%), and a combination of EK and chemical additives (48V + 4%), the soil blocks showed only slight turbidity upon hydrolysis, with no visible diffusion in the turbid state. These treated soil samples were classified as non-dispersive. The dispersivity of the soil treated with a combination of EK and additives was lower than that of the soil treated with MICP, EK, or additives alone. For dispersive soil, the presence of substantial fine particles, small pores, and low permeability hinders the movement of microorganisms and water, limiting the supply of essential nutrients necessary for microbial growth and reproduction. Consequently, the inadequate microbial mineralization reaction restricts the formation of induced calcium carbonate crystals, which may not sufficiently fill the pores. As a result, the calcium carbonate only cements a fraction of the soil particles, leading to suboptimal soil mineralization. This, in turn, means that the soil dispersivity does not decrease to a minimum.
The optimal scheme for each of the four treatments was selected for ESP analysis, and the experimental results are illustrated in
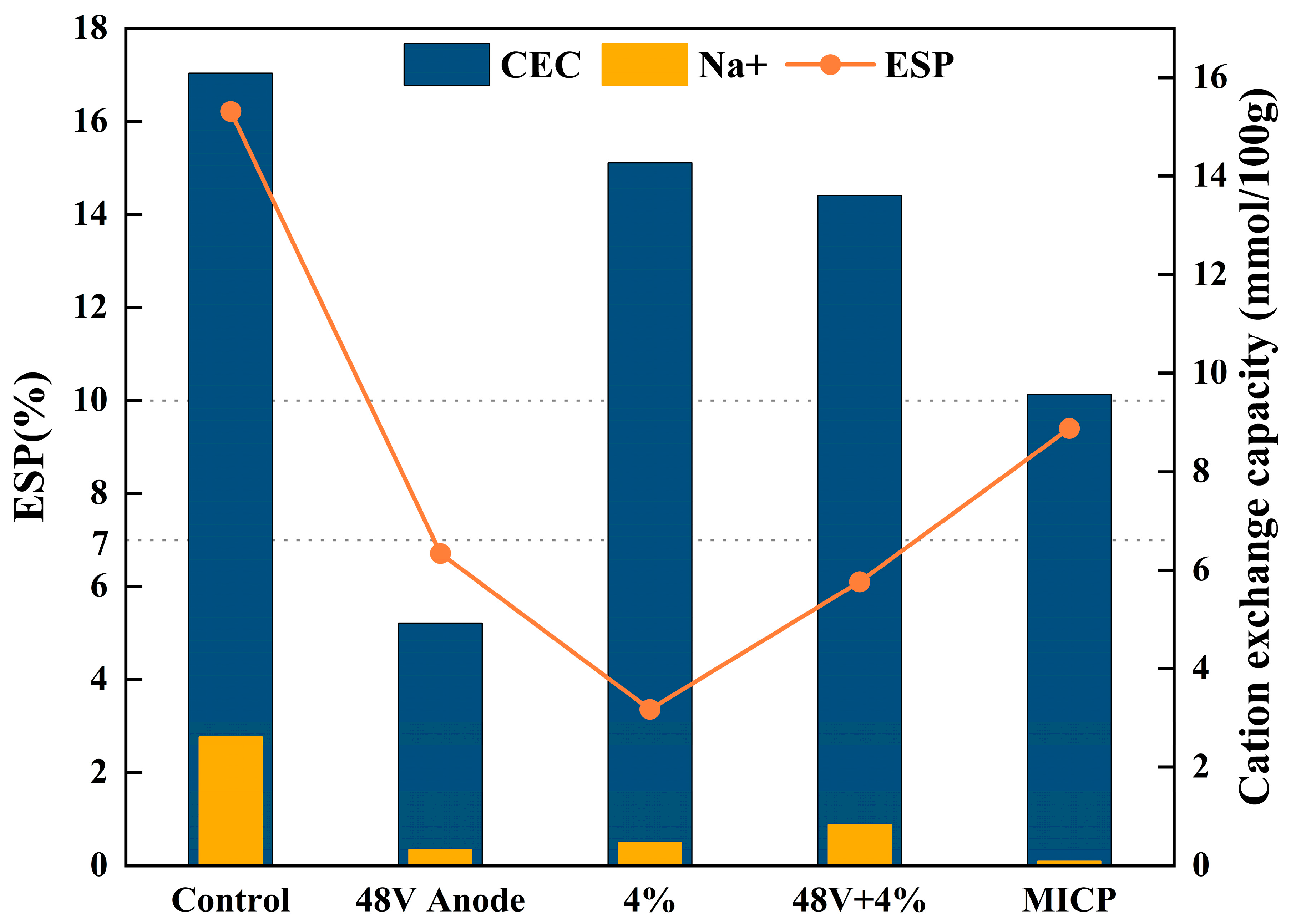
Figure 6. The concentrations of exchangeable sodium ions and the cation exchange capacity in the treated soil were significantly reduced. The soil treated with EK, additives, and the combination method all had an ESP value of less than 7%, classifying them as non-dispersive soil. In contrast, the soil treated with MICP had an ESP value of 9.4%, classifying it as transitional soil.
The dispersivity of samples collected from the vicinity of the anode is generally lower than that of samples from the cathode region, regardless of whether a curing agent is added to the EK (Electrokinetics) treatment. As pore water migrates from the anode to the cathode, cations, including sodium ions, also move in the same direction. This migration of cations, particularly sodium ions, results in their subsequent drainage along with the pore water. Consequently, the dispersivity in the anode region decreases more rapidly compared to the cathode region. Upon completion of the treatment, the samples from the cathode exhibit higher dispersivity than those from the anode, highlighting the limitations of single EK treatment. The addition of curing agents to the EK process can help alleviate these limitations.
3.2. pH
A high pH value is one of the primary factors contributing to soil dispersivity [
45]. The pH value influences the dispersibility of soil particles by altering their surface charge state [
46]. As the pH changes, the surface charge state of the soil particles also varies [
47]. Additionally, the adsorption of simple organic molecules on the surface of clay particles can enhance their negative charge, and this enhancement is also affected by the pH value [
48,
49].
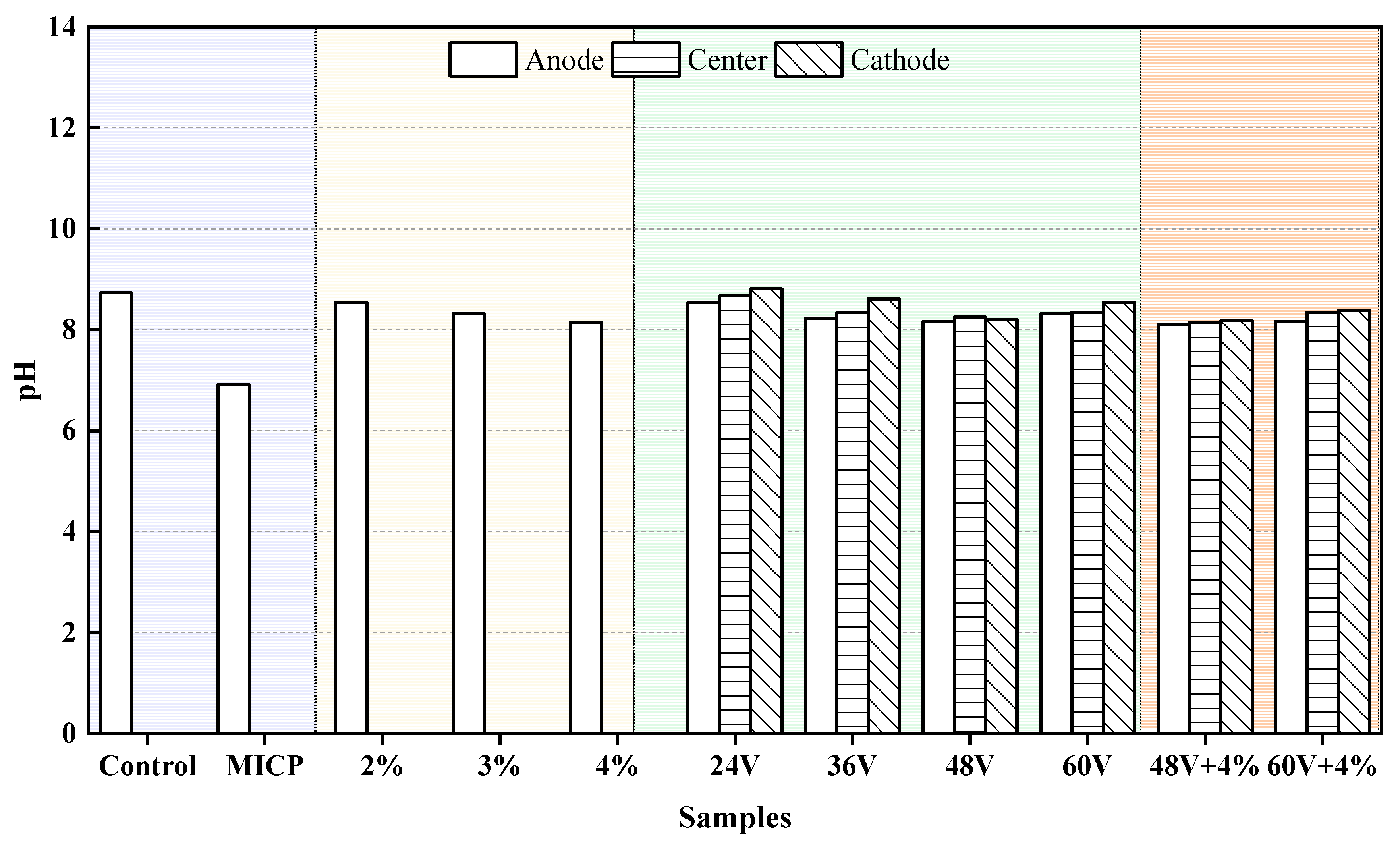
Figure 7 illustrates the pH values of the dispersive soil before and after treatment.
The treated samples showed significant variations in pH reduction depending on the method used. Among the various treatment methods, MICP (Microbially Induced Calcite Precipitation) had the most pronounced effect on pH, achieving a nearly neutral value of 6.91. This is attributed to the precipitation of calcium carbonate through microbial mineralization and associated biochemical reactions. A decrease in soil pH can reduce soil dispersivity. In the case of EK (Electrokinetics) treatment, the pH initially decreases and then increases with voltage, reaching a minimum at 48V. Under each voltage, the pH follows the pattern: anode < center < cathode. This pattern occurs because the anode undergoes hydrolysis, producing H⁺ ions, while the cathode generates OH⁻ ions, leading to a lower pH at the anode. For additive treatment, the pH decreases with increasing additive content [
2]. Compared to EK and additive methods, the combination of EK and additives results in a more significant reduction in pH.
3.3. Atterberg Limits
Plasticity reflects the sensitivity of the interaction between water and soil particles and is commonly represented by the liquid limit, plastic limit, and plasticity index. Moravej et al. [
24] tested dispersed soil treated with a bacterial solution and found a significant reduction in both the PI and LL. In this study, Atterberg limit tests for the dispersive soil were conducted according to the standard GB/T 50123-2019 [
38].
Figure 8 illustrates the plasticity of the dispersive soil before and after treatment.
Figure 8a demonstrates that the point representing the plasticity index (PI) and liquid limit (LL) of the natural dispersive soil lies above the A-line in the plasticity chart, classifying the soil as 'CL' (clay of low to medium plasticity). Among the various treatment methods, MICP (Microbially Induced Calcite Precipitation) exhibits the most significant reduction in plasticity. The soil sample treated with a 4% additive also lies above the A-line but with a lower PI, indicating that the additive can reduce plasticity. The plasticity of the soil sample treated by EK (Electrokinetics) decreases with increasing voltage and shows more remarkable changes compared to the additive treatment. The electrokinetics reaction alters the inherent structure of the soil, thereby reducing the plasticity index. The plasticity index initially increases and then decreases with voltage, suggesting that higher voltage does not always result in a more effective electrokinetics response. Notably, the combination treatment results in the most significant increase in plasticity. Despite these changes in plasticity, all the treated soils remain classified as 'CL'. Additionally, only the soils treated by EK show an improvement in the plastic limit, as illustrated in
Figure 8b. All the treated samples decline in the liquid limit, as illustrated in
Figure 8a. Overall, different treatment methods result in varying degrees of reduction in the plasticity index.
3.4. Mechanical Property
3.4.1. UCS
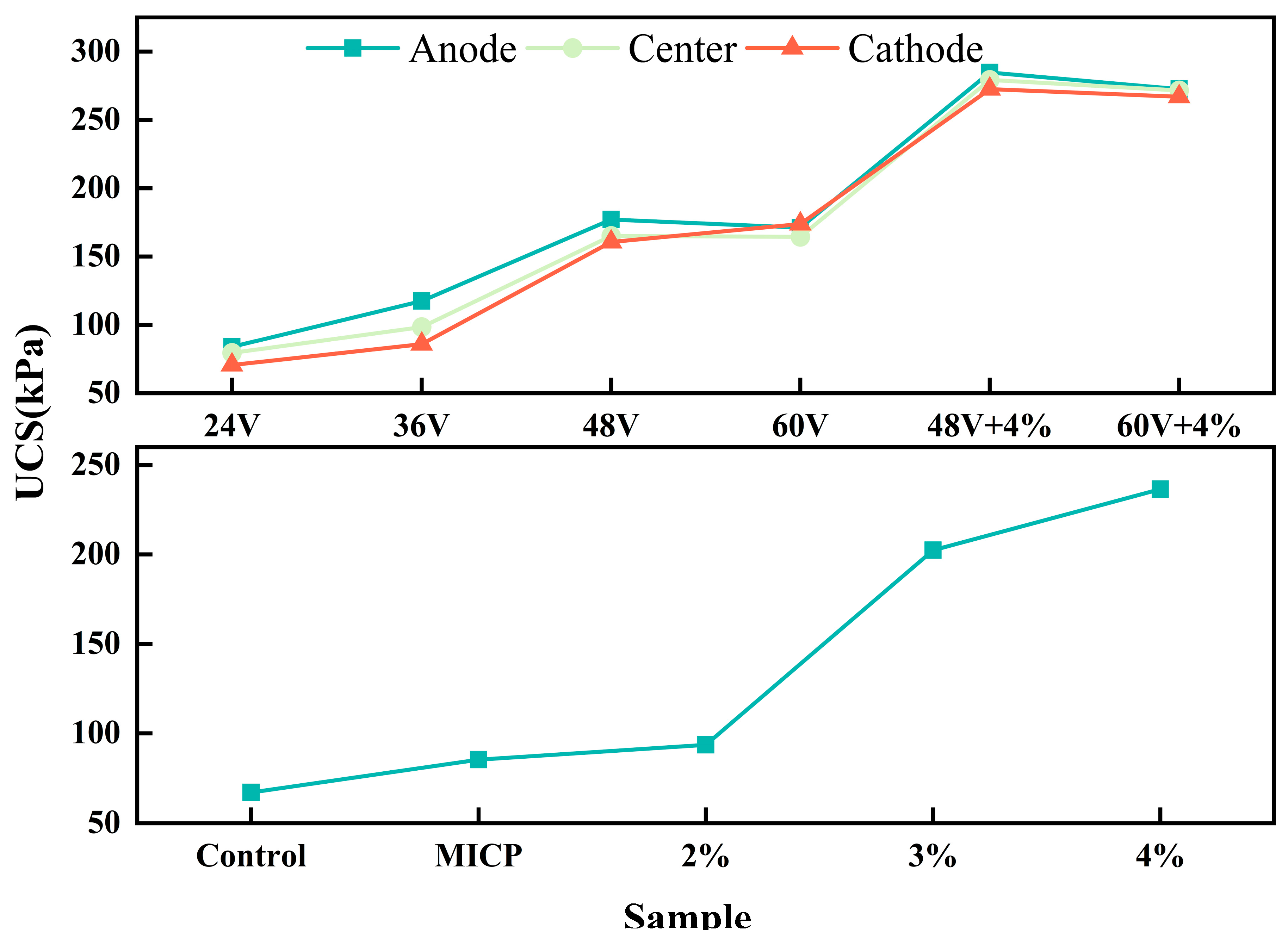
Figure 9 illustrates the results of the unconfined compressive strength (UCS) tests. The UCS of the soil samples treated by EK (Electrokinetics) treatment initially increases and then decreases with voltage, reaching a maximum at 48V. Notably, the soil near the anode exhibits a more significant increase in UCS compared to the soil near the cathode and central regions. The addition of additives dramatically increases the UCS from 93.69 kPa to 236.6 kPa, which is a more significant improvement than that achieved by the EK method alone. This phenomenon can be attributed to the involvement of the additive in physicochemical reactions, such as ion adsorption and exchange with the soil matrix. These reactions alter the connectivity at the particle-particle interfaces, reducing the thickness of the double electric layer and promoting cohesive behavior within the soil. Furthermore, the new substances produced from the chemical reactions occupy the voids within the soil matrix, enhancing the bonding strength among soil particles and further increasing the overall strength of the soil, as characterized by the increase in UCS. The combination treatment results in the most significant enhancement of UCS. Under the same additive concentration, the most significant increase in UCS is observed at 48V. In contrast, the MICP (Microbially Induced Calcite Precipitation) treatment shows little improvement in UCS.
3.4.2. Direct Shearing Test
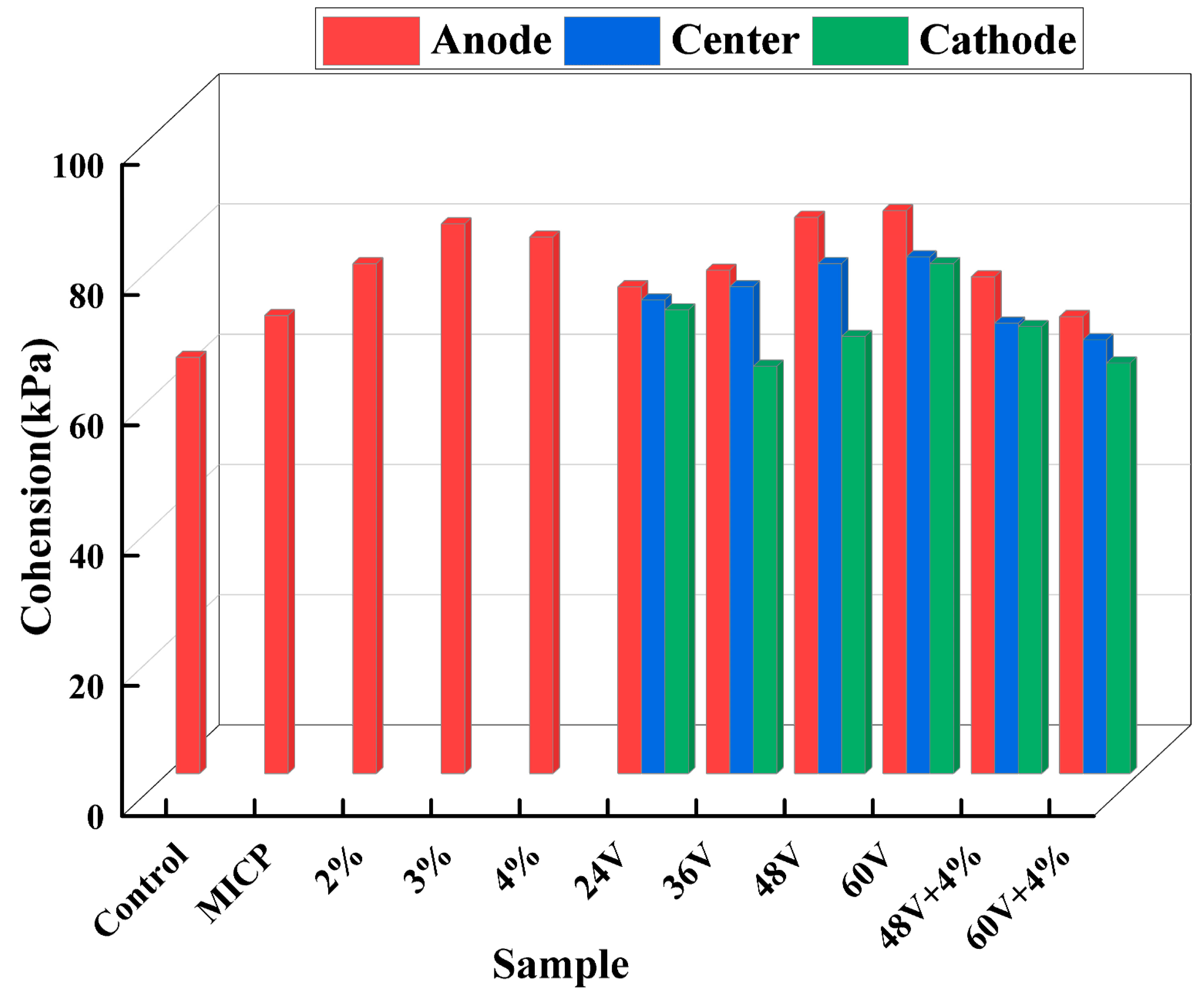
Figure 10 illustrates the cohesion of dispersive soil before and after treatment. Under EK (Electrokinetics) treatment with a 200 kPa load, the cohesive force increases with voltage, and the anode region exhibits higher cohesion than the middle and cathode regions. The maximum cohesion was observed at the anode at 60V, while the minimum cohesion was found at the cathode at 36V.In the case of additive treatment, the cohesion initially increases and then decreases with increasing additive concentration, reaching a maximum at a 3% additive concentration. When the additive concentration increases from 2% to 3%, the cohesion increases from 73.81 kPa to 84.41 kPa due to the enhanced bonding forces between particles. However, as the concentration continues to increase, the cohesion decreases from 84.41 kPa to 82.38 kPa. This indicates that the addition of an appropriate amount of additives can improve the cohesion of dispersive soil to a certain extent, but excessive additive content can lead to the expansion of cracks in the soil, forming weak surfaces and reducing the cohesion between soil particles.
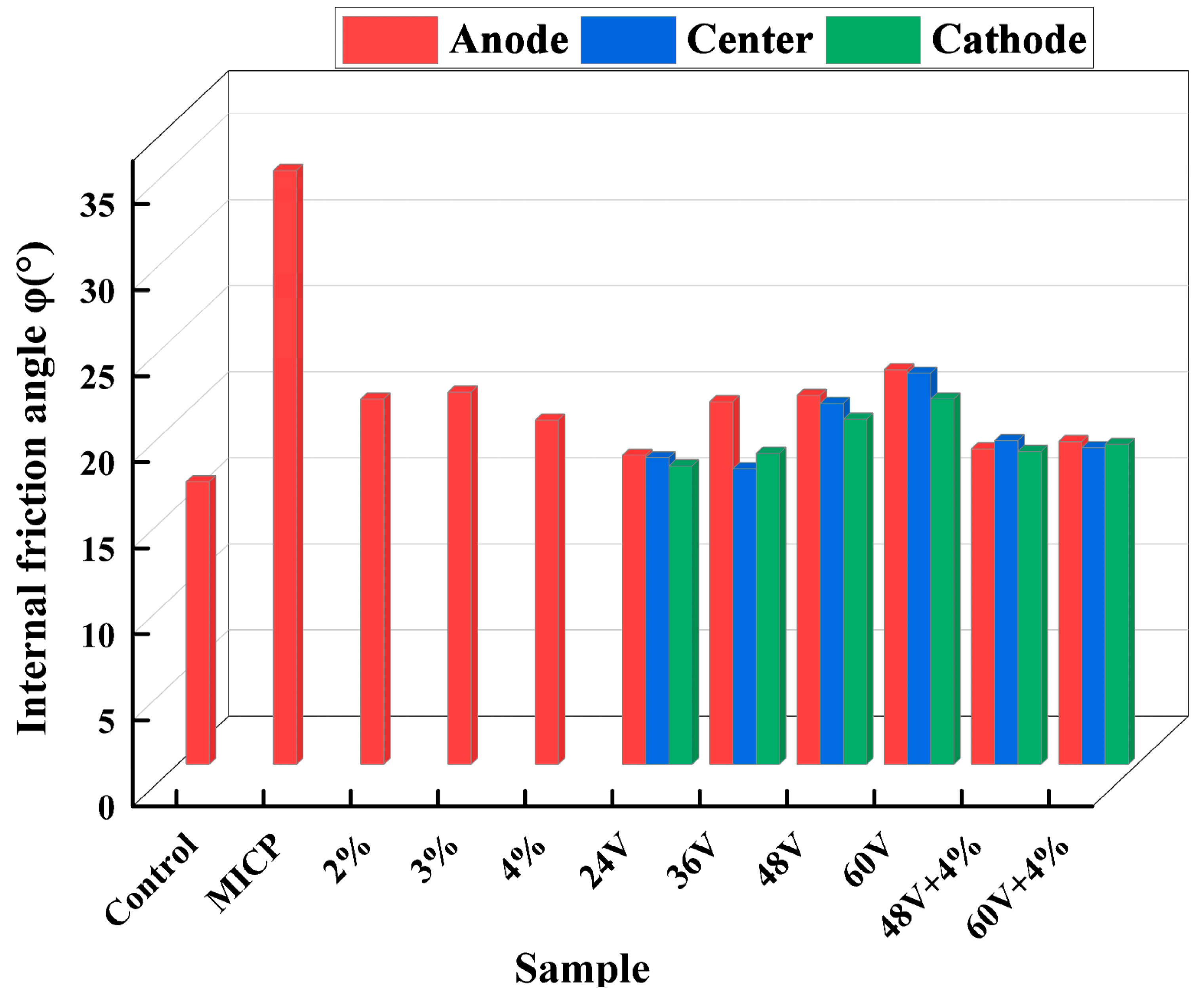
Figure 11 indicates that the internal friction angle increases after treatment in a manner similar to cohesion. EK (Electrokinetics) treatment results in a slightly higher increase in the internal friction angle compared to the additive method and the combination treatment. Among the various treatment methods, MICP (Microbially Induced Calcite Precipitation) provides the most significant increase in the internal friction angle. This is due to the cementation effect of calcium carbonate crystals formed between soil particles. The calcium carbonate induced by microorganisms exerts a binding and filling effect, which improves the particle size distribution and surface roughness of the soil granules. The calcium carbonate crystals formed between soil particles enhance the interlocking forces among the particles through an interlocking mechanism, thereby improving the internal friction angle of the dispersive soil.
3.5. Microstructure
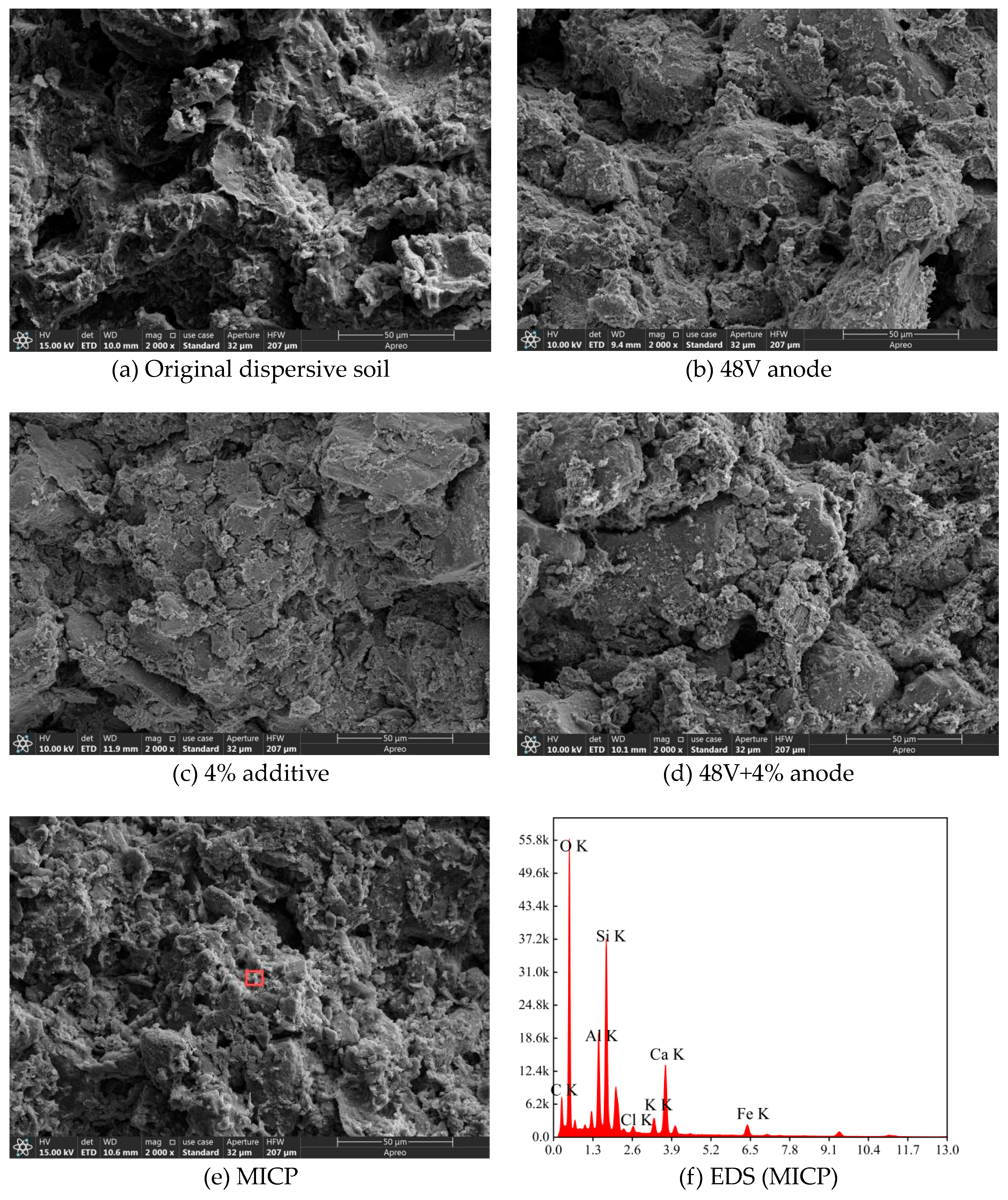
Figure 12 presents microstructural photographs at 2000× magnification of dispersive soil before and after various treatments. The untreated dispersive soil samples contain a high abundance of fine particles, resulting in a loose soil skeleton with large pores. Additionally, pronounced cracks form within the samples, indicating strong connectivity between the pores and cracks.
Figure 12(b) shows that after EK (Electrokinetics) treatment, the number of pores, as well as the length and width of the cracks, is significantly reduced.
Figure 12(c) illustrates that, with the addition of 4% additives, there is a substantial reduction in porosity and the dimensions of the cracks, accompanied by an increase in particle agglomeration. The additives effectively fill the pores, leading to a denser soil structure, and the enhanced bonding between particles improves the integrity and strength of the soil.
Figure 12(d) demonstrates that the microscopic morphology after the combination treatment differs markedly from that after either EK treatment or additive treatment alone. In
Figure 12(e), it is observed that a significant number of calcium carbonate crystals fill the pores following MICP (Microbially Induced Calcite Precipitation) treatment. This enhances the cohesion among soil particles and increases the soil's resistance to dispersivity.
Figure 12(f) shows the EDS (Energy-Dispersive X-ray Spectroscopy) results of the soil samples after MICP treatment, indicating high levels of oxygen (O), aluminum (Al), silicon (Si), and calcium (Ca). This confirms that the filler produced by MICP is primarily composed of calcium carbonate (CaCO₃).
4. Conclusions
This study conducted laboratory experiments to investigate the effects of electrokinetics (EK) treatment, additives, Microbially Induced Calcite Precipitation (MICP), and a combination of EK and additives on the improvement of dispersive soil. The research involved experimental studies and a comparison of the basic physical characteristics, dispersivity, unconfined compressive strength (UCS), pH, shear strength, and microstructural features of the dispersive soil before and after treatment. The primary aim was to explore the effectiveness and comparative performance of the four improvement methods. The key conclusions drawn from this research are as follows:
(1) Following the enhancement of dispersive soil through methods such as Microbially Induced Calcite Precipitation (MICP), additives, EK (Electrokinetics) treatment, and a combination of EK and additives, varying degrees of reduction in dispersivity were observed. The results from crumb tests, double hydrometer tests, pinhole tests, and exchangeable sodium ion tests indicated that these four methods effectively reduce soil dispersivity to varying extents. Furthermore, the combined method resulted in the most significant improvement in dispersivity.
(2) The combination of EK (Electrokinetics) treatment and additives significantly influences the mechanical properties of the soil. The Unconfined Compressive Strength (UCS) of the dispersive soil increased substantially by approximately 325% after treatment with the combined EK and additives method, compared to the original dispersive soil. This increase in UCS was markedly more significant in the dispersive soil treated with the combined method than in soil treated solely with MICP (Microbially Induced Calcite Precipitation), EK, or additives.However, regarding the internal friction angle, the treatment effect of MICP was the most significant, followed closely by the EK method. The results of tests involving EK treatment indicate that the enhancement effect on soil located near the anode is more pronounced than that observed near the cathode.
(3) By integrating the results from ESP (Electrostatic Potential) and SEM (Scanning Electron Microscopy) with all experimental data, it was observed that EK (Electrokinetics) treatment reduces Na⁺ concentration and pH through ion migration and hydrolysis reactions near the electrodes. The additive contains a high concentration of Ca²⁺, which facilitates ion exchange with Na⁺ in the dispersive soil, thereby reducing the thickness of the double electric layer and ultimately decreasing soil dispersion. The additive also includes C₂S (Calcium Silicate) and C₃S (Tricalcium Silicate), which generate significant amounts of hydration products. This process leads to the formation of a structural gel and the development of cementation between soil particles, thereby enhancing the soil's mechanical strength.
(4) MICP (Microbially Induced Calcite Precipitation) method does not significantly improve the dispersivity of dispersive soil, it does enhance the shear strength of the treated soil, with a particularly notable increase in the internal friction angle. The detailed mechanisms underlying these improvements require further in-depth study and discussion.
Author Contributions
Conceptualization, Methodology, Supervision, Writing -review& editing, Proof reading the article, Pinghui Liu. Validation, Writing -original and Investigation, Ruimeng Zhu. Investigation, Data analysis, Feiyan Zhao. Methodology, editing, Yang Zhao.
Funding
This research was funded by the Young Innovative Talent Project of North China University of Water Resources and Electric Power and Natural Science Foundation of Henan Province (232300421208).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data, models, or code generated or used during the study are available from the corresponding author upon request.
Acknowledgments
The authors gratefully acknowledge the financial supports from the Young Innovative Talent Project of North China University of Water Resources and Electric Power and Natural Science Foundation of Henan Province (232300421208).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Turgut, A.; Isik, N. S.; Kasapoglu, K. E. Investigation of factors affecting the dispersibility of clays and estimation of dispersivity. Bull. Int. Assoc. Eng. Geol. 2016, 76, 1051–1073. [Google Scholar] [CrossRef]
- Shoghi, H.; Ghazavi, M.; Ganjian, N. Pilot-scale electrokinetic treatment of dispersive soil and feasibility study of sodium ion transport across the soil by electric field relocation. Arabian J. Geosci. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Desir, G.; Gutiérrez, M. Causes of the catastrophic failure of an earth dam built on gypsiferous alluvium and dispersive clays (Altorricón, Huesca Province, NE Spain). Environ. Geol. 2003, 43, 842–851. [Google Scholar] [CrossRef]
- Fatima, B.; Alshameri, B.; Hassan, W.; Maqsood, Z.; Jamil, S. M.; Madun, A. Sustainable incorporation of Plaster of Paris kiln dust for stabilization of dispersive soil: A potential solution for construction industry. Constr. Build. Mater. 2023, 397. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, X.; Chen, H. e.; Shi, Z.; Shi, B.; Niu, C. , et al. A Study on the Durability of Dispersive Soils Improved by Alum in Western Jilin Province of China. Geofluids 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Fan, H.; Kong, L. Empirical equation for evaluating the dispersivity of cohesive soil. Can. Geotech. J. 2013, 50, 989–994. [Google Scholar] [CrossRef]
- Abbaslou, H.; Hadifard, H.; Ghanizadeh, A. Effect of cations and anions on flocculation of dispersive clayey soils. Heliyon 2020, 6, e03462. [Google Scholar] [CrossRef]
- Yang, J.; Song, Y.; Fu, R.; Lu, C.; Liu, H.; Zhang, X. Experimental Study on the Comprehensive Identification and Improvement of Dispersive Soil in Western Jilin Province, China. Geofluids 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- McElroy, C. H.,The use of chemical additives to control the erosive behavior of dispersed clays.ASCE.Symposium on Engineering Aspects of Soil Erosion, Dispersive Clays, and Loess, Atlantic City, .29 Apr 1987, New Jersey (USA),.
- Zhang, L.; Qiu, W.; Yang, X.; Fan, H.; Zhang, S.; Zhang, A. Dispersivity Identification and Modification with Lime of Soil in Huaaopao’s Water Conservancy Project. Geotech. Geol. Eng. 2022, 40, 5347–5359. [Google Scholar] [CrossRef]
- Türköz, M.; Savaş, H.; Tasci, G. The effect of silica fume and lime on geotechnical properties of a clay soil showing both swelling and dispersive features. Arabian J. Geosci. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Mohanty, S.; Roy, N.; Singh, S. P.; Sihag, P. Strength and durability of flyash, GGBS and cement clinker stabilized dispersive soil. Cold Reg. Sci. Tech. 2021, 191. [Google Scholar] [CrossRef]
- Shoghi, H.; Ghazavi, M.; Ganjian, N. The effects of chemical admixtures and physical factors on the treatment of dispersive soils. Arabian J. Geosci. 2017, 10. [Google Scholar] [CrossRef]
- Hassan, W.; Alshameri, B.; Haider, A.; Maqsood, Z.; Jamil, S. M.; Shahzad, A. A novel technique for the construction industry to mitigate dispersibility and internal erosion problems of sodium rich clays by using Water-Soluble potassium rich ions material. Constr. Build. Mater. 2023, 400, 132780. [Google Scholar] [CrossRef]
- Fattah, M. Y.; Ismael, R. H.; Aswad, M. F. Dispersion characteristics of MgO-treated dispersive clay. Arabian J. Geosci. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Abbasi, N.; Farjad, A.; Sepehri, S. The Use of Nanoclay Particles for Stabilization of Dispersive Clayey Soils. Geotech. Geol. Eng. 2017, 36, 327–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhao, W.; Jiang, X.; Chen, Y.; Hou, J.; et al. Influence of multi-scale fiber on residual compressive properties of a novel rubberized concrete subjected to elevated temperatures. J. Build. Eng. 2023, 65. [Google Scholar] [CrossRef]
- Lu, D.; Shi, X.; Zhong, J. Understanding the role of unzipped carbon nanotubes in cement pastes. Cem. Concr. Compos. 2022, 126. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, T.; Ren, G.; Zhu, Z.; Gao, Y.; Shi, M. , et al. Reusing waste coal gangue to improve the dispersivity and mechanical properties of dispersive soil. J. Clean Prod. 2023, 404. [Google Scholar] [CrossRef]
- Zhao, G.; Xie, S.; Zhang, Z.; Ren, G.; Ding, Y.; Wu, T. , et al. Engineering properties optimization of dispersive soil by calcium silicate waste-The role of steel-making slag. J. Environ. Manage. 2024, 365, 121563. [Google Scholar] [CrossRef]
- Zhao, G.; Yan, D.; Ren, G.; Zhu, Z.; Wu, T.; Ding, S. , et al. Influence of ground granulated blast furnace slag on the dispersivity and mechanical property of dispersive soil. Constr. Build. Mater. 2023, 409. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Z.; Wu, T.; Ren, G.; Zhao, G. A potential way for improving the dispersivity and mechanical properties of dispersive soil using calcined coal gangue. J. Mater. Res. Technol-JMRT 2024, 29, 3049–3062. [Google Scholar] [CrossRef]
- Xu, X.; Lei, H.; Wang, Q.; Yuan, X.; Guo, L.; Yu, Z. Polyaluminum chloride (PAC) modification of dispersive soil: A comprehensive study on dispersivity, mechanical properties, and microscale mechanisms. Constr. Build. Mater. 2024, 425. [Google Scholar] [CrossRef]
- Moravej, S.; Habibagahi, G.; Nikooee, E.; Niazi, A. Stabilization of dispersive soils by means of biological calcite precipitation. Geoderma 2018, 315, 130–137. [Google Scholar] [CrossRef]
- Li, C.; Shi, G.-y.; Wu, H.-m.; Wang, C.; Gao, Y. Experimental study on bio-mineralization for dispersed soil improvement based on enzyme induced calcite precipitate technology. Rock Soil Mech 2021, 42, 333–342. [Google Scholar] [CrossRef]
- Tang, C.-S.; Yin, L.-y.; Jiang, N.-j.; Zhu, C.; Zeng, H.; Li, H.; et al. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: a review. Environ. Earth Sci. 2020, 79. [Google Scholar] [CrossRef]
- Ouhadi, V. R.; Goodarzi, A. R. Assessment of the stability of a dispersive soil treated by alum. Eng. Geol. 2006, 85, 91–101. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S.; Puppala, A. J. Engineering properties and microstructural characteristics of foundation silt stabilized by lignin-based industrial by-product. KSCE J. Civ. Eng. 2016, 20, 2725–2736. [Google Scholar] [CrossRef]
- Vakili, A. H.; Ghasemi, J.; bin Selamat, M. R.; Salimi, M.; Farhadi, M. S. Internal erosional behaviour of dispersive clay stabilized with lignosulfonate and reinforced with polypropylene fiber. Constr. Build. Mater. 2018, 193, 405–415. [Google Scholar] [CrossRef]
- Joga, J. R.; B. J.S, V. Effect of xanthan gum biopolymer on dispersive properties of soils. World J. Eng. 2020, 17, 563–571. [Google Scholar] [CrossRef]
- Jayasekera, S. Electrokinetics to Modify Strength Characteristics of Soft Clayey Soils: A Laboratory Based Investigation. Electrochim. Acta 2015, 181, 39–47. [Google Scholar] [CrossRef]
- Abdullah, W. S.; Al-Abadi, A. M. Cationic–electrokinetic improvement of an expansive soil. Appl. Clay Sci. 2010, 47, 343–350. [Google Scholar] [CrossRef]
- Vakili, A. H.; Kaedi, M.; Mokhberi, M.; Selamat, M. R. b.; Salimi, M. Treatment of highly dispersive clay by lignosulfonate addition and electroosmosis application. Appl. Clay Sci. 2018, 152, 1–8. [Google Scholar] [CrossRef]
- Sihag, P.; Suthar, M.; Mohanty, S. Estimation of UCS-FT of Dispersive Soil Stabilized with Fly Ash, Cement Clinker and GGBS by Artificial Intelligence. Iran. J. Sci. Technol.-Trans. Civ. Eng. 2019, 45, 901–912. [Google Scholar] [CrossRef]
- ASTM. Test Methods for Identification and Classification of Dispersive Clay Soils by the Pinhole Test, (n.d.).2020, D 4647-20.
- ASTM. Standard Test Methods for Determining Dispersive Characteristics of Clayey Soils by the Crumb Test.2021,D 4221.
- ASTM. Test Method for Dispersive Characteristics of Clay Soil by Double Hydrometer.2018,D 6572.
- GB/T 50123-2019. Standard for geotechnical testing method.
- ASTM Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). D 2487–17.
- Lauchnor, E. G.; Topp, D. M.; Parker, A. E.; Gerlach, R. Whole cell kinetics of ureolysis by Sporosarcina pasteurii. J Appl Microbiol 2015, 118, 1321–1332. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, Z.; Lv, J.; Shen, W.; Xu, R. A Novel Approach to Enhance the Urease Activity of Sporosarcina pasteurii and its Application on Microbial-Induced Calcium Carbonate Precipitation for Sand. Geomicrobiol. J. 2019, 36, 819–825. [Google Scholar] [CrossRef]
- Li, C.; Yao, D.; Liu, S.; Zhou, T.; Bai, S.; Gao, Y. , et al. Improvement of Geomechanical Properties of Bio-remediated Aeolian Sand. Geomicrobiol. J. 2017, 35, 132–140. [Google Scholar] [CrossRef]
- Khodaparast, M.; Rajabi, A. M.; Moein, B.; Bazargan, J. Identifying dispersive soils by modification of chemical criterion, validated based on data from Northwest and Central Iran. Arabian J. Geosci. 2021, 14. [Google Scholar] [CrossRef]
- Singh, B.; Gahlot, P.; Purohit, D. Dispersive soils-characterization, problems and remedies. Int. J. Eng. Technol 2018, 5, 2478–2484. [Google Scholar]
- Chorom, M.; Rengasamy, P.; Murray, R. Clay dispersion as influenced by pH and net particle charge of sodic soils. Soil Res. 1994, 32, 1243–1252. [Google Scholar] [CrossRef]
- Sumner, M. E. Sodic soils - New perspectives. Soil Res. 1993, 31, 683–750. [Google Scholar] [CrossRef]
- Suarez, D. L.; Rhoades, J. D.; Lavado, R.; Grieve, C. M. Effect of pH on Saturated Hydraulic Conductivity and Soil Dispersion. Soil Sci. Soc. Am. J. 1984, 48, 50–55. [Google Scholar] [CrossRef]
- Oades, J. M. Soil organic matter and structural stability: mechanisms and implications for management. Plant Soil 1984, 76, 319–337. [Google Scholar] [CrossRef]
- Goldberg, S.; Forster, H. Flocculation of reference clays and arid-zone soil clays. Soil Sci. Soc. Am. J. 1990, 54, 714–718. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).