Submitted:
22 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Post-COVID-19 Functional Status Scale
2.1.2 Short Form 36 (SF 36)
2.2. Data Analysis and Statistical Methods
3. Results
| Outpatient (n=67) |
General ward (n=47) |
Intensive care unit (n=17) |
pa | |
|---|---|---|---|---|
| n(%) | n(%) | n(%) | ||
| PCFS scale Pre-COVID-19 | ||||
| 0 | 59 (88,1) | 30 (63,8) | 5 (29,4) | <0,001** |
| 1 | - | - | - | |
| 2 | 8 (11,9) | 15 (31,9) | 11 (64,7) | |
| 3 | - | 2(4,3) | 1(5,9) | |
| 4 | - | - | - | |
| PCFS scale at diagnosis | ||||
| 0 | 2 (3,0) | - | - | <0,001** |
| 1 | 8 (11,9) | 2 (4,3) | - | |
| 2 | 10 (14,9) | 1 (2,1) | - | |
| 3 | 42 (62,7) | 37 (78,7) | 9 (52,9) | |
| 4 | 5 (7,5) | 7 (14,9) | 8 (47,1) | |
| PCFS scale at 1. month post-diagnosis | ||||
| 0 | 7 (10,4) | 7 (14,9) | 1 (5,9) | <0,001** |
| 1 | 4 (6,0) | - | - | |
| 2 | 11 (16,4) | 6 (12,8) | - | |
| 3 | 44 (65,7) | 32 (68,1) | 9 (52,9) | |
| 4 | 1 (1,5) | 2 (4,3) | 7 (41,2) | |
| PCFS scale at 3rd month post-diagnosis | ||||
| 0 | 17 (25,8) | 14 (29,8) | 1 (5,9) | 0,003** |
| 1 | 9 (13,6) | 1 (2,1) | - | |
| 2 | 17 (25,8) | 9 (19,1) | 4 (23,5) | |
| 3 | 23 (34,8) | 22 (46,8) | 10 (58,8) | |
| 4 | - | - | 2 (11,8) | |
| D | - | 1(2,1) | - | |
| PCFS scale at 6th month post-diagnosis | ||||
| 0 | 26 (40,0) | 17 (36,2) | 2 (11,8) | 0,121 |
| 1 | 9 (13,8) | 2 (4,3) | 1 (5,9) | |
| 2 | 17 (26,2) | 15 (31,9) | 5 (29,4) | |
| 3 | 13 (20,0) | 11 (23,4) | 8 (47,1) | |
| 4 | - | 1 (2,2) | 1 (5,9) | |
| D | - | 1(2,1) | - | |
| PCFS scale at 12th month post-diagnosis | ||||
| 0 | 36 (55,4) | 16 (34,8) | 2 (11,8) | 0,003** |
| 1 | 7 (10,8) | 2 (4,3) | 1 (5,9) | |
| 2 | 16 (24,6) | 18 (39,1) | 8 (47,1) | |
| 3 | 6(9,2) | 7(15,2) | 6(35,3) | |
| 4 | - | - | - | |
| D | - | 3(6,5) | - |
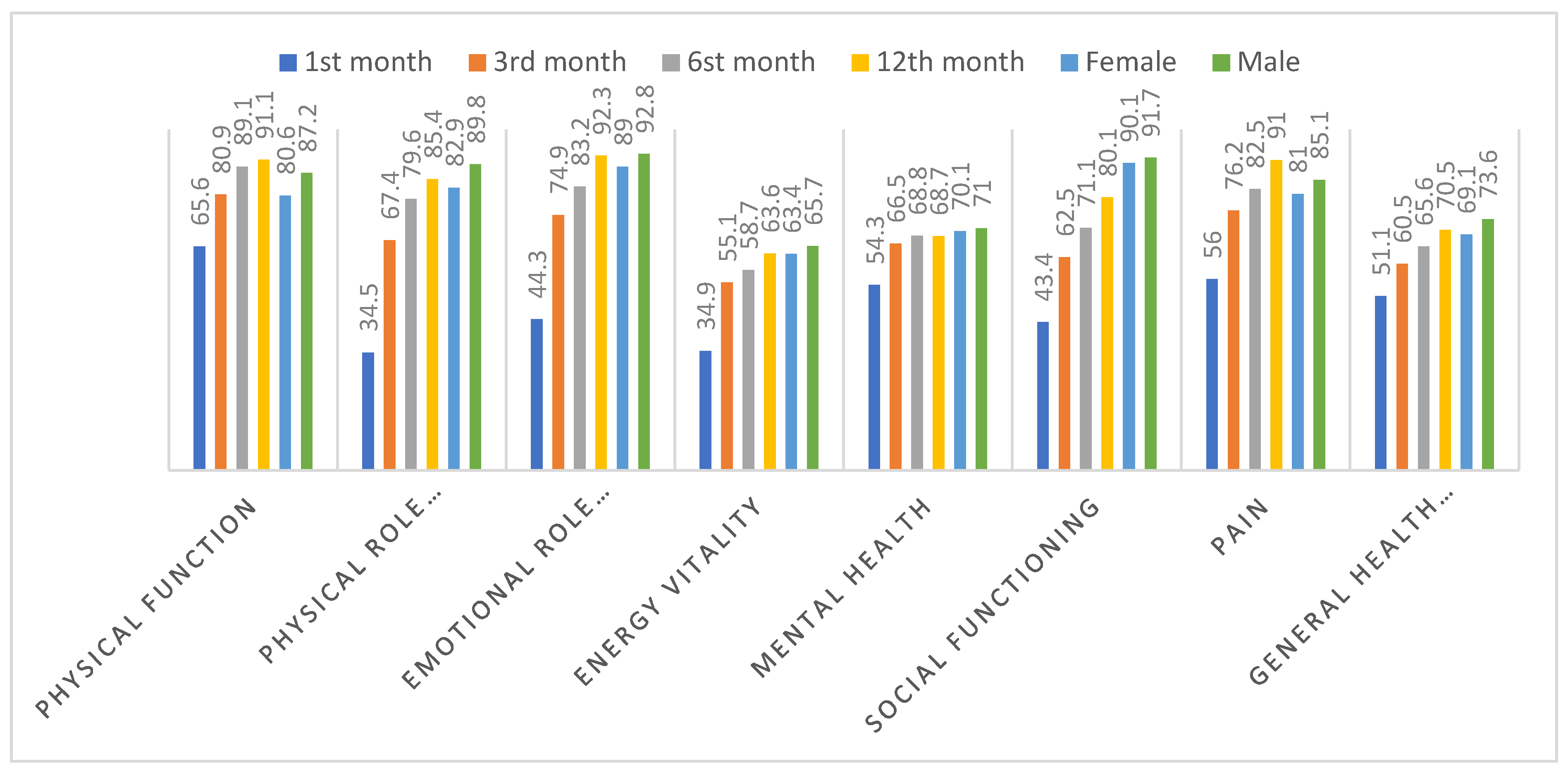
| 1st month | 3rd month | 6th month | 12th month | |
|---|---|---|---|---|
| (mean ± SD) |
(mean ± SD) |
(mean ± SD) |
(mean ± SD) | |
| Physical Function | 65,6±32,9 | 80,9±24,5 | 89,1±18,7 | 91,1±14,2 |
| Physical Role Difficulty | 34,5±42,9 | 67,4±42,3 | 79,6±35,1 | 85,4±30,9 |
| Emotional Role Difficulty | 44,3±46,3 | 74,9±40,4 | 83,2±33,8 | 92,3±25,8 |
| Energy vitality | 34,9±23,8 | 55,1±21,4 | 58,7±20,9 | 63,6±15,2 |
| Mental health | 54,3±22,3 | 66,5±17,7 | 68,8±17,2 | 68,7±12,6 |
| Social Functioning | 43,4±31,8 | 62,5±28,9 | 71,1±27,1 | 80,1±18,2 |
| Pain | 56,0±33,8 | 76,2±26,7 | 82,5±23,9 | 91,0±17,7 |
| General Health Perception | 51,1±21,1 | 60,5±18,6 | 65,6±18,3 | 70,5±15,5 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han Q, Zheng B, Daines L, Sheikh A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens. 2022,11, 269. [CrossRef]
- Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020,324,603-605. [CrossRef]
- Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS) outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med . 2020,52, 1-11.
- Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022,22, e102-e107.
- Nalbandian A, Desai AD, Wan EY. Post-COVID-19 Condition. Annu Rev Med. 2023,74, 55-64.
- Klok FA, Boon GJAM, Barco S, Endres M, Miranda Geelhoed JJ, Knauss S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020,56,2001494. [CrossRef]
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992,30, 473-483.
- Lopez-Leon S, Wegman-Ostrosky T, Perelman C. More than 50 Long-term effects of COVID- 19: a systematic review and meta-analysis. medRxiv 2021. [CrossRef]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2023, 401, e21-e33.
- Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021,27,1507-1513. [CrossRef]
- Khoddami SM, Aghadoost S, Aghajanzadeh M, Molazeinal Y. The Health-related Quality of Life and Voice Handicap Index in Recovered COVID-19 Patients in Comparison to Healthy Subjects. J Voice. 2023. [CrossRef]
- McFann K, Baxter BA, LaVergne SM, et al. Quality of Life (QoL) Is Reduced in Those with Severe COVID-19 Disease, Post-Acute Sequelae of COVID-19, and Hospitalization in United States Adults from Northern Colorado. Int J Environ Res Public Health. 2021,18,11048. [CrossRef]
- Van den Borst B, Peters JB, Brink M, et al. Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2021,73,e1089-e1098. [CrossRef]
- Malesevic S, Sievi NA, Baumgartner P, et al. Impaired health-related quality of life in long-COVID syndrome after mild to moderate COVID-19. Sci Rep. 2023,13, 7717. [CrossRef]
- Van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021,176, 106272.
- Benkalfate N, Eschapasse E, Georges T, et al. Evaluation of the Post-COVID-19 Functional Status (PCFS) Scale in a cohort of patients recovering from hypoxemic SARS-CoV-2 pneumonia. BMJ Open Respir Res. 2022, 9, e001136.
- Vaes AW, Goërtz YMJ, Van Herck M, et al. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021,7,00141-2021. [CrossRef]
- Taboada M, Cariñena A, Moreno E, et al. Post-COVID-19 functional status six-months after hospitalization. J Infect. 2021,82,e31-e33. [CrossRef]
- Mohamed Hussein AA, Saad M, Zayan HE, et al. Post-COVID-19 functional status: Relation to age, smoking, hospitalization, and previous comorbidities. Ann Thorac Med. 2021,16,260-265.
| Total | Outpatient (n=67) | General ward (n=47) | Intensive care unit (n=17) | pa | |
| n(%) | n(%) | n(%) | n(%) | ||
| Gender | |||||
| Female | 60(45,8) | 44 (65,7) | 14 (29,8) | 2 (11,8) | <0,001** |
| Male | 71(54,2) | 23 (34,3) | 33 (70,2) | 15 (88,2) | |
| Comorbidities | 81(61,8) | 30 (44,8) | 36 (76,6) | 15 (88,2) | <0,001** |
| Diabetes mellitus | 15(11,5) | 3 (4,5) | 8 (17,0) | 4 (23,5) | 0,029* |
| Hypertension | 23(17,6) | 6 (9,0) | 13 (27,7) | 4 (23,5) | 0,028* |
| Cardiovascular disease | 18(13,7) | 3 (4,5) | 8 (17,0) | 7 (41,2) | <0,001** |
| COPD/Asthma | 14(10,7) | 3 (4,5) | 5 (10,6) | 6 (35,3) | 0,001** |
| Obesity | 8(6,1) | 3 (4,5) | 3 (6,4) | 2 (11,8) | 0,531 |
| Chronic Renal Failure | 2(1,5) | 1 (1,5) | 1 (2,1) | - | 0,828 |
| HIV infection | 15(11,5) | 13 (19,4) | 1 (2,1) | 1 (5,9) | 0,013* |
|
Outpatient (n=67) |
General ward (n=47) |
Intensive care unit (n=17) |
pb | ||
|
(mean ± SD) Med (Min-Max) |
(mean ± SD) Med (Min-Max) |
(mean ± SD) Med (Min-Max) |
|||
| Age | 43,7±15,3 45(18-83) |
36,4±13,2 31 (18-76) |
48,9±13,2 51 (20-83) |
58,0±12,1 57 (32-81) |
<0,001** |
| At diagnosis | 1st month | 3rd month | 6st month | 12th month | |
|---|---|---|---|---|---|
| n(%) | n(%) | n(%) | n(%) | n(%) | |
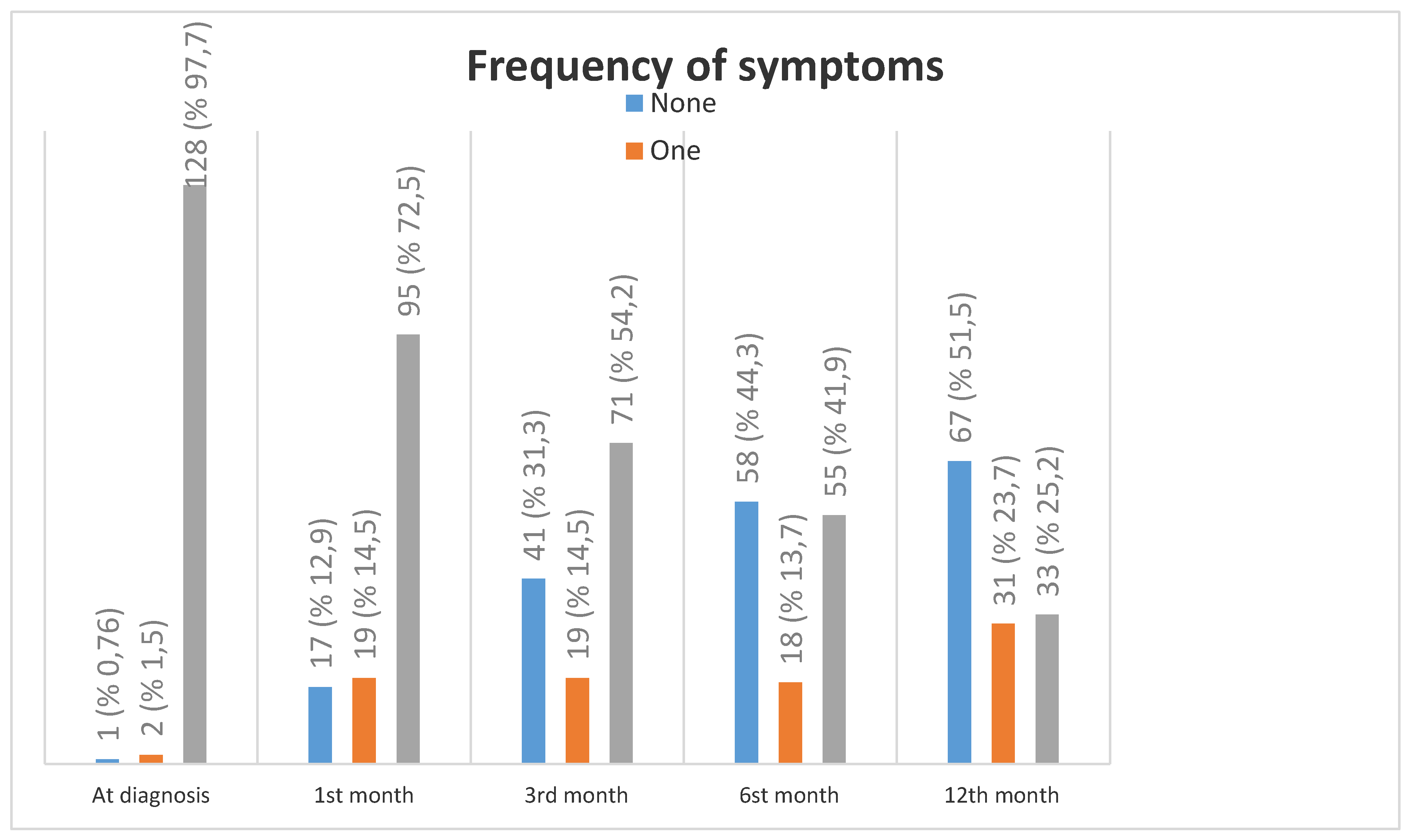
| Symptoms | 127 (96,9) | ||||
| Symptoms | |||||
| Fever | 74 (56,5) | 5 (3,8) | - | - | - |
| Weakness and Fatigue | 113 (86,3) | 74 (56,5) | 52 (39,7) | 34 (26,0) | 21 (16,0) |
| Easy fatigue during exertion | 92 (70,2) | 98 (74,8) | 66 (50,4) | 50 (38,2) | 48 (36,6) |
| Cough | 91 (69,5) | 34 (26,0) | 11 (8,4) | 6 (4,6) | 3 (2,3) |
| Dyspnea | 66 (50,4) | 35 (26,7) | 14 (10,7) | 8 (6,1) | 2 (1,5) |
| Myalgia | 93 (71,0) | 45 (34,4) | 24 (18,3) | 14 (10,7) | 9 (6,9) |
| Arthralgia | 76 (58,0) | 36 (27,5) | 17 (13,0) | 9 (6,9) | 9 (6,9) |
| Palpitation | 42 (32,1) | 25 (19,1) | 14 (10,7) | 10 (7,6) | 12 (9,2) |
| Headache | 72 (55,0) | 25 (19,1) | 17 (13,0) | 7 (5,3) | 3 (2,3) |
| Sleep disturbance | 64 (48,9) | 31 (23,7) | 17 (13,0) | 15 (11,5) | 3 (2,3) |
| Forgetfulness | 48 (36,6) | 32 (24,4) | 29 (22,1) | 18 (13,7) | 6 (4,6) |
| Difficulty concentrating | 39 (29,8) | 26 (19,8) | 11 (8,4) | 10 (7,6) | 4 (3,1) |
| Loss of appetite | 77 (58,8) | 20 (15,3) | 8 (6,1) | 5 (3,8) | 4 (3,1) |
| Loss of smell | 79 (60,3) | 32 (24,4) | 19 (14,5) | 12 (9,2) | 3 (2,3) |
| Loss of taste | 76 (58,0) | 24 (18,3) | 12 (9,2) | 9 (6,9) | 2 (1,5) |
| Diarrhea | 28 (21,4) | 4 (3,1) | 1 (0,8) | - | 1 (0,8) |
| Skin findings | 6 (4,6) | - | - | - | - |
| Vision problems | 3 (2,3) | 1 (0,8) | 1 (0,8) | - | 1 (0,8) |
| Diagnostic Findings |
At diagnosis | 1st month | 3rd month | 6th month | 12th month |
|---|---|---|---|---|---|
| n(%) | n(%) | n(%) | n(%) | n(%) | |
| Fever | |||||
| Outpatient (n=67) | 30 (44,8) | 1 (1,5) | - | - | - |
| General ward (n=47) | 32 (68,1) | 3 (6,4) | - | - | - |
| Intensive care unit (n=17) | 12 (70,6) | 1 (5,9) | - | - | - |
| p | 0,021* | 0,363 | |||
| Weakness and Fatigue | |||||
| Outpatient (n=67) | 54 (80,6) | 31 (46,3) | 23 (34,3) | 16 (23,9) | 9 (13,4) |
| General ward (n=47) | 45 (95,7) | 29 (61,7) | 18 (38,3) | 10 (21,3) | 6 (12,8) |
| Intensive care unit (n=17) | 14 (82,4) | 14 (82,4) | 11 (64,7) | 8 (47,1) | 6 (35,3) |
| p | 0,061 | 0,018* | 0,071 | 0,099 | 0,067 |
| Easy fatigue during exertion | |||||
| Outpatient (n=67) | 41 (61,2) | 45 (67,2) | 30 (44,8) | 15 (22,4) | 13 (19,4) |
| General ward (n=47) | 37 (78,7) | 38 (80,9) | 22 (46,8) | 22 (46,8) | 21 (44,7) |
| Intensive care unit (n=17) | 14 (82,4) | 15 (88,2) | 14 (82,4) | 13 (76,5) | 14 (82,4) |
| p | 0,066 | 0,100 | 0,018* | <0,001** | <0,001** |
| Cough | |||||
| Outpatient (n=67) | 41 (61,2) | 15 (22,4) | 4 (6,0) | 2 (3,0) | 1 (1,5) |
| General ward (n=47) | 38 (80,9) | 11 (23,4) | 3 (6,4) | 3 (6,4) | 1 (2,1) |
| Intensive care unit (n=17) | 12 (70,6) | 8 (47,1) | 4 (23,5) | 1 (5,9) | 1 (5,9) |
| p | 0,080 | 0,103 | 0,054 | 0,668 | 0,555 |
| Dyspnea | |||||
| Outpatient (n=67) | 25 (37,3) | 12 (17,9) | 3 (4,5) | 1 (1,5) | - |
| General ward (n=47) | 27 (57,4) | 12 (25,5) | 5 (10,6) | 4 (8,5) | - |
| Intensive care unit (n=17) | 14 (82,4) | 11 (64,7) | 6 (35,3) | 3 (17,6) | 2 (11,8) |
| p | 0,002** | <0,001** | 0,001** | 0,032* | 0,001** |
| Myalgia | |||||
| Outpatient (n=67) | 49 (73,1) | 21 (31,3) | 14 (20,9) | 5 (7,5) | 4 (6,0) |
| General ward (n=47) | 33 (70,2) | 18 (38,3) | 7 (14,9) | 6 (12,8) | 3 (6,4) |
| Intensive care unit (n=17) | 11 (64,7) | 6 (35,3) | 3 (17,6) | 3 (17,6) | 2 (11,8) |
| p | 0,783 | 0,741 | 0,715 | 0,405 | 0,691 |
| Arthralgia | |||||
| Outpatient (n=67) | 41 (61,2) | 15 (22,4) | 8 (11,9) | 2 (3,0) | 4 (6,0) |
| General ward (n=47) | 27 (57,4) | 16 (34,0) | 6 (12,9) | 4 (8,5) | 5 (10,6) |
| Intensive care unit (n=17) | 8 (47,1) | 5 (29,4) | 3 (17,6) | 3 (17,6) | - |
| p | 0,571 | 0,383 | 0,821 | 0,088 | 0,304 |
| Palpitation | |||||
| Outpatient (n=67) | 23 (34,3) | 14 (20,9) | 7 (10,4) | 2 (3,0) | 3 (4,5) |
| General ward (n=47) | 13 (27,7) | 7 (14,9) | 4 (8,5) | 6 (12,8) | 4 (8,5) |
| Intensive care unit (n=17) | 6 (35,3) | 4 (23,5) | 3 (17,6) | 2 (11,8) | 5 (29,4) |
| p | 0,720 | 0,639 | 0,577 | 0,121 | 0,006** |
| Headache | |||||
| Outpatient (n=67) | 41 (61,2) | 16 (23,9) | 9 (13,4) | 2 (3,0) | 1 (1,5) |
| General ward (n=47) | 24 (51,1) | 7 (14,9) | 6 (12,8) | 4 (8,5) | 1 (2,1) |
| Intensive care unit (n=17) | 7 (41,2) | 2 (11,8) | 2 (11,8) | 1 (5,9) | 1 (5,9) |
| p | 0,266 | 0,346 | 0,982 | 0,432 | 0,555 |
| Sleep disturbance | |||||
| Outpatient (n=67) | 31 (46,3) | 13 (19,4) | 8 (11,9) | 3 (4,5) | - |
| General ward (n=47) | 23 (48,9) | 8 (17,0) | 5 (10,6) | 5 (10,6) | 2 (4,3) |
| Intensive care unit (n=17) | 10 (58,8) | 10 (58,8) | 4 (23,5) | 7 (41,2) | 1 (5,9) |
| p | 0,652 | 0,001** | 0,374 | <0,001** | 0,186 |
| Forgetfulness | |||||
| Outpatient (n=67) | 24 (35,8) | 15 (22,4) | 13 (19,4) | 9 (13,4) | 2 (3,0) |
| General ward (n=47) | 16 (34,0) | 9 (19,1) | 10 (21,3) | 3 (6,4) | 3 (6,4) |
| Intensive care unit (n=17) | 8 (47,1) | 8 (47,1) | 6 (35,3) | 6 (35,3) | 1 (5,9) |
| p | 0,622 | 0,062 | 0,365 | 0,012* | 0,668 |
| Difficulty concentrating | |||||
| Outpatient (n=67) | 24 (35,8) | 13 (19,4) | 6 (9,0) | 6 (9,0) | 2 (3,0) |
| General ward (n=47) | 10 (21,3) | 7 (14,9) | 2 (4,3) | 2 (4,3) | 2 (4,3) |
| Intensive care unit (n=17) | 5 (29,4) | 6 (35,3) | 3 (17,6) | 2 (11,8) | - |
| p | 0,247 | 0,194 | 0,227 | 0,512 | 0,682 |
| Loss of appetite | |||||
| Outpatient (n=67) | 35 (52,2) | 7 (10,4) | 3 (4,5) | 4 (6,0) | 1 (1,5) |
| General ward (n=47) | 31 (66,0) | 8 (17,0) | 2 (4,3) | - | 2 (4,3) |
| Intensive care unit (n=17) | 11 (64,7) | 5 (29,4) | 3 (17,6) | 1 (5,9) | 1 (5,9) |
| p | 0,297 | 0,139 | 0,103 | 0,234 | 0,538 |
| Loss of smell | |||||
| Outpatient (n=67) | 51 (76,1) | 22 (32,8) | 16 (23,9) | 10 (14,9) | 3 (4,5) |
| General ward (n=47) | 23 (48,9) | 8 (17,0) | 2 (4,3) | 1 (2,1) | - |
| Intensive care unit (n=17) | 5 (29,4) | 2 (11,8) | 1 (5,9) | 1 (5,9) | - |
| p | <0,001** | 0,066 | 0,008** | 0,058 | 0,231 |
| Loss of taste | |||||
| Outpatient (n=67) | 46 (68,7) | 15 (22,4) | 9 (13,4) | 7 (10,4) | 2 (3,0) |
| General ward (n=47) | 25 (53,2) | 8 (17,0) | 2 (4,3) | 1 (2,1) | - |
| Intensive care unit (n=17) | 5 (29,4) | 1 (5,9) | 1 (5,9) | 1 (5,9) | - |
| p | 0,010* | 0,279 | 0,218 | 0,221 | 0,379 |
| Diarrhea | |||||
| Outpatient (n=67) | 12 (17,9) | 4 (6,0) | - | - | - |
| General ward (n=47) | 13 (27,7) | - | 1 (2,1) | - | - |
| Intensive care unit (n=17) | 3 (17,6) | - | - | - | - |
| p | 0,422 | 0,139 | 0,406 | ||
| Skin findings | |||||
| Outpatient (n=67) | 6 (9,0) | - | - | - | - |
| General ward (n=47) | - | - | - | - | - |
| Intensive care unit (n=17) | - | - | - | - | - |
| p | 0,050 | ||||
| Vision problems | |||||
| Outpatient (n=67) | 3 (4,5) | 1 (1,5) | 1 (1,5) | - | 1 (1,5) |
| General ward (n=47) | - | - | - | - | - |
| Intensive care unit (n=17) | - | - | - | - | - |
| p | 0,231 | 0,618 | 0,618 | 0,618 | |
| Grade | Frequency (n) | Percentage (%) |
|---|---|---|
| PCFS scale at pre-COVID-19 | ||
| 0 | 94 | 71,8 |
| 1 | 0 | 0 |
| 2 | 34 | 26 |
| 3 | 3 | 2,2 |
| 4 | 0 | 0 |
| PCFS scale at diagnosis | ||
| 0 | 2 | 1,5 |
| 1 | 10 | 7,6 |
| 2 | 11 | 8,4 |
| 3 | 88 | 67,2 |
| 4 | 20 | 15,3 |
| PCFS scale at 1. month post-diagnosis | ||
| 0 | 15 | 11,5 |
| 1 | 4 | 3,1 |
| 2 | 17 | 13,0 |
| 3 | 85 | 64,9 |
| 4 | 10 | 7,6 |
| PCFS scale at 3rd month post-diagnosis | ||
| 0 | 32 | 24,6 |
| 1 | 10 | 7,7 |
| 2 | 30 | 23,1 |
| 3 | 55 | 42,3 |
| 4 | 2 | 1,5 |
| D | 1 | 0,8 |
| PCFS scale at 6th month post-diagnosis | ||
| 0 | 45 | 34,9 |
| 1 | 12 | 9,3 |
| 2 | 37 | 28,7 |
| 3 | 32 | 24,8 |
| 4 | 2 | 1,6 |
| D | 1 | 0,8 |
| PCFS scale at 12th month post-diagnosis | ||
| 0 | 54 | 42,2 |
| 1 | 10 | 7,8 |
| 2 | 42 | 32,8 |
| 3 | 19 | 14,8 |
| 4 | 0 | 0 |
| D | 3 | 2,3 |
| 1st month | 12th month | Delta (12th -1st ) | |
|---|---|---|---|
| (mean ± SD) | (mean ± SD) |
||
| Physical Function | |||
| Outpatient (n=67) | 77,2±24,1 | 93,9±11,6 | 17,1±21,5 |
| General ward (n=47) | 63,7±30,7 | 89,5±14,0 | 24,9±26,5 |
| Intensive care unit (n=17) | 25,6±37,3 | 84,1±20,6 | 58,5±35,6 |
| p | <0,001** | 0,001** | 0,001** |
| Physical Role Difficulty | |||
| Outpatient (n=67) | 44,8±44,1 | 90,8±24,4 | 46,2±42,7 |
| General ward (n=47) | 30,3±41,7 | 84,3±32,3 | 52,9±48,9 |
| Intensive care unit (n=17) | 5,88±24,3 | 67,6±43,1 | 61,8±45,2 |
| p | 0,001** | 0,025* | 0,758 |
| Emotional Role Difficulty | |||
| Outpatient (n=67) | 44,8±44,0 | 92,8±23,9 | 46,7±48,2 |
| General ward (n=47) | 51,1±49,1 | 90,7±29,4 | 36,4±52,4 |
| Intensive care unit (n=17) | 23,5±43,7 | 94,1±24,3 | 70,6±46,9 |
| p | 0,106 | 0,907 | 0,049* |
| Energy Vitality | |||
| Outpatient (n=67) | 38,6±21,9 | 63,8±16,3 | 24,9±25,1 |
| General ward (n=47) | 33,8±24,9 | 63,1±15,7 | 27,8±23,9 |
| Intensive care unit (n=17) | 23,2±25,2 | 64,1±9,7 | 40,9±23,7 |
| p | 0,028* | 0,607 | 0,064 |
| Mental health | |||
| Outpatient (n=67) | 54,9±19,5 | 68,2±13,8 | 12,2±8,6 |
| General ward (n=47) | 57,4±23,9 | 69,8±11,5 | 10,7±24,1 |
| Intensive care unit (n=17) | 43,8±26,6 | 68,2±11,1 | 24,5±26,5 |
| p | 0,121 | 0,877 | 0,060 |
| Social Functioning | |||
| Outpatient (n=67) | 50,4±25,6 | 83,3±18,1 | 31,5±32,5 |
| General ward (n=47) | 41,6±37,1 | 78,2±19,1 | 35,7±36,5 |
| Intensive care unit (n=17) | 20,6±28,6 | 72,8±13,4 | 52,2±29,4 |
| p | 0,001** | 0,031* | 0,061 |
| Pain | |||
| Outpatient (n=67) | 55,5±32,7 | 91,9±14,8 | 36,3±31,8 |
| General ward (n=47) | 55,9±35,6 | 88,1±22,5 | 31,3±36,4 |
| Intensive care unit (n=17) | 58,5±34,8 | 95,3±13,4 | 36,8±32,1 |
| p | 0,968 | 0,245 | 0,335 |
| General Health Perception | |||
| Outpatient (n=67) | 57,5±21,1 | 72,9±13,9 | 14,3±18,1 |
| General ward (n=47) | 48,4±17,5 | 68,9±17,5 | 19,9±18,1 |
| Intensive care unit (n=17) | 33,5±19,2 | 65,3±14,7 | 31,8±17,0 |
| p | <0,001** | 0,078 | <0,001** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





