1. Introduction
Microplastics are becoming persistent pollutants that are of growing concern [
1], due to their potential toxicity to aquatic biota and human health, as well as their association with pathogenic bacteria, and affects on the food web [
2,
3]. Microplastics have become ubiquitous worldwide, in the ocean, on land, and even in polar glaciers where human activity is minimal, and are regarded as a rising threat to ecological and environmental sustainability [
4]. Occurrence of MP in water is an emerging worldwide environmental issue, with an estimated annual release of approximately 0.7 to 1.8 million tonnes of MP into the environment being reported in Europe [
5]. Microplastic particles have been reported in seafood, processed food, and beverages [
6]. The large surface area and small volume (particle size is usually less than 5 mm) of MP can lead to the adsorption and transfer of various environmental pollutants such as PAH, pesticides, polybrominated diphenyl ethers, pharmaceutical and personal care products, and heavy metals including chromium (CrVI), nickel (NiII), copper (CuII), zinc (Zn), cadmium (Cd), lead (Pb), and titanium (Ti), from environmental media to aquatic food webs. Furthermore, pollutants carried in MP may have an impact on aquatic organisms when discharged into the aquatic environment [
7,
11]. Plasticizers have been identified within MP, which also demonstrate a significant ability to adsorb toxic chemical compounds, including polycyclic aromatic hydrocarbons (PAH) and dichlorodiphenyltrichloroethane (DDT). These substances can be transferred to organisms and impact human health through the food chain [
12]. In the past few years, research on MP in aquatic environments has received considerable attention, with aquatic MP pollution being listed as one of the top ten environmental challenges to be solved globally, partly due to the negative impact of MP particles on a range of aquatic organisms, such as phytoplankton, zooplankton, and fish [
4,
13]. To date, MP have been found in honey, sugar, beer, milk, table salt, bottled water, tap water, seafood, and rice, among other foods and beverages [
14,
18].
Microplastics contaminate a wide range of environmental compartments, which includes oceans, rivers, lakes, soils, and atmosphere, as well as sources of drinking water [
19,
20]. Furthermore, rivers play a fundamental role in the transportation of plastic particles into lakes, seas, and oceans [
21]. Microplastics have the potential to affect soil fauna via a variety of mechanisms, including ingestion by soil living organisms, release of toxic plastic additives, and serving as vectors for transport of pathogenic bacteria [
20,
22]. Additionally, pesticides have the potential to be transported by MP, which accumulate in soil or sediment and serve as a pesticide sink [
23,
24]. Moreover, freshwater sediments act as a sink for MP and source for toxic chemicals, and as a result, benthic organisms can be exposed to possibly significant amounts of MP [
25,
26]. Small and lightweight MP accumulate downstream in rivers, however MP are also often detected in riverbed sediment, showing persistence over time [
27].
Polycyclic aromatic hydrocarbons (PAH) are a large class of aromatic compounds produced through inadequate combustion or pyrolysis activities, such as automobile exhaust, cigarette smoking, industrial processes, consumption of grilled and smoked food, fossil fuel, and forest fires. Consequently, PAH have been found in high quantities in air, water, and soil [
28,
29]. Pesticides are chemical mixtures that are used to prevent, remove, repel, or neutralize organisms that cause issues in agriculture, such as microorganisms (bacteria, fungi, and viruses), insects, animals, and weeds [
30]. Pesticides have adverse implications in ecosystems that vary depending on the pollutant concentration, quantity, and exposure time. Pesticides pose a significant hazard due to their persistence in the environment and tendency to accumulate in organisms [
30], leading to contamination of water and soil, even when used at low rates, and with poor biodegradability [
31]. For example, 1,3- dichloropropene is frequently used because of its effectiveness in controlling pathogenic nematodes and weeds [
32]. Herbicides are synthetic chemicals that aid in the management and elimination of unwanted weeds [
30]. atrazine is a commonly used, highly effective, and low-cost pesticide for controlling broadleaf and grassy weeds. It is a major pollutant of soil and aquatic systems, and it has been shown to disrupt aquatic flora and animal reproduction, which can have a negative impact on community structure [
33,
34].
Nematodes are the most abundant and diverse group of metazoans found in sediment and soil, and they play a critical role in the food webs of benthic and soil environments [
35]. Nematodes are non-segmented roundworms that live in almost every ecosystem on the planet and have considerable potential as environmental indicators. They are used as bioindicators in assessments of anthropogenic pollution because of their ecological significance, widespread prevalence, and exceptionally high individual densities [
36]. Nematodes have been used to study gene expression in relation to environmental challenges, as well as in laboratory ecotoxicity assays, and
in situ monitoring of the ecological impact of a variety of enviromental problems [
36,
37].
Entomopathogenic nematodes (EPN) from the families Steinernematidae (genus
Steinernema) and Heterorhabditidae (genus
Heterorhabditis) of the Phylum Nematoda are soil-dwelling, lethal insect parasites which can be an alternative to chemical insecticides, and are easily mass produced
in vivo and
in vitro [
38,
39]. Their symbiotic bacteria are released into their insect host hemocoel by nematode free-living infective juveniles (IJ), which penetrate insect hosts through natural body openings and pores in the cuticle. The subsequent resulting bacterial septicemia kills the host quickly, and its cadaver is changed to a nutritious broth for the nematodes, mediated by the nematode symbiotic bacteria. By creating bacteriocins, antimicrobials, and other antibiotics, the bacteria protect the cadaver resource from rivals. Infective juveniles feed on the bacterial broth andmature into adults, which can give rise to multiple generations until resources are depleted. Then, new IJ emerge from the cadaver and disperse in the soil, looking for new hosts to infect [
40,
42].
Caenorhabditis elegans is a model organism that has been widely used to study genotoxic reactions to environmental contaminants from molecular to organismal levels [
43,
44]. Because of its small size, rapid life cycle (3-4 days), short average lifetime (2-3 weeks), and ease of rearing, this transparent, free-living nematode offers numerous benefits for genetic investigations [
45,
46].
Caenorhabditis elegans has shown a low-dose response to several chemicals after being used in assessing the toxicity of a range of environmental pollutants, including zinc oxide (ZnO) nanoparticles, which are commonly found in toothpaste, beauty products, sunscreens, and textiles, as well as bisphenol A (BPA), which is widely used in the manufacture of plastic products [
46,
48]. Brenner (1974) emphasized the advantages of biological testing with
C. elegans.
Caenorhabditis elegans displays rapid growth on agar plates or in liquid culture at temperatures ranging from 15 to 25°C, using
Escherichia coli as a food source. Its fast growth is evidenced by a half-day doubling time and a generation period lasting only 3 to 5 days. Additionally, advantages of using
C. elegans include the possibility of self- or cross-fertilization [
45].
Caenorhabditis elegans is one of the species that has been used to study the toxicity of MP [
19,
49]. In a number of previous studies, MP have been shown to have negative physical and biological impacts on
C. elegans [
19,
44,
46,
47,
48,
49,
50].
However, there is limited research on the impact of MP on other nematode indicators, such as EPN, apart from the present study involving testing
C. elegans and EPN as sentinels of MP pollution. For assessing sediment quality, nematodes have been used as an effective alternative to the existing conventional methods for this task, such as chemical analyses [
25]. Both morphological and molecular (DNA-based) methods have been employed in the identification of nematodes in order to facilitate an effective assessment of biodiversity [
51,
52]. In the 1970s, Zullini (1976) studied the relationship between nematode community structure and sediment pollution (anthropogenic contamination with heavy metals and organic pollutants) and discovered that nematode community structure of river sediments was related to pollution and site structure [
53,
54].
Hence, the aims of the current study were (1) to improve understanding of MP properties and their interactions with organic pollutants, (2) to determine the toxicity effects of MP particles using three nematode species (C. elegans, Steinernema feltiae (SB12(1) and Steinernema carpocapsae) as sentinel organisms, and (3) to observe possible effects of MP on nematode communities from Irish river sediments via high-throughput sequencing of nematode 18S rDNA for taxa identification.
4. Discussion
To the best of our knowledge, this is the first study to evaluate possible toxic effects of MP pollutants in EPN. Previous studies [
50,
69,
70] in relation to MP toxicity have used
C. elegans mostly and the freshwater amphipod
Gammarus duebeni as sentinel organisms, while focusing more on the impacts of MP upon ingestion, reproduction, oxidative stress, with less emphasis on assessing mortality. In this study, it was observed that contaminated MP were able to uptake toxicants such as atrazine, resulting in the mortality of nematodes. Similar studies in relation to MP as carriers of toxicants, have also shown that MP act as vectors of various environmental pollutants such as polycyclic aromatic hydrocarbons (PAH), or hydrophobic persistent organic pollutants (POP), pesticides, and heavy metals, thus affecting test organisms, including nematodes [
11,
49]. Furthermore, [
43,
46] have also found that MP cause mortality of
C. elegans, as well as inhibiting their growth and reproduction [
43,
46]. This study is the first to evaluate the effects of MP on the three nematode species tested. The findings can now be compared to other studies that have investigated the impact of MP on nematodes. Previous research also examined how MP affect
C. elegans, concluding that MP are causing significant issues in this particular nematode species [
19,
70]. By comparing the results of this study to these previous findings, a more comprehensive understanding of the impact of MP on nematodes can be achieved.
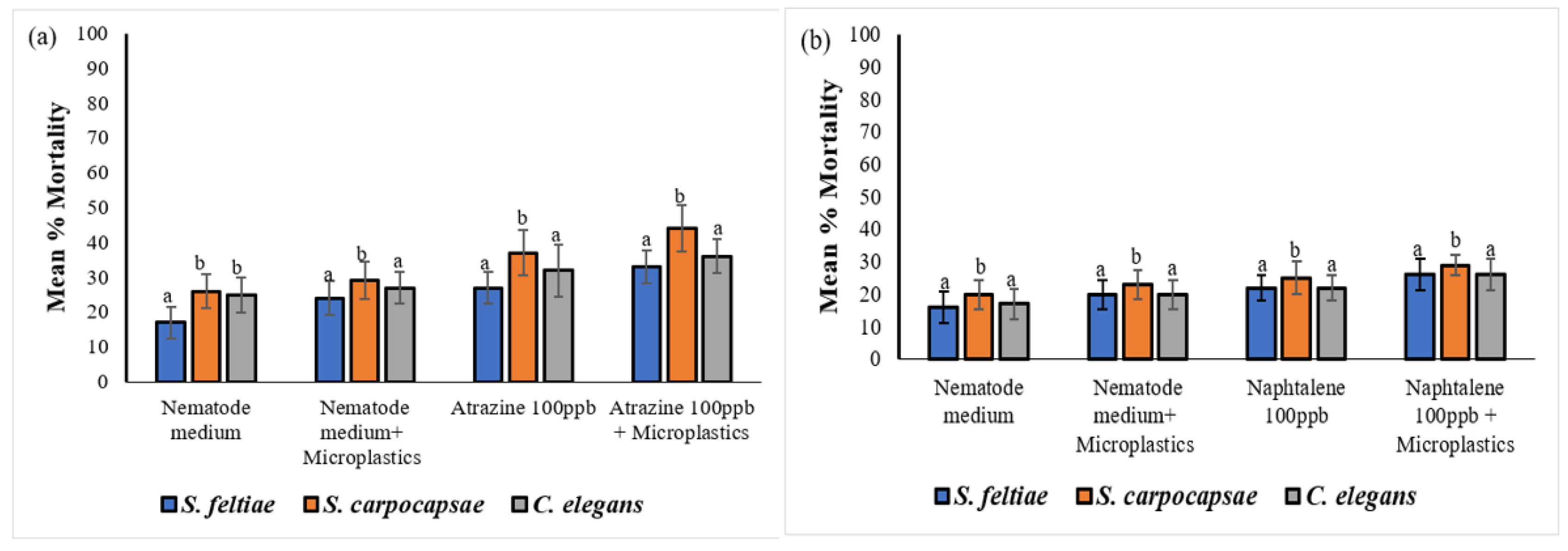
This study demonstrated that increasing MP exposure time from 72 to 96 hours increased the mortality of nematodes exposed to the various treatments. In the case of naphthalene, these increases were from 26 to 30% (
S. feltiae SB 12(1) IJ), 29 to 40% (
S. carpocapsae IJ) and 26 to 35% (
C. elegans J3). Nematode mortality was shown to increase prominently in the presence of MP treated with atrazine and 1,3 – dichloropropene than that in the presence of MP treated with naphthalene and fluorene. [
19,
46] have also reported the impacts of MP on
C. elegans. When comparing the three species, the results of this paper clearly showed that
S. carpocapsae IJ and
C. elegans J3 responded differently to all the treatments containing MP compared to
S. feltiae SB 12(1) IJ. In addition, the
S. carpocapsae IJ and
C. elegans J3 mean percentage mortality levels were significantly higher (
P < 0.05) compared to that in
S. feltiae SB 12(1) IJ. From the three species used
C. elegans is usually the most sensitive test organism [
19]. However,
C. elegans J3 mortality was lower compared to that in
S. carpocapsae IJ. This presents a possible justification for the utility of
S. carpocapsae IJ as sentinel organisms in future toxicology assays, and research based evidence suggests that
S. carpocapsae can also be used as a sentinel organism alongside
C. elegans. This study demonstrated that the toxicity of MP on the three nematode species differed depending on the treatments and exposure time. The cause of mortality in nematodes could be as a result of leaching of MP additives as this was also oberved by [
19]. Similar findings indicated that toxic chemicals absorbed by MP may potentially have very high impact on
C. elegans tissues inducing toxic effects [
44,
49,
50].
This study is also the first to fill the gap of knowledge on MP pollution and its environmental impact on the RB sediment nematodes communities. The majority of similar studies focused on the impacts of MP on nematode (namely
C. elegans) growth, reproduction, and survival [
19,
71], as well as MP ingestion by benthic organisms such as freshwater amphipods
Gammarus duebeni [
72]. Studies [
20,
25] showed that nematodes found in soil and river sediment can act as bioindicators of MP environmental pollution. Recently, the authors of this study confirmed the presence of MP in RB sediment samples [
68]. A similar study by [
69] made similar observation confirming plastic microfibres widely present in freshwater sediments.
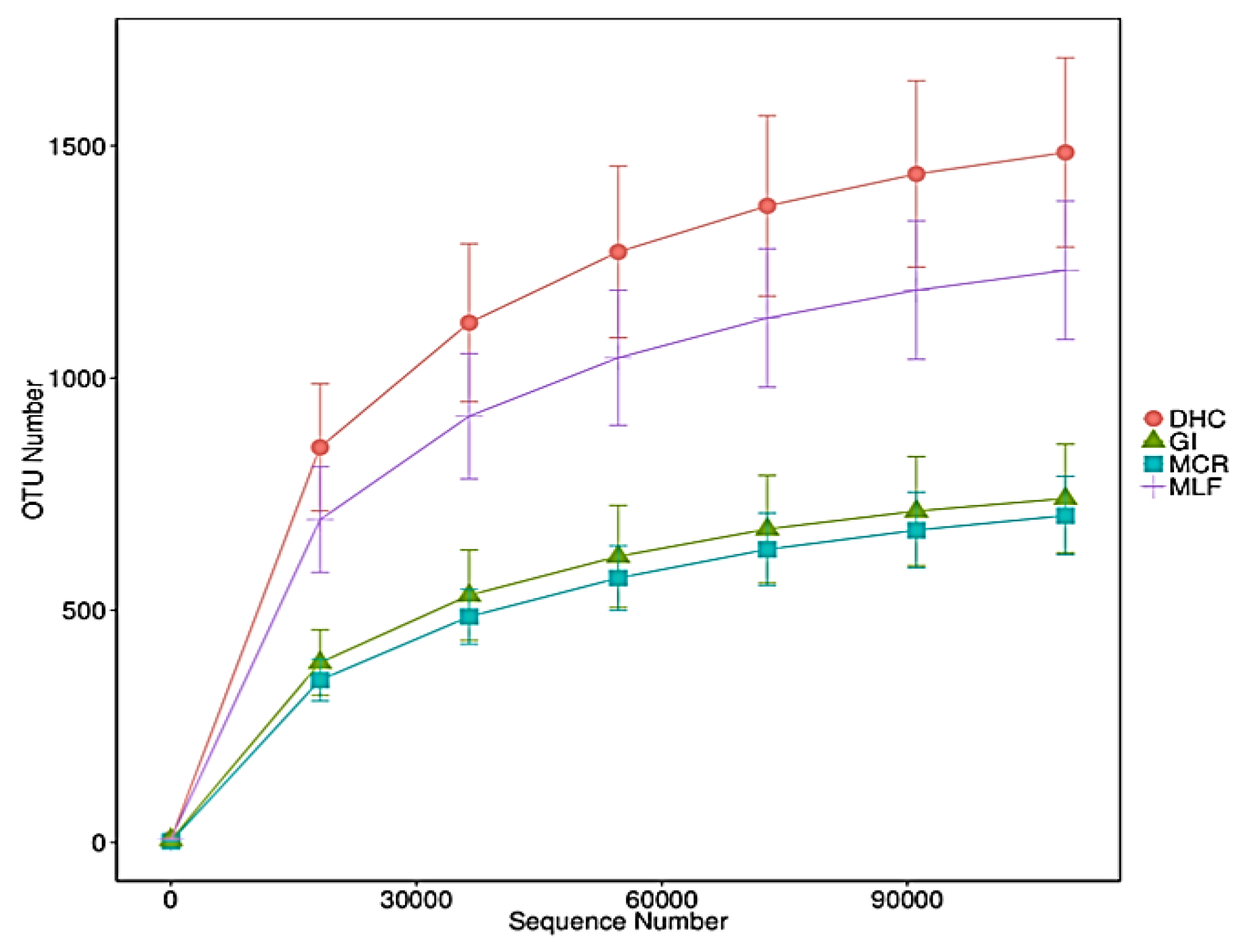
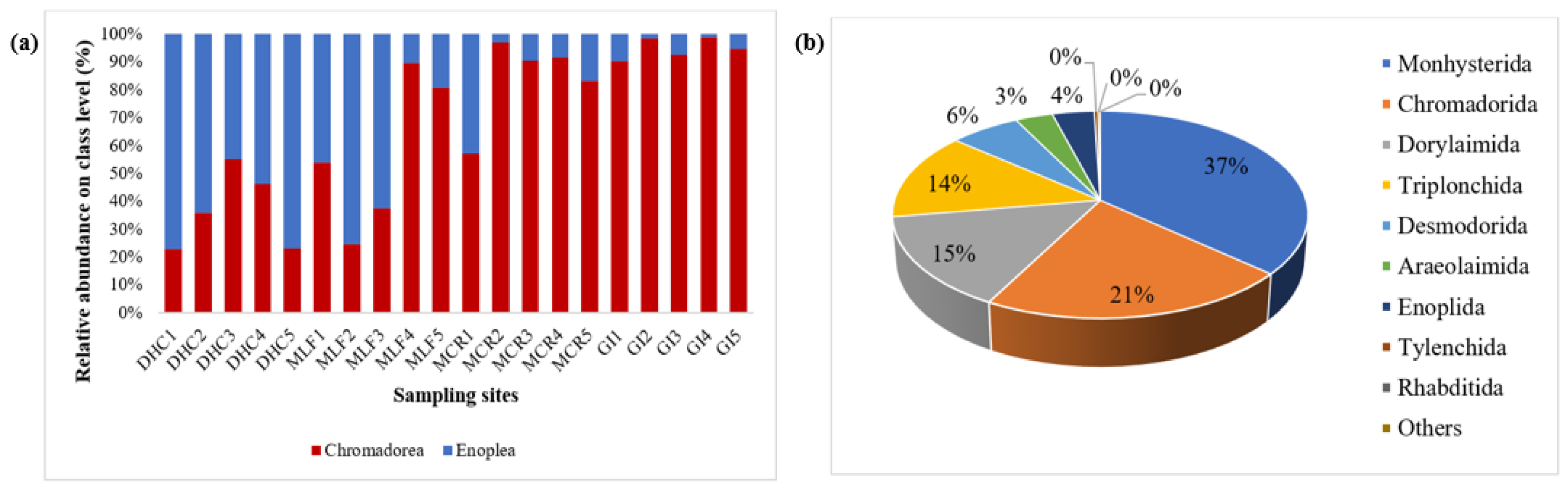
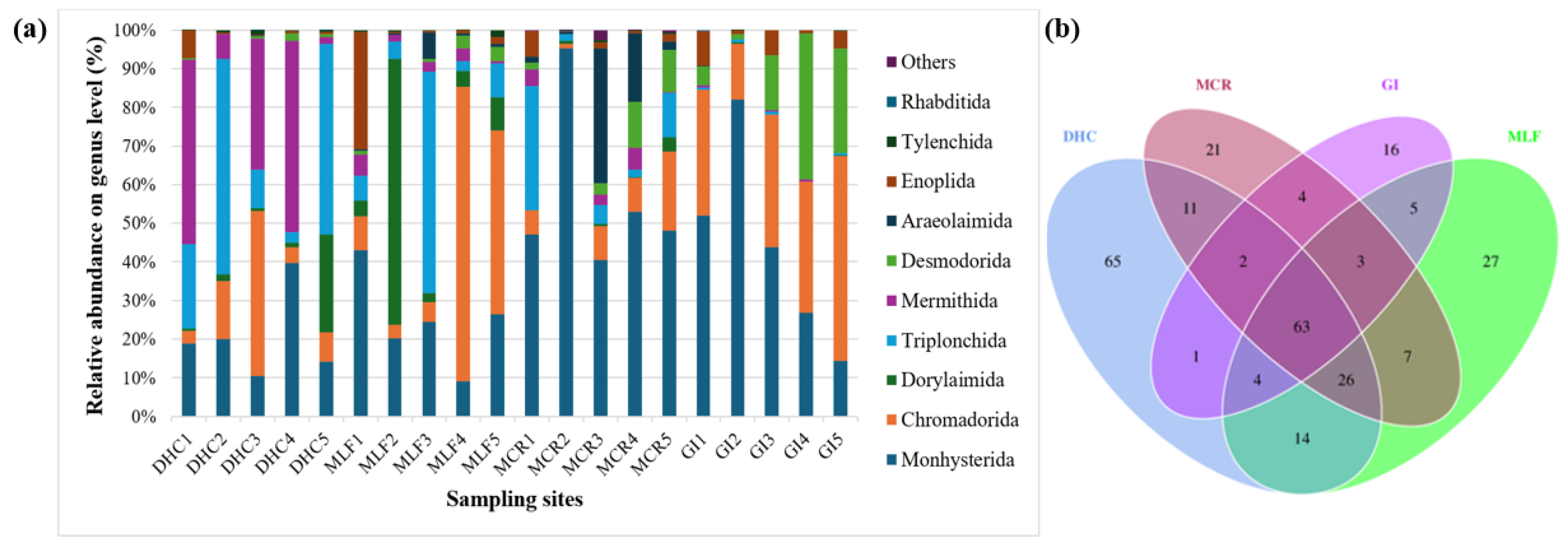
The higher number of reads obtained in the MCR samples are likely due to the abundance of Monhysterida in this particular location (57 ± 22 %) (
Figure 10a). The average read length was 301 bp which was larger than 99% of Good`s coverage, as defined in the scikit-bio documentation (
http://scikit-bio.org/docs/latest/generated/skbio.diversity.alpha.goods_coverage.html#skbio.diversity.alpha.goods_coverage) for the 18 S rRNA gene region, with an average GC content of 46%. According to rarefaction curves, a common method for assessing the biodiversity of samples, OTU saturation was achieved at approximately 60,000 reads per sample.The dilution curves of the sediment samples tended to be flat at all four locations, while the overall OTU coverage of the samples appeared to have reached saturation, suggesting that the sequencing depth adequately reflected the structure of the nematode community (
Figure 9).
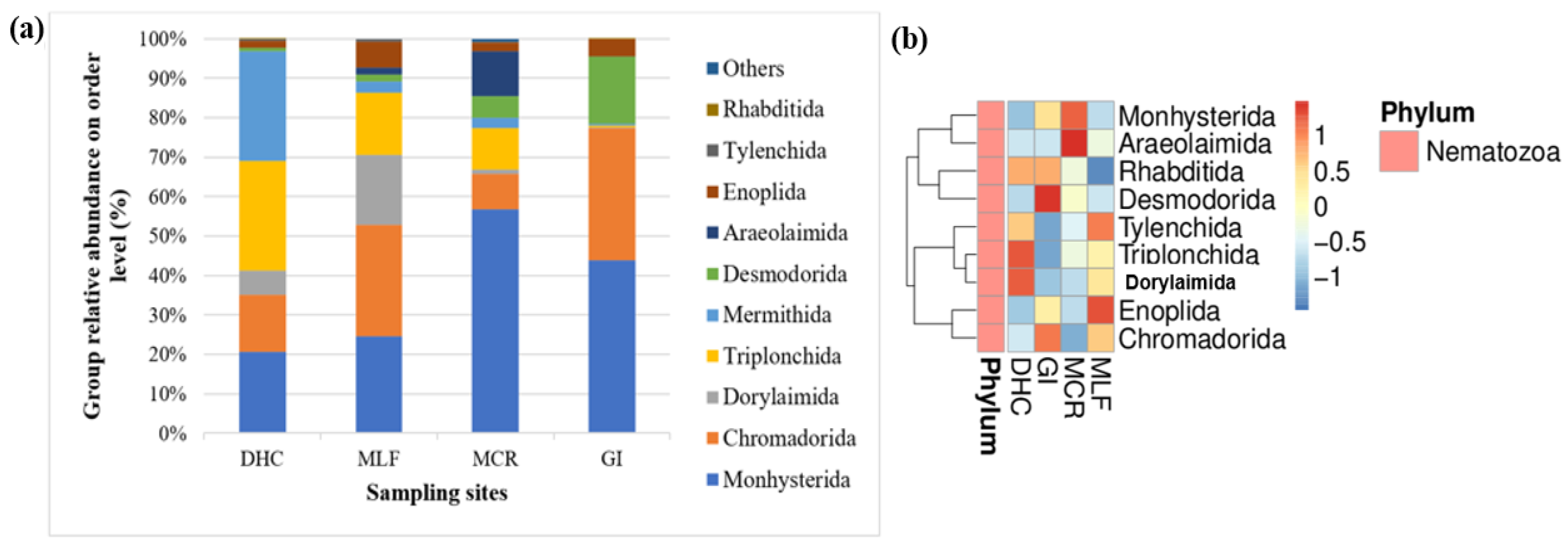
The four locations selected in this study revealed significant differences in nematode communities. For example, the DHC location was sandy/gravel, the MLF location was gravel, the MCR location was muddy/fine grain size, and the GI location was muddy/fine grains, which were the notable differences across the study locations. The smaller the grain size, the less diverse a nematode assemblage is [
73]. For instance, compared to the sediment samples from the other three locations, the GI location's sediment samples had higher abundances of only three nematode orders. The three main orders observed from the GI sediment samples were Monhysterida, followed by Chromadorida, and Dorylamida. Monhesterida were among the most numerous groups of nematodes in the sediment samples according to the data of this study. According to [
19], MP and their associated toxicants have the potential to adversely impact soil nematodes. As many of the harmful chemicals introduced into waters bind to settling particles, soft sediments are frequently very polluted [
74]. This could potentially explain the difference in nematode feeding types and communities observed across the four locations.[
75] similarly observed that sediment grain size and organic matter content have strong correlations with nematode communities.
In this study, it was observed that the coarser the sediments the more diverse the communities. This was particularly observed in DHC and MLF samples compared to MCR and GI samples, as the diversity and abundance of sediment nematodes significantly decreased in MCR and GI samples compared to DHC and MLF. It has been also shown via assessment of MP in Irish river sediment, that GI was the most polluted location with MP compared to the rest of the locations used in this current work [
68]. There is a clear evidence that MP have significantly affected the nematode communities as the number of nematode orders in the MCR and GI were lower compared to that in the DHC and MLF samples (
Figure 12b). Similar findings were observed by [
76].
The increasing MP pollutants have been considered as an emerging threat to biodiversity and ecosystem functioning while exerting significant negative effects on the abundance of nematodes [
20,
77]. Furthermore, the authors’ recent paper [
68] revealed that finer sediments act as a sink for MP deposition, which clearly explained the decline in the species abundance and nematode feeding types in the MCR and GI locations. These two particular sites were found to have fine sediments with more deposited MP compared to sediment samples from DHC and MLF locations. [
77] confirmed that the presence of various MP in the sediments can reduce nematode feeding types and alter the sediment pH. Despite all this evidence however, the impacts of MP pollution on nematode communities of sediments in the RB in other counties in Ireland remains largely unknown.
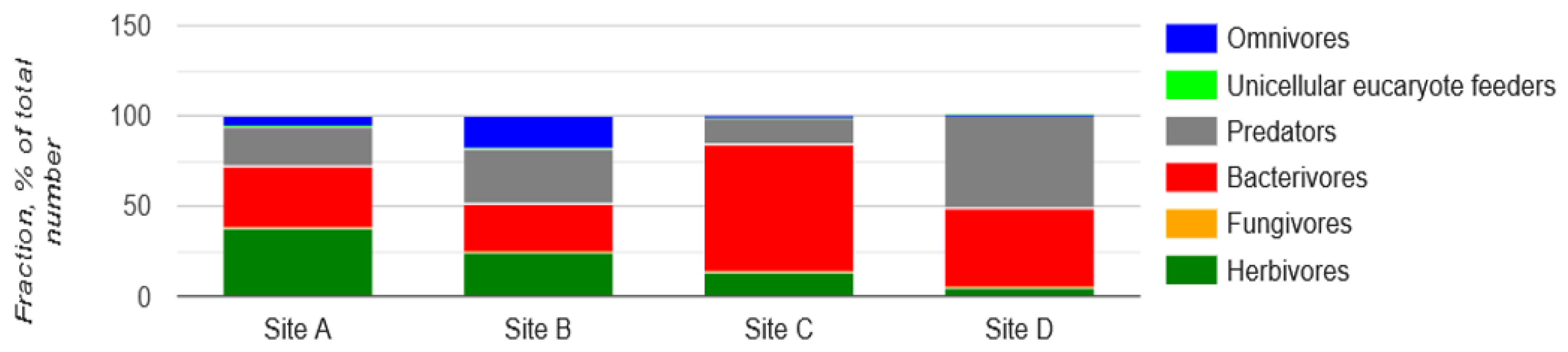
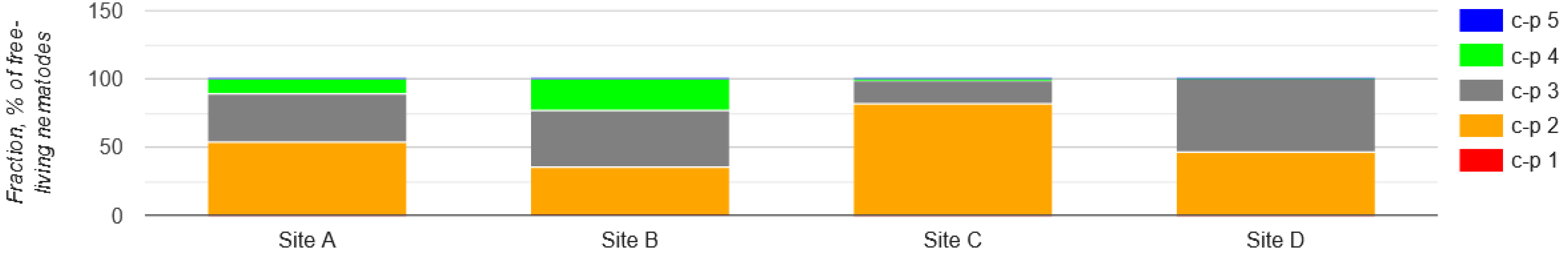
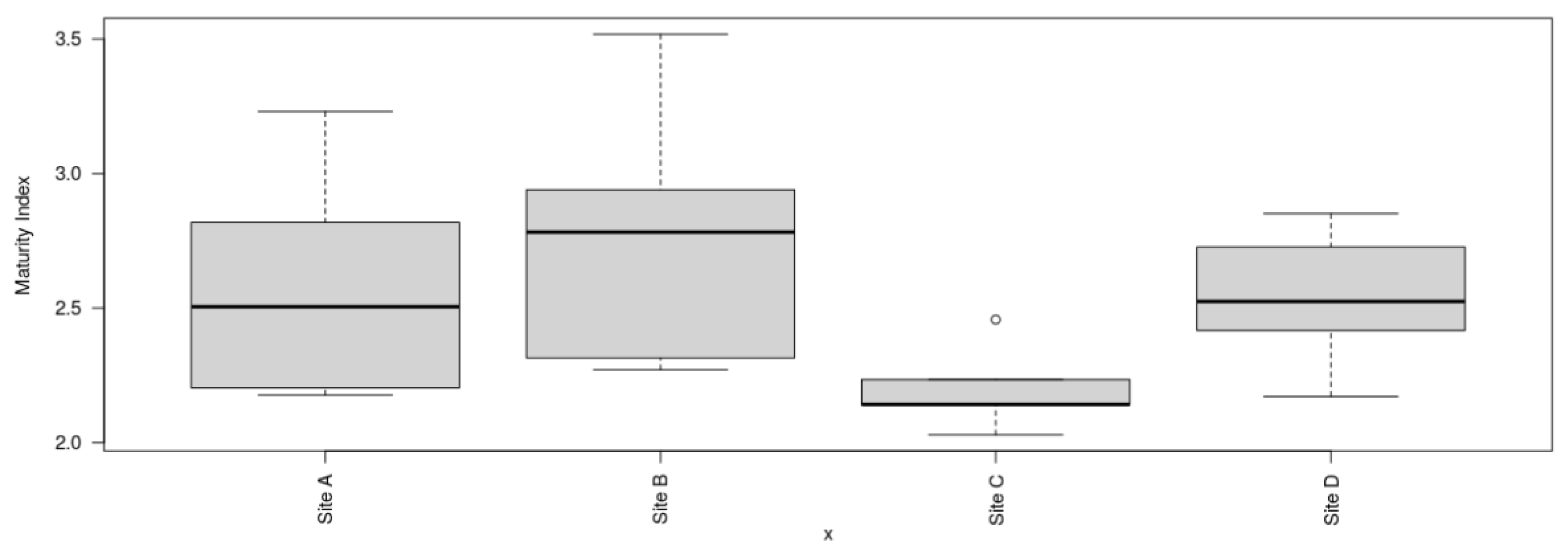
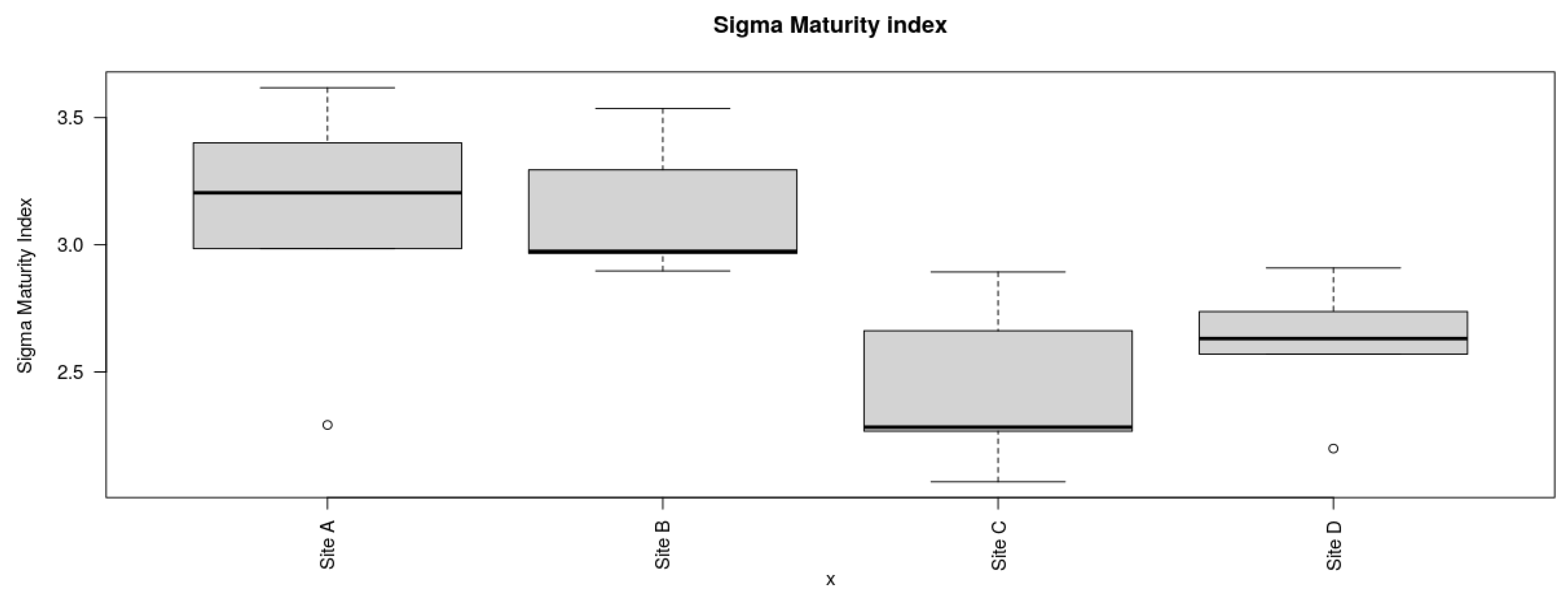
The nematodes community structure is indicative of the condition or health of the soil/sediment in which nematodes live, as they respond rapidly to disturbance and enrichment [
51]. The impact of MP contamination in the river sediment that caused the disruption of nematode communities was evaluated using a number of indices. For free-living nematodes, the maturity and sigma maturity indices were used to assess the environmental disruption caused by MP. Nematodes were assigned to c-p values according to Bongers (1990). The most resistant nematode taxa to MP pollution were found to be those in class c-p2. [
64] also reported similar results, however, it was regarding heavy metals rather than MP. Among the nematode feeding type composition, there was more variation in sites A - DHC and D - MLF compared to sites C - MCR and D - GI. Bacterivores were the most dominant, followed by predators and herbivores. The abundance of bacterivores was high in sites C (MCR) and D (GI). A plausible explanation is that the presence of environmental factors, including organic/inorganic carbon, nitrogen, phosphorus, and other minerals in the environment may modify the direct negative impact of MP. [
2,
20] also made similar observations. On the other hand, omnivores were the lowest in abundance. Sites C - MCR and D - GI had the highest level of MP compared to the two other locations. The disturbance in nematode feeding types could potentially be caused by the presence of MP. Therefore, these indices can be used as guidelines by the EU Water Framework Directive (WFD) for monitoring MP pollution in the RB sediments. Lower maturity and sigma maturity indices in river sediment have also been observed throughout the four locations, particularly in locations with a high number of MP, including MCR and GI, indicating a disturbed sediment nematode community by MP. The findings reported here support the hypothesis that perturbation generally leads to a lower maturity indices [
63]. The disturbance caused by MP likely disrupts the natural habitat and food sources of sediment nematodes, ultimately impacting their development and maturity levels. Thus, the findings from this study provide further evidence linking perturbation, specifically from MP, to lower maturity indices in sediment nematode communities.
Analysis of river sediment samples from the four locations along the RB shows that MP do affect the nematode communities, causing a decline in nematode diversities, feeding type, and maturity index. Therefore, based on the findings of this study, it can be inferred that MP pollution of river sediment in the four locations along the RB is evident. This statement is supported by the observed decrease in nematode diversities, changes in feeding type composition, and lower maturity indices in sediment nematode communities. The disruption caused by MP contamination likely alters the natural habitat and food sources of sediment-dwelling nematodes, ultimately influencing their development and maturity levels. The results from this research provide further evidence of the relationship between disturbance, particularly from MP, and the reduced maturity indices in sediment nematode communities. This in turn indicates that the health and condition of the river sediment in the four locations are indeed affected by MP pollution, as suggested by the observed changes in nematode communities. Nematode communities are at risk as a result of the accumulation of MP contaminants in river sediments. Nematode communities were mostly affected by the presence of MP in samples from the MCR and GI locations. A decrease in the number of nematode feeding types, and maturity indices was also observed in the MCR and GI locations, potentially associated with the high number of MP. Furthermore, sediment MP contamination can modify the composition of feeding types in nematode communities, both directly and indirectly, due to differences in the sensitivity of various nematode species [
78,
79]. The low sediment web maturity indices observed can be attributed to the ongoing rise in MP within the RB sediments [
68]. Therefore, it is important for ecologists to use these indices as a means to improve the sediment quality of the RB by reducing the use of non-biodegradable plastics and thereby mitigating the environmental effects of MP. Stakeholders such as policymakers, industry, government agencies, and the general public can also play a role in reducing non-biodegradable inputs and mitigating the environmental impacts of MP. Implementing regulations on the use of plastics, promoting recyclable and biodegradable materials, and educating the public on the importance of reducing plastic use and pollution can all contribute to improving the sediment quality of the RB. Collaborative efforts between environmental activists, policymakers, industry, and the public are essential in addressing the issue of MP contamination and promoting environmental sustainability. The impact of this research is significant, as it highlighted the negative effects of MP on beneficial nematodes and nematode biodiversity in river sediments, ultimately affecting the overall ecological health of aquatic ecosystems. The findings of this research can be implemented in several ways: (1) Environmental policy and regulation: The data on the impacts of MP on nematode communities can inform policymakers and regulators about the potential ecological risks posed by MP in aquatic environments. This information can be used to develop strategies to reduce MP pollution and protect benthic ecosystems. (2) Eco-friendly product development: The identification of commercial cosmetic products as a potential source of MP underscored the importance of developing eco-friendly alternatives that do not contain harmful pollutants. Relevant industry can use this information to reformulate their products and reduce their environmental impact. (3) Further research: The study highlighted the need for more research on the pathways of nanoparticles in organisms and the effects of MP on different types of organisms. Future studies can build upon these findings to deepen understanding of the ecological implications of MP pollution. By addressing the issue of MP pollution and its effects on benthic organisms, such as nematodes, steps can be taken to protect and preserve aquatic ecosystems for future generations. This study will contribute to the limited work on the impacts of MP pollutants in the RB sediments.
5. Conclusions
This paper presents for the first time the toxic effects of MP pollutants on EPN. Attenuated total reflectance Fourier transform infrared spectroscopy showed that atrazine accumulated on the surface of polyethylene MP. There was some evidence of interaction between these MP and both 1,3–dichloropropene and naphthalene, while fluorene did not appear to be retained on the MP surface. The present study showed that S. carpocapsae IJ and C. elegans J3, when exposed in vitro to MP for 72 to 96 hours, exhibited significant effects in all treatments. The mortality percentages were notably higher compared to S. feltiae (SB12(1) IJ. Furthermore, continuous exposure of nematodes to MP led in increasing nematodes mortality. Therefore, experiments using the three species of nematodes as test organisms demonstrated that MP treated with a range of toxicants affected the survival percentages of the nematodes. The results demonstrated the toxicity of MP among the three species, confirming the hypothesis that MP particles act as vector of contaminants for nematodes.
This work explored the capacity of MP to absorb and transfer chemical pollutants into nematodes, providing experimental insights and considerations for studying EPN as model organisms. This suggests that EPN can serve as excellent models for studying the transfer of chemical pollutants via MP in aquatic ecosystems. Microplastics have the ability of absorbing toxic substances. Therefore, limiting the release of MP may reduce their ability to damage organisms and ecosystems. Thus, these findings raise a great concern regarding the long-term exposure of nematode species to MP and highlight the urgent need of more studies. Subsequent research may consider using S. carpocapsae as a sentinel organism in toxicological studies due to the extensive use of C. elegans in such assays. This is justified by the significant findings observed in this study, where both S. carpocapsae and C. elegans showed notable responses to exposure to MP. The mortality rates of both nematode species were higher compared to S. feltiae, indicating the potential toxicity of MP to nematodes. Given the prevalence of research focusing on C. elegans in toxicological assessments, incorporating S. carpocapsae as a sentinel organism could provide a more comprehensive understanding of the impacts of MP on nematode communities. This approach would also broaden the scope of research on the effects of MP on various nematode species, ultimately contributing to a more thorough assessment of the ecological implications of MP pollution.
This study additionally examines the distribution of sediment-dwelling nematode communities within the RB. The abundance and diversity of sediment nematodes were significantly affected by the presence of MP. In conclusion, MP have negative impacts on nematode communities. This work gave an insight into the status on the community structure of nematodes in river sediment from the DHC, MLF, MCR, and GI locations. The differences in sediment types and MP occurrence can shape the meiofaunal nematode communities. This study revealed that the highest nematode richness and abundance were observed in the DHC location, followed by MLF, while the lowest nematode richness and abundance were found in the MCR and GI locations. Monhysterida was the predominant order across all the samples, particularly in the MCR samples. The differences in nematode communities in the various sediments indicate significant MP impacts on the nematode community composition.
The composition of the nematode communities in the sediments, the trophic groups recorded and the maturity index can be used in ecological monitoring of the RB. The accumulation of MP in river sediments, as demonstrated by [
68] poses a threat to nematode communities. Therefore, it could be useful to assess the preliminary ecosystem functions of the RB sediment in order to mitigate the potential impact of MP accumulation on nematode communities. This assessment can be conducted by analysing the composition of nematode communities, trophic groups, and maturity index as indicators for ecological monitoring of the RB. Preliminary ecosystem functions refer to the initial assessment of the ecological processes and functions in a particular ecosystem, such as the distribution and diversity of sediment-dwelling nematode communities. By analysing the composition of nematode communities, trophic groups, and maturity index as indicators for ecological monitoring, one can gain valuable insights into the status of the ecosystem and potential impacts of MP accumulation on nematode communities. This preliminary assessment can help in understanding the baseline conditions of the ecosystem and in recognising any changes that may occur due to factors such as MP accumulation. This information can then be used to develop strategies to mitigate the negative impact of MP on nematode communities, such as implementing measures to reduce MP pollution and restore the ecosystem balance.
The findings from this work should facilitate further research concerning MP pollutants in the RB at different counties in Ireland. This will provide better indications of the stability of the sediments that benthic nematode communities inhabit and provide better insights into how such communities may respond to anthropogenic disturbance which includes MP. Finally, based on the findings from this study, sediment nematodes can serve as bioindicators of MP environmental pollution.
Author Contributions
Conceptualization, L.M., J.C., K.G. and T.K.D.; methodology, L.M., J.C., and T.K.D.; software, L.M.; validation, L.M., J.C., K.G. and T.K.D.; formal analysis, L.M., J.C., K.G. and T.D.K.; investigation, L.M., J.C., K.G., and T.K.D.; writing—original draft preparation, L.M.; writing—review and editing, L.M. and T.K.D.; visualization, L.M., J.C., K.G., and T.K.D; supervision, J.C., K.G., and T.K.D. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Seeded NGM plates.
Figure 1.
Seeded NGM plates.
Figure 2.
Rearing of entomopathogenic nematodes.
Figure 2.
Rearing of entomopathogenic nematodes.
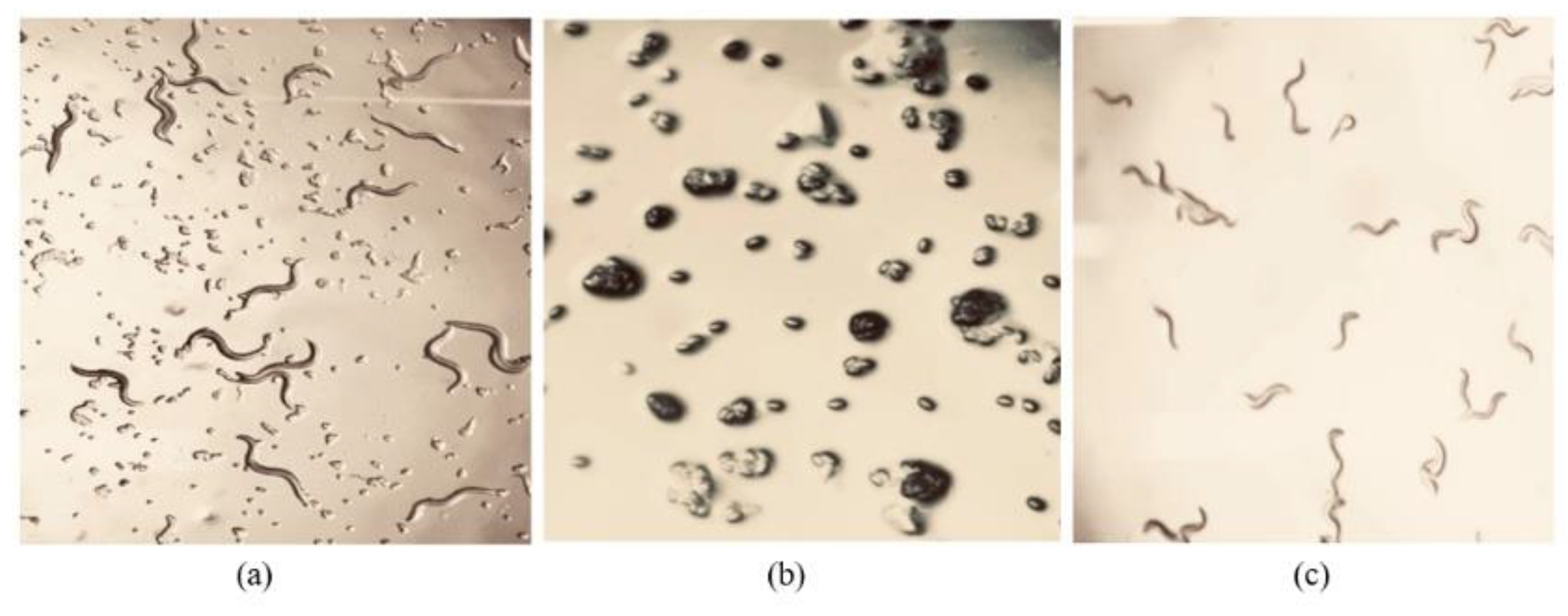
Figure 3.
Caenorhabditis. elegans age synchronization. (a) Various C. elegans stages on an agar culture plate; the substrate was pre-inoculated with the nematode food, Escherichia coli OP50; (b) Hatched eggs on unseeded NGM plates; (c) Age synchronised N2 C. elegans J3 at 20 °C after 3 days.
Figure 3.
Caenorhabditis. elegans age synchronization. (a) Various C. elegans stages on an agar culture plate; the substrate was pre-inoculated with the nematode food, Escherichia coli OP50; (b) Hatched eggs on unseeded NGM plates; (c) Age synchronised N2 C. elegans J3 at 20 °C after 3 days.
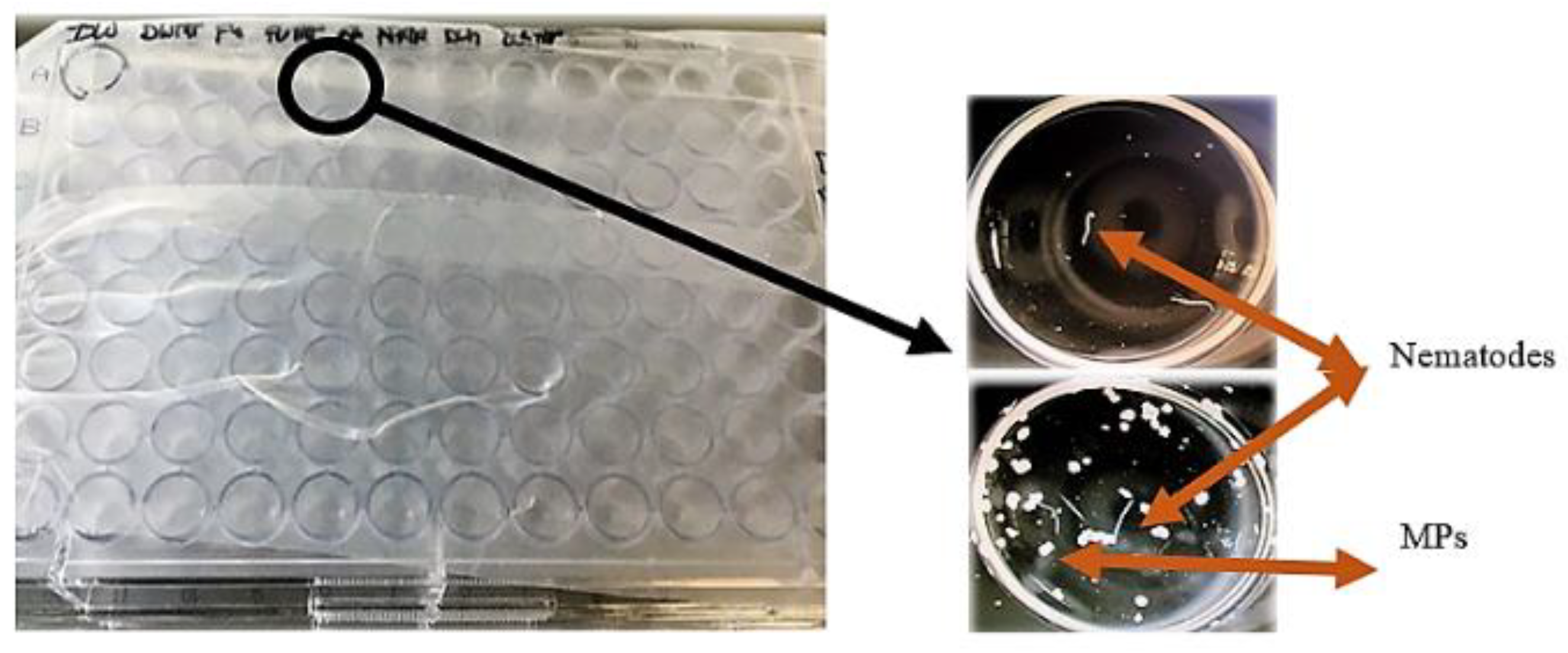
Figure 4.
The assessment of nematode mortality was done using a stereoscope. The tests were done in 96 well plates containing various treatments and 10 nematodes in each well.
Figure 4.
The assessment of nematode mortality was done using a stereoscope. The tests were done in 96 well plates containing various treatments and 10 nematodes in each well.
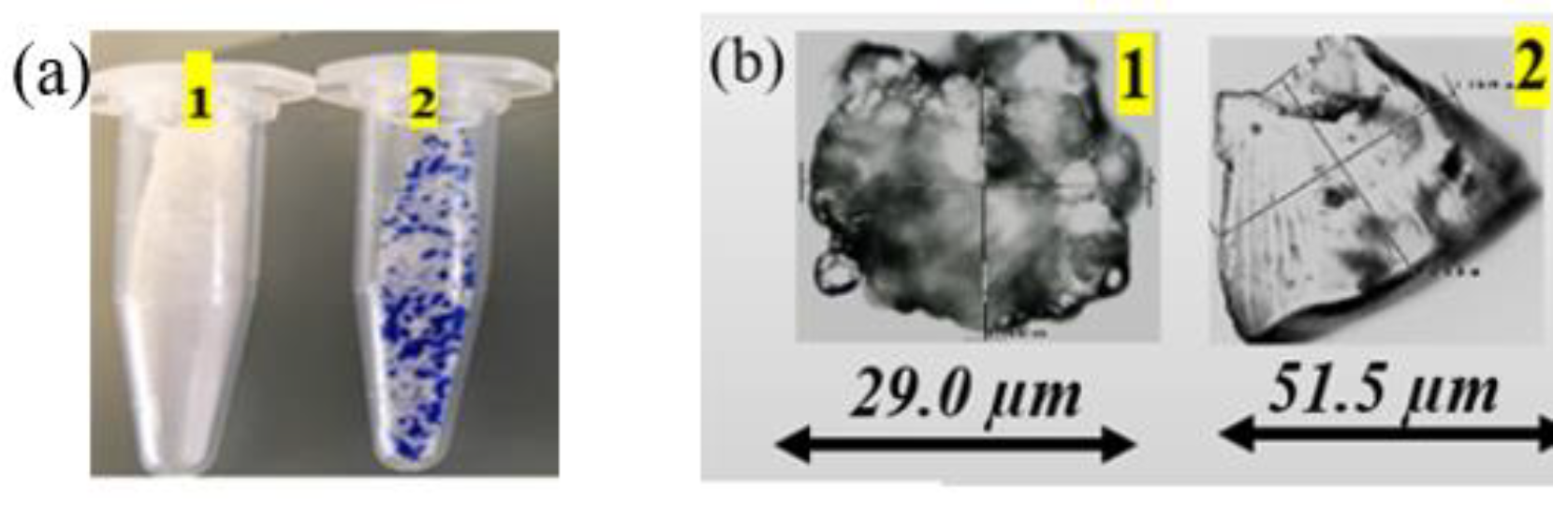
Figure 5.
(a) Microplastics extracted from cosmetic products; (b) Stereoscope images of the extracted MP.
Figure 5.
(a) Microplastics extracted from cosmetic products; (b) Stereoscope images of the extracted MP.
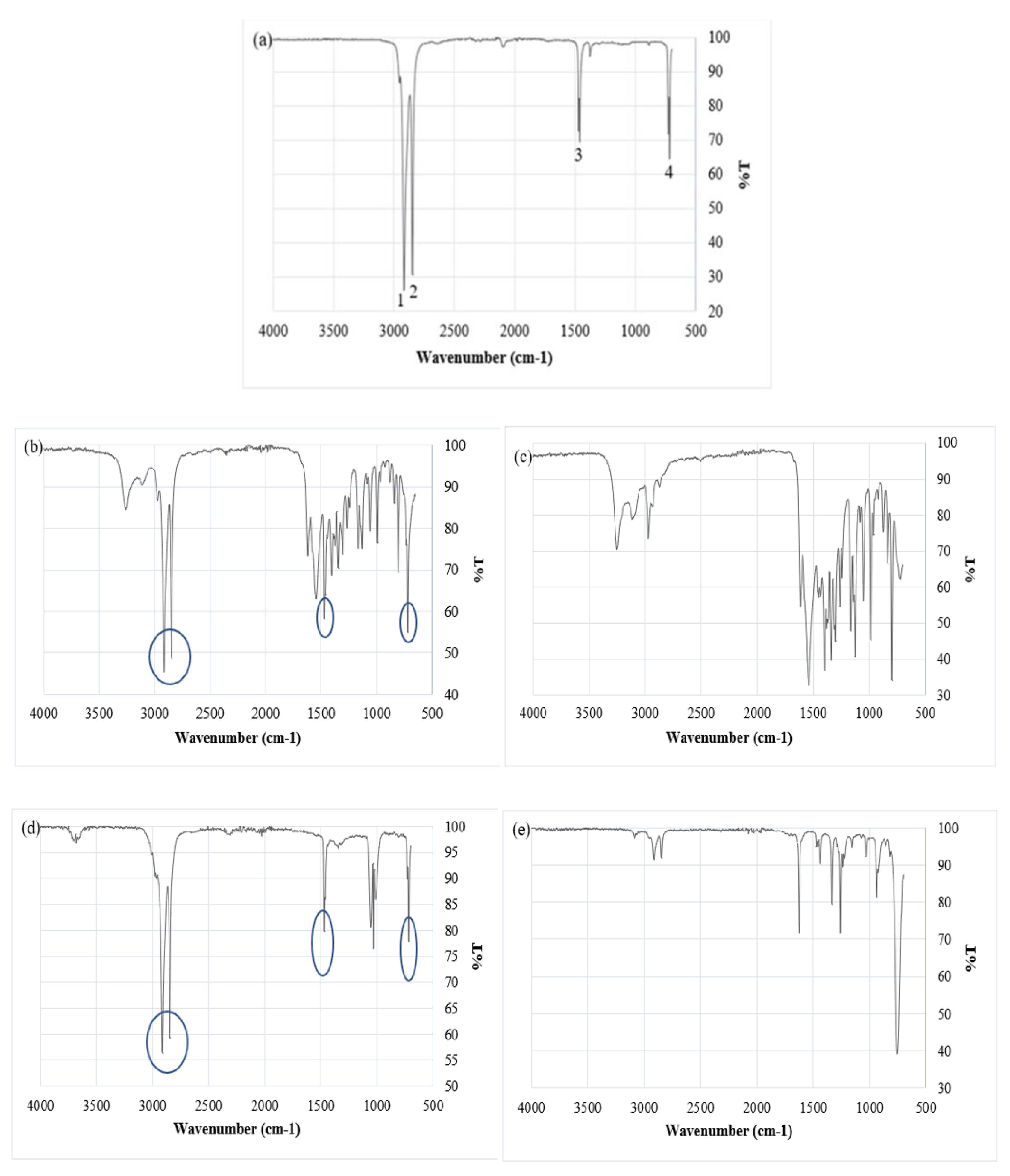
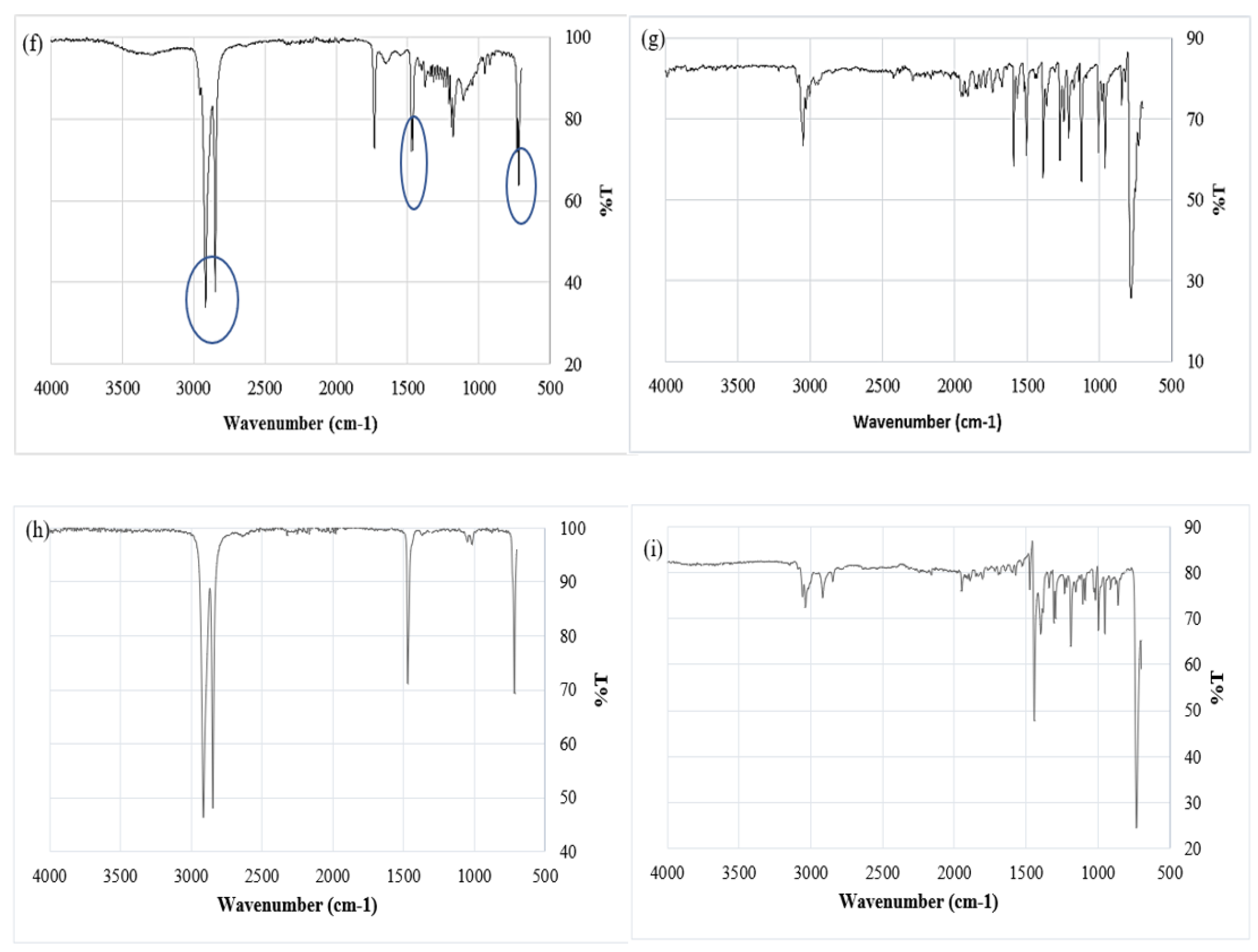
Figure 6.
(a) ATR-FTIR spectrum of commercial clear polyethylene; ATR-FTIR spectra of MP after immersion in: (b) 100ppb atrazine; (c) atrazine reference spectrum; (d) 100 ppb 1,3–dichloropropene; (e) 1,3–dichloropropene reference spectrum; (f) 100 ppb naphthalene; (g) naphthalene reference spectrum; (h) 100 ppb fluorene; (i) fluorene reference spectrum, showing MP absorption of toxic chemicals. The blue circles on the spectra represent original peaks before MP exposure to treatments.
Figure 6.
(a) ATR-FTIR spectrum of commercial clear polyethylene; ATR-FTIR spectra of MP after immersion in: (b) 100ppb atrazine; (c) atrazine reference spectrum; (d) 100 ppb 1,3–dichloropropene; (e) 1,3–dichloropropene reference spectrum; (f) 100 ppb naphthalene; (g) naphthalene reference spectrum; (h) 100 ppb fluorene; (i) fluorene reference spectrum, showing MP absorption of toxic chemicals. The blue circles on the spectra represent original peaks before MP exposure to treatments.
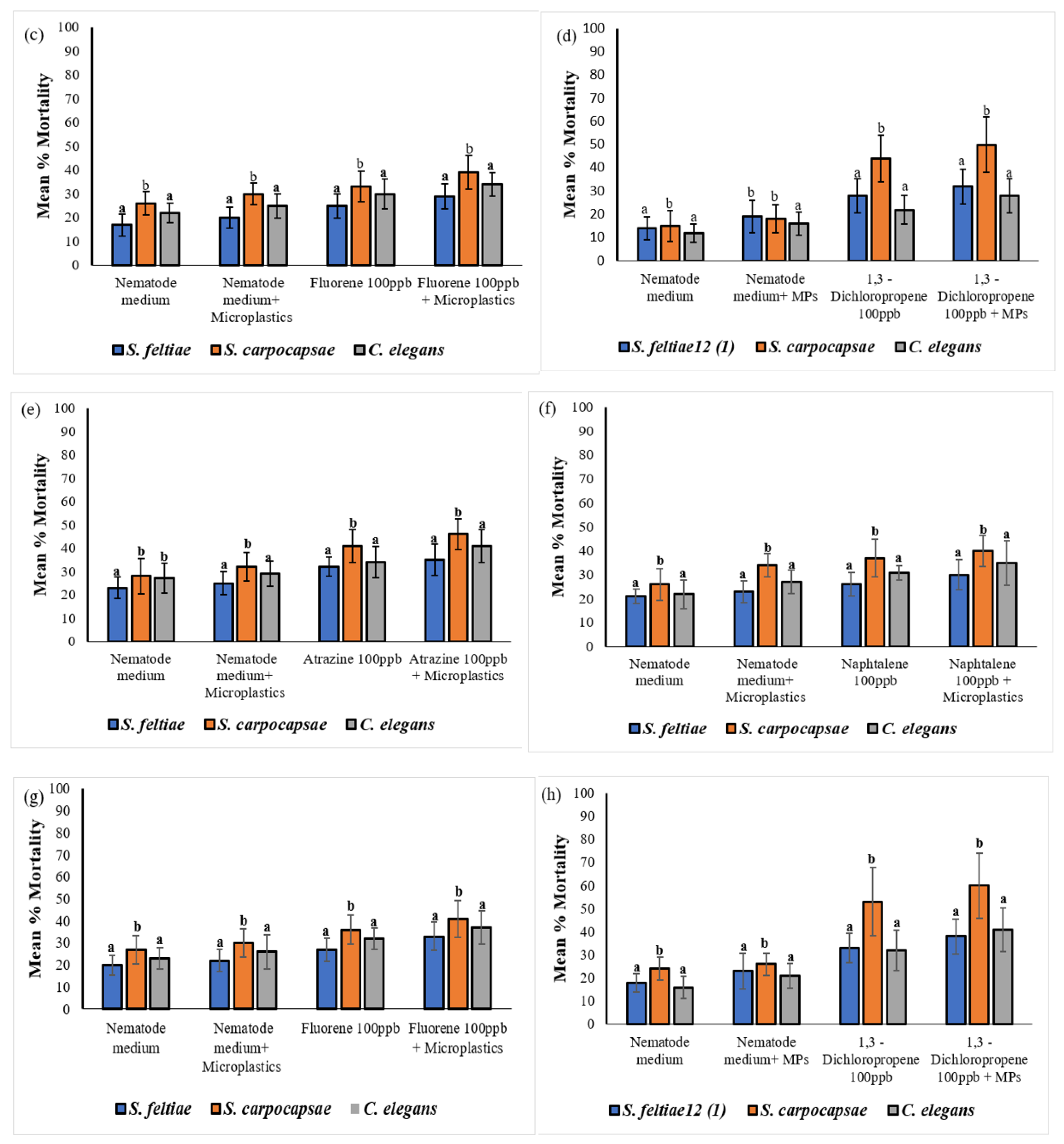
Figure 7.
Comparison of mortality of S. feltiae SB 12(1) IJ, S. carpocapsae IJ and C. elegans J3 exposed to a mixture of MP in various treatments after 72 hours (a-d), 96 hours (e-h).
Figure 7.
Comparison of mortality of S. feltiae SB 12(1) IJ, S. carpocapsae IJ and C. elegans J3 exposed to a mixture of MP in various treatments after 72 hours (a-d), 96 hours (e-h).
Figure 10.
River sediment nematode abundances. (a) Relative abundance of sediment nematode classes in samples from the locations of the study ; (b) The relative distribution of sequences in the nematode dataset within different nematode orders for all sediment samples.
Figure 10.
River sediment nematode abundances. (a) Relative abundance of sediment nematode classes in samples from the locations of the study ; (b) The relative distribution of sequences in the nematode dataset within different nematode orders for all sediment samples.
Figure 11.
River sediment nematode community composition. (a) The most frequently occurring nematode genera in the study sites and (b) Heatmap representing the abundances of the nine most prevalent nematode orders among the four locations. Location group name DHC: Dolmen Hotel – Carlow; GI: Great Island – Wexford; MCR: Macmurroughs – Wexford; and MLF: Milford – Carlow The colour-coded indicates the heatmap scale from 1 to –1.
Figure 11.
River sediment nematode community composition. (a) The most frequently occurring nematode genera in the study sites and (b) Heatmap representing the abundances of the nine most prevalent nematode orders among the four locations. Location group name DHC: Dolmen Hotel – Carlow; GI: Great Island – Wexford; MCR: Macmurroughs – Wexford; and MLF: Milford – Carlow The colour-coded indicates the heatmap scale from 1 to –1.
Figure 12.
Relative abundance of the various orders in the nematode communities at the various study sites. (a) The relative distribution of sequences in the nematode dataset within different nematode orders for each of the 20 analysed sediment samples and (b) Venn diagram displaying the overlap of shared OTUs among the four locations (at 97% similarity).
Figure 12.
Relative abundance of the various orders in the nematode communities at the various study sites. (a) The relative distribution of sequences in the nematode dataset within different nematode orders for each of the 20 analysed sediment samples and (b) Venn diagram displaying the overlap of shared OTUs among the four locations (at 97% similarity).
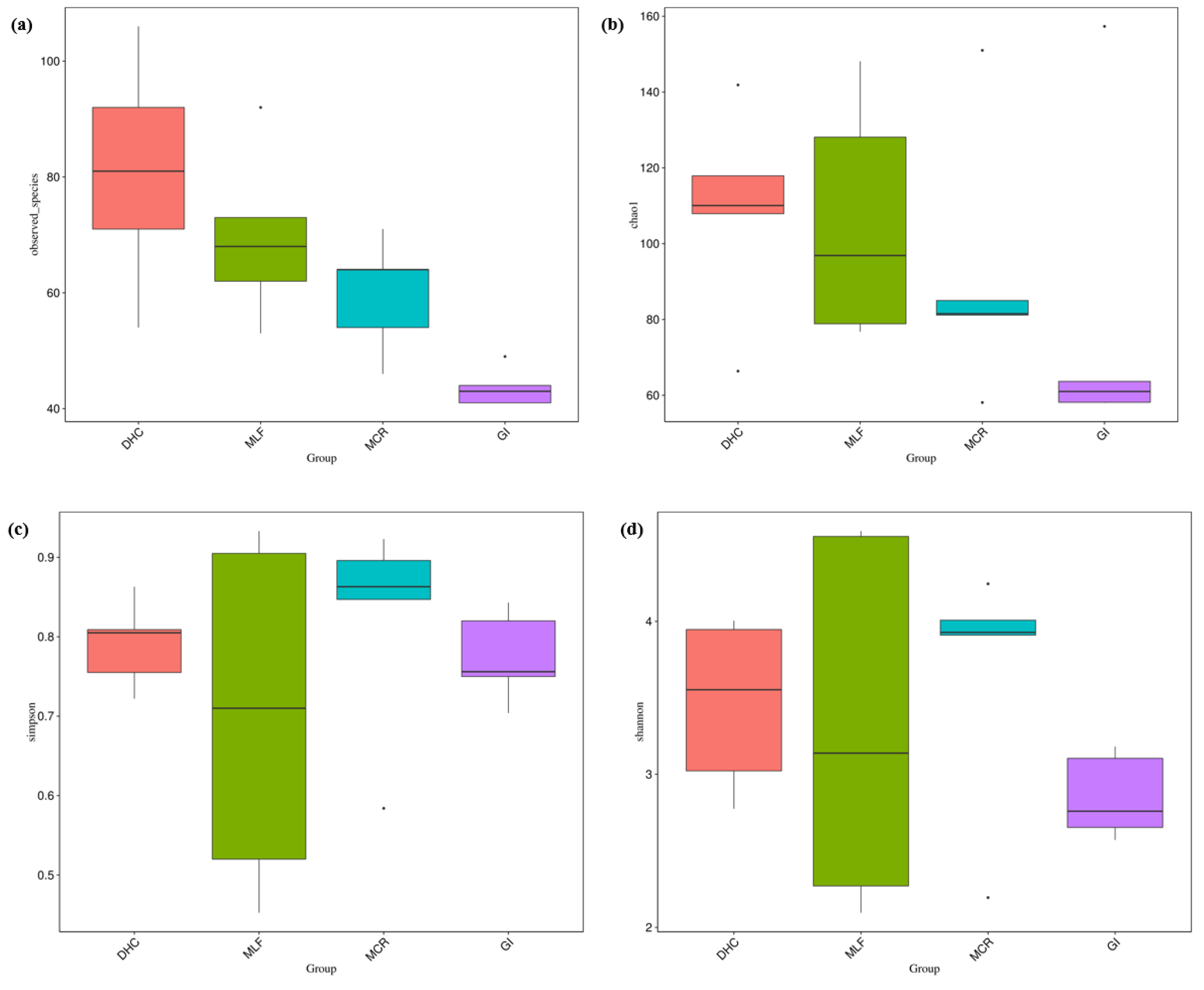
Figure 13.
Alpha diversities of the nematode communities associated with the river sediment samples from DHC, MLF, MCR, and GI; observed species, Chao1, Simpson , and Shannon are displayed in a, b, c, and d respectively.
Figure 13.
Alpha diversities of the nematode communities associated with the river sediment samples from DHC, MLF, MCR, and GI; observed species, Chao1, Simpson , and Shannon are displayed in a, b, c, and d respectively.
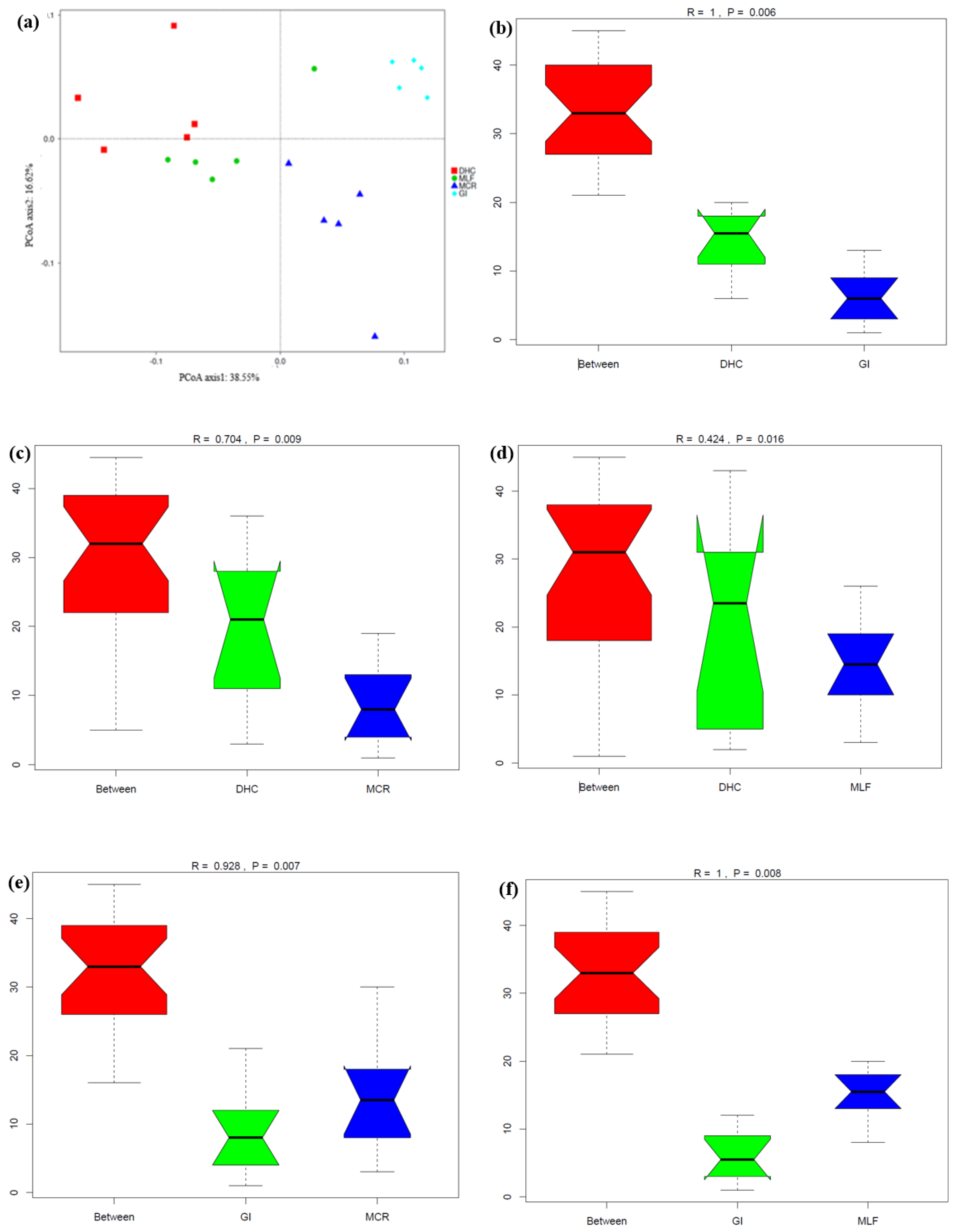
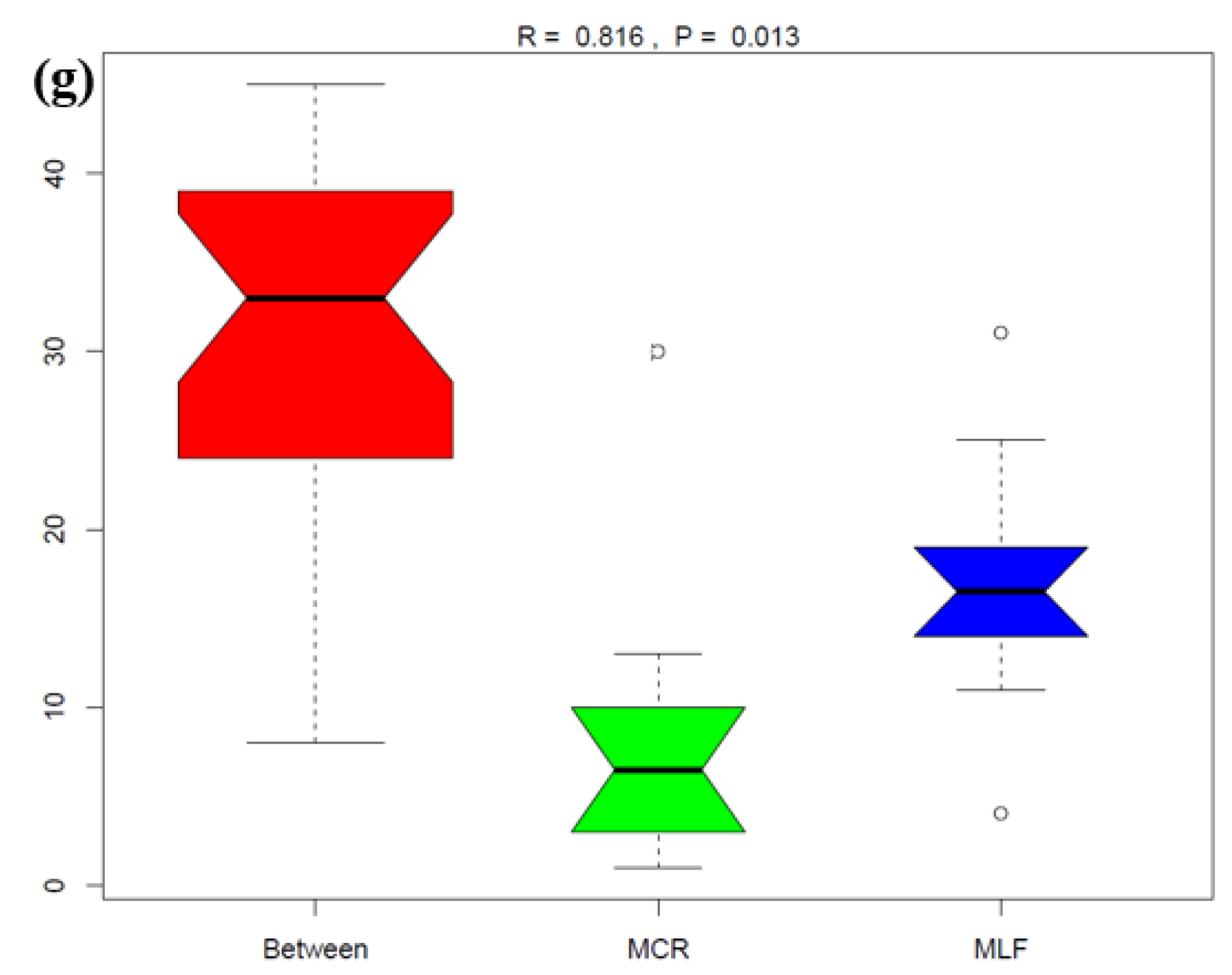
Figure 14.
Beta diversities of the nematode communities associated with the river sediment (a) Principal coordinate analysis (PCoA) of sediment nematode communities based on Bray–Curtis distances; PCoA plots are based on Bray-Curtis distances at the OTU level (97% sequence similarity). Red are Dolmen Hotel – Carlow samples, Green are Milford samples, Blue are Macmurroughs samples, and cyan are great island samples. (b) to (g) Anosim results of samples .
Figure 14.
Beta diversities of the nematode communities associated with the river sediment (a) Principal coordinate analysis (PCoA) of sediment nematode communities based on Bray–Curtis distances; PCoA plots are based on Bray-Curtis distances at the OTU level (97% sequence similarity). Red are Dolmen Hotel – Carlow samples, Green are Milford samples, Blue are Macmurroughs samples, and cyan are great island samples. (b) to (g) Anosim results of samples .
Figure 15.
Nematode abundance of various feeding (trophic) groups (guilds) across locations. Feeding type composition of nematode assemblage in the River Barrow sediment samples. Site A (DHC), site B (MLF), site C (MCR), and site D (GI). The percentage was determined by summing the abundances of all samples collected at each site and subsequently presenting the total cumulative abundance of all samples within each respective site.
Figure 15.
Nematode abundance of various feeding (trophic) groups (guilds) across locations. Feeding type composition of nematode assemblage in the River Barrow sediment samples. Site A (DHC), site B (MLF), site C (MCR), and site D (GI). The percentage was determined by summing the abundances of all samples collected at each site and subsequently presenting the total cumulative abundance of all samples within each respective site.
Figure 16.
Relative abundance (%) of nematode assemblage C–P groups at four different locations. The percentage was determined by adding up the abundances of all samples at each site and presenting the total cumulative abundance of all samples at each site.
Figure 16.
Relative abundance (%) of nematode assemblage C–P groups at four different locations. The percentage was determined by adding up the abundances of all samples at each site and presenting the total cumulative abundance of all samples at each site.
Figure 17.
Comparison of the mean (± SD) maturity index values of the four locations for nematodes.
Figure 17.
Comparison of the mean (± SD) maturity index values of the four locations for nematodes.
Figure 18.
Comparison of the mean sigma maturity index values of the four locations for nematodes.
Figure 18.
Comparison of the mean sigma maturity index values of the four locations for nematodes.
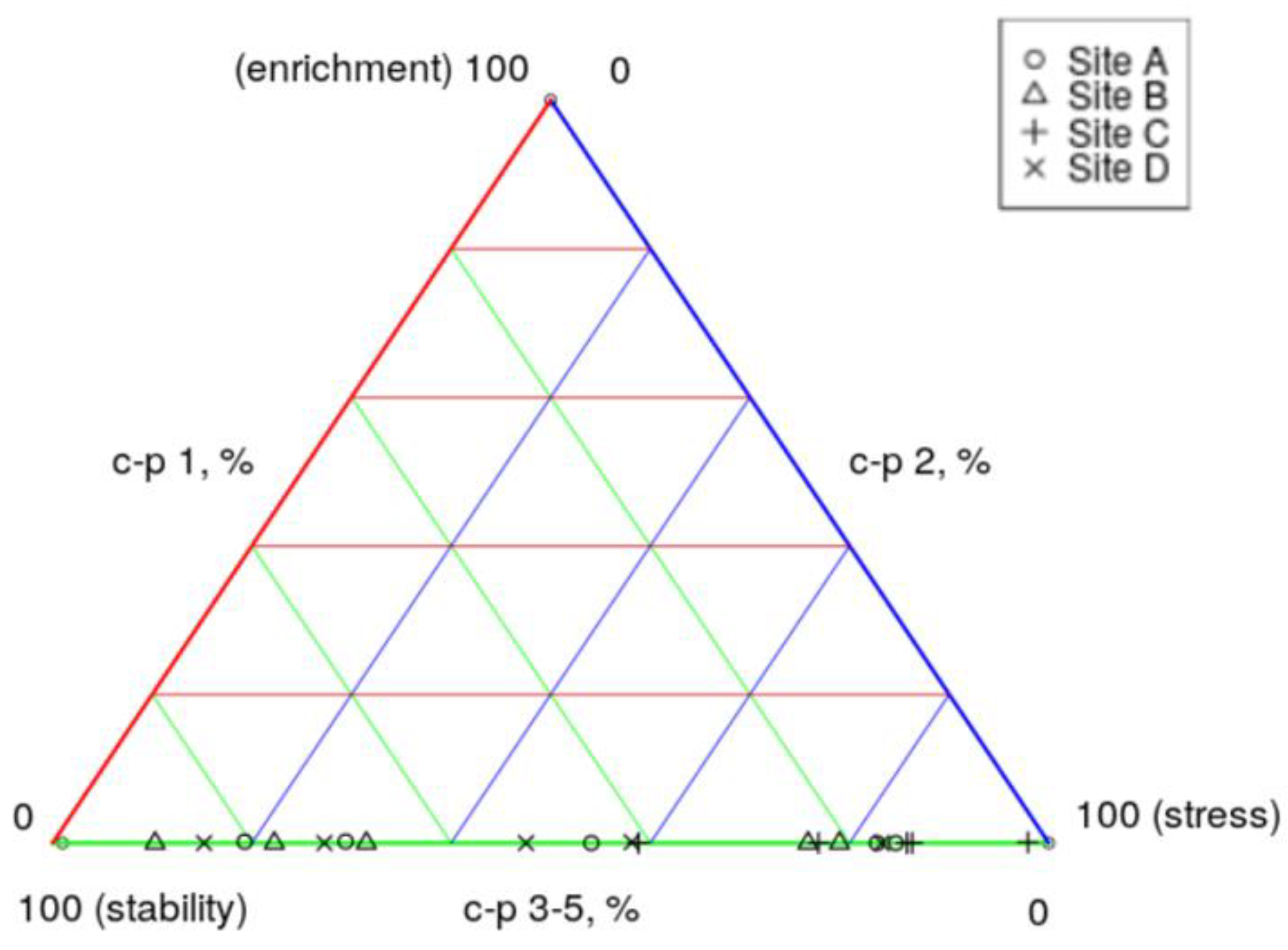
Figure 19.
Colonizer-persister triangle depicting river sediment status regarding nematodes c-p groups in the River Barrow.
Figure 19.
Colonizer-persister triangle depicting river sediment status regarding nematodes c-p groups in the River Barrow.
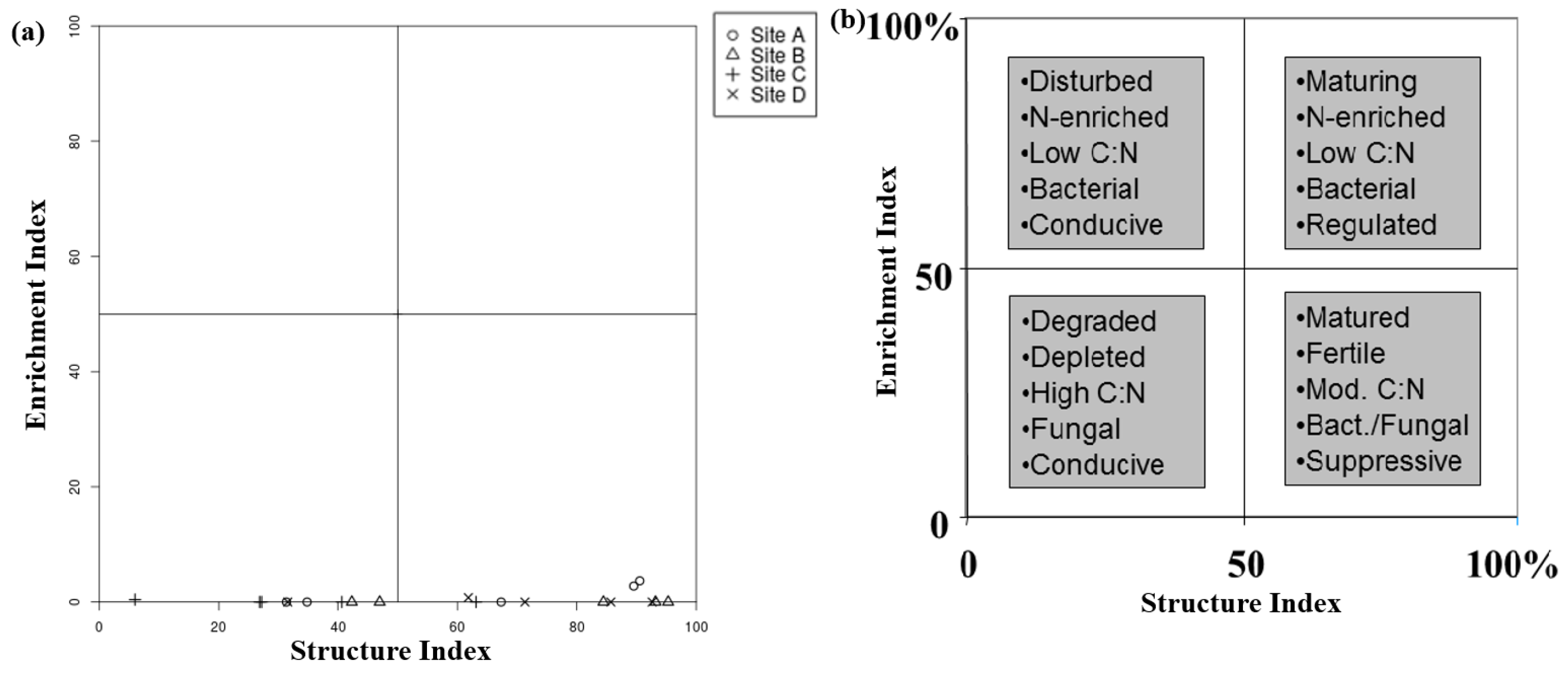
Figure 20.
(a) Food web analysis of nematode communities at different River Barrow locations sampled and (b) Food web analysis interpretation scheme.
Figure 20.
(a) Food web analysis of nematode communities at different River Barrow locations sampled and (b) Food web analysis interpretation scheme.
Table 1.
Nematode growth medium (NGM).
Table 1.
Nematode growth medium (NGM).
| Reagent |
Amount to be added (1 L) |
| Bacteriological agar (Nº3) |
17.0 g |
| Bacteriological peptone |
1.25 g |
| Calcium chloride (CaCl2) |
1.0 mL |
| Sodium chloride (NaCl) |
3.0 g |
| 5 mg/mL Cholesterol in ethanol |
1.0 mL |
| Potassium phosphate buffer (KH2PO4/K2HPO4) pH 6.0 |
mL |
Table 2.
Alkaline hypochlorite solution (20%) (15 mL).
Table 2.
Alkaline hypochlorite solution (20%) (15 mL).
| Reagent |
Amount to be added (15 mL) |
| Sodium hydroxide (NaOH) |
3.75 mL |
| Bleach |
3.0 mL |
| Double deionised water |
8.25 mL |
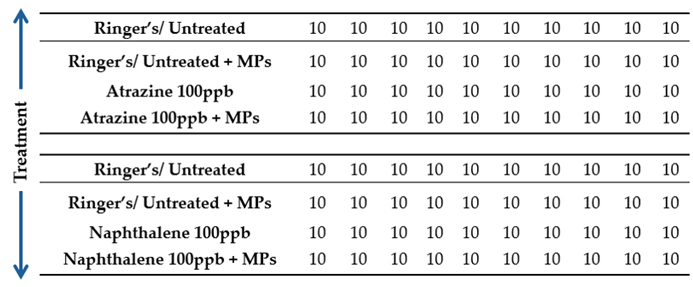
Table 3.
Experimental design. The toxicity bioassays were run in 96 well plates.
Table 3.
Experimental design. The toxicity bioassays were run in 96 well plates.
Table 4.
DNA concentration from Irish river sediment samples, and sampling coordinates (August 2021).
Table 4.
DNA concentration from Irish river sediment samples, and sampling coordinates (August 2021).
| Sampling coordinates |
Sampling sites |
MP Samples |
Ds DNA (ng/µL) |
| 52°49'58.5"N 6°55'30.0"W |
Dolmen Hotel - Carlow |
1 DHC |
56 |
| 2 DHC |
59 |
| 3 DHC |
51 |
| 4 DHC |
55 |
| 5 DHC |
57 |
| 52°47'04.7"N 6°57'60.0"W |
Milford - Carlow |
1 MLF |
53 |
| 2 MLF |
59 |
| 3 MLF |
57 |
| 4 MLF |
39.4 |
| 5 MLF |
55 |
| 52°25'13.0"N 6°56'04.9"W |
Macmurroughs - Wexford |
1MCR |
39 |
| 2 MCR |
31.7 |
| 3 MCR |
29.3 |
| 4 MCR |
40.9 |
| 5 MCR |
28.3 |
| 52°16'58.9"N 6°59'52.3"W |
Great Island - Wexford |
1 GI |
46.9 |
| 2 GI |
42.3 |
| 3 GI |
45.4 |
| 4 GI |
42.6 |
| 5 GI |
49.4 |
Table 5.
ATR-FTIR peak data and assignments for polyethylene.
Table 5.
ATR-FTIR peak data and assignments for polyethylene.
| Polymer |
Absorption bands ((cm-1) |
Assignment |
| Polyethylene (PE) |
2915 |
C-H stretch |
| 2848 |
C-H stretch |
| 1472 |
CH2 bend |
| 717 |
CH2 rock |
Table 6.
F and P-Value of nematode mortality in various treatments.
Table 6.
F and P-Value of nematode mortality in various treatments.
| Treatments |
Exposure time (hours) |
F |
P-Value |
| Nematode medium, atrazine and MP |
96 |
0.964 |
0.527931 |
| Nematode medium, naphthalene and MP |
72 |
0.938 |
0.563178 |
| Nematode medium, naphthalene and MP |
96 |
1.954 |
0.008807 |
| Nematode medium, fluorene and MP |
72 |
1.645 |
0.039446 |
| Nematode medium, fluorene and MP |
96 |
0.639 |
0.913678 |
| Nematode medium, 1,3-dichloropropene and MP |
72 |
0.718 |
0.843136 |
| Nematode medium, 1,3-dichloropropene and MP |
96 |
0.948 |
0.548654 |
Table 7.
Alpha diversity indices of nematode communities from river sediment samples (n = 5). The data shows the averages and ± standard deviations.
Table 7.
Alpha diversity indices of nematode communities from river sediment samples (n = 5). The data shows the averages and ± standard deviations.
| Sample name |
Observed number of species |
Shannon |
Simpson |
Chao1 |
ACE |
Goods coverage |
| DHC |
80.8 ± 19.84 |
3.46 ± 0.55 |
0.7908 ± 0.054 |
108.82 ± 27.295 |
114.798 ± 34.818 |
0.980 ± 0.006 |
| MLF |
69.6 ± 14.57 |
3.33 ± 1.202 |
0.7042 ± 0.218 |
105.741 ± 31.369 |
107.27 ± 21.354 |
0.9818 ± 0.004 |
| MCR |
59.8 ± 9.81 |
3.66 ± 0.828 |
0.8226 ± 0.137 |
91.347 ± 35.021 |
81.037 ± 16.566 |
0.9874 ± 0.004 |
| GI |
43.6 ± 3.29 |
2.85 ± 0.274 |
0.7746 ± 0.056 |
79.628 ± 43.50 |
72.519 ± 15.127 |
0.9874 ± 0.003 |
Table 8.
Feeding types and colonizer-persistent class assignments of nematode taxa in river sediment.
Table 8.
Feeding types and colonizer-persistent class assignments of nematode taxa in river sediment.
| Family |
c-p class |
p-p class |
Feeding type |
| Trichodoridae |
0 |
4 |
Herbivores – ectoparasites |
| Tylenchidae |
0 |
2 |
Herbivores – epidermal/root hair feeders |
| Monhysteridae |
2 |
0 |
Bacterivores |
| Plectidae |
2 |
0 |
Bacterivores |
| Rhabditidae |
1 |
0 |
Bacterivores |
| Achromadoridae |
3 |
0 |
Predators |
| Cyatholaimidae |
3 |
0 |
Predators |
| Dorylaimidae |
4 |
0 |
Omnivores |