1. Introduction
Monoamines including catecholamines (dopamine, epinephrine, norepinephrine), serotonin, melatonin, histamine, and trace amines (
TAs) are a group of mammalian neuroactive compounds produced by both host cells and microbiota. TAs are neuromodulators of classical neurotransmitters (
NTs), including dopamine, noradrenalin, and serotonin [
1]. However, the recent discovery of TA-associated receptors (
TAARs) suggests that TAs may also function as NTs in vertebrates [
2,
3]. While TAs activate these receptors with nanomolar affinities, classical NTs require micromolar concentrations for TAAR activation [
4]. From this point forward, for simplicity, we will refer to all mentioned monoamines as NTs.
The main enzymes responsible for production of these NTs are categorized as aromatic L-amino acid decarboxylases (
AADCs) [
1].The bacterial AADCs differ from human ones with respect to substrate specificity and kinetics of activity [
5,
6]. Different kinds of microbial AADCs have been reported so far. Tyrosine decarboxylase (
TDC) with the ability to decarboxylate tyrosine, dihydroxy phenylalanine (
L-Dopa), and/or phenylalanine is the most common one among enterococci and other lactic acid bacteria [
7,
8,
9,
10,
11,
12,
13,
14,
15].
Ruminococcus gnavus, an anaerobic Gram-positive gut bacterium from the
Clostridiales family, utilizes a tryptophan decarboxylase to primarily produce tryptamine (
TRY), but also phenylethylamine (
PEA), and tyramine (
TYM). The same enzyme has also been reported in another gut species,
Clostridium sporogenes [
6]. The AADC enzyme from
Bacillus atrophaeus shows broad substrate activity, with tryptophan at 610%, tyrosine at 12%, L-Dopa at 24%, and 5-hydroxytryptophan (5-HTP) at 71%, all relative to its activity towards phenylalanine [
16]. The AADC enzyme from
Pseudomonas putida is exclusively active towards L-Dopa [
17]. A highly unspecific AADC, SadA, has been described in certain
Staphylococcus species belonging to the skin and gut microbiota. This enzyme is able to produce all three TAs (TRY, PEA, and TYM), as well as serotonin (
SER), and dopamine (
Dopa). However, the production of NTs varies significantly among
Staphylococcus species, with some strains, such as
S. pseudintermedius, exhibiting higher levels of production, while others produce considerably lower amounts [
18,
19]. Metagenomic analysis revealed the presence of SadA homologs in at least 7 phyla such as
Bacillota,
Actinobacteria,
Proteobacteria,
Bacteroidetes,
Acidobacteria, and in 23 genera of the phylum
Bacillota, including species within the skin and the gut microbiota such as
Clostridiaceae,
Enterococcaceae,
Lactobacillaceae or
Ruminococcaceae [
19].
Despite the high levels of monoamines in stool and skin samples from healthy human subjects [
20,
21], it is difficult to determine the extent to which microbiota contribute to the NT content of the body. However, compelling evidence from mouse studies suggests that the gut microbiota significantly contributes to the NT content in the brain, blood, gut, and other tissues [
22,
23,
24,
25].
The advantages of monoamine production by bacteria are not fully understood; however, the AADC activity seems to be an acid resistance mechanism by consuming excessive cytoplasmic protons and producing alkaline products to control intracellular pH homeostasis [
7,
10,
26,
27,
28]. Monoamines have also been shown to increase bacterial adherence and internalization into epithelial cells, thereby providing a colonization advantage [
18,
20,
29]. Moreover, these metabolites may act as signaling molecules to interact with the host niche [
30]. The effects of TRY on gut motility [
31], the anti-inflammatory effects of TRY and its metabolite, indole acetic acid, on macrophages and hepatocytes [
32], and the immunomodulatory effects of TYM on enterocytes [
29] are examples of monoamine-mediated interactions between microbiota and host. In addition, NT-producing
Staphylococcus epidermidis, which belongs to the normal skin microbiota, was found to accelerate wound healing [
21]. Microbiota-derived NTs are also assumed to affect the mental state and may be associated with neurodegenerative diseases [
4,
33,
34].
Monoamines produced by human microbiota can, therefore, enter the bloodstream and exert their effects beyond the original niche. However, compared to gut microbiota, less attention has been given to the role of skin and nasal microbiota in NT production. Given the ability of TAs to passively diffuse through cell membranes [
2] and the widespread distribution of TAARs throughout the body [
35], the skin microbiota may significantly contribute to the microbiota-derived fraction of NTs in the body.
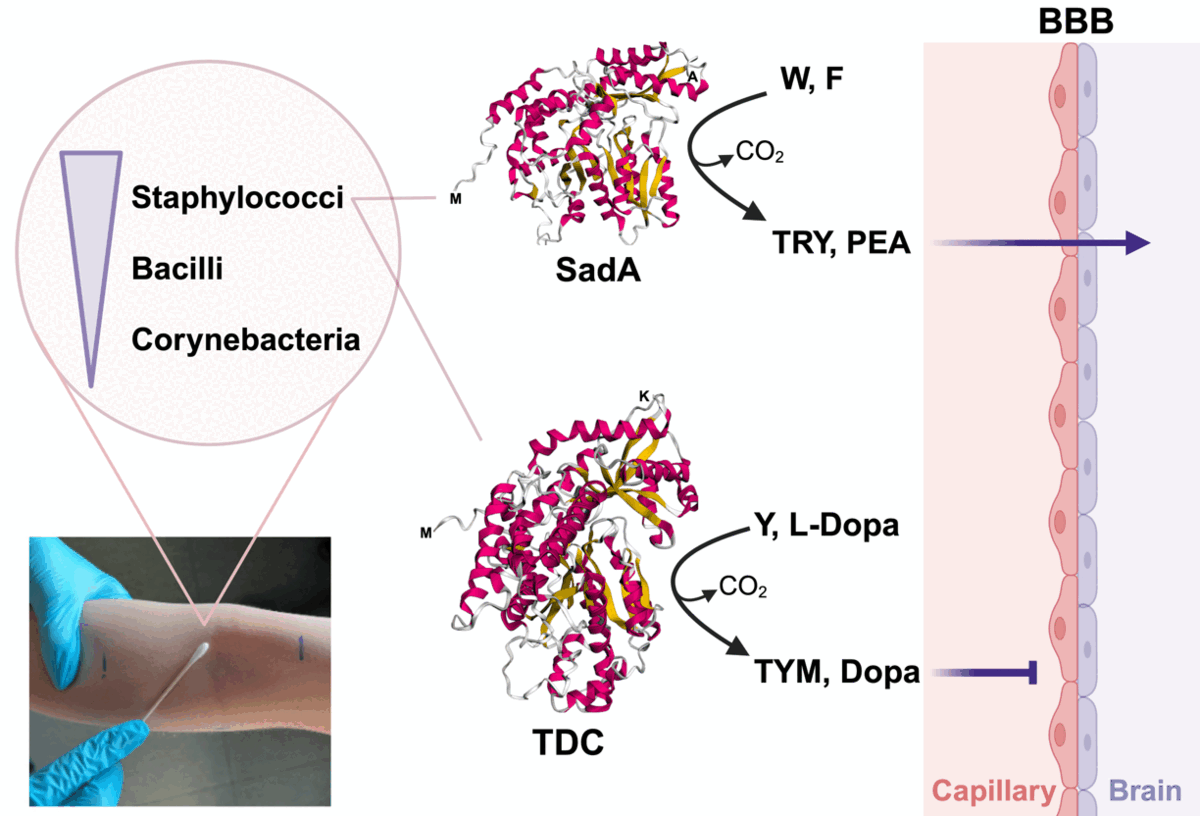
In this study, we aimed to investigate the spectrum of NT production by the skin microbiota. We identified two types of producers with distinct product profiles: the SadA-type and the TDC-type, implying that the skin microbiota defines the spectrum and content of NTs on our skin.
2. Results
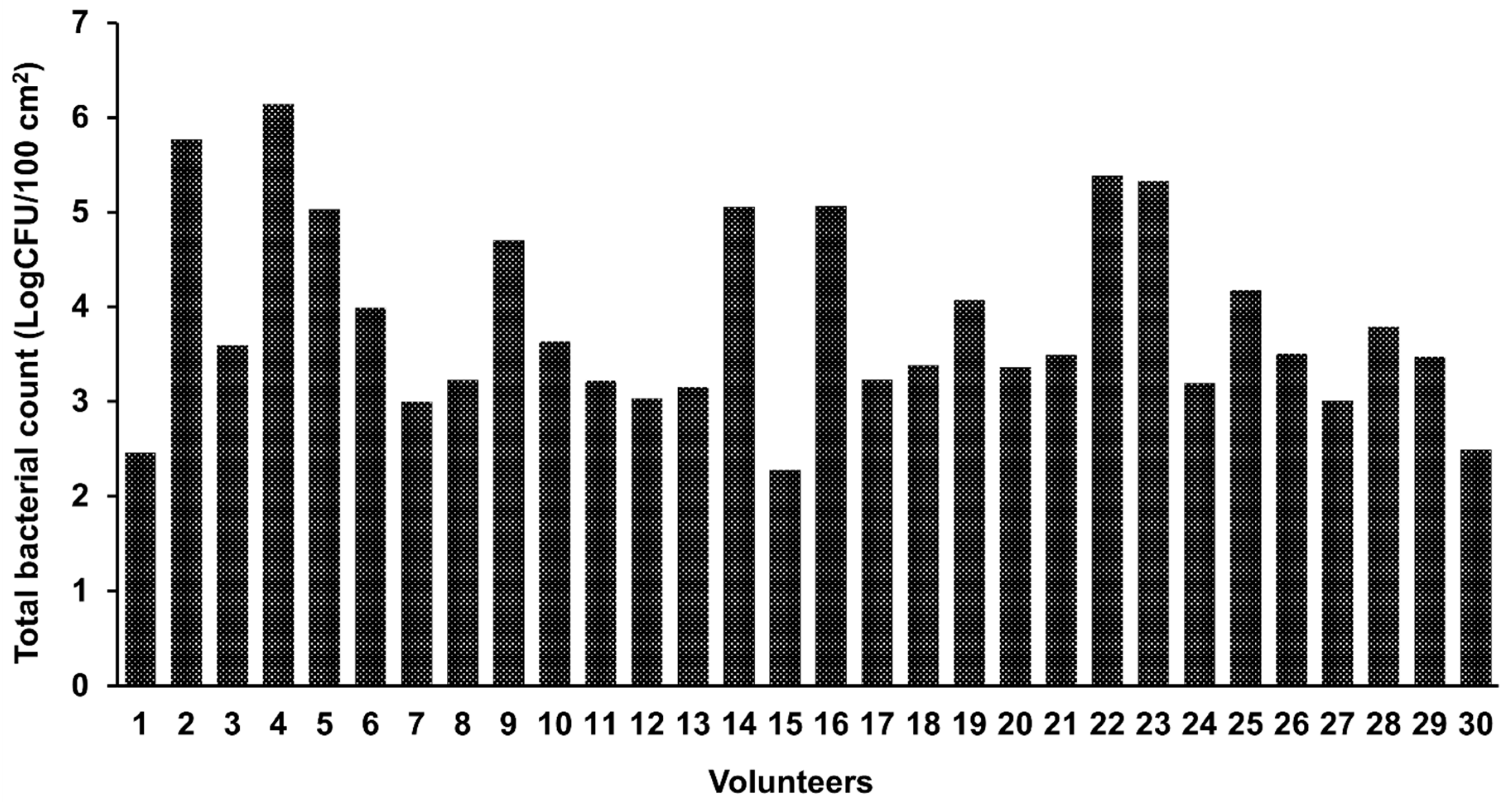
Strain collection of bacteria from human skin. Swab samples were taken from the antecubital fossa skin of 30 healthy volunteers to isolate aerobic and anaerobic bacterial species on agar medium as detailed in Materials and Methods. The colony forming unit (
CFU) counts varied tremendously across samples and ranged from 2.28 to 6.14 log CFU/100 cm
2 skin area (
Figure 1 and
Table S1). No statistically significant differences were found between samples derived from men and women with respect to the number of CFUs (
P = 0.151). There was no significant correlation between age and CFU counts (r = -0.185,
P = 0.329). Following the isolation and purification of colonies with different morphologies, a diverse strain collection of 1909 skin bacteria was established. No lactic acid bacteria (
LABs) were isolated from MRS and M17 plates incubated under anaerobic conditions. Based on 16S rRNA sequencing, the spectrum of predominantly Gram (+) bacteria mainly comprised the genera
Staphylococcus,
Bacillus, and
Corynebacterium, with the vast majority of the isolates belonging to the genus
Staphylococcus (
Table 1).
Identification of neurotransmitter (NT) producers. The identification of NT producers was performed in two steps. In a first screening, all 1909 isolates were cultivated in TSB overnight, and the resulting culture supernatants were then analyzed for the ability to convert aromatic amino acids (
AA) to their decarboxylation products by RP-HPLC [
18]. From previous work, we knew that TSB has a relatively high content of tryptophan, phenylalanine, and tyrosine (367, 613, and 355 μg/ml, respectively), which serve as substrates of AADC enzymes [
18]. This analysis enabled us to identify NT producers – mainly staphylococcal strains – in our isolate collection as shown in
Table 2. Most staphylococcal NT-producers belonged to the species
S. epidermidis, followed by
Staphylococcus saccharolyticus,
Staphylococcus coagulans,
Staphylococcus capitis,
Staphylococcus hominis, and
Staphylococcus pragensis.
To reduce the noise of the media components in RP-HPLC analyses, a different method for sample preparation was implemented. We washed the overnight cultures twice with 1 x PBS and then resuspended the pellets in 5 x PBS/10% glucose, together with all respective substrates (1 mg/ml each), L-Dopa, tryptophan (
W), phenylalanine (
F), and tyrosine (
Y). Representative RP-HPLC analyses are shown for an
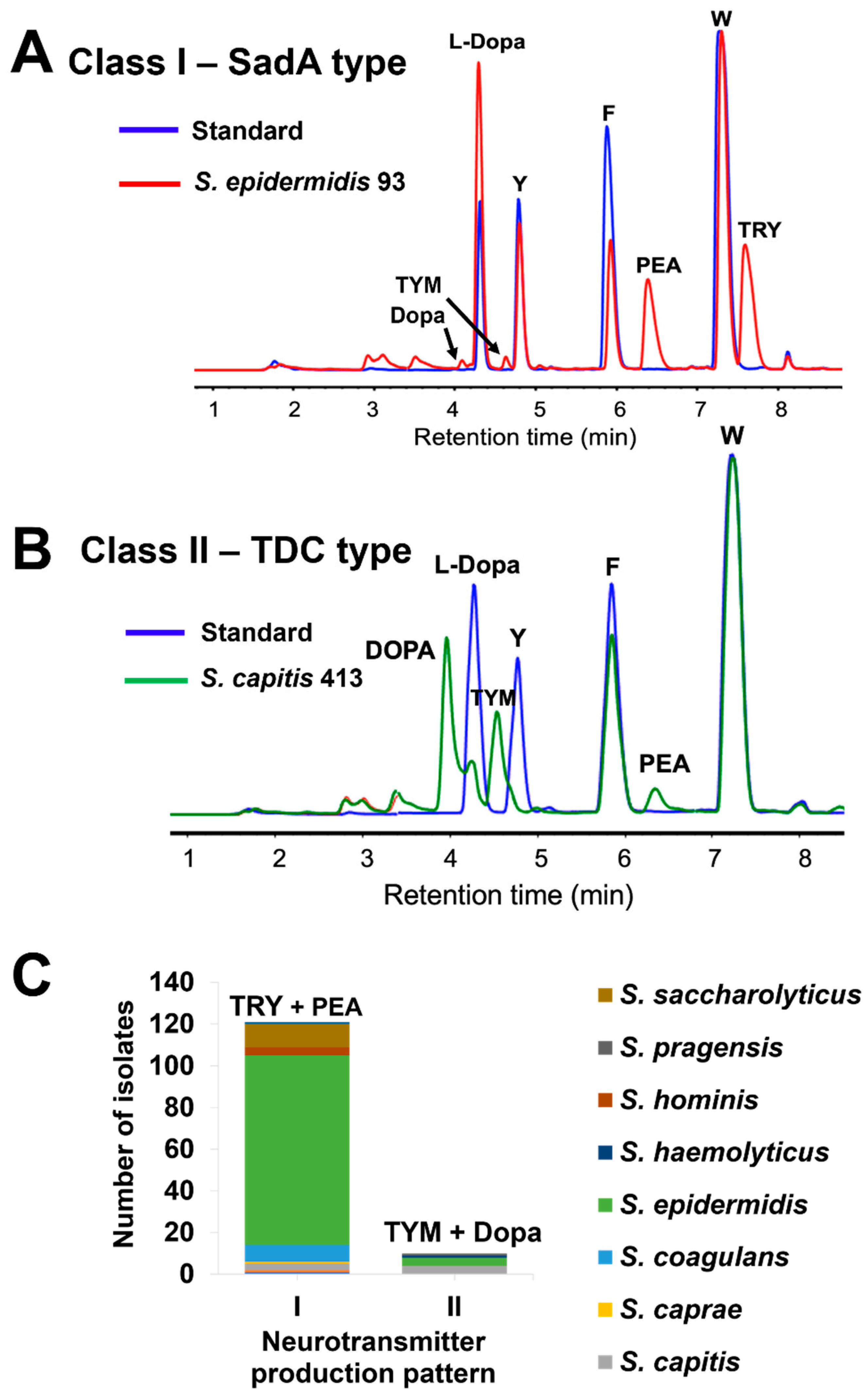
S. epidermidis isolate, which mainly produces PEA and TRY and little TYM and Dopa (
Figure 2A), and an
S. capitis isolate, which mainly produces Dopa and TYM and little PEA (
Figure 2B). With this refined production process, we could clearly distinguish between two classes of NT-producers: class I members predominantly produced TRY and PEA and little TYM and Dopa, whereas class II members predominantly produced TYM and Dopa and little PEA (
Figure 2C). Class I made up the largest group with 118 isolates, while class II comprised only 10 isolates. Of the 1909 skin isolates, 128 (6.7%) were able to produce TAs (
Table S2).
SadA and TDC enzymes are responsible for NT production. The class I production pattern had already been reported in staphylococcal isolates from gut and skin and is known to be mediated by the enzyme SadA [
18]. It was, therefore, reasonable to hypothesize that the same enzyme is responsible for NT production in the isolates investigated in this study. To test this hypothesis, we confirmed the presence of the
sadA gene in the isolates with class I production pattern by PCR using
sadA-specific primer sets (
Table S3). The observed class II production pattern resembled that previously reported for another AADC enzyme type, TDC that is common among food-related lactic acid bacteria, especially
Enterococcus sp. [
7,
8,
9,
10,
11,
12,
13,
14,
15]. The presence of the
tdc gene in skin isolates exhibiting class II production pattern was confirmed using a consensus primer set with high specificity for various Gram (+) bacteria, yielding the expected amplicon size of 335 bps [
36].
SadA homologues are more prevalent in Staphylococcus spp. Following a BLASTp search of the NCBI NR protein sequence database within the
Staphylococcus genus, using a threshold of 90% coverage and 50% identity, SadA and TDC homologues were found in 6,098 staphylococcal strains (
Table 3). Among these, 5,900 strains had SadA homologues, 176 contained TDC homologues, and 22 strains carried both (
Tables S4–S6).
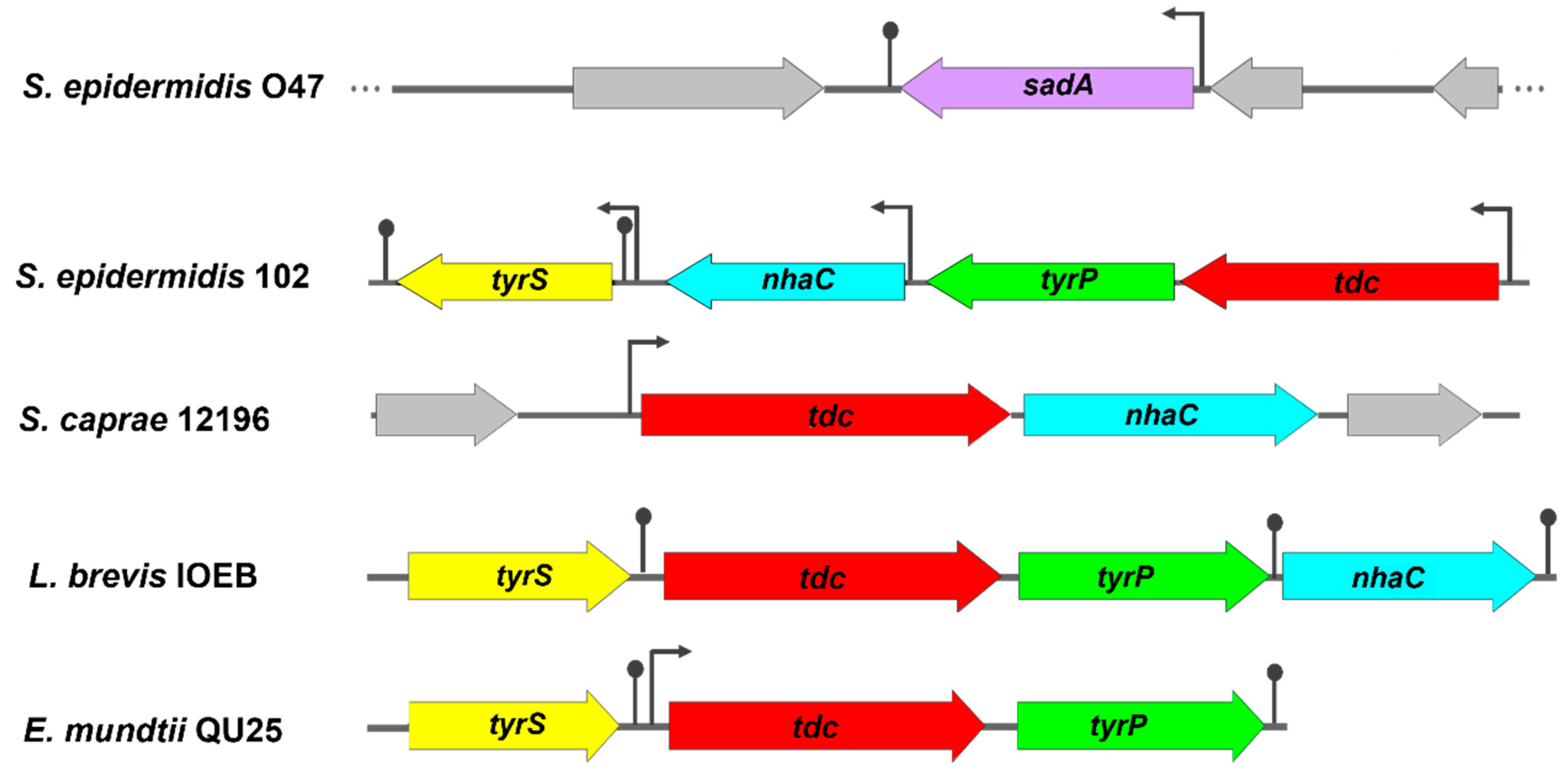
Identification of the tdc operon in an S. epidermidis skin isolate. Among the NT-producing isolates harboring the
tdc gene,
S. epidermidis 102 was selected for further characterization. Analysis of the whole genome sequence of this isolate revealed the presence of a 6409 bp
tdc operon consisting of 4 contiguous open reading frames (ORFs) oriented in the same direction, but with a slightly different gene arrangement compared to previously reported
tdc operons (
Figure 3). BLASTp analysis showed high amino acid sequence homology with the previously described
tdc operon from
Levilactobacillus brevis IOEB (
Table 4).
The same
tdc operon was also found in other TDC-harboring staphylococcal species retrieved from the NCBI database, except in
S. caprae strains. The
tdc operon of
S. caprea strains consists of only two genes,
tdc and
nhaC (
Figure 3). Based on the phylogenetic tree, all TDC enzymes were closely related but exhibited a substantial genetic distance from the SadA enzyme (
Figure 4A). Multiple alignments of the aa sequences indicated high similarity between the TDC enzyme from
S. epidermidis 102 and those from
Enterococcus durans IPLA 655,
E. faecalis JH2-2,
E. mundtii QU 25, and
L. brevis IOEB strains (
Figure S1). In addition, the 3D structure models of these TDC proteins revealed a comparable folding pattern which clearly differs from the SadA protein type (
Figure 4B). The alignment of aa sequences and the 3D structures of TDC and SadA enzymes from
S. epidermidis species showed relatively low similarity, with a root mean square deviation (RMSD) value of 4.410 Å (Figure S2 and
Figure 4C). A further difference between SadA and TDC orthologues is their size: SadA enzymes have an average monomer size of approximately 475 aa, whereas TDC enzymes are larger with 616-635 aa.
In
S. epidermidis 102, downstream of
tdc are three other ORFs encoding tyrosine-tyramine permease (
tyrP), Na+/H+ antiporter (
nhaC), and tyrosyl-tRNA synthetase (
tyrS) (
Figure 3). Putative promoters and Rho-independent terminators were found upstream of the start codons of
tdc,
nhaC, and
tyrS, but not between
tdc and
tyrP. This suggests that
tdc and
tyrP might be co-transcribed independently of
tyrS and
nhaC. The
nhaC gene, which is generally found as a flanking gene upstream of
tyrP [
7,
37], is located between
tyrP and
tyrS in the
tdc operon of
S. epidermidis 102. However, some
tdc operons such as those from
E. faecalis JH2-2 [
38],
E. mundtii [
9]
, L. lactis IPLA 655 [
8] lack this gene. TyrS is a class I aminoacyl tRNA synthetase. characterized by the conserved HIGH and KMSKS motifs. In TyrS of
S. epidermidis 102, the KMSKS motif is replaced by KFGKT as in TryS of
E. faecalis,
E. mundtii,
L. brevis, and
L. lactis [
8,
9,
11,
38].
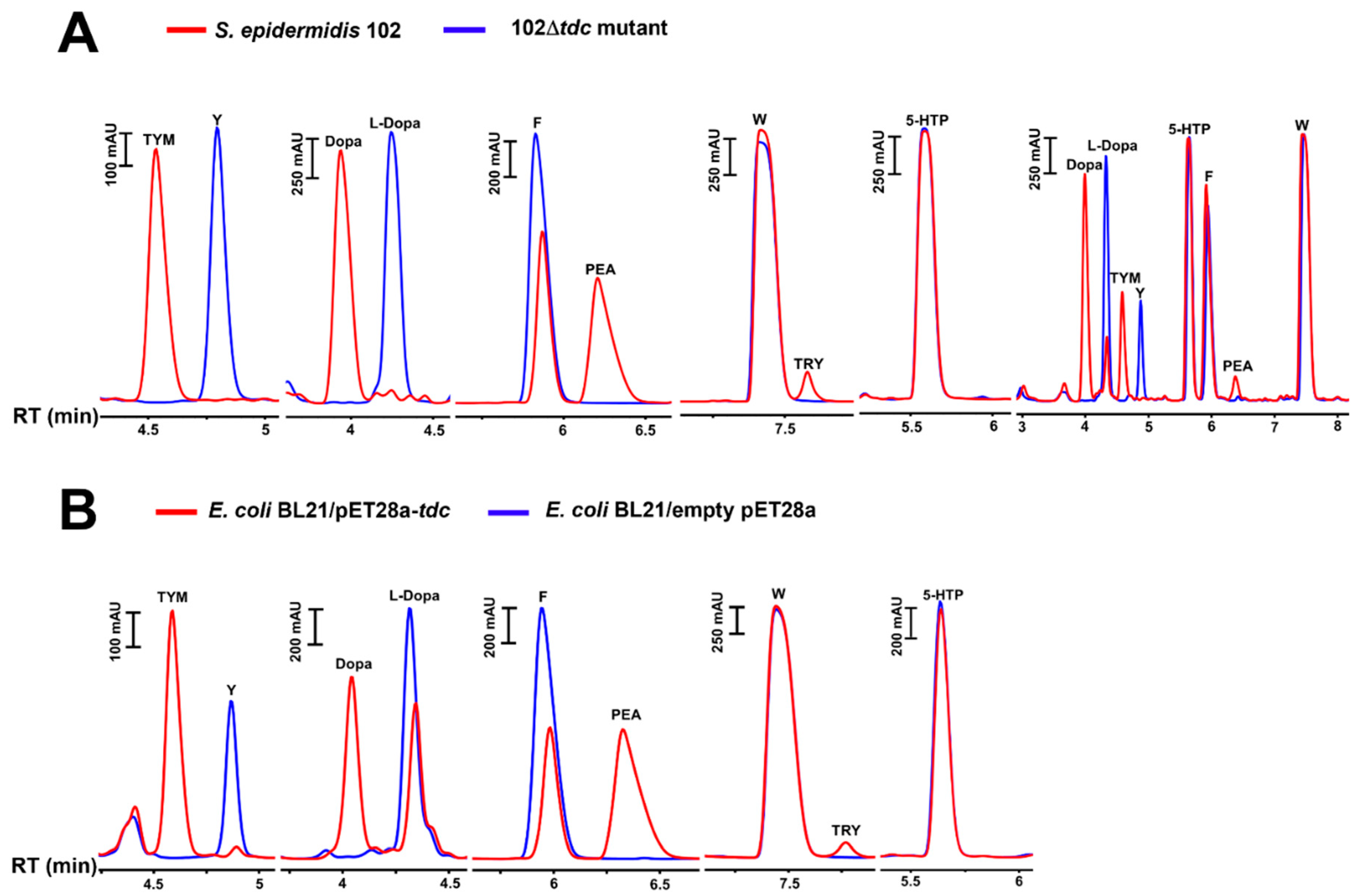
TDC is responsible for NT production in S. epidermidis 102. To confirm the role of TDC in NT production in
S. epidermidis 102, we generated the deletion mutant 102Δ
tdc and compared its NT production to that of the WT strain. After overnight incubation of the WT and mutant cells with individual or a combination of substrates (W, F, Y, L-Dopa, and 5-HTP), the culture supernatants were collected and analyzed by HPLC. No NT production activity was observed following the deletion of the
tdc gene. In contrast, the WT strain exhibited decarboxylase activity primarily towards Y and L-dopa, but also towards F. It was, however, unable to decarboxylate 5-HTP and W to SER and TRY. In the absence of Y and L-dopa, there was a higher accumulation of PEA. Production of TRY and SER was negligible even when W and 5-HTP were the only available substrates (
Figure 5A). The same production pattern was found for the TDC-harboring
E. faecalis strain (ATCC 19433), although the production of Dopa and PEA was lower due to the slower growth rate of
E. faecalis compared to
S. epidermidis (
Figure S3A).
The
tdc genes from
S. epidermidis 102 and
E. faecalis strain were then cloned (
Figure 6A) and overexpressed in
E. coli. The resulting transformants were fed with individual substrates (W, F, Y, L-Dopa, and 5-HTP). No production was found in the
E. coli strain with the empty vector, whereas the
E. coli strains expressing either of the
tdc genes showed AADC activity comparable to the original strains (Figures 5B and S3B).
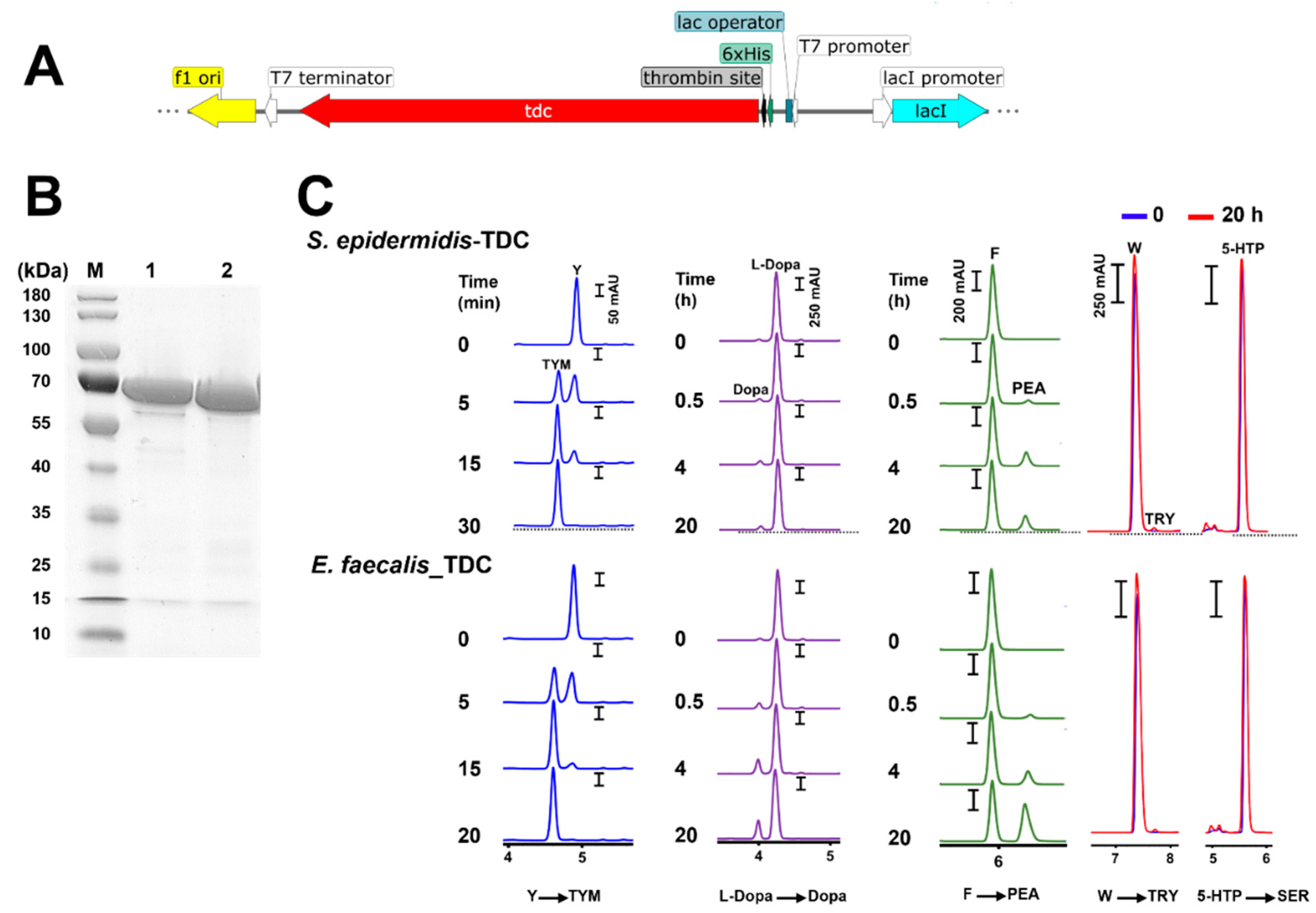
In
S. epidermidis 102, the
tdc gene encodes a protein of 616 aa with an estimated molecular mass of 69.9 kDa which was confirmed by a single band at approximate molecular weight of 70 kDa in SDS-PAGE analysis of the purified enzyme (
Figure 6B). The TDC enzyme from
E. faecalis ATCC 19433 consisting of 620 amino acids with a molecular weight of 70 kDa was cloned in
E. coli and used as a control. The decarboxylase activity of the purified enzymes was time-dependent. Total conversion of Y (2 mM) to TYM occurred within less than 30 min, while the accumulation of Dopa and PEA was steady and gradual over 20 h. The respective production of TRY and SER by both enzymes was negligible or undetectable (
Figure 6C).
3. Discussion
Metagenomic profiling of the human skin microbiome revealed that SadA homologs are widely distributed throughout almost the entire bacterial kingdom [
19]. In this study, skin swabs were collected from the antecubital fossa of 30 volunteers and the isolated bacterial strains were examined for their ability to produce NTs. We created a strain collection of 1909 pure cultures, ensuring the inclusion of a diverse range of colony morphologies, although the applied culturing method did not capture the full bacterial diversity observed in metagenomic studies [
39,
40,
41,
42,
43]. One possible explanation for the discrepancy between metagenomic and cultivation estimates of bacterial abundance on the skin [
44,
45] is that traditional metagenomic approaches and 16S rRNA sequencing do not differentiate between DNA from viable and non-viable bacteria, leading to an overestimation of both the richness and diversity of the skin microbiome [
46].
Out of the 1909 skin isolates, only 6.7% were identified as NT producers. All NT-producing isolates belonged to the genus
Staphylococcus, with
S. epidermidis being the most prevalent species which can be attributed to its high abundance on the skin compared to other staphylococcal species [
41,
42,
47]. Previous studies have shown that not all
Staphylococcus species produce NTs. Of the 44 species representatives tested, only 12 (27%) produced NTs [
18]. Additionally, current evidence indicates considerable diversity in NT-producing abilities among different strains of the same species. Therefore, the absence of NT-producers in bacterial genera other than
Staphylococcus does not necessarily indicate that they lack strains harboring AADC enzymes. Indeed, AADC activity has been reported in taxonomically distant species available in our strain collection, including
Bacillus cereus [
48],
B. atrophaeus [
16], and
Streptococcus thermophilus [
14].
NT-producing staphylococci were categorized into two production classes: class I which produces mainly TRY and PEA and only little TYM and DOPA, and class II which produces mainly TYM and Dopa and only little PEA (
Figure 2A,B). The class I product pattern was found to be the result of SadA activity, while class II was attributed to the activity of TDC.
The class II type (TDC enzyme) is underrepresented in our skin isolates; only 10 (7.8%) of the 128 NT-producing staphylococcal skin isolates showed this type (
Figure 2C,
Table S2). We found that the TDC-type is also underrepresented in the published staphylococcal genomes with a share of only 3.2% (
Table S5). The dataset only partially represents the staphylococci found on human skin, as many species such as
S. pseudintermedius,
S. delphinii, and
S. aureus do not inhabit this environment. If these three species are excluded, the dominance of SadA becomes significantly less pronounced: With 1031 SadA-encoding strains compared to 191 TDC-encoding strains, the latter account for around 16% of the retrieved strains. Our results suggest that TRY and PEA are likely to be dominant on the human skin. However, in a previous study, TYM was found to be very abundant on the human skin [
21]. This discrepancy suggests that with the applied growth conditions, we probably missed species carrying the
tdc-type gene, an aspect that will be investigated in future studies. On the other hand, in contrast to TYM and Dopa, the main products of TDC enzymes, the products of the SadA-type enzyme (TRY and PEA) can cross the blood-brain-barrier (
BBB) [
49]. Therefore, SadA-harboring staphylococci may have the advantage of better interacting with the central nervous system (
CNS) through the skin-brain axis.
Although all AADCs are PLP-dependent homodimeric enzymes [
33], SadA and TDC orthologues differ in size, phylogenetic relationship, and predicted 3D structure. While the SadA-type monomer is ~ 475 aa in length, the TDC-type comprises ~ 620 aa. The phylogenetic tree of TDC enzymes from various staphylococcal and non-staphylococcal species shows that these proteins are closely related and distinct from the SadA cluster (
Figure 4A). The separation of SadA orthologues from TDC enzymes in the phylogenetic tree is also reflected in the AlphaFold 2 structure predictions (
Figure 4B).
The genomic localization of
sadA has been analyzed in 15 staphylococcal species [
18]. There was neither a common insertion site, nor was the gene flanked by mobile elements, indicating a lack of horizontal transfer of
sadA. In contrast,
tdc is flanked by genes such as
nhaC,
tyrP, or
tyrS, that are also present in unrelated bacteria, including different staphylococcal species,
E. durans,
E. faecalis,
E. faecium,
E. hirae, E. mundtii, and
L. brevis (
Figure 3 and
Table 4) [
7,
8,
9,
10,
11,
13], suggesting that the
tdc gene cluster has spread by horizontal gene transfer. Whether these flanking genes form a functional unit, is not entirely understood. Besides their essential catalytic roles in protein biosynthesis, aminoacyl-tRNA synthetases like TyrS also contribute to other functions, such as the regulation of gene expression [
8]. For example, in enteric bacteria, the expression of histidine biosynthetic genes is regulated by histidyl-tRNA synthetase as a sensor for the intracellular histidine pool [
50]. The
tyrS gene in the
tdc operon was proposed to regulate decarboxylation in response to tyrosine concentration and extracellular pH [
8,
51]. Like TYM producing
E. faecalis V583 and
L. brevis ATCC 357 [
8],
S. epidermidis 102 has a related TyrS paralog with 54% identity to the TyrS in the
tdc operon, supporting the regulatory role of the protein encoded in the operon. The role of NhaC in the biosynthesis of TYM has yet to be determined [
52]. However, as part of the
tdc operon, it might also be involved in maintaining cytoplasmic pH homeostasis by uptaking Na+ in exchange for proton extrusion [
28]. The permease TyrP catalyzes electrogenic exchange of tyrosine and TYM [
53]. In all bacteria with a
tyrP-containing
tdc operon,
tdc and
tyrP are co-transcribed. It is assumed that amino acid decarboxylation coupled with amino acid/amine antiport generates proton motive force at the cell membrane [
53,
54]. The generation of metabolic energy due to the combined action of histidine decarboxylase and histidine/histamine antiporter has been reported in
Lactobacillus buchneri, suggesting an indirect proton pumping mechanism as means of producing metabolic energy in bacteria [
55]. Although we expressed only the
tdc gene without
tyrP from
S. epidermidis 102 in
E. coli, there was no difference in the product spectrum. Since
E. coli BL21 has a
tyrosine transporter (TyrP, GenBank accession no. ACT43729.1), the role of TyrP as a necessary TYM transporter remains unclear. TYM, PEA, and TRY have been shown to cross synthetic lipid bilayer membranes by simple diffusion [
56]. TYM can pass through the intestinal epithelial cells via passive diffusion but cannot cross the BBB, whereas PEA is able to cross the BBB [
2]. Further studies are, therefore, required to address the possible role of transporters in the release of TAs. This will help determine whether variations in NT production spectra among bacteria are due to differences in AADC enzyme specificity or the available transport systems.
The specificity of the SadA enzyme has already been studied, and as previously mentioned, the main products of SadA are TRY and PEA [
18]. In this study, the TDC enzymes of
S. epidermidis 102 and
E. faecalis ATCC 19433 were purified from
E. coli and tested for their substrate spectrum. Both enzymes showed the same substrate specificity: the highest conversion rate was observed with the substrates Y, followed by F, and then L-Dopa. Hardly any turnover was detected with W and 5-HTP, the precursor of serotonin. Bacterial TDC enzymes show different NT production spectra; while all show high affinity for Y, their affinities for other substrates vary. TDC orthologues from
L. brevis CGMCC 1.2028,
E. faecalis, and
E. faecium show 2-10 fold higher catalytic efficiency towards Y compared to L-Dopa [
5,
15,
57], whereas the TDC from
L. brevis IOEB 9809 cannot decarboxylate L-Dopa [
58]. Enterococcal TDCs can also produce PEA, albeit with a very low conversion rate and yield [
54,
59]. The TDC enzymes from
L. brevis strains (CGMCC 1.2028 and IOEB 9809) are, however, unable to produce PEA [
15,
58].
Comparison between
S. epidermidis 102 and its Δ
tdc mutant indicates that its TDC (
Figure 5A), similar to that of
E. faecalis, is capable of decarboxylating Y, L-Dopa, and phenylalanine. However, unexpectedly, the purified enzymes from both
S. epidermidis 102 and
E. faecalis exhibited lower activity towards L-Dopa compared to the parent strains or the corresponding
E. coli transformants. In this study, the enzymatic assays were conducted at pH 6.8, while the maximum activity of TDC enzymes occurs at lower pH levels. The purified TDC enzyme from
Lactobacillus brevis CGMCC 1.2028 (GenBank accession no. AFP73381.1) was shown to have the highest activity at pH 5.0, with activity decreasing at pH levels above 6.0 [
15]. A similar optimum pH range has been reported for TDC enzymes from
E. faecalis,
E. faecium, and
L. brevis IOEB 9809 [
5,
57,
58]. Since AADCs are cytoplasmic enzymes, their activity should be assessed at intracellular pH (pH
i) levels. In LABs, the intracellular pH can drop to as low as 5 in response to a decrease in extracellular pH [
28,
60,
61]. In contrast, staphylococci are able to maintain a more neutral pH
i in acidic environment [
62,
63,
64]. However, to thrive in acidic niches like the skin (pH 4.2 to 5.9) [
64], staphylococci may possess mechanisms that enhance AADC activity, as increasing decarboxylation activity is a bacterial strategy to overcome environmental acid stress [
7,
10,
26,
27,
28]. Moreover, in our experiments, the bacterial cells were incubated with 10% glucose. Glucose fermentation acidifies the cytoplasm, which can then induce tyrosine decarboxylation process to stabilize the internal pH [
28].
5. Materials and Methods
Materials. All media and chemicals were of either analytical or ultrapure grade from Merck Co. (Darmstadt, Germany) unless otherwise stated. Q5 High-Fidelity DNA polymerase and NEBuilder HiFi DNA Assembly Master Mix were from New England Biolabs Inc. (Beverly, MA, USA). Restriction enzymes were provided by Thermo Fisher Scientific (Waltham, MA, USA). Glass beads were purchased from Carl Roth GmbH + Co. (Karlsruhe, Germany).
Skin swab samples collection and cultivation. The skin swab samples were collected from 30 healthy volunteers aged between 22-76 years (15 Females and 15 Males). None of the subjects had a history of skin disease or had received any treatment within at least 3 months before this study. A pre-moistened cotton swab was used to sample a 100 cm
2 area of antecubital fossa. Swabs were then vortexed in PBS (pH 7.2), diluted up to 10,000-fold and the bacterial suspensions were inoculated onto agar media. Tryptic soy broth-agar (TSBA, TSB plus 15 g L
-1 agar) plates were used to isolate a wide variety of aerobic species and incubated for 2 days at 30
oC under aerobic conditions. To isolate LABs, the samples were inoculated onto De Man–Rogosa–Sharpe (MRS) and M17 plates and incubated at 37
oC under anaerobic conditions for 3 days [
66]. Furazolidone-Tween 80-Oil red O (FTO) agar plates, incubated at 37
oC under aerobic atmosphere with 5% CO
2, were used to isolate corynebacteria [
67]. Colonies with different morphologies from TSA, MRS, and M17 plates, along with orange-pink circular colonies from FTO plates, were subcultured into pure cultures using the same agar plates and under the same incubation conditions. The bacterial count in the sampled area was determined as log CFU/100 cm
2 using TSBA cultures.
Identification of NT producers. For primary screening, colonies from TSBA plates were inoculated into TSB and incubated at 37
oC under aerobic conditions for 2 days with shaking at 150 rpm. Corynebacteria isolates were cultivated in brain heart infusion broth containing 1% tween 80 under aerobic atmosphere with 5% CO
2 at 37
oC for 2 days. The supernatants were collected every 24 h and analyzed using reversed-phase HPLC (RP-HPLC) at room temperature with a Poroshell 120 EC-C18 HPLC column (Agilent; 4.6×150 mm, 2.7 µm). Linear gradient elution was performed at a flow rate of 1 mL/min, transitioning from 100% of 0.1% phosphoric acid to 100% of 50% acetonitrile over 15 min, with an injection volume of 10 µL. The diode array detector was set at 210 nm, with 360 nm used as the reference [
21]. Cell pellets from overnight cultures of producing isolates were washed with PBS (pH 7.2) and resuspended in 5xPBS (pH 7.2) supplemented with 10% glucose and 1 mg/mL of substrates (W, F, Y, and L-Dopa) at an OD of 50 [
18]. After overnight incubation under the same conditions, the supernatants were subjected to RP-HPLC analysis.
S. epidermidis O47 and
E. faecalis ATCC 19433 were used as positive controls. The NT peaks were confirmed by spiking the samples with NT’s standards and comparing retention times and UV spectra.
The producing isolates were then identified at the species level by amplifying a 1525 bp fragment of the 16S rRNA gene using the universal primers E8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and E1541R (5′-AAGGAGGTGATCCANCCRCA-3′) [
68]. Amplification was conducted in a 50 µL reaction mixture containing 10 µL of 5x Q5 reaction buffer, 0.2 mM dNTPs, 0.5 µM of each primer, 1 unit of Q5 DNA polymerase, and 50 to 100 ng of DNA template. The thermal cycling program consisted of an initial denaturation step of 30 s at 98°C, followed by 35 cycles of 10 s at 98°C, 30 s at 50°C, and 90 s at 72°C, with a final elongation at 72°C for 2 min. PCR products were sequenced at both ends at Eurofins Genomics (Ebersberg, Germany). The 16S rRNA sequences were then aligned against the EzTaxon database (
http://www.ezbiocloud.net/eztaxon) for species identification.
To gain an overview of the spectrum of bacteria isolated from the skin, a selection of non-producing isolates with varying morphologies was also identified.
Identification of sadA and tdc genes in NT producers. To detect
sadA gene in NT-producing isolates, three
sadA-specific primer sets were designed by aligning sequences from the NCBI database using Geneious Prime 2019.1.3 (Biomatters Limited, Auckland, New Zealand) (
Table S3).
DEC3 (5′-CCGCCAGCAGAATATGGAAYRTANCCCAT-3′) and DEC5 (5′-CGTTGTTGGTGTTGTTGGCACNACNGARGARG-3′) primers were used for detection of
tdc gene [
36].
The PCR conditions were identical to those used for the 16S rRNA PCR except that the elongation step was set to 24 s.
Construction of tdc deletion mutant. The temperature-sensitive shuttle vector pBASE6 was used to construct the
S. epidermidis 102 mutant lacking the
tdc gene (102Δ
tdc) [
18]. The 1-kb flanking regions of the
tdc gene were amplified from
S. epidermidis 102 genomic DNA with primer pairs KOS1F/KOS1R and KOS2F/KOS2R (
Table S7). The linearized plasmid (
EcoRV restriction site) was then ligated with the fragments using Hi-Fi DNA Assembly Master Mix. The constructed plasmid was first transformed into
E. coli DC10B and then into
S. epidermidis 102 by electroporation. Positive transformants were identified by colony PCR using the KOS3F/KOS3R primer pair. Mutagenesis was carried out by the method of Bae and Schneewind [
69] and mutants were confirmed by sequence analysis. The NT-production of the 102Δ
tdc strain was investigated as mentioned above.
Overexpression and purification of TDC. The
tdc genes from
S. epidermidis 102 and
E. faecalis ATCC 19433 were amplified with primer pairs 78S/79S and 32S/33S, respectively (
Table S7). The amplicons were then ligated into a
BmtI and
NotI digested pET28a using Hi-Fi DNA Assembly Master Mix. The pET28a plasmids encoding N-terminal His-tagged
tdc genes were first transformed into
E. coli DC10B and then into
E. coli BL21 expression strain. The transformants were confirmed by colony PCR using primer sets 81S/82S and 42S/44S and sequencing.
For expression, overnight cultures of recombinant BL21 strains were diluted in fresh LB medium supplemented with kanamycin (30 µg/mL) to an OD
600 of 0.1. The cultures were grown to the exponential phase (OD
600 of 0.6) and induced with 0.2 mM Isopropyl-
β-D-1-thiogalactopyranoside (IPTG) at 15
oC for 16-18 h. The cells were then collected, resuspended in lysis buffer (300 mM NaCl, 10 mM imidazole, 50 mM KPO
4, protease inhibitor mix; pH 7.5), mixed with a mixture of 0.1- and 1-mm glass beads (1:1), and lysed using a Precellys
® Evolution homogenizer (Bertin Technologies). The lysate was cleared by centrifugation at 40000×g at 4
oC for 1 h. The 6×His tagged proteins were purified using a nickel-nitrilotriacetic acid (Ni-NTA) agarose matrix (Qiagen, Hilden, Germany) as described by van Kessel et al. [
70]. Protein concentrations were measured using a NanoPhotometer
® NP80 spectrophotometer (Implen, Munich, Germany), with the predicted extinction coefficient and molecular weight from ExPASy ProtParam tool (
www.web.expasy.org/protparam/). The purified proteins were confirmed by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
In vitro enzymatic assay. The catalytic activity of the purified enzymes towards W, F, Y, L-DOPA, or 5-HTP was determined in a reaction mixture of 100 µL containing 50 mM sodium phosphate (pH 6.8), 300 mM NaCl, 40 µM PLP, 150 nM enzyme, and 2 mM substrate. The reactions were quenched at different time intervals with methanol or ethyl acetate and analyzed by RP-HPLC [
18].
In silico analyses. A BLASTp search of the NCBI NR protein sequence database was conducted on the 15th of May 2023 to identify homologues of the SadA enzymes from S. epidermidis O47 (GenBank accession no. QKN61770.1) and S. schleiferi NCTC 12218 (GenBank accession no. CAD7359406.1), as well as the TDC enzyme from E. faecalis JH2-2 (GenBank accession no. AAM46082), within the genus Staphylococcus. Alignments with a minimum identity of 50% over at least 90% of the protein length were considered putative homologues.
Genome sequencing of
S. epidermidis 102 was conducted using Illumina shotgun sequencing at the Göttingen Genomics Laboratory. DNA libraries were prepared using the Nextera XT DNA sample preparation kit and then sequenced on a MiSeq platform (Illumina, San Diego, CA, USA) utilizing the v3 reagent kit with 600 cycles. Raw reads were trimmed using Trimmomatic version 0.39. The assembly of reads into contigs was performed using the SPAdes genome assembler software (version 3.15.2). The coverage and quality of the assembled genome were estimated using Qualimap version 2.2.1. The draft genome sequence was deposited in GenBank under the accession number JBIENV000000000. Gene prediction and annotation were performed with RAST [
71].
A maximum likelihood phylogenetic tree of AADC enzymes (SadA and TDC) was constructed using the MEGA_11.0.13 package with 1000 bootstrap replicates. The 3D structures of the enzymes were predicted using AlphaFold 2 and subsequently aligned using the PyMOL alignment command (version 3.0.5).
Statistical analysis. Data were analyzed using IBM SPSS Statistics (version 29.0.2; IBM Corp., Armonk, NY). The CFU count comparison between samples derived from men and women was performed using an independent-samples t-test. Pearson’s correlation coefficient was calculated to assess the correlation between age and CFU counts. The level of significance was set at 0.05.
Figure 1.
Total bacterial count in the antecubital fossa area from 30 healthy subjects.
Figure 1.
Total bacterial count in the antecubital fossa area from 30 healthy subjects.
Figure 2.
Representative RP-HPLC chromatograms of neurotransmitter production by skin isolates with two different patterns in 5xPBS buffer/10% glucose and all the substrates (1 mg/mL each): A) S. epidermidis 93 produces primarily TRY and PEA, with almost no TYM and Dopa; B) S. capitis 413 produces mainly TYM and Dopa, with little PEA and no TRY; C) Species distribution of neurotransmitter producing skin isolates (n=128) with two production patterns: (I) production of TRY and PEA as main products, with TYM and Dopa at lower levels; (II) production of TYM and Dopa as main products, with PEA at lower levels. 5xPBS buffer/10% glucose supplemented with substrates (1 mg/mL each) was used as the standard. W, tryptophan; F, phenylalanine; Y, tyrosine; TRY, tryptamine; PEA, phenethylamine; TYM, tyramine; Dopa, dopamine.
Figure 2.
Representative RP-HPLC chromatograms of neurotransmitter production by skin isolates with two different patterns in 5xPBS buffer/10% glucose and all the substrates (1 mg/mL each): A) S. epidermidis 93 produces primarily TRY and PEA, with almost no TYM and Dopa; B) S. capitis 413 produces mainly TYM and Dopa, with little PEA and no TRY; C) Species distribution of neurotransmitter producing skin isolates (n=128) with two production patterns: (I) production of TRY and PEA as main products, with TYM and Dopa at lower levels; (II) production of TYM and Dopa as main products, with PEA at lower levels. 5xPBS buffer/10% glucose supplemented with substrates (1 mg/mL each) was used as the standard. W, tryptophan; F, phenylalanine; Y, tyrosine; TRY, tryptamine; PEA, phenethylamine; TYM, tyramine; Dopa, dopamine.
Figure 3.
Genetic organization of the tyrosine decarboxylase operons in S. epidermidis 102 (this study), S. caprae NCTC 12196 (GenBank accession no. AF446085.5), Levilactobacillus brevis IOEB (GenBank accession no. AF446085.5), and E. mundtii QU 25 (GenBank accession no. AP013036.1) in comparison with sadA gene location in S. epidermidis O47 (GenBank accession no. CP040883.1). Putative promoters are represented by broken arrows and transcription terminator regions by lollipops. The surrounding genes are shown in gray. sadA, staphylococcal aromatic amino acid decarboxylase; tdc, tyrosine decarboxylase; tyrP, tyrosine-tyramine permease; nhaC, Na+/H+ antiporter; tyrS, tyrosyl-tRNA synthetase.
Figure 3.
Genetic organization of the tyrosine decarboxylase operons in S. epidermidis 102 (this study), S. caprae NCTC 12196 (GenBank accession no. AF446085.5), Levilactobacillus brevis IOEB (GenBank accession no. AF446085.5), and E. mundtii QU 25 (GenBank accession no. AP013036.1) in comparison with sadA gene location in S. epidermidis O47 (GenBank accession no. CP040883.1). Putative promoters are represented by broken arrows and transcription terminator regions by lollipops. The surrounding genes are shown in gray. sadA, staphylococcal aromatic amino acid decarboxylase; tdc, tyrosine decarboxylase; tyrP, tyrosine-tyramine permease; nhaC, Na+/H+ antiporter; tyrS, tyrosyl-tRNA synthetase.
Figure 4.
A) Maximum likelihood dendrogram of TDC enzymes from S. epidermidis 102 (this study), S. aureus (GenBank accession no. HDA2154217.1), S. capitis (GenBank accession no. WVQ24098.1), S. caprae NCTC 12196 (GenBank accession no. AF446085.5), S. haemolyticus (GenBank accession no. MBW5904582.1), S. pasteuri (NCBI Reference Sequence WP_154840952.1), Enterococcus durans IPLA 655 (GenBank accession no. CAF33980), E. faecalis JH2-2 (GenBank accession no. AAM46082), E. hirae 508 (GenBank accession no. AAQ73505.1), E. mundtii QU 25 (GenBank accession no. BAO05941.1), Levilactobacillus brevis IOEB (GenBank accession no. AAN77279), and Streptococcus thermophilus 1TT45 (GenBank accession no. CBW46640.1) strains and of SadA enzymes from S. aureus (GenBank accession no. WP_032605060.1), S. capitis Sc1516941 (GenBank accession no. WP_032605060.1), S. caprae N99 (GenBank accession no. MBU5272604.1), S. carnosus NCTC 13825 (GenBank accession no. SUL89052.1), S. coagulans DSM 6628 (GenBank accession no. PNZ10338.1), S. condimenti NCTC 13827 (GenBank accession no. VEG62997.1), S. cornubiensis NW1 (GenBank accession no. WP_086428678.1), S. debuckii SDB 2975 (GenBank accession no. AYU54119.1), S. delphini NCTC 12225 (GenBank accession no. VED61613.1), S. epidermidis O47 (GenBank accession no. QKN61770.1), S. haemolyticus acroh (GenBank accession no. MCI2935196.1), S. hominis SDD3 (GenBank accession no. TRM05964.1), S. intermedius NCTC 11048 (GenBank accession no. SUM45602.1), S. lugdunensis (GenBank accession no. MDU3709123.1), S. lutrae ATCC 700373 (GenBank accession no. ARJ50788.1), S. petrasii P5404 (GenBank accession no. TGE14952.1), S. piscifermentans NCTC 13836 (GenBank accession no. SNU95601.1), S. pragensis CCM 8529 (GenBank accession no. GGG93612.1), S. pseudintermedius ED99 (GenBank accession no. WP_014612792.1), S. schleiferi NCTC 12218 (GenBank accession no. CAD7359406.1), S. simulans G12 (GenBank accession no. MCD8915340.1), and S. ureilyticus 498-2 (GenBank accession no. MEX6179459.1). The tree was generated using the MEGA_11.0.13 package with 1000 bootstrap replicates. Bootstrap values greater than 60% are listed as percentages at the nodes, and the scale bar represents genetic distance; B) The 3D structure of TDC enzymes from S. epidermidis 102 (this study), S. caprae NCTC 12196 (GenBank accession no. AF446085.5), Enterococcus durans IPLA 655 (GenBank accession no. CAF33980), E. faecalis JH2-2 (GenBank accession no. AAM46082), E. hirae 508 (GenBank accession no. AAQ73505.1), E. mundtii QU 25 (GenBank accession no. BAO05941.1), Levilactobacillus brevis IOEB (GenBank accession no. AAN77279), and Streptococcus thermophilus 1TT45 (GenBank accession no. CBW46640.1) strains in comparison with SadA enzymes from S. epidermidis O47 (GenBank accession no. QKN61770.1), S. pseudintermedius ED99 (GenBank accession no. WP_014612792.1), and S. schleiferi NCTC 12218 (GenBank accession no. CAD7359406.1) using AlphaFold 2.0. The TDC enzyme from Lactiplantibacillus plantarum IR BL0076 (GenBank accession no. AFQ52525.1) was not included in these analyses due to its 100% identity with that of L. brevis IOEB; C) The alignment of 3D structures of TDC enzyme from S. epidermidis 102 (cyan) and SadA enzyme from S. epidermidis O47 (GenBank accession no. QKN61770.1; magenta) using the PyMOL software (version 3.0.5). The enzymes showed low structural similarity, with a root mean square deviation (RMSD) value of 4.410 Å.
Figure 4.
A) Maximum likelihood dendrogram of TDC enzymes from S. epidermidis 102 (this study), S. aureus (GenBank accession no. HDA2154217.1), S. capitis (GenBank accession no. WVQ24098.1), S. caprae NCTC 12196 (GenBank accession no. AF446085.5), S. haemolyticus (GenBank accession no. MBW5904582.1), S. pasteuri (NCBI Reference Sequence WP_154840952.1), Enterococcus durans IPLA 655 (GenBank accession no. CAF33980), E. faecalis JH2-2 (GenBank accession no. AAM46082), E. hirae 508 (GenBank accession no. AAQ73505.1), E. mundtii QU 25 (GenBank accession no. BAO05941.1), Levilactobacillus brevis IOEB (GenBank accession no. AAN77279), and Streptococcus thermophilus 1TT45 (GenBank accession no. CBW46640.1) strains and of SadA enzymes from S. aureus (GenBank accession no. WP_032605060.1), S. capitis Sc1516941 (GenBank accession no. WP_032605060.1), S. caprae N99 (GenBank accession no. MBU5272604.1), S. carnosus NCTC 13825 (GenBank accession no. SUL89052.1), S. coagulans DSM 6628 (GenBank accession no. PNZ10338.1), S. condimenti NCTC 13827 (GenBank accession no. VEG62997.1), S. cornubiensis NW1 (GenBank accession no. WP_086428678.1), S. debuckii SDB 2975 (GenBank accession no. AYU54119.1), S. delphini NCTC 12225 (GenBank accession no. VED61613.1), S. epidermidis O47 (GenBank accession no. QKN61770.1), S. haemolyticus acroh (GenBank accession no. MCI2935196.1), S. hominis SDD3 (GenBank accession no. TRM05964.1), S. intermedius NCTC 11048 (GenBank accession no. SUM45602.1), S. lugdunensis (GenBank accession no. MDU3709123.1), S. lutrae ATCC 700373 (GenBank accession no. ARJ50788.1), S. petrasii P5404 (GenBank accession no. TGE14952.1), S. piscifermentans NCTC 13836 (GenBank accession no. SNU95601.1), S. pragensis CCM 8529 (GenBank accession no. GGG93612.1), S. pseudintermedius ED99 (GenBank accession no. WP_014612792.1), S. schleiferi NCTC 12218 (GenBank accession no. CAD7359406.1), S. simulans G12 (GenBank accession no. MCD8915340.1), and S. ureilyticus 498-2 (GenBank accession no. MEX6179459.1). The tree was generated using the MEGA_11.0.13 package with 1000 bootstrap replicates. Bootstrap values greater than 60% are listed as percentages at the nodes, and the scale bar represents genetic distance; B) The 3D structure of TDC enzymes from S. epidermidis 102 (this study), S. caprae NCTC 12196 (GenBank accession no. AF446085.5), Enterococcus durans IPLA 655 (GenBank accession no. CAF33980), E. faecalis JH2-2 (GenBank accession no. AAM46082), E. hirae 508 (GenBank accession no. AAQ73505.1), E. mundtii QU 25 (GenBank accession no. BAO05941.1), Levilactobacillus brevis IOEB (GenBank accession no. AAN77279), and Streptococcus thermophilus 1TT45 (GenBank accession no. CBW46640.1) strains in comparison with SadA enzymes from S. epidermidis O47 (GenBank accession no. QKN61770.1), S. pseudintermedius ED99 (GenBank accession no. WP_014612792.1), and S. schleiferi NCTC 12218 (GenBank accession no. CAD7359406.1) using AlphaFold 2.0. The TDC enzyme from Lactiplantibacillus plantarum IR BL0076 (GenBank accession no. AFQ52525.1) was not included in these analyses due to its 100% identity with that of L. brevis IOEB; C) The alignment of 3D structures of TDC enzyme from S. epidermidis 102 (cyan) and SadA enzyme from S. epidermidis O47 (GenBank accession no. QKN61770.1; magenta) using the PyMOL software (version 3.0.5). The enzymes showed low structural similarity, with a root mean square deviation (RMSD) value of 4.410 Å.
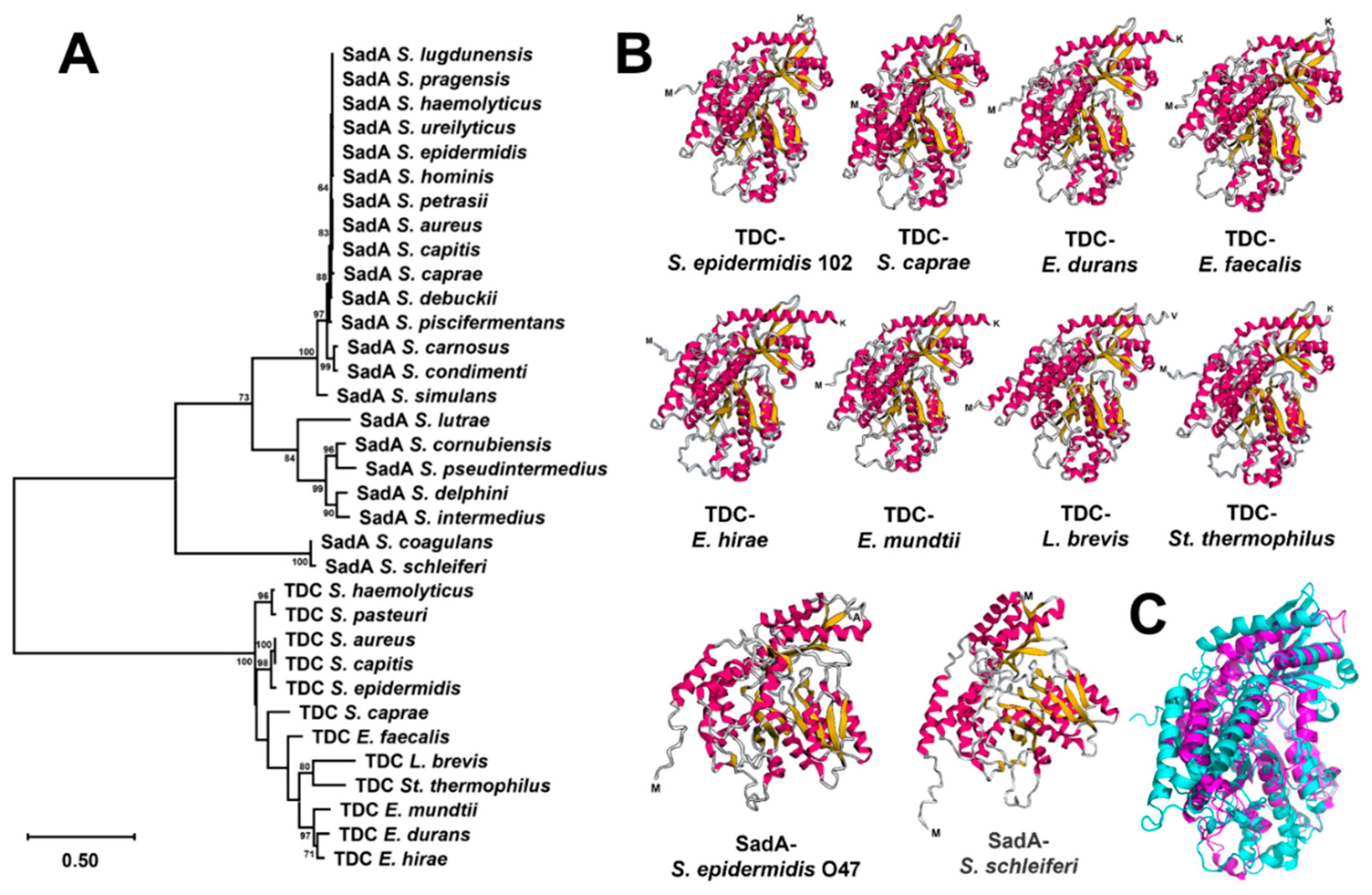
Figure 5.
RP-HPLC chromatograms of neurotransmitter production by: A) S. epidermidis 102 and its tdc deletion mutant (102Δtdc) after overnight incubation in 5xPBS buffer/10% glucose containing individual or a combination of substrates (1 mg/mL each); B) E. coli BL21 transformants harboring tdc gene from S. epidermidis 102 in the vector pET28a after overnight incubation in 5xPBS buffer/10% glucose containing individual substrates (1 mg/mL each) in comparison with transformants harboring empty plasmid. 5-HTP, 5-hydroxytryptophan; W, tryptophan; F, phenylalanine; Y, tyrosine; TRY, tryptamine; PEA, phenethylamine; Dopa, dopamine; TYM, tyramine.
Figure 5.
RP-HPLC chromatograms of neurotransmitter production by: A) S. epidermidis 102 and its tdc deletion mutant (102Δtdc) after overnight incubation in 5xPBS buffer/10% glucose containing individual or a combination of substrates (1 mg/mL each); B) E. coli BL21 transformants harboring tdc gene from S. epidermidis 102 in the vector pET28a after overnight incubation in 5xPBS buffer/10% glucose containing individual substrates (1 mg/mL each) in comparison with transformants harboring empty plasmid. 5-HTP, 5-hydroxytryptophan; W, tryptophan; F, phenylalanine; Y, tyrosine; TRY, tryptamine; PEA, phenethylamine; Dopa, dopamine; TYM, tyramine.
Figure 6.
Product pattern with purified TDC enzymes from a Staphylococcus and Enterococcus strain. A) Section of the pET28a vector with tdc gene from S. epidermidis 102 or Enterococcus faecalis ATCC 19433, expressed with N-terminal His-tag in E. coli BL21; B) SDS–PAGE analysis of purified TDC enzymes from the E. coli clones: M, molecular weight marker; Lane 1, E. faecalis ATCC 19433 (GenBank accession no. WP_002355450.1); Lane 2, S. epidermidis 102; C) RP-HPLC chromatograms of tyramine (TYM), dopamine (Dopa), phenethylamine (PEA), tryptamine (TRY), and serotonin (SER) production by N-terminal His-tagged TDCs of S. epidermidis 102 and E. faecalis ATCC 19433 cloned into pET28a and purified from E. coli BL21 transformants in 50 mM sodium phosphate/300 mM NaCl buffer (pH 6.8) containing 150 nM enzyme, 40 µM pyridoxal phosphate (PLP), and 2 mM substrate at 37°C. 5-HTP, 5-hydroxytryptophan; W, tryptophan; F, phenylalanine; Y, tyrosine.
Figure 6.
Product pattern with purified TDC enzymes from a Staphylococcus and Enterococcus strain. A) Section of the pET28a vector with tdc gene from S. epidermidis 102 or Enterococcus faecalis ATCC 19433, expressed with N-terminal His-tag in E. coli BL21; B) SDS–PAGE analysis of purified TDC enzymes from the E. coli clones: M, molecular weight marker; Lane 1, E. faecalis ATCC 19433 (GenBank accession no. WP_002355450.1); Lane 2, S. epidermidis 102; C) RP-HPLC chromatograms of tyramine (TYM), dopamine (Dopa), phenethylamine (PEA), tryptamine (TRY), and serotonin (SER) production by N-terminal His-tagged TDCs of S. epidermidis 102 and E. faecalis ATCC 19433 cloned into pET28a and purified from E. coli BL21 transformants in 50 mM sodium phosphate/300 mM NaCl buffer (pH 6.8) containing 150 nM enzyme, 40 µM pyridoxal phosphate (PLP), and 2 mM substrate at 37°C. 5-HTP, 5-hydroxytryptophan; W, tryptophan; F, phenylalanine; Y, tyrosine.
Table 1.
Spectrum of Gram (+) bacteria isolated from the antecubital fossa skin of 30 healthy subjects.
Table 1.
Spectrum of Gram (+) bacteria isolated from the antecubital fossa skin of 30 healthy subjects.
| Bacillus |
Staphylococcus |
Corynebacterium |
| B. agri |
S. capitis |
C. bouchesdurhonense |
| B. albus |
S. caprae |
C. curieae |
| B. altitudinis |
S. coagulans |
C. gottingense |
| B. atrophaeus |
S. epidermidis |
C. kefirresidentii |
| B. canaveralius |
S. haemolyticus |
C. meitnerae |
| B. cereus |
S. hominis |
C. mucifaciens |
| B. haynesii |
S. petrasil |
C. parakroppenstedtii |
| B. licheniformis |
S. pragensis |
C. pilbarense |
| B. mobilis |
S. saccharolyticus |
C. tuberculostearicum |
| B. paramycoides |
S. saprophyticus |
C. ureicelerivorans |
| B. siamensis |
|
|
| B. tequilensis |
Cutibacterium |
Peribacillus |
| B. tyonensis |
Cut. acnes |
P. butanolivorans |
| B. velezensis |
Cut. avidum |
P. frigoritolerans |
| B. wiedmannii |
|
P. simplex |
| |
|
|
| Kocuria |
Nialia |
Roseomonas |
| K. arsenatis |
N. circulans |
R. mucosa |
| |
|
|
| Micrococcus |
Paeniibacillus |
Streptococcus |
| M. endophyticus |
P. etheri |
St. anginosus |
| M. luteus |
|
St. thermophilus |
Table 2.
Spectrum of trace amine production in 128 skin isolates from healthy subjects after overnight incubation in TSB.
Table 2.
Spectrum of trace amine production in 128 skin isolates from healthy subjects after overnight incubation in TSB.
| Production pattern |
Species |
No. of isolates |
| TRY+PEA+TYM |
S. capitis |
1 |
| S. coagulans |
8 |
| S. epidermidis |
75 |
| S. hominis |
4 |
| S. saccharolyticus |
11 |
| Total |
99 |
| TRY + PEA |
S. capitis |
2 |
| S. caprae |
1 |
| S. epidermidis |
16 |
| Total |
19 |
| PEA + TYM |
S. epidermidis |
3 |
| S. haemolyticus |
1 |
| S. pragensis |
1 |
| Total |
5 |
| TYM |
S. capitis |
4 |
| |
S. epidermidis |
1 |
| |
Total |
5 |
Table 3.
Distribution of SadA and TDC homologues in available sequence data from Staphylococcus spp.
Table 3.
Distribution of SadA and TDC homologues in available sequence data from Staphylococcus spp.
| Enzyme |
Species |
| S. aureus |
S. capitis |
S. caprae |
S. carnosus |
S. coagulans |
S. condimenti |
S. cornubiensis |
S. debuckii |
S. delphini |
S. epidermidis |
S. haemolyticus |
S. hominis |
S. intermedius |
S. lugdunensis |
S. lutrae |
S. pasteuri |
S. petrasii |
S. piscifermentans |
S. pragensis |
S. pseudintermedius |
S. schleiferi |
S. simulans |
S. ureilyticus |
Staphylococcus sp.* |
Total |
| SadA |
676 |
1 |
3 |
14 |
63 |
9 |
1 |
1 |
42 |
798 |
17 |
14 |
5 |
2 |
1 |
ND |
1 |
1 |
1 |
4188 |
55 |
2 |
3 |
24 |
5922 |
| TDC |
1 |
21 |
30 |
ND |
ND |
ND |
ND |
ND |
ND |
125 |
14 |
ND |
ND |
ND |
ND |
1 |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
6 |
198 |
Table 4.
BLASTp sequence homology analyses of tdc operons from Staphylococcus epidermidis 102 and Levilactobacillus brevis IOEB.
Table 4.
BLASTp sequence homology analyses of tdc operons from Staphylococcus epidermidis 102 and Levilactobacillus brevis IOEB.
| tdc operon |
Blastp |
| S. epidermidis 102 |
|
L. brevis IOEB |
|
| Protein |
Length (aa) |
GenBank accession No. |
Length (aa) |
Coverage (%) |
E value |
Identity (%) |
| Tyrosyl-tRNA synthetase (TyrS) |
417 |
AAQ83557.1 |
418 |
99 |
0.0 |
69 |
| Tyrosine decarboxylase (TDC) |
616 |
AAN77279.2 |
635 |
95 |
0.0 |
71 |
| Tyrosine-tyramine permease (TyrP) |
479 |
AAQ83558.1 |
473 |
99 |
0.0 |
66 |
| Na+/H+ antiporter (NhaC) |
461 |
AAQ83559.1 |
476 |
96 |
2e-142 |
55 |