Submitted:
21 October 2024
Posted:
24 October 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Phenotypic Tests
2.3. Genotypic Identification by 16S rRNA Gene SEquencing
2.4. Genome Sequencing, Assembly, and Annotation
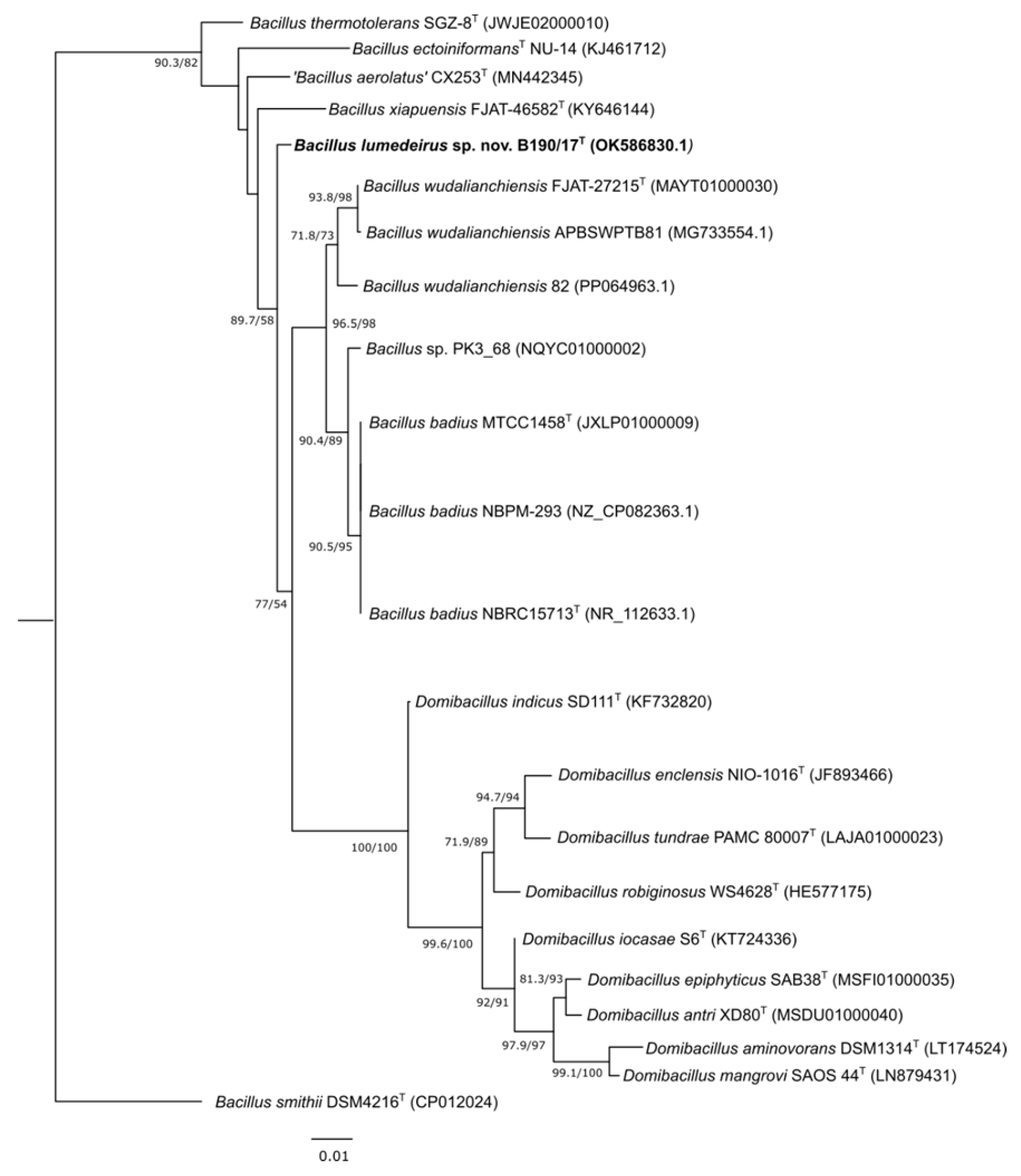
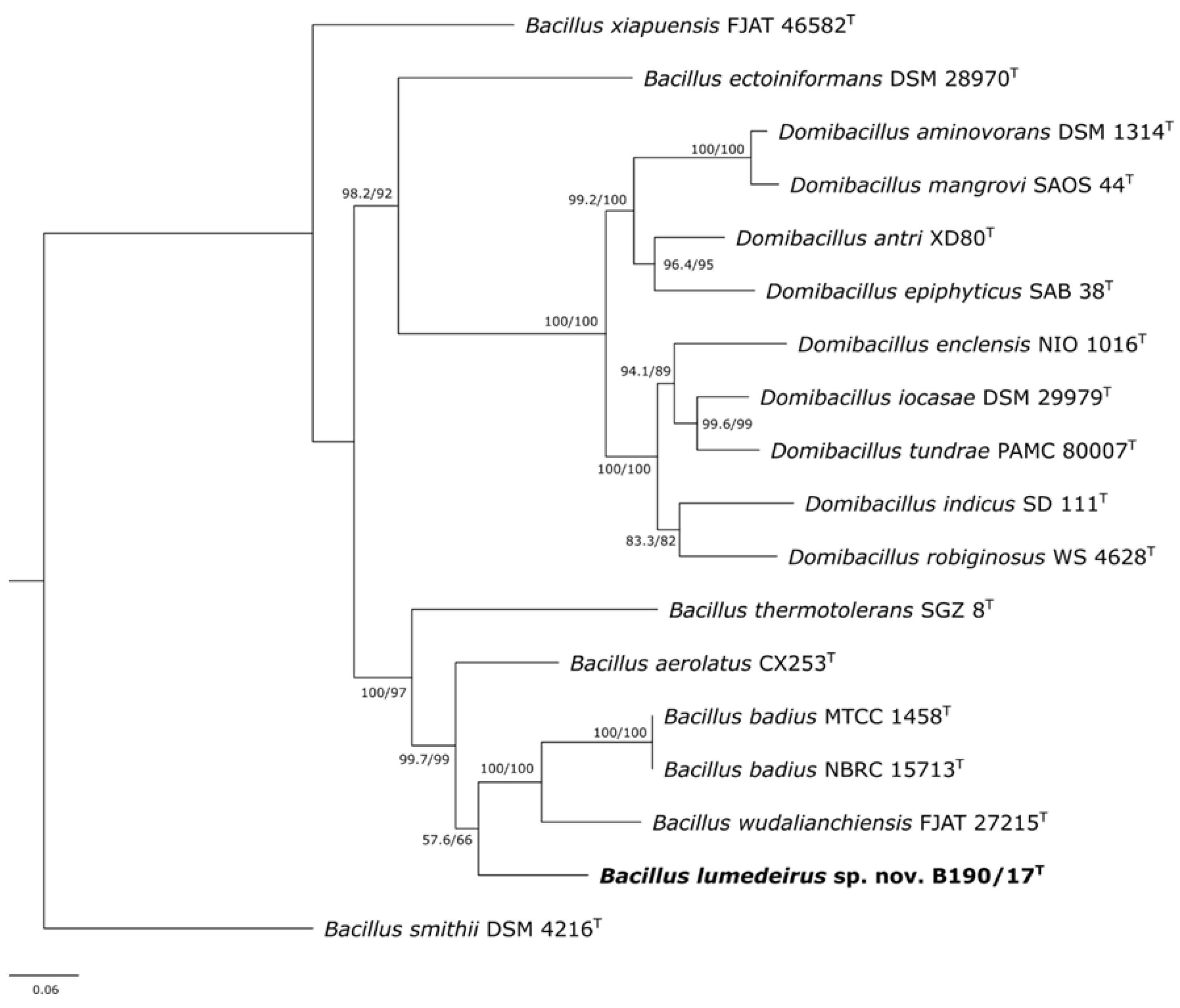
2.5. Phylogenetic Analysis of 16S rRNA, rpoB and gyrB Genes
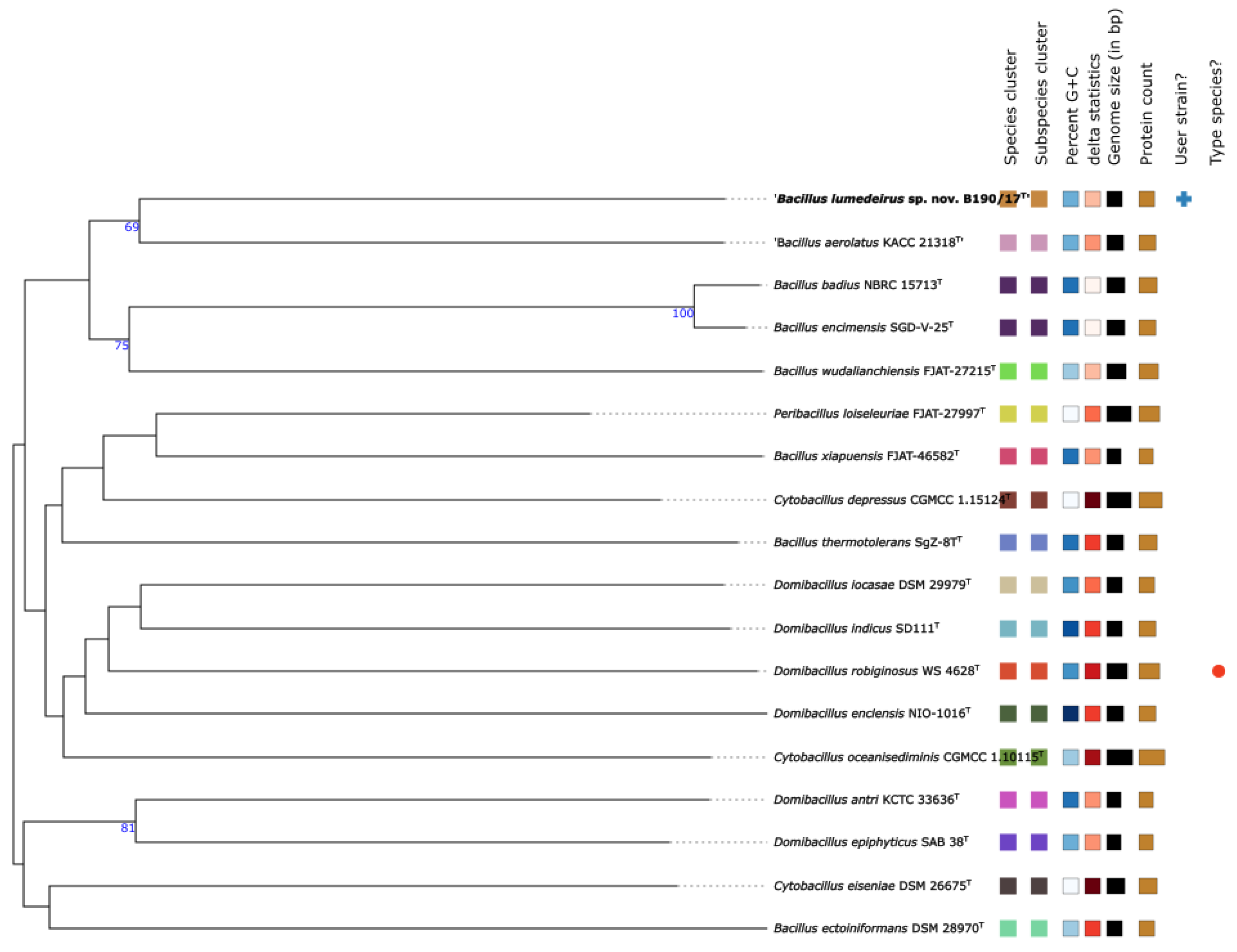
2.6. Genomic Taxonomy Analysis
2.7. Genome Sequence Deposit
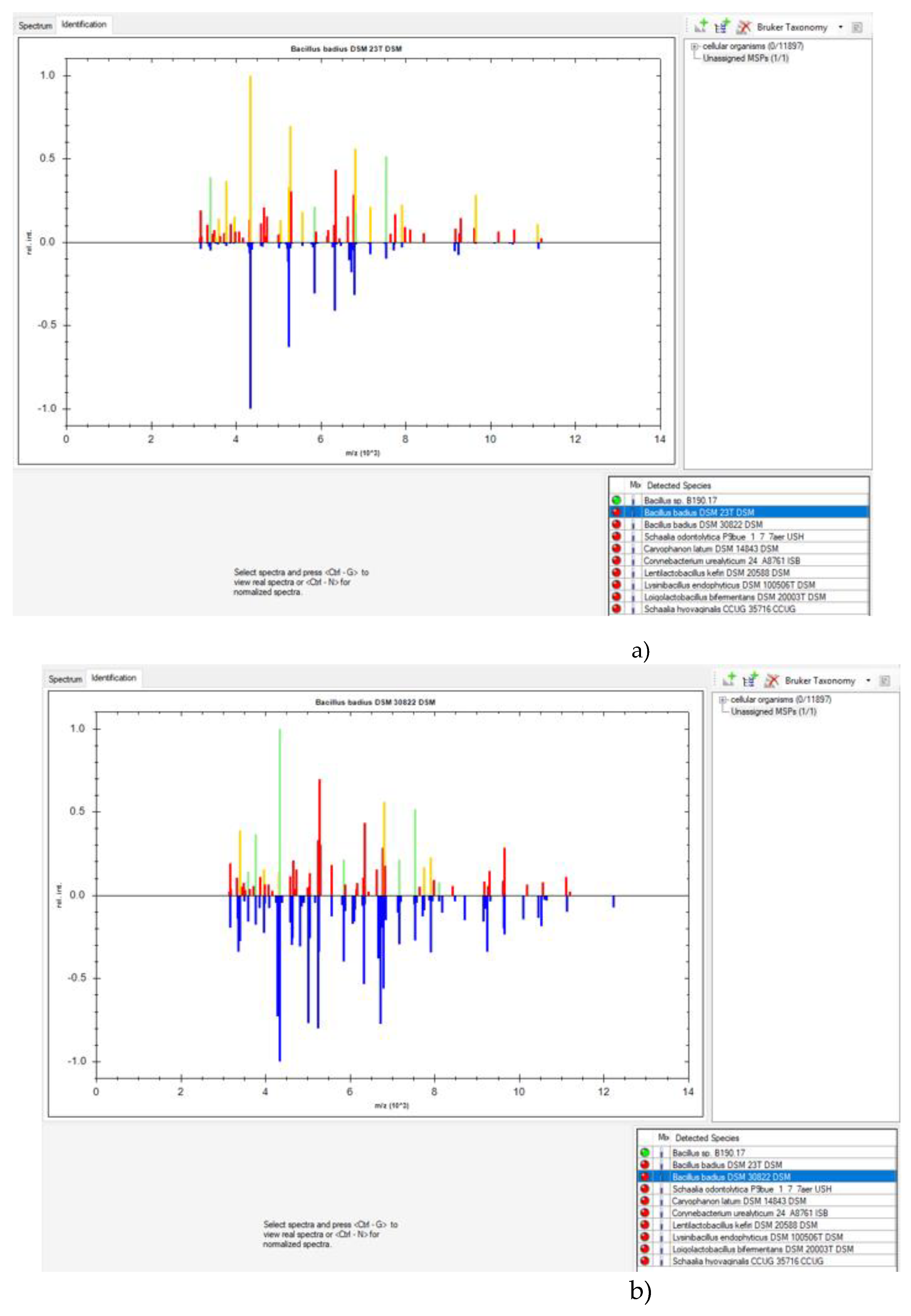
2.8. Addition of B190/17 spectra to the VITEK® MS RUO and MALDI Biotyper® database
3. Results and Discussion
Description of Bacillus lumedeirus sp. nov.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, L.V.D.; Miranda, R.V.D.S.L.; Reis, C.M.F.; Andrade, J.M.; Cruz, F.V.; Frazão, A.M.; Fonseca, E.L.; Ramos, J.N.; Brandão, M.L.L.; Vieira, V.V. MALDI-TOF MS database expansion for identification of Bacillus and related genera isolated from a pharmaceutical facility. J. Microbiol. Methods 2022, 203, 106625. DOI: 10.1016/j.mimet.2022.106625. [CrossRef]
- Song, M.; Li, Q.; Liu, C.; Wang, P.; Qin, F.; Zhang, L.; Fan, Y.; Shao, H.; Chen, G.; Yang, M. A comprehensive technology strategy for microbial identification and contamination investigation in the sterile drug manufacturing facility—a case study. Front. Microbiol. 2024, 15, 1327175. DOI: 10.3389/fmicb.2024.1327175. [CrossRef]
- European Medicines Agency. European Medicines Agency. European Union guidelines for good manufacturing practice for medicinal products for human and veterinary use. In The Rules Governing Medicinal Products in the European Union; Annex 1: Manufacture of Sterile Medicinal Products; European Medicines Agency: Brussels, Belgium, 2022; Volume 4.
- Miranda, R.V.D.S.L.; da Costa, L.V.; Albuquerque, L.S.; Dos Reis, C.M.F.; Braga, L.M.P.D.S.; de Andrade, J.M.; Ramos, J.N.; Mattoso,J.M.V.; Forsythe, S.J.; Brandão, M.L.L. Identification of Sutcliffiella horikoshii strains in an immunobiological pharmaceutical industry facility. Lett. Appl. Microbiol. 2023, 76, ovad056. DOI: 10.1093/lambio/ovad056. [CrossRef]
- Mattoso, J.M.V.; Costa, L.V.C.; Vale, B.A.; Reis, C.M.F.; Andrade, J.M.; Braga, L.M.P.S.; Conceição, G.M.S.; Costa, P.B.M.; Silva, I.B.; Rodrigues, L.A.P.; Anjos, J.P.; Brandão, M.L.L. Quantitative and qualitative evaluation of microorganism profile identified in bioburden analysis in a biopharmaceutical facility in Brazil: Criteria for classification and management of results. PDA J. Pharm. Sci. Technol. 2024, 78, 3, 1. DOI: 10.5731/pdajpst.2023.012883. [CrossRef]
- Stamatoski, B.; Ilievska, M.; Babunovska, H.; Sekulovski, N.; Panov, S. Optimized genotyping method for identification of bacterial contaminants in pharmaceutical industry. Acta Pharm. 2020, 66, 2, 289-295. DOI: 10.1515/acph-2016-0011. [CrossRef]
- Caldeira, N.G.S.; de Souza, M.L.S.; de Miranda, R.V.D.S.L.; da Costa, L.V.; Forsythe, S.J.; Zahner, V.; Brandão, M.L.L. Characterization by MALDI-TOF MS and 16S rRNA gene sequencing of aerobic endospore-forming bacteria isolated from pharmaceutical facility in Rio de Janeiro, Brazil. Microorganisms 2024, 12, 4, 724. DOI: 10.3390/microorganisms12040724. [CrossRef]
- Costa, L.V.D.; Miranda, R.V.D.S.L.; Fonseca, E.L.; Gonçalves, N.P.; Reis, C.M.F.; Frazão, A.M.; Cruz, F.V.; Brandão, M.L.L.; Ramos, J.N.; Vieira, V.V. Assessment of VITEK® 2, MALDI-TOF MS and full gene 16S rRNA sequencing for aerobic endospore-forming bacteria isolated from a pharmaceutical facility. J. Microbiol. Methods 2022, 194, 106419. DOI: 10.1016/j.mimet.2022.106419. [CrossRef]
- Husni, A.A.A.; Ismail, S.I.; Jaafar, N.M.; Zulperi, D. Current classification of the Bacillus pumilus group species, the rubber-pathogenic bacteria causing trunk bulges disease in Malaysia as assessed by MLSA and multi rep-PCR approaches. Plant. Pathol. J. 2021, 37, 3, 243. DOI: 10.5423/PPJ.OA.02.2021.0017. [CrossRef]
- Qi, H.Y.; Wang, D.; Han, D.; Song, J.; Ali, M.; Dai, X.; Zhang, X.; Chen, J. Unlocking antagonistic potential of Bacillus amyloliquefaciens KRS005 to control gray mold. Front. Microbiol. 2023, 14, 1189354. DOI 10.3389/fmicb.2023.1189354.
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. DOI: 10.1099/ijsem.0.004475. [CrossRef]
- Patel, S.; Gupta, R.S. A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus Bacillus: Proposal for six new genera of Bacillus species, Peribacillus gen. nov., Cytobacillus gen. nov., Mesobacillus gen. nov., Neobacillus gen. nov., Metabacillus gen. nov. and Alkalihalobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 406–438. DOI: 10.1099/ijsem.0.003775. [CrossRef]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. DOI: 10.1099/ijsem.0.004332. [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617.. DOI: 10.1099/ijsem.0.001755. [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tse, H.; Yuen, K-Y. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infec. 2008, 14, 10, 908-934. DOI: 10.1111/j.1469-0691.2008.02070.x. [CrossRef]
- Xiang, C-Y.; Gao, F.; Jakovlic, I.; Lei, H-P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G-T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, e87. DOI: 10.1002/imt2.87. [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Res. 2020, 20, 1, 348–355. DOI: 10.1111/1755-0998.13096. [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 4, 772–780. DOI: 10.1093/molbev/mst010. [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 6, 587–589. DOI: 10.1038/nmeth.4285. [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268-274. DOI: 10.1093/molbev/msu300. [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinformatics 2020, 70, 1, e102. DOI: 10.1002/cpbi.102. [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; Meyer, F.; Olsen, G.J.; Olson, R.; Osterman, A.L.; Overbeek, R.A.; McNeil, L.K.; Paarmann, D.; Paczian, T.; Parrello, B.; Pusch, G.D.; Reich, C.; Stevens, R.; Vassieva, O.; Vonstein, V.; Wilke, A.; Zagnitko, O. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 2008, 9, 1-15. DOI: 10.1186/1471-2164-9-75. [CrossRef]
- Lee, I.; Kim, Y.O.; Park, S.C.; Chun, J. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–3. DOI: 10.1099/ijsem.0.000760. [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 1, 2182. DOI 10.1038/s41467-019-10210-3.
- bioMerieux. Guide for VITEK® 2 BCL card, v. 045519- 02 - 2019-03. 2019, 1-24.
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.; Meyer, S.D.; Trujillo, M.E. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol 2018, 68, 1, 461-466. DOI: 10.1099/ijsem.0.002516. [CrossRef]
- Chen, P.; Wang, D. ; Ren, Q. ; Wu, J.; Jiang, Y.; Wu, Z.; Pan, Y.; Zhong, Y.; Guan, Y.; Chen, K.; Zhang, G. Bacillus aerolatus sp. nov., a novel member of the genus Bacillus, isolated from bioaerosols in a school playground. Arch Microbiol 2020, 202, 2373-2378. DOI: 10.1007/s00203-020-01955-3. [CrossRef]
- Verma, A.; Pal, Y.; Ojha, A.K.; Kumari, M.; Khatri, I.; Rameshkumar, N.; Schumann, P.; Dastager, S.G.; Mayilraj, S.; Subramanian, S.; Krishnamurthi, S. Taxonomic insights into the phylogeny of Bacillus badius and proposal for its reclassification to the genus Pseudobacillus as Pseudobacillus badius comb. nov. and reclassification of Bacillus wudalianchiensis Liu et al., 2017 as Pseudobacillus wudalianchiensis comb. nov. Syst. Appl. Microbiol. 2019, 42, 3, 360-372. DOI: 10.1016/j.syapm.2019.03.003. [CrossRef]
- Riesco, R.; Trujillo, M.E. Update on the proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2024, 74, 3, 006300. DOI: 10.1099/ijsem.0.006300. [CrossRef]
- Bruker. Species/Entry List MBT Compass Library Revision K. 2022, 1-75.
| Biochemical test | Result | Biochemical test | Result | Biochemical test | Result | Biochemical test | Result | Biochemical test | Result | Biochemical test | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BXYL | - | LysA | - | AspA | - | LeuA | + | PheA | + | ProA | - |
| BGAL | - | PyrA | - | AGAL | - | AlaA | + | TyrA | + | BNAG | - |
| APPA | + | CDEX | - | dGAL | - | GLYG | - | INO | - | MdG | - |
| ELLM | + | MdX | - | AMAN | - | MTE | - | GlyA | - | dMAN | - |
| dMNE | - | dMLZ | - | NAG | - | PLE | - | IRHA | - | BGLU | - |
| BMAN | - | PHC | - | PVATE | - | AGLU | - | dTAG | - | dTRE | - |
| INU | - | dGLU | - | dRIB | - | PSCNa | - | NaCI 6.5% | - | KAN | - |
| OLD | - | ESC | - | TTZ | - | POLYB_R | - |
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Cell shape | rod | rod | rod | ovoid | rod | rod |
| Motility | - | + | + | - | + | + |
| Optimum temperature for growth (ºC) | 37 | 37 | 30 | 50 | 37 | 25 |
| Catalase | + | + | + | + | + | + |
| Starch | - | - | - | - | - | - |
| L-arabinose | - | - | - | - | - | - |
| L-rhamnose | - | - | - | - | - | ND |
| Lactose | - | - | - | - | - | - |
| Glucose | - | + | - | - | - | + |
| L-sorbose | - | - | - | + | - | ND |
| Mannitol | - | + | - | - | - | - |
| Sucrose | - | + | - | - | - | - |
| Amygdalin | - | + | - | - | - | ND |
| Inositol | - | + | - | - | - | - |
| G + C content (%) | 41.6 | 42.3 | 41.2 | 44.4 | 44.0 | 44.2 |
| Genera | Number of species/group of species in VITEK® 2 database | Number of species described |
|---|---|---|
| Alicyclobacillus | 1 | 29 |
| Aneurinibacillus | 1 | 9 |
| Bacillus | 21 | 111 |
| Brevibacillus | 8 | 33 |
| Geobacillus | 4 | 12 |
| Lysinibacillus | 1 | 22 |
| Paenibacillus | 14 | 310 |
| Virgibacillus | 2 | 34 |
| Strains | 16S rRNA (%) | rpoB (%) | gyrB 的(%) | Ortho ANI (%) | GGDC (%) | Mol GC distance (%) |
|---|---|---|---|---|---|---|
| ‘Bacillus aerolatus’ CX253T | 98.28 | 87.50 | 85.43 | 80.01 | 24.00 | 0.70 |
| Bacillus badius NBRC 15713T | 97.96 | 86.91 | 80.90 | 76.97 | 21.60 | 2.33 |
| Bacillus thermotolerans SGZ8T | 97.21 | 84.09 | NSSF | 73.73 | 20.10 | 2.81 |
| Bacillus wudalianchiensis FJAT 27215T | 98.51 | 87.38 | 81.17 | 78.22 | 22.50 | 0.39 |
| Bacillus xiapuensis FJAT 46582T | 97.63 | 82.91 | NSSF | 72.82 | 21.10 | 2.63 |
| Characteristics | B190/17 |
|---|---|
| Estimated genome size (pb) | 3,434,160 |
| Cover | 73x |
| G+C content (%) | 41.6 |
| N50 | 219177 |
| L50 | 4 |
| Number of contigs | 89 |
| Number of Subsystems | 305 |
| Number of Coding Sequences | 3544 |
| Number of RNA genes | 159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





