1. Introduction
The small ubiquitin-like modifier (SUMO) pathway plays a crucial role in various cellular processes, including cell cycle regulation, transcription, and protein trafficking[
1,
2]. SUMO-specific proteases, such as Ulp1 (Ubiquitin-like-specific protease 1), are enzymes that cleave SUMO from SUMO-conjugated proteins, thereby reversing SUMO modifications [
3,
4]. In Saccharomyces cerevisiae, Ulp1 has been extensively studied and found to be essential for cell viability, as its absence leads to G2/M cell cycle arrest[
5,
6]. Similarly, in the methylotrophic yeast Pichia pastoris, Ulp1 has also been identified as a key regulator of the cell cycle. Pichia pastoris is a widely used host for the production of recombinant proteins due to its efficient secretion system [
7,
8,
9]. However, the functions of Ulp1 in Pichia pastoris have not been extensively explored. Understanding the role of Ulp1 in Pichia pastoris cell cycle regulation could provide valuable insights into improving recombinant protein production in this yeast.
The cell cycle is a highly regulated process that ensures the accurate replication and segregation of genetic material into daughter cells. In eukaryotes, the cell cycle is composed of four main phases: G1 (gap 1), S (DNA synthesis), G2 (gap 2), and M (mitosis) [
10]. Progression through the cell cycle is controlled by a complex network of checkpoint mechanisms and regulatory proteins, such as cyclins and cyclin-dependent kinases (CDKs) [
11,
12,
13]. In yeast, the cell cycle is similarly regulated, and various studies have identified key players in this process, including the SUMO pathway and its associated enzymes like Ulp1 [
5,
14,
15].
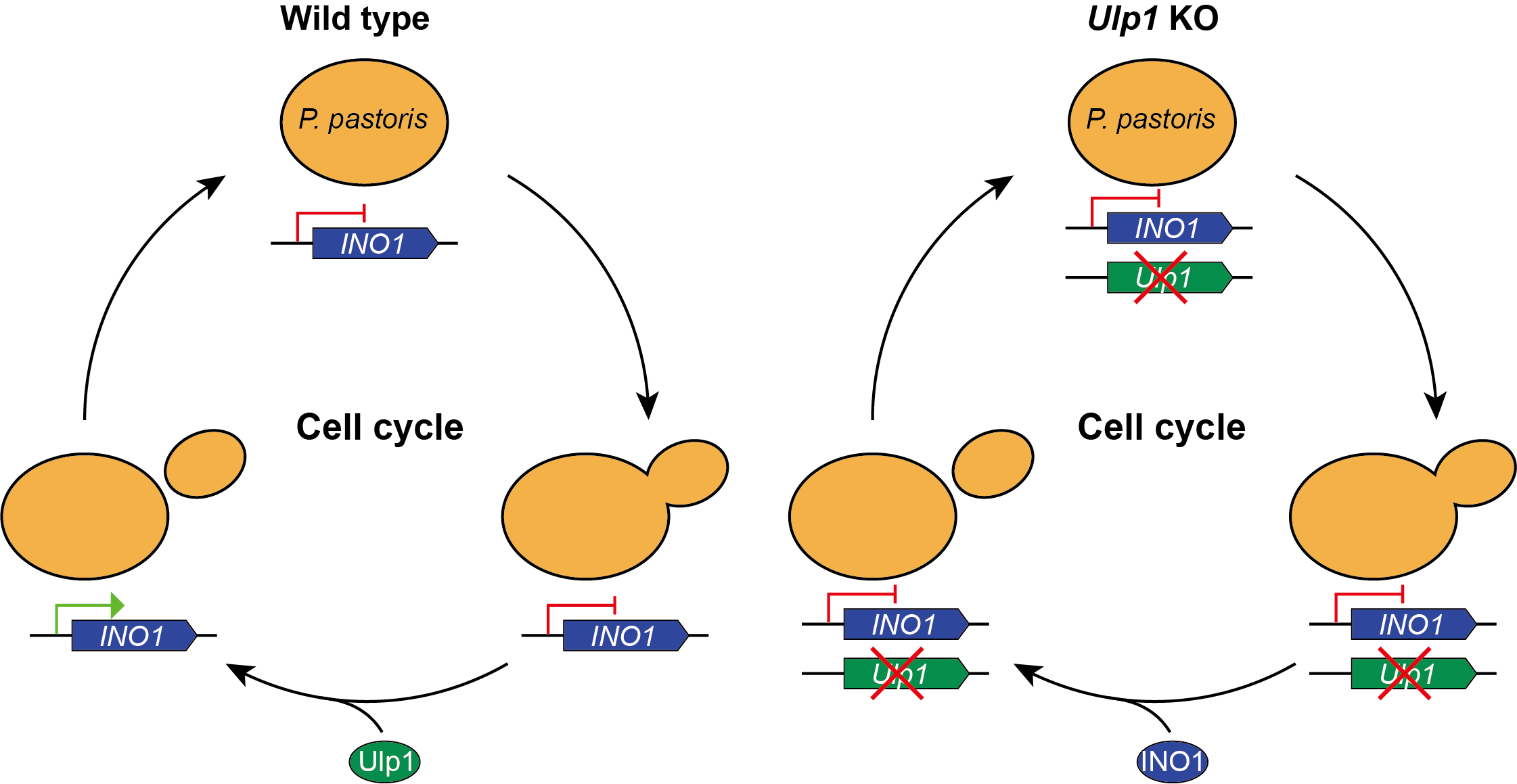
In this study, we investigated the functions of Ulp1 in Pichia pastoris and its impact on cell cycle regulation. We identified three Ulp1-like proteins in Pichia pastoris and focused on the role of the pUlp1 homolog, which is the functional equivalent of Ulp1 in Saccharomyces cerevisiae. By generating Ulp1 knockout strains in Pichia pastoris, we observed G2 phase stalling, similar to the phenotype reported in Saccharomyces cerevisiae [
5]. Additionally, our transcriptome analysis revealed significant changes in the expression of several genes, including INO1, which is involved in inositol biosynthesis. Interestingly, overexpression of INO1 in Ulp1 knockout cells restored cell proliferation, suggesting that Ulp1 regulates cell proliferation through the modulation of INO1 expression. These findings provide new insights into the role of Ulp1 in the cell cycle and raise intriguing questions about the complex regulatory mechanisms governing yeast cell division.
2. Materials and Methods
2.1. Identification of Ubiquitin-Like-Specific Proteases in Pichia pastoris
The Ulp1 sequence from Saccharomyces cerevisiae (Uniprot ID: Q02724) was downloaded and used as a query in a tblastn search to identify ubiquitin-like-specific proteases in the Pichia pastoris (Komagataella pastoris) NCBI genome database. We identified three Ulp1-like protein coding genes, which we named pUlp1, pUlp2, and pUlp3 based on their sequence similarity to the Ulp1 protein from Saccharomyces cerevisiae.
2.2. Construction of Specific Pichia pastoris Strains
Plasmids pPICZ-FLP-DouH5, pPICZ-FLP-DouH3, pPICZ-FLP-DouH3a, pUFRT-GFP-Avi, and pUFRT-GFP-Avi-INO1 were constructed according to the manual of the Seamless Cloning Kit (Beyotime, D7010M). Newly prepared yeast competent cells were made using 1 mol/L sorbitol. Plasmids were linearized with appropriate primer pairs and transformed into yeast competent cells using the BIO-RAD MicroPulser, Fungi2-Pic at 2 kV. The cells were then cultured at 28°C for 2-3 days. Clones were picked and validated by PCR.
For the FLP inducible cutting steps[
16], cells were cultured in Glycerol medium (BMGY) for 24 hours, then transferred to 1% Methanol medium (BMMY) and induced for 48-72 hours, with extra methanol added to the culture every 24 hours. The strategies are outlined in
Figure 1C,
Figure 2B and
Figure 3D.
2.3. Library Preparation for Transcriptome Sequencing
Total RNA was used as the input material for RNA sample preparations. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in First Strand Synthesis Reaction Buffer (5X). First strand cDNA was synthesized using random hexamer primers and M-MuLV Reverse Transcriptase (RNase H-). Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of the 3’ ends of DNA fragments, adaptors with hairpin loop structures were ligated to prepare for hybridization.
To select cDNA fragments preferentially 370-420 bp in length, the library fragments were purified with the AMPure XP system. PCR was then performed with Phusion High-Fidelity DNA Polymerase, Universal PCR primers, and Index (X) Primer. Finally, PCR products were purified using the AMPure XP system, and library quality was assessed on the Agilent Bioanalyzer 2100 system.
2.4. RNA-Seq Data Analysis
Raw data (raw reads) in FASTQ format were first processed using fastp software. During this step, clean data (clean reads) were obtained by removing reads containing adapters, reads containing poly-N, and low-quality reads from the raw data. Additionally, Q20, Q30, and GC content of the clean data were calculated. All downstream analyses were based on the high-quality clean data. The reference genome and gene model annotation files were downloaded directly from the genome website. The index of the reference genome was built using Hisat2, and paired-end clean reads were aligned to the reference genome using Hisat2[
17]. Gene read counts were calculated using HTSeq, and differentially expressed genes were identified using DESeq2.
3. Results
3.1. Ulp1 Is Essential for Pichia pastoris Proliferation
Several laboratories have reported the essential role of Ulp1 in the mitosis of
Saccharomyces cerevisiae[
5]. However, there is little research on the functions of Ulp1 in
Pichia pastoris. In this study, we performed a tblastn search using the Ulp1 protein sequence of
Saccharomyces cerevisiae (scUlp1) to identify homologs in
Pichia pastoris [
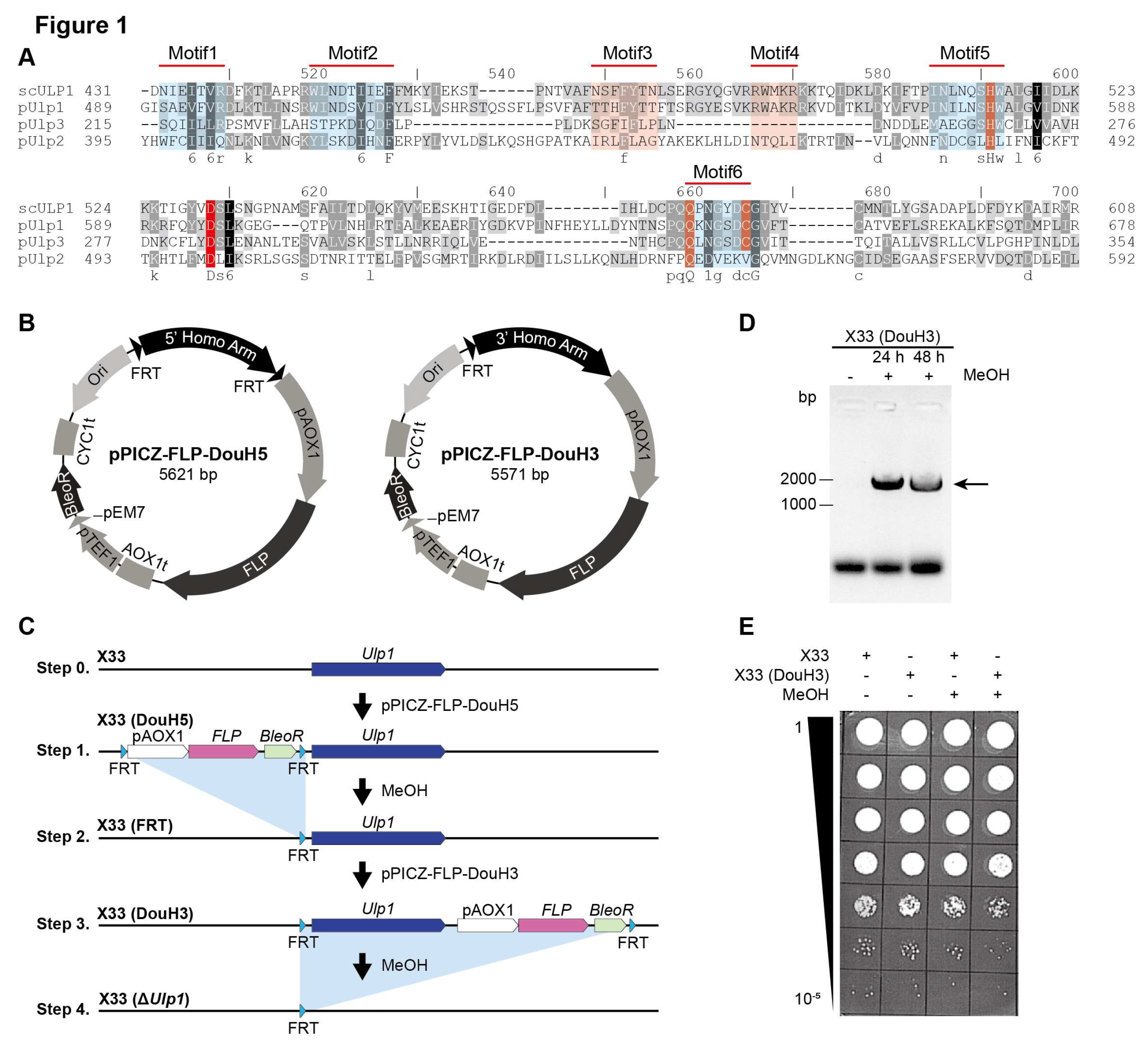
18]. We identified three Ulp1-like proteins, which we designated as pUlp1, pUlp2, and pUlp3 (
Figure 1A,
Figure S1). And We found six conserved motifs and amino acids in the homologs of scUlp1, pUlp1, indicating that pUlp1 is the functional equivalent of scUlp1 in Pichia pastoris (
Figure 1A).
Ulp1 is an enzyme responsible for cleaving SUMO/Smt3 tags from SUMO-tagged proteins, which prevents the secretion of SUMO-tagged foreign proteins from yeast[
19]. Based on this knowledge, we decided to delete
pUlp1 in
Pichia pastoris. In the subsequent sections of this article, we will refer to
pUlp1 as
Ulp1. To replace
Ulp1, we performed a double crossover using BleR and FLP. However, we were unable to obtain any BleR-positive clones (data not shown). To address the possibility of low recombination efficiency, we performed two single crossover assays to eliminate
Ulp1 (
Figure 1B and C). PCR results demonstrate that
Ulp1 can be eliminated following methanol induction. However, we were unable to achieve any
Ulp1 knockout clones (BleoR negative) (
Figure 1D and E), indicating that
Ulp1 is essential for
Pichia pastoris.
3.2. The Recombination Rate of FLP Is Decreased in Ulp1 Conditional Knockout Pichia pastoris
We observed a slight decrease in the number of X33(DouH3) clones following methanol induction (
Figure 1E). To further investigate the viability of yeast cells lacking
Ulp1, we introduced a reporter gene, GFP, which would be expressed upon
Ulp1 elimination (
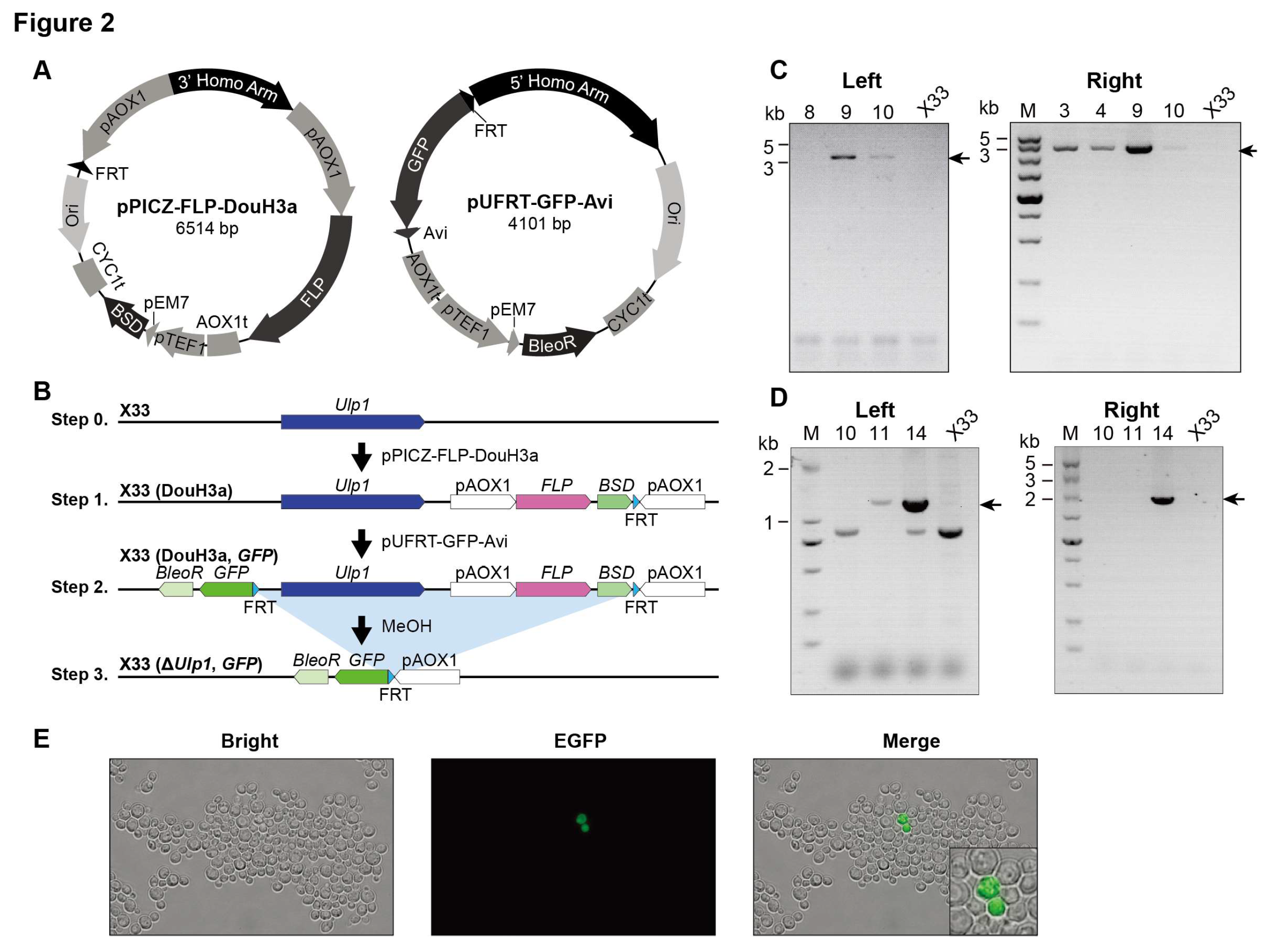
Figure 2A and B). Using PCR, we confirmed the correctness of the X33(DouH3a) #9 clones (
Figure 2C) and the X33(DouH3a, GFP) #14 clones (
Figure 2D).
Following methanol induction, GFP expression was observed in only about 2‰ (0.2%) of the clones (
Figure 2E). This observation indicates a significant decrease in FLP recombination efficiency in the Ulp1 conditional knockout strain of Pichia pastoris. The reduced number of GFP-expressing clones suggests that while some cells can initiate the knockout process, the complete elimination of Ulp1 severely impacts cell viability and proliferation.
Our findings imply that the complete ablation of Ulp1 from Pichia pastoris is not feasible under the conditions tested. Although the yeast cells lacking Ulp1 can survive to some extent, they are unable to undergo successful cell division. This inability to proliferate might be attributed to the essential role of Ulp1 in the cell cycle, particularly in transitioning through the G2 phase.
3.3. Overexpression of INO1 Rescues Cell Proliferation in Ulp1 Knockout Pichia pastoris
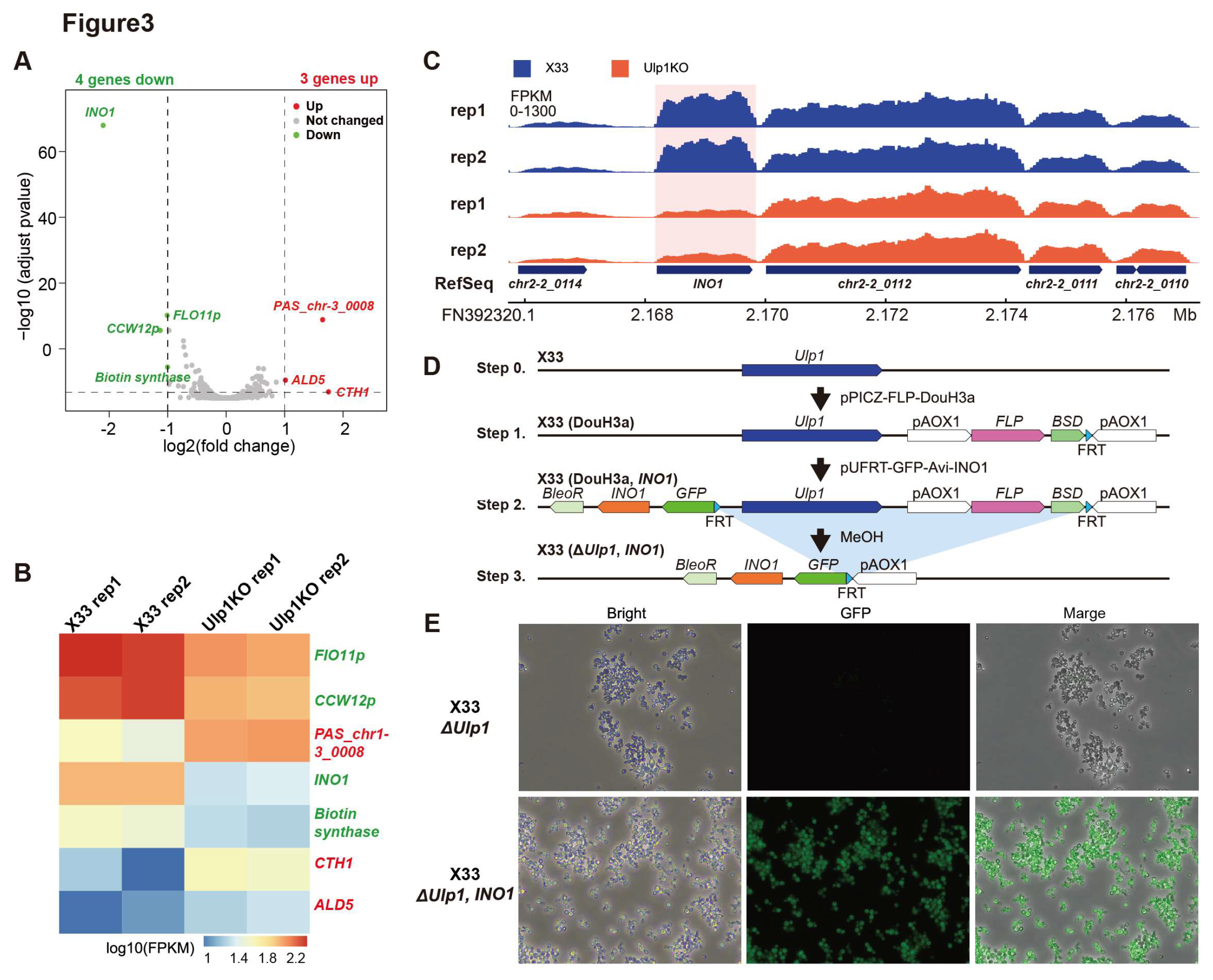
Ulp1 is essential for yeast cell cycles, though the underlying mechanism remains unknown. To uncover this mechanism, we performed RNA-seq on X33 (WT) and
Ulp1 knockout yeast strains. RNA-seq data showed that the expression of four genes, including INO1, was downregulated, consistent with previous reports in
Saccharomyces cerevisiae [
20], and three genes were upregulated (
Figure 3A-C).
INO1 encodes the inositol-3-phosphate synthase enzyme, which catalyzes the rate-limiting step in the biosynthesis of inositol. Deletion of
INO1 results in inositol auxotrophy, meaning the yeast cells require exogenous inositol for growth and survival. Whether Ulp1 regulates cell proliferation through
INO1 expression is still unknown. To address this question, we constructed the
INO1 overexpressed Pichia pastoris strain X33 (DouH3a,
INO1), in which
Ulp1 knockout induces GFP expression and the extra
INO1 expression is controlled by the AOX1 promoter (
Figure 3D). To our surprise, we observed that
Ulp1 knockout cells restored cell proliferation in the X33 (
ΔUlp1,
INO1) strain (
Figure 3E). These data suggest that INO1 can rescue the cell proliferation phenotype of
Ulp1 knockout cells, indicating that Ulp1 regulates cell proliferation through INO1.
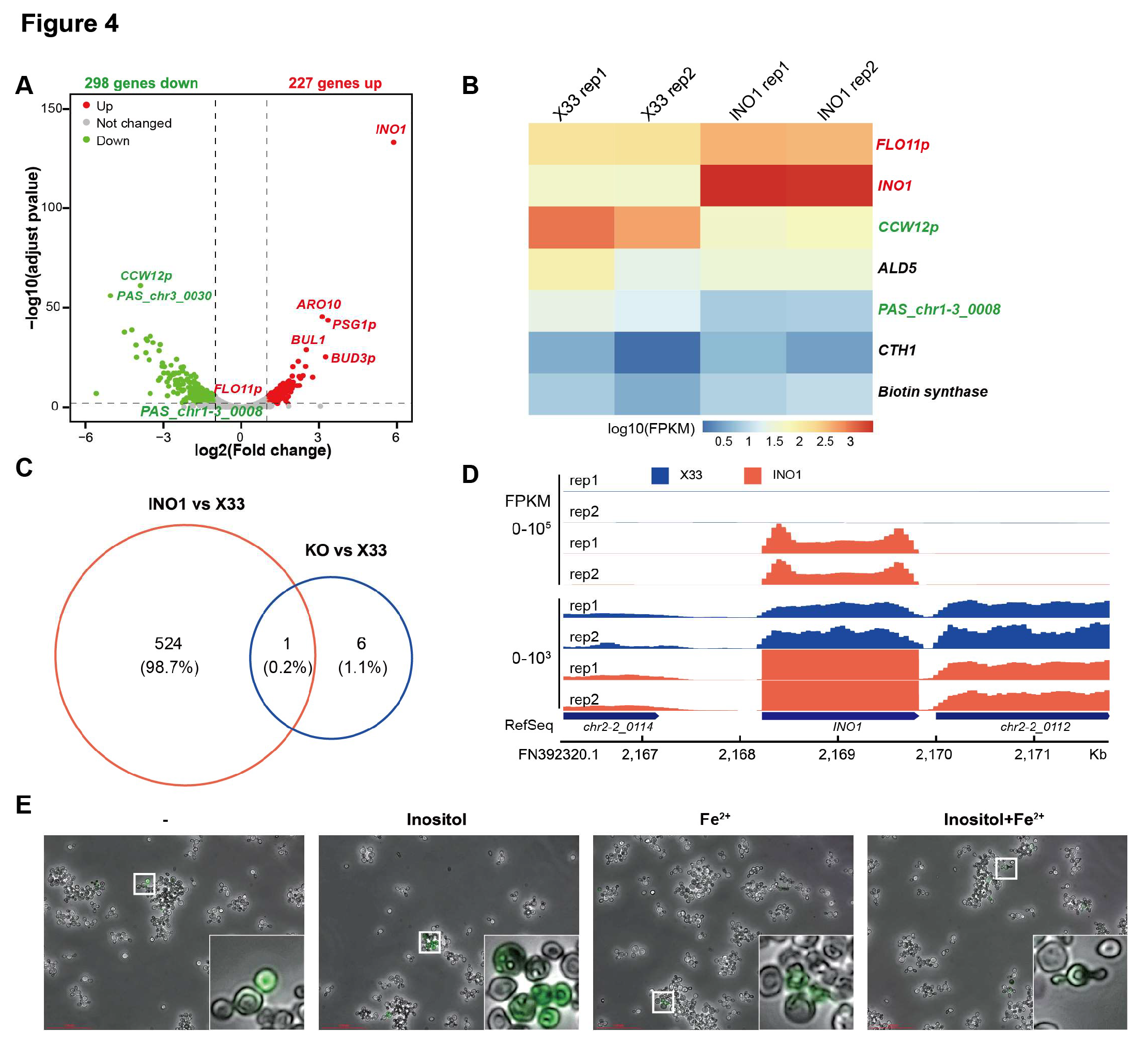
3.4. Addition of Extra Inositol and Fe2⁺ Does Not Rescue Cell Proliferation in Ulp1 Knockout Pichia pastoris
As mentioned before, INO1 catalyzes the rate-limiting step in the biosynthesis of inositol. To understand the mechanism by which INO1 regulates
Pichia pastoris cell proliferation, we employed RNA-seq on X33 (WT) and X33 (
ΔUlp1,
INO1) strains. RNA-seq data showed that the expression of 298 genes was downregulated and 227 genes, including
INO1, were upregulated (
Figure 4A). We found that the expression of six genes, except
CCW12p, was rescued when we overexpressed
INO1 in
Ulp1 knockout cells (
Figure 4B, C). Since
AOX1 promoter is a strong inducible promoter, we observed
INO1 upregulated more than 100-fold compared to X33 (
Figure 4D). Among these rescued genes, we identified
CTH1.
CTH1 encodes an RNA-binding protein that regulates the expression of iron-responsive genes[
21,
22]. The targets of CTH1 include genes involved in iron-containing proteins, iron uptake, and iron utilization pathways. By downregulating these iron-dependent genes, CTH1 helps the cell prioritize the use of limited iron resources during times of iron scarcity. To further investigate, we added extra inositol and Fe
2⁺ to
Ulp1 knockout cells. We observed that additional inositol, Fe
2⁺, and the combination of inositol and Fe
2⁺ were not able to rescue the phenotype of
Ulp1 knockout cells (
Figure 4E). These data suggest that INO1 regulates cell proliferation through the inositol synthesis pathway independently.
4. Discussion
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted. Ulp1 has been reported to regulate cell cycles in yeast for more than two decades, but the mechanisms of how Ulp1 regulates cell cycle progression are still not well understood. Previous research has shown that Ulp1-mediated desumoylation is a critical regulatory mechanism that controls multiple key transitions and events throughout the yeast cell cycle, from the G2/M phase transition to mitotic spindle assembly, chromosome segregation, and mitotic exit. However, in our study, we report that Ulp1 regulates the cell cycle primarily through the desumoylation of INO1 in Pichia pastoris. Overexpression of INO1 in Ulp1-deficient cells restored normal cell proliferation, suggesting that INO1 is the most important target for Ulp1 in regulating cell cycle progression.
To further elucidate the mechanisms, we investigated how INO1 regulates the cell cycle. INO1 is essential for inositol synthesis, and its absence results in inositol auxotrophy[
23]. Initially, we hypothesized that the cell cycle arrest observed in
Ulp1 knockout cells was due to inositol depletion. However, to our surprise, overexpressing
INO1 could rescue the cell cycle arrest phenotype, while the addition of exogenous inositol was unable to do so. Additionally, we found that the major myo-inositol transporter Itr1p is present in
Pichia pastoris, suggesting that INO1 regulates the cell cycle through an inositol-independent pathway. Further investigations are necessary to uncover the precise mechanisms by which INO1 modulates cell cycle progression.
Another important question is whether the desumoylation of proteins other than those regulating
INO1 expression, such as Scs2[
20], is essential for cell cycle control. In Pichia pastoris, the overexpression of
INO1 could rescue the cell cycle arrest phenotype, suggesting that the desumoylation of key cell cycle regulators, such as the mitotic kinase Cdc28/Cdk1[
24,
25], may not be essential. However, we cannot exclude the possibility that INO1 may serve as a bridge to recruit Ulp1, Ulp2, or Ulp3 to target proteins, thereby facilitating their desumoylation. Additional experiments are required to determine the significance of the desumoylation of specific cell cycle regulators.
Furthermore, while some studies in
Saccharomyces cerevisiae have reported that inositol can rescue the phenotypes of
INO1 knockout cells[
26,
27], our findings in
Pichia pastoris indicate that inositol is unable to rescue
INO1 knockdown cells. This suggests that the functions of Ulp1 in Pichia pastoris may differ from those in
Saccharomyces cerevisiae, and that Ulp1 may regulate the cell cycle through different mechanisms in these two yeast species. Further comparative studies between these species are necessary to elucidate these differences.
In summary, our study has found that Ulp1 regulates cell proliferation through INO1 in Pichia pastoris, providing a new perspective on the functions of Ulp1 in cell cycle regulation. However, this discovery also raises additional questions about the diverse mechanisms by which yeast cells regulate their cell cycles.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Amino acid sequence alignment of scUlp1, pUlp1, pUlp2, and pUlp3.
Author Contributions
Junjie Yang , Bo Zhong : performed the experiments, analyzed the data, formal analysis, Data curation, wrote the manuscript. Lan Yang :performed the experiments . Zhan luo, Lei Jia, Kaixi Zheng, Wenjie Tang, Wennan Shang: did plasmids’ construction. Xiaofeng Jiang, Zhengbing Lyu: helped edit and modify the manuscript. Jianqing Chen and Guodong Chen: designed and conceptualized the project, and wrote the manuscript. All authors have read and approved the final manuscript.
Funding
Please add: Project supported by Zhejiang Provincial Public Welfare Technology Application Research Project, China LGN21C010001 and Hunan Provincial Natural Science Foundation of China S2024JJYXQN0312.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The raw data is available in NCBI GEO: GSE274307.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Kroetz, M.B. SUMO: A ubiquitin-like protein modifier. The Yale journal of biology and medicine 2005, 78, 197. [Google Scholar]
- Dohmen, R.J. SUMO protein modification. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2004, 1695, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Kunz, K.; Piller, T.; Müller, S. SUMO-specific proteases and isopeptidases of the SENP family at a glance. J. Cell Sci. 2018, 131, jcs211904. [Google Scholar] [CrossRef]
- Békés, M. The regulation of global deSUMOylation by human SUMO-specific proteases: University of California, San Diego; 2010.
- Li, S.-J.; Hochstrasser, M. A new protease required for cell-cycle progression in yeast. Nature 1999, 398, 246–251. [Google Scholar] [CrossRef]
- Dobson, M.J.; Pickett, A.J.; Velmurugan, S.; Pinder, J.B.; Barrett, L.A.; Jayaram, M.; et al. The 2μm plasmid causes cell death in Saccharomyces cerevisiae with a mutation in Ulp1 protease. Molecular and cellular biology 2005, 25, 4299–4310. [Google Scholar] [CrossRef]
- Damasceno, L.M.; Huang, C.-J.; Batt, C.A. Protein secretion in Pichia pastoris and advances in protein production. Appl. Microbiol. Biotechnol. 2012, 93, 31–39. [Google Scholar] [CrossRef]
- Li, P.; Anumanthan, A.; Gao, X.-G.; Ilangovan, K.; Suzara, V.V.; Düzgüneş, N.; Renugopalakrishnan, V. Expression of Recombinant Proteins in Pichia Pastoris. Appl. Biochem. Biotechnol. 2007, 142, 105–124. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Hunt T, Nasmyth K, Novák B. The cell cycle. The Royal Society; 2011. p. 3494-7.
- Johnson, D.G.; Walker, C.L. Cyclins and cell cycle checkpoints. Annual review of pharmacology and toxicology 1999, 39, 295–312. [Google Scholar] [CrossRef]
- Nigg, E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. BioEssays 1995, 17, 471–480. [Google Scholar] [CrossRef]
- Łukasik, P.; Załuski, M.; Gutowska, I. Cyclin-Dependent Kinases (CDK) and Their Role in Diseases Development–Review. Int. J. Mol. Sci. 2021, 22, 2935. [Google Scholar] [CrossRef] [PubMed]
- Dasso, M. Emerging roles of the SUMO pathway in mitosis. Cell Div. 2008, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Sumoylation regulates diverse biological processes. Cell. Mol. Life Sci. 2007, 64, 3017–3033. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.J.; Proteau, G.A.; Beatty, L.G.; Sadowski, P.D. The FLP recombinase of the 2μ circle DNA of yeast: Interaction with its target sequences. Cell 1985, 40, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, S.; Madden, T.L. BLAST: At the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004, 32, W20–W25. [Google Scholar] [CrossRef]
- Elmore, Z.C.; Donaher, M.; Matson, B.C.; Murphy, H.; Westerbeck, J.W.; Kerscher, O. Sumo-dependent substrate targeting of the SUMO protease Ulp1. BMC Biol. 2011, 9, 1–20. [Google Scholar] [CrossRef]
- Felberbaum, R.; Wilson, N.R.; Cheng, D.; Peng, J.; Hochstrasser, M. Desumoylation of the Endoplasmic Reticulum Membrane VAP Family Protein Scs2 by Ulp1 and SUMO Regulation of the Inositol Synthesis Pathway. Mol. Cell. Biol. 2012, 32, 64–75. [Google Scholar] [CrossRef]
- Martínez-Pastor, M.; Vergara, S.V.; Puig, S.; Thiele, D.J. Negative Feedback Regulation of the Yeast Cth1 and Cth2 mRNA Binding Proteins Is Required for Adaptation to Iron Deficiency and Iron Supplementation. Mol. Cell. Biol. 2013, 33, 2178–2187. [Google Scholar] [CrossRef]
- Puig, S.; Vergara, S.V.; Thiele, D.J. Cooperation of two mRNA-binding proteins drives metabolic adaptation to iron deficiency. Cell Metab. 2008, 7, 555–564. [Google Scholar] [CrossRef]
- Shaldubina, A.; Ju, S.; Vaden, D.L.; Ding, D.; Belmaker, R.H.; Greenberg, M.L. Epi-inositol regulates expression of the yeast INO1 gene encoding inositol-1-P synthase. Mol. Psychiatry 2002, 7, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Enserink, J.M.; Chymkowitch, P. Cell Cycle-Dependent Transcription: The Cyclin Dependent Kinase Cdk1 Is a Direct Regulator of Basal Transcription Machineries. Int. J. Mol. Sci. 2022, 23, 1293. [Google Scholar] [CrossRef] [PubMed]
- Dorison, H. Sumo-Directed Control of the Resolvase Yen1 in Mitotic Cells: Université Paris-Saclay; 2021.
- Deranieh, R.M.; He, Q.; Caruso, J.A.; Greenberg, M.L. Phosphorylation regulates myo-inositol-3-phosphate synthase: a novel regulatory mechanism of inositol biosynthesis. Journal of Biological Chemistry 2013, 288, 26822–26833. [Google Scholar] [CrossRef] [PubMed]
- Deranieh, R.; He, Q.; Caruso, J.; Greenberg, M. Phosphorylation regulates myo-inositol 3-phosphate synthase: A novel regulatory mechanism of inositol biosynthesis (LB190). FASEB J. 2014, 28, LB190. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).