1. Introduction
The use of Cannabis sativa in veterinary medicine has garnered increasing interest in recent years, mainly due to its potential therapeutic effects on various animal conditions. The endocannabinoid system, discovered in the late 1980s, has been identified as a significant target for treatment in both human and veterinary medicine, leading to a resurgence in research regarding the medicinal properties of cannabis (Holst, 2024). Cannabis sativa, commonly known for its psychoactive properties, contains a variety of cannabinoids, including cannabidiol (CBD), which has been shown to exhibit anti-inflammatory, analgesic, and anxiolytic effects in animals (NAWROT & SOROKO-DUBROVINA, 2022). Research indicates that CBD can effectively reduce pain and inflammation in horses, suggesting its potential applicability in other veterinary contexts (NAWROT & SOROKO-DUBROVINA, 2022). Moreover, studies have demonstrated that dogs can tolerate oral doses of CBD without significant adverse effects, indicating its safety in veterinary applications (Akinola et al., 2018). This is particularly relevant given the increasing reports from dog owners regarding the perceived benefits of unlicensed cannabis products for managing conditions such as anxiety and chronic pain in their pets (Holst, 2024; Banach, 2023). The pharmacological effects of Cannabis sativa are not solely attributed to cannabinoids; terpenes present in the plant also play a crucial role in enhancing its therapeutic effects. For instance, specific terpenes have been shown to interact with cannabinoid receptors, potentially amplifying the analgesic properties of cannabinoids (LaVigne et al., 2021). This synergistic effect between cannabinoids and terpenes underscores the complexity of Cannabis sativa's pharmacology and its potential for diverse veterinary applications. Furthermore, the antimicrobial properties of Cannabis sativa have been documented, with studies revealing that extracts from the plant exhibit antibacterial activity against various pathogenic strains ("In vitro antibacterial activity of Cannabis sativa leaf extracts to some selective pathogenic bacterial strains," 2014). This characteristic could be beneficial in treating infections in animals, further expanding the therapeutic scope of cannabis in veterinary medicine. Despite the promising findings, the legal and regulatory landscape surrounding the use of Cannabis sativa in veterinary medicine remains complex and varies significantly across different regions. In some countries, the use of cannabis for veterinary purposes is still prohibited, which poses challenges for veterinarians and pet owners seeking alternative treatments (Banach, 2023). The urgent need for accuracy and consistency in the products derived from Cannabis sativa for veterinary uses is a critical concern encompassing various aspects of product formulation, dosing, quality control, and regulatory compliance. As the interest in utilizing cannabinoids, particularly cannabidiol (CBD), in veterinary medicine grows, ensuring that the products available to veterinarians and pet owners are safe, effective, and reliable is imperative. The formulation of cannabis-derived products for veterinary use must adhere to strict standards to ensure consistency in cannabinoid concentrations and the presence of other active compounds. Variability in the concentration of cannabinoids, terpenes, and other phytochemicals can lead to inconsistent therapeutic effects and potential adverse reactions in animals. For instance, a study by McGrath et al. (2021) highlighted the discrepancies in cannabinoid content across various commercially available CBD products, emphasizing the need for standardized formulations to ensure that veterinarians can confidently prescribe these products (Holst, 2024).
Quality control measures are essential to ascertain the purity and potency of cannabis-derived products. Third-party testing for contaminants, such as heavy metals, pesticides, and microbial pathogens, is crucial to ensure the safety of these products for animal consumption. The American Veterinary Medical Association (AVMA) has recommended that veterinarians only use products undergoing rigorous testing and quality assurance processes (NAWROT & SOROKO-DUBROVINA, 2022). This is particularly important given the potential for harmful substances in unregulated products. Accurate dosing is vital for the effective use of cannabis products in veterinary medicine. The lack of established dosing guidelines for different species and conditions can lead to underdosing or overdosing, both of which can compromise the therapeutic outcomes. Research by Kogan et al. (2020) suggests that veterinarians should rely on evidence-based dosing protocols and adjust dosages based on the individual animal's response and condition (Akinola et al., 2018). Furthermore, developing clear dosing guidelines will enhance the consistency of treatment outcomes across different veterinary practices. The regulatory landscape surrounding cannabis products for veterinary use is complex and varies by jurisdiction. In many regions, cannabis remains a controlled substance, and the legal framework for its use in animals is still evolving. The U.S. Food and Drug Administration (FDA) has not yet approved any cannabis-derived products for veterinary use, which raises concerns about the legality and safety of these products (Banach, 2023). As such, manufacturers must comply with existing regulations, and veterinarians must stay informed about the legal status of cannabis products in their respective areas. Veterinarians must be adequately educated about the use of cannabis products in their practice. This includes understanding the pharmacology of cannabinoids, potential interactions with other medications, and the legal implications of prescribing such products. Continuing education programs and resources provided by veterinary associations can help practitioners stay informed about the latest research and best practices in cannabis use for veterinary purposes (LaVigne et al., 2021). The urgent need for accuracy and consistency in cannabis-derived products for veterinary use cannot be overstated. Ensuring standardized formulations, rigorous quality control, clear dosing guidelines, regulatory compliance, and ongoing education for veterinarians is essential for safe and effective use of these products. As research continues to elucidate the therapeutic potential of Cannabis sativa in veterinary medicine, addressing these concerns will be crucial for fostering trust and promoting the responsible use of cannabis in animal health care. The determination of accuracy and consistency in cannabis-derived products for veterinary use is paramount, especially as the demand for these products increases among pet owners and veterinarians. The validation of an accurate analytical method for analyzing cannabinoids in commercially available products intended for veterinary use is essential for ensuring product safety and efficacy. High-Performance Liquid Chromatography (HPLC) is a widely accepted technique for this purpose, allowing for the precise quantification of cannabinoids such as cannabidiol (CBD) and tetrahydrocannabinol (THC). This work aims to establish a validated HPLC method and compare the analytical results with the declared cannabinoid concentrations on product labels.
2. Materials and Methods
2.1. Chemical and Reagents
Distilled water (LC Grade), Methanol (LC Grade), and Acetonitrile (LC Grade) were purchased from Merck KGaA, Germany; certified reference material, which contains 12 components of phytocannabinoid Mixture 10 (CRM) Cayman, was purchased from Chemical Co. 1180 E. Ellsworth Rd. Ann Arbor, MI 48108, USA.
2.2. HPLC-Analysis
The analysis was conducted using the Agilent 1260 Infinity II with binary pump (BP), which comprises two pumps responsible for the flow of the mobile phase through the instrument at a maximum working pressure of 600 bar. Chromatographic separation was performed using an Agilent column (Poroshell 120 EC-18, 150x30mm, 2.7µm). The column temperature is 30°C, and the autosampler temperature is ambient. The separation is isocratic, with a flow rate of 0.6 ml/min, with two mobile phases: mobile phase A 25%: water (LC-MS purity) with 0.1% formic acid and mobile phase B 75%: acetonitrile with 0.1% formic acid (Table 7). The injection volume is ten μl. This ensured the elution of all cannabinoids with baseline separation in as little as twelve minutes. The last cannabinoid, THCA, was eluted at approximately 11.28 minutes (see Figure 1).
2.3. Sample Preparation
With a volumetric automatic pipette, carefully was taken 100 µL of the homogeneously mixed Cannabis oil. The pipette is carefully wiped from the outside without touching the tip of the pipette, and the measured oil is transferred to a 10 mL volumetric flask. The flask is filled halfway with methanol, closed, and placed in an ultrasonic bath. The bath is set to a temperature of 30°C, a time of 5 minutes, the sweep option is selected, and the start button is clicked. After completion, it is checked whether the oil is completely dissolved and the flask is topped up to the mark. Then, the solution is stirred and filtered through a 0.2 µm RC filter in a glass flask. 100 µL is taken from the filtrate and transferred to a vial, supplemented with 900 µL of methanol. The vial is vortexed, capped, stirred, and placed on the vial rack of an Agilent 1260 HPLC. The final sample for analysis in the vial has a dilution factor of 1000.
To create a calibration curve, we prepared eight standards with different concentrations. These standards were made from the first standard, Std stock, with a concentration of approximately 100 µg/mL. The calibration curve is created for each cannabinoid separately, so we made eight solutions of a standard with specific concentrations ranging from approximately 0.1 µg/mL to 85 µg/mL. After preparing the standards, we need to calculate the exact concentration of each cannabinoid in the solutions, considering the potency declared in the quality certificate of the CRM used. For example, if we take 100 µL from a CBD CRM with a potency of 99.42%, the final solution will have a 99.42 µg/mL concentration. The calculated concentrations for each cannabinoid are then inserted into the calibration table for each level. A crucial requirement for constructing a precise and accurate calibration curve is that the correlation factor must be greater than or equal to 0.999 (R2≥0.999) for each cannabinoid. We verify the calibration curve through the System Suitability Standard, which must have a concentration of approximately 25 µg/mL as read from the calibration curve. If a significantly higher or lower value is obtained, then it is mandatory to construct a new calibration curve with newly prepared standards.
3. Results
The method for determining the cannabinoid content, described in the German Pharmacopoeia, used to analyze cannabinoids in different types of cannabis was introduced and validated according to the ICH guidelines. This research focused on developing a new analytical method for determining CBD in hemp oil products and their compliance with the declared value.
Table 1,
Table 2 and
Table 3 show the method validation parameters: system suitability, linearity, specificity, accuracy and precision (repeatability and mean precision), detection limit, and quantification.
3.1. Linearity
The linearity of a method is its ability to produce test results that are directly proportional to the concentration or amount of sample within a given range. The relationship between the detector response (peak area) and the sample concentration is used for HPLC methods to determine linearity. The linear representation of this dependence function implies drawing a regression line with the lowest possible residual value for the selected concentration levels. Values on the x-axis (concentration/amount) are considered values with a tiny error justified by the low variability in the preparation of analytical standards about the response of the analytical method represented on the y-axis (area of chromatographic peaks). All standards are prepared by diluting directly from the first standard, Std stock with C≈100µg/mL. The calibration curve is constructed for each cannabinoid component separately. For this purpose, it is necessary to prepare eight standard solutions containing all 12 cannabinoids, with 0.1-85 µg/mL concentrations. The method was found to be linear for all eight different concentrations. Correlation coefficients (R2) for each cannabinoid individually were close to 1, showing good linear correlation (R2 >0.999), as shown in
Table 1.
3.2. Limit of Detection (LOD) and Limit of Quantification (LOQ)
The limit of detection is the smallest amount in the test sample that can be reliably detected using the appropriate method. The detection limit is determined according to the formula:
LOD =3N/s
The ratio between the noise level (N) and the peak height of the cannabinoid is determined from the chromatogram itself, where the peak signal from the cannabinoid should be three times the noise signal (where s is the standard deviation of the regression line and is the slope coefficient of the calibration curve).
The limit of quantification is usually taken to be ten times the signal-to-noise ratio:
LOQ =10N/s
It should also be emphasized that the quoted LOD and LOQ values do not consider the possibility of achieving lower detection limits by over-concentration of the obtained extracts. The limit of detection (LOD) and limit of quantification (LOQ) for each cannabinoid was calculated using the mean, standard deviation, and slope from the regression analysis (
Table 2). The obtained values are comparable to the results of similar methods described in the literature, confirming the suitability and sensitivity of the technique.
3.3. Accuracy
The accuracy of the analytical method is defined as the match between the mean value obtained during the experiment and the accepted reference value. Determining this parameter allows for estimating the effect of the systematic error of the method on the final result. The analytical yield expresses the accuracy when no certified reference material exists. The analytical yield is obtained when enriching negative samples with a standard solution at three concentration levels. It represents the ratio between the obtained and added concentration values, expressed in percent. The accuracy is determined by analyzing the enrichment of oil samples with six repetitions at three different concentrations. The results are shown in
Table 3. Analytical yield percentages ranged from 98-102% for all cannabinoids (acceptance criteria: 90-110% according to ICH guidelines). Analytical yield data show the high extraction efficiency for cannabinoids from CBD oil, according to the applied method.
3.4. Precision
According to ICH, precision is the closeness between the results obtained from a series of measurements made on samples from the same homogeneous source under prescribed conditions. The precision can be expressed as repeatability – where analyses are performed under identical conditions in a short period; intermediate precision – where additional random effects of the working environment are included over a more extended period (days, weeks); and reproducibility – as precision between different laboratories. From the data obtained for six repetitions, mean value, standard deviation, and relative standard deviation are calculated for all analyzed samples. The RSD was 0.82 for (cannabigerol -CBG) while for (cannabidivarin CBDV), 1.59 %, with the results shown in
Table 2.
3.5. Suitability of the HPLC System
1.0 mL CBD standard solution was diluted with 10 mL acetonitrile in a volumetric flask (10 μg/mL CBD concentration standard mixture) used for the assay method. This solution was used to test the system's suitability, where six injections were made, which determined the number of theoretical floors, peak spreading factor, resolution between peaks, and reproducibility (percent RSD of retention time, area, and height of the peak for six injections). The values obtained for the resolution between the peaks of CBD, Δ9-THC, and Δ8-THC in the chromatograms after applying the solution to check the system's suitability indicate an excellent separation of the components (
Table 3). The repeatability of the system is satisfactory (RSD≤1%).
3.6. Analysis of oil Samples
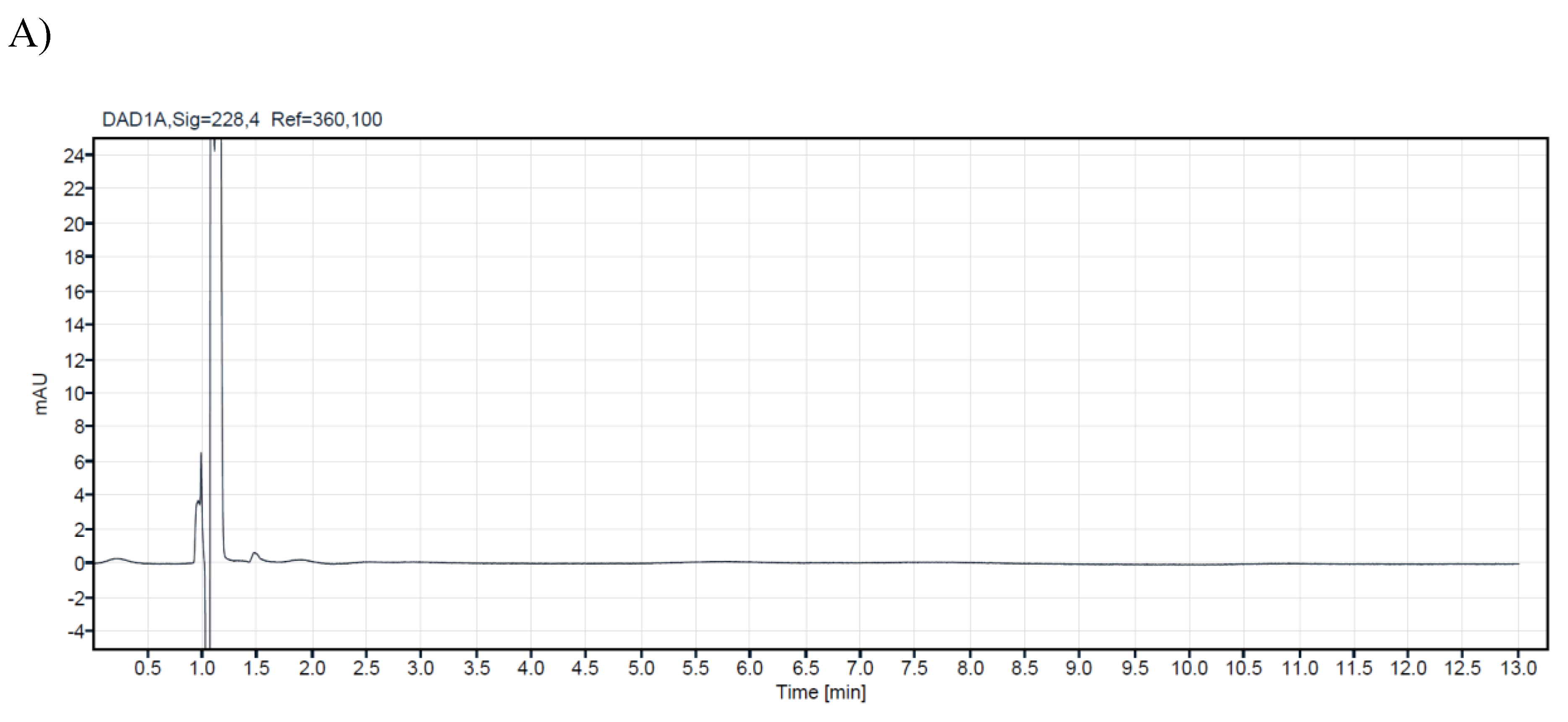
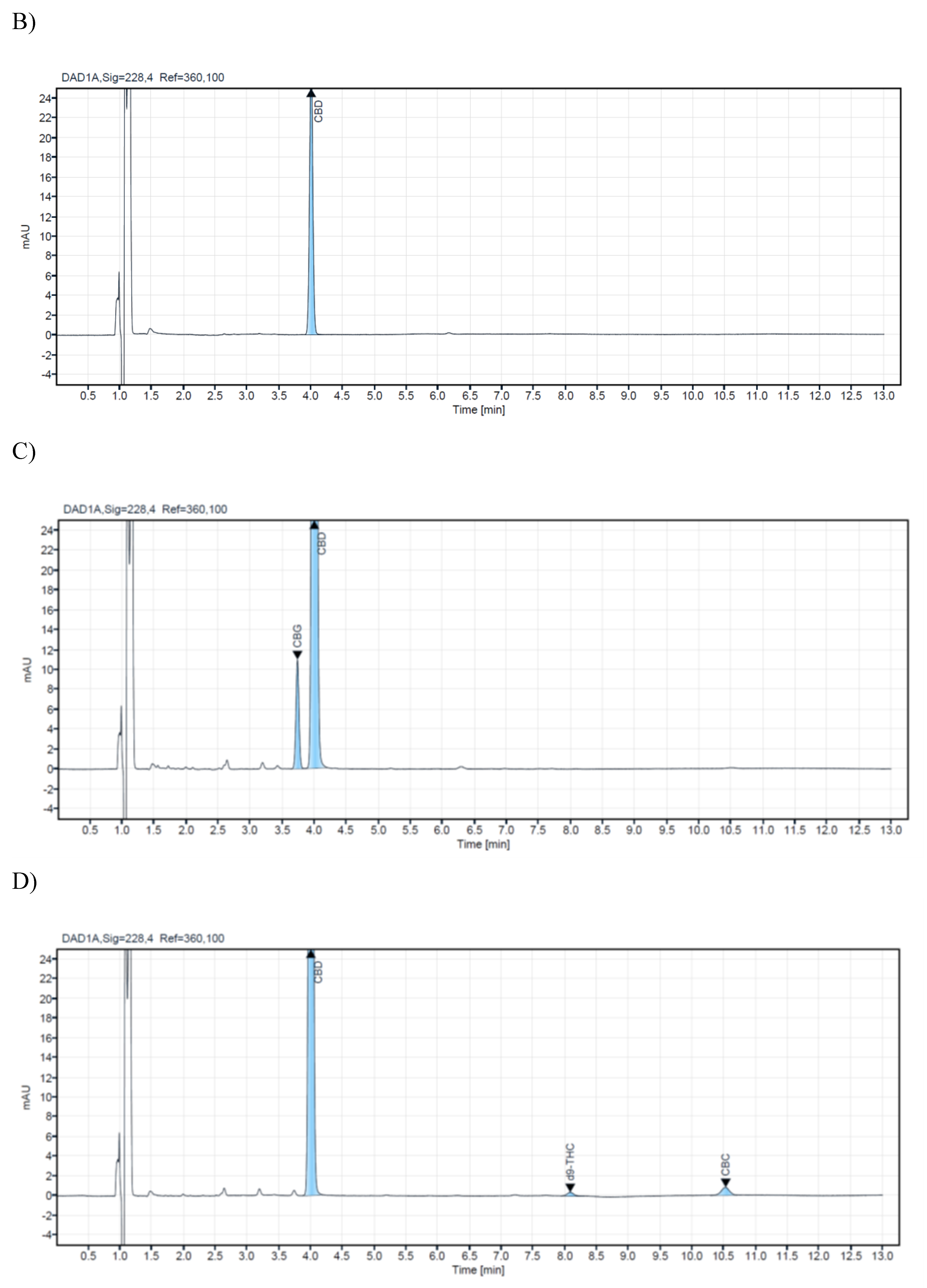
A research study analyzed 14 commercial samples of CBD oils intended for veterinary use. The samples were purchased from local veterinary pharmacies and stored in their original bottles at +4⁰C until analysis. All samples were analyzed using a validated HPLC method to ensure accurate and precise results. The CBD concentration level of the analyzed samples for veterinary oils ranges from 1.4% to 21.20%. In the samples with ordinal numbers 3,4,7,8,13, and 14, low concentrations of CBG were detected (0.54%-0.03%). For CBC in samples 13 and 14, 0.05%, while d9-THC was detected in concentrations of 0.04% in samples 13 and 14. Chromatograms of a blank sample and CBD oil extracts containing different concentrations of cannabinoids are presented in
Figure 2. Cannabinoid concentrations observed in veterinary oil samples ranged from non-detectable (N/D) to deficient concentrations for CBC, CBG, and d9-THC. This may not be too surprising since it is common knowledge that some of these cannabinoids are present at relatively low levels in cannabis and hemp. The obtained results from the analyzed samples in this research are shown in (
Table 4).
4. Discusion
FDA-approved drugs provide patients with confidence and safety in the products. Without regulatory scrutiny, many small and large companies are entering the business of manufacturing medical hemp products even though there are no documented clinical benefits from such treatments (U.S. Food and Drug Administration (FDA). (2021). The "product" is often an extract of an unproven compound that may or may not be beneficial or safe for the patient, especially in the absence of regulatory standards for cannabinoid content, pesticide, or heavy metal contamination.
Our goal in this paper was to analyze a representative set of commercial veterinary CBD oils to determine if the labeled content is accurate. Also, in the absence of any regulatory control, we were interested in knowing whether the THC content of these samples was within legally permissible limits.
The final composition of commercial hemp oil will depend on the type of extraction used and any subsequent purification or treatment of the hemp extract. The relative amounts of these chemicals will also rely on the hemp variety selected (A Hazekamp 2012). On the other hand, it should be emphasized that cannabinoid receptors are different in humans and animals. In all mammals, these receptors are found in the brain, large organs, and bones. While the human body can produce its endocannabinoids to regulate functions, sometimes their production is disrupted, leading to various diseases. Exogenous cannabinoids, such as CBD oil, can help restore balance. Animals have more receptors in the brain and are, therefore, more sensitive to cannabinoids. They can become toxic more quickly, primarily THC, even at much lower doses. Due to their sensitivity, CBD oils for pets must contain zero THC (AVMA) (2020). Only products without detectable THC content are registered as veterinary products by the Institute for State Control of Veterinary Biologicals and Medicines in the Czech Republic (SCVBM). Vets using CBD in their practice emphasize the importance of zero THC content.
On the other hand, there are different analytical techniques for determining and quantifying cannabinoids. Gas chromatography (GC) is the preferred method for cannabinoid analysis. However, chemical derivatization is necessary to prevent the decarboxylation of acidic cannabinoids. Liquid chromatography (LC) has gained popularity with the advent of high-performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC) Citti, C et al., 2016). It enables the determination of cannabinoids in both neutral and acidic forms without the need for derivatization. LC, HPLC, and UHPLC can be paired with various detectors, including fluorescence, mass spectrometry (MS), diode-array detection (DAD), or ultraviolet (UV) detectors (20). While MS enhances the selectivity and sensitivity of analyses when coupled with HPLC and UHPLC, it comes with higher costs. It requires more specialized expertise to operate while DAD offers a range of detection. DAD can help to improve specificity because acidic and neutral cannabinoids have different absorption spectrums (Leghissa et al. 2018a). Thus, Peschel, Politi (Andreae et al., 2015) used HPLC-DAD to differentiate between Cannabis sativa chemotypes, and extracts of different polarity and profile extracts.
This paper comprehensively optimizes the HPLC-DAD method, sample preparation, and analysis conditions. We achieved excellent separation of all 12 cannabinoids within 12 minutes, demonstrating baseline resolution (R > 1.0) between CBD and CBG. Utilizing a carefully selected analytical column and optimized analytical conditions, the isocratic mobile phase consisting of 0.1% aqueous formic acid (A) and 0.1% acetonitrile (B) effectively ensured superior separation of CBD, CBG, and THC, the primary components of hemp oil. Comprehensive validation confirmed the method's high precision and accuracy, including specificity, linearity, accuracy, precision, LOD, LOQ, system suitability, and analytical yield. This validated method represents a significant advancement in cannabinoid analysis.
Twelve cannabinoids were analyzed in 14 CBD oil samples, with total CBD concentrations ranging from 16.25 to 206.768 mg/g. CBD was the dominant cannabinoid in all oils, averaging 92.3% of the cannabinoid profile. Declared CBD concentrations ranged from 1.5 to 20%, while measured CBD concentrations ranged from 1.47 to 21.25%. All CBD products prominently declare the concentration of CBD on the packaging, often with higher concentrations offered at higher prices.
Based on the data presented in this paper, the 14 samples analyzed have CBD concentrations in the range provided by the manufacturers' certificates (-4.4%<c<12%). An overview of the determined deviations is shown in Table 5. Regarding the determined content of THC in the products, it was determined that the legal limit of 0.3% was not exceeded in any of them.
The findings of this analysis have significant implications for the veterinary use of CBD products. Given that CBD is often marketed for various therapeutic applications in animals, ensuring the accuracy of cannabinoid concentrations is crucial for effective treatment. In summary, our results show that cannabis oil products do not show much variation in terms of cannabinoid content, purity, and labeling.
References
- Akinola O, Ogbeche EO, Olumoh-Abdul HA, Alli-Oluwafuyi AO, Oyewole AL, Amin A, AbdulMajeed WI, Olajide OJ, Nafiu AB, Njan AA, Olorundare OE, Gbotosho GO. Oral Ingestion of Cannabis sativa: Risks, Benefits, and Effects on Malaria-Infected Hosts. Cannabis Cannabinoid Res. 2018 Nov 26;3(1):219-227. [CrossRef] [PubMed] [PubMed Central]
- American Veterinary Medical Association (AVMA), (2023) Cannabis in veterinary medicine, 1931 N. Meacham Road, Schaumburg, IL 60173 ISBN [979-8-9877127-3-3] Version 2023.0.1.
- American Veterinary Medical Association (AVMA). (2020). "Guidelines for the Use of Cannabinoids in Veterinary Medicine." Retrieved from AVMA website.
- Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, Abrams DI, Prasad H, Wilsey B, Indyk D, Johnson M, Sacks HS. Inhaled cannabis for chronic neuropathic pain: A meta-analysis of individual patient data. Journal of Pain. 2015;16(12):1121–1232. [CrossRef]
- Atalay, & Jarocka-Karpowicz, & Skrzydlewska,. (2019). Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants. 9. 21. 10.3390/antiox9010021. Banach D, Ferrero P. Cannabis and pathologies in dogs and cats: first survey of phytocannabinoid use in veterinary medicine in Argentina. J Cannabis Res. 2023 Nov 29;5(1):39. [CrossRef] [PubMed] [PubMed Central]
- Bo´csa, P. Ma´the´, L. Hangyel, (1997). Effect of nitrogen on tetrahydrocannabinol (THC) content in hemp (Cannabis sativa L.) leaves at different positions, J Int Hemp Assoc 4, 80-81.
- Carrozza A., “Medical Marijuana Research Remains Top Priority for Veterinarians American Veterinarian” https://www.americanveterinarian.com/news/medical-marijuana-research-remains-top-priority-for-veterinarians (2018).
- Citti, C., Ciccarella, G., Braghiroli, D., Parenti, C., Vandelli, M.A., Cannazza, G., 2016. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high-performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method 128, 201–209. [CrossRef]
- COMMISSION REGULATION (EU) 2015/1933 of 27 October 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in cocoa fiber, banana chips, food supplements, dried herbs, and dried spices.
- COMMISSION REGULATION (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 regarding maximum levels for polycyclic aromatic hydrocarbons in foodstuffs.
- de Meijer EP, Bagatta M, Carboni A, et al. (2003). The inheritance of chemical phenotype in Cannabis sativa L. Genetics. 163(1):335-346. [CrossRef] [PubMed] [PubMed Central]
- Doran CE, McGrath S, Bartner LR, Thomas B, Cribb AE, Gustafson DL. Drug-drug interaction between cannabidiol and phenobarbital in healthy dogs. Am J Vet Res. 2021 Nov 1;83(1):86-94. [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), 2018, Medical use of cannabis and cannabinoids: questions and answers for policymaking, Publications Office of the European Union. [CrossRef]
- Fischedick JT, Hazekamp A, Erkelens T, Choi YH, Verpoorte R. (2010). Metabolic fingerprinting of Cannabis sativa L., cannabinoids, and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 71(17-18):2058-73. [CrossRef] [PubMed]
- Gurley BJ, Murphy TP, Gul W, Walker LA, ElSohly M. (2020) Content versus Label Claims in Cannabidiol (CBD)-Containing Products Obtained from Commercial Outlets in Mississippi. J Diet Suppl. 17(5):599-607. [CrossRef] [PubMed]
- Hazekamp A, Fischedick JT. (2012). Cannabis - from cultivar to chemovar. Drug Test Anal. 4(7-8):660-7. [CrossRef] [PubMed]
- Holst P, Kristensen AT, Arendt ML (2024). Danish dog owners’ use and the perceived effect of unlicensed cannabis products in dogs. PLoS ONE 19(1): e0296698. [CrossRef]
- KAROLINA NAWROT, MARIA SOROKO-DUBROVINA (2022). The application of cannabidiol in horses' treatment and health prevention. Med. Weter. 78 (9), 431-433, 2022. [CrossRef]
- Kinghorn A. D., Falk, H. Gibbons S., Kobayashi J. (2017) Phytocannabinoids, Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa, Springer International Publishing, SAD, ISBN: 978-3-319-45539-6.
- LaVigne, J.E., Hecksel, R., Keresztes, A. et al. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci Rep 11, 8232 (2021). [CrossRef]
- Leghissa A., Hildenbrand Z.L., Schug K.A. (2018) A review of methods for the chemical characterization of cannabis natural products. J. Sep. Sci. 41:398–415. [CrossRef]
- Liebling JP, Clarkson NJ, Gibbs BW, Yates AS, O'Sullivan SE. (2022) An Analysis of Over-the-Counter Cannabidiol Products in the United Kingdom. Cannabis Cannabinoid Res. 7(2):207-213. [CrossRef] [PubMed] [PubMed Central]
- Patel B., Wene D., Fan Z.T. (2017) Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm Biomed. Anal. 146:15–23. [CrossRef]
- Radwan M.M., Wanas A.S., Chandra S., ElSohly M.A. (2017) Cannabis sativa L.-Botany and Biotechnology. 1st ed. Springer International Publishing AG; Cham, Switzerland: Natural Cannabinoids of Cannabis and Methods of Analysis; pp. 161–182.
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amЕnding Regulation (EU) No 1169/2011 of the European Parliament and the Council and repealing Regulation (EC) No 258/97 of the European Parliament and the Council and Commission Regulation (EC) No 1852/2001 [displayed 22 March 2019] https://eur-lex.europa.eu/eli/reg/2015/2283/oj Available at.
- Rhee M.H., Vogel Z., Barg J., Bayewitch M., Levy R., Hanuš L., Breuer A., Mechoulam R., (1997) Cannabinol Derivatives: Binding to Cannabinoid Receptors and Inhibition of Adenylyl cyclase, Journal of Medicinal Chemistry, 40(20), 3228–3233. [CrossRef]
- UNODC, World Drug Report 2012 (United Nations) publication, Sales No. E.12.XI.1.
- Wood T. B., Spivey W. T. N., and Easterfield T. H., (1998) J. Chem. Soc., Trans., 75, 20. [CrossRef]
- U.S. Food and Drug Administration (FDA). (2021). "Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research." Retrieved from FDA website.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).