Submitted:
22 October 2024
Posted:
23 October 2024
You are already at the latest version
Abstract
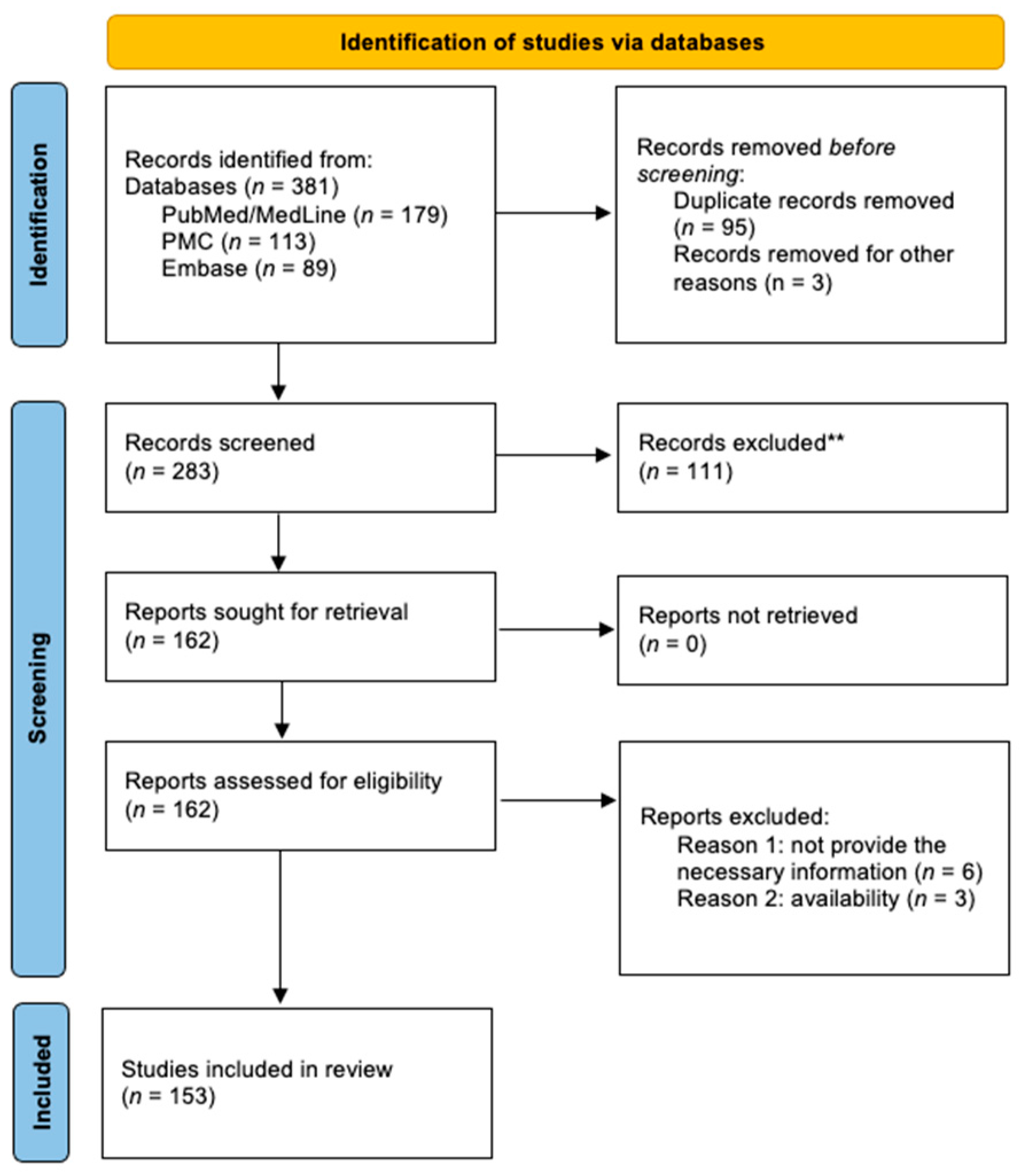
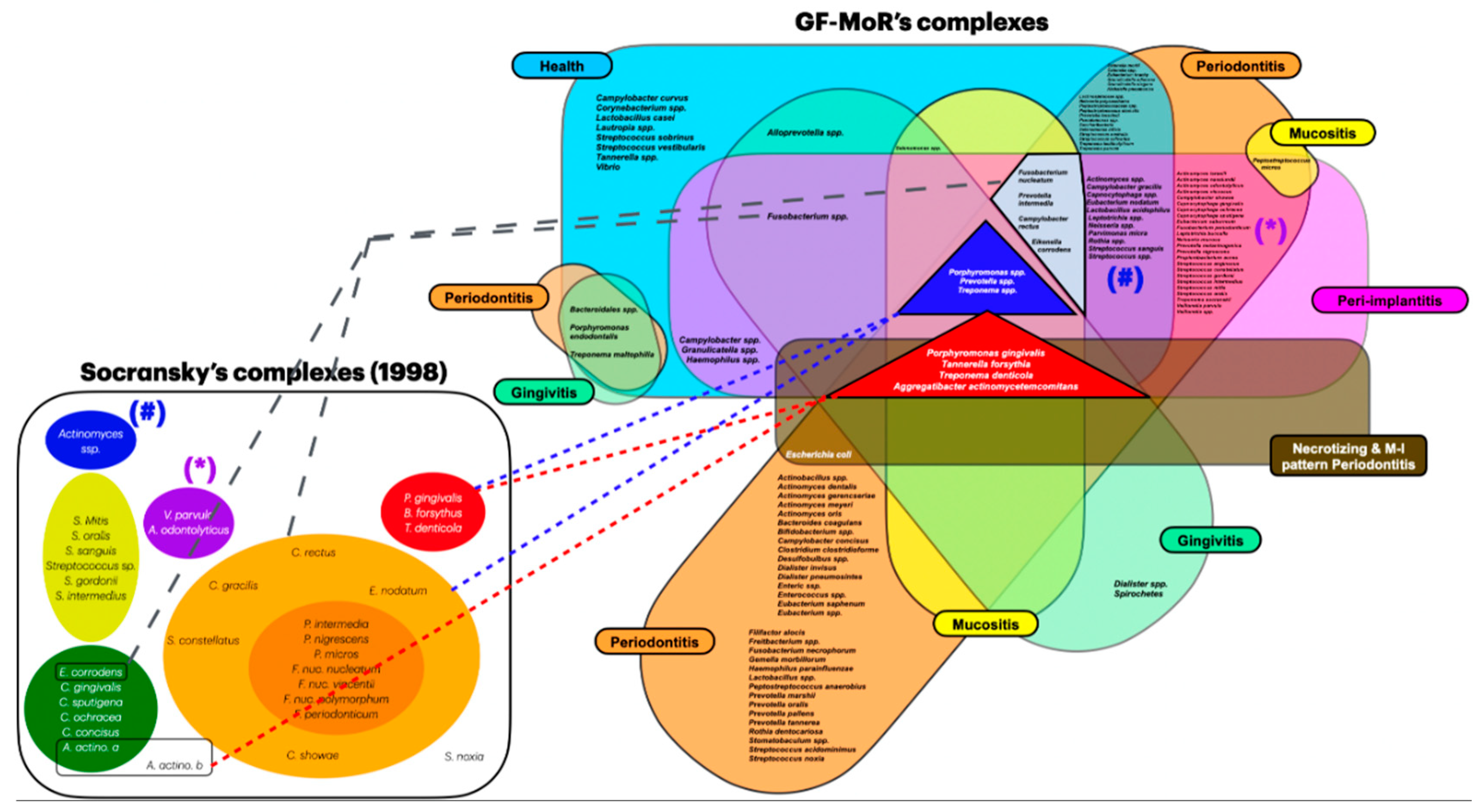
Objective: The aim of this review was to identify newly discovered bacteria from individuals with periodontal/peri-implant diseases and organize them in new clusters (GF-MoR complexes) to update Socransky’s complexes (1998). Methods: The focus question was developed based on the PCC (Population, Concept, Context) strategy: “In patients with periodontal and/or peri-implant disease, what bacteria (microorganisms) were detected through laboratory assays?” The search strategy was applied to PubMed/MEDLINE, PubMed Central, and Embase. The search keyterms, combined with Boolean markers, were: (1) bacteria, (2) microbiome, (3) microorganisms, (4) biofilm, (5) niche, (6) native bacteria, (7) gingivitis), (8) periodontitis, (9) peri-implant mucositis, and (10) peri-implantitis. The search was restricted to 1998–2024 and English language. The bacteria groups in the oral cavity obtained/found were retrieved and were included in the GF-MoR complexes which were based on the disease/condition, presenting six groups: (1) Health, (2) Gingivitis, (3) Peri-implant mucositis, (4) Periodontitis, (5) Peri-implantitis, and (6) Necrotizing and Molar-Incisor (M-O) pattern Periodontitis. The percentual found per group refers to the number of times a specific bacterium was found associated with a particular disease. Results: A total of 381 articles were found; 162 articles were eligible for full-text reading (k=0.92); 9 articles were excluded with justification, and 153 were included in this review (k=0.98). Most of the studies reported results for Health condition, Periodontitis, and Peri-implantitis (3 out of 6 GF-MoR clusters), limiting the number of bacteria found in the other groups. Therefore, it is essential to understand that bacterial colonization is a dynamic process, and the bacteria present in one group can also be present in other one or others, such as observed with the bacteria found in all groups (Porphyromonas gingivalis, Tannarela forsythia, Treponema denticola, and Aggregatibacter actinomycetemcomitans) (GF-MoR’s red Triangle); the second most observed bacteria were grouped at GF-MoR’s blue Triangle: Porphyromonas spp., Prevotela spp., and Treponema spp., present in 5 of the six groups; and the third most detected bacteria were clustered in the grey Polygon (GF-MoR’s grey Polygon): Fusobacterium nucleatum, Prevotella intermedia, Campylobacter rectus, and Eikenella corrodens. These three geometric shapes had the most relevant bacteria for periodontal and peri-implant diseases. Specifically, per group, GF-MoR’s Health had 58 species; GF-MoR’s Gingivitis presented 16 bacteria; the GF-MoR’s Peri-implant mucositis included 17 bacteria; GF-MoR’s Periodontitis group had 101 different bacteria; GF-MoR’s Peri-implantitis presented 61 bacteria; and the last group was a combination of Necrotizing diseases and Molar-Incisor (M-I) pattern Periodontitis, with seven bacteria. Observing the top seven bacteria of all groups, all were gram-negative; Groups 4 and 5 (Periodontitis and Peri-implantitis) presented the same top seven bacteria. Conclusion: For the first time in the literature, GF-MoR’s complexes are presented, gathering the bacteria according to the condition found and including more bacteria than Socransky’s complexes. On this understanding, this study can drive future research into treatment options for periodontal and peri-implant diseases, guiding future studies and collaborating to prevent and worsen systemic conditions. Moreover, it permits the debate about the evolution of the bacterial clusters correlated to periodontal and peri-implant diseases and conditions.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
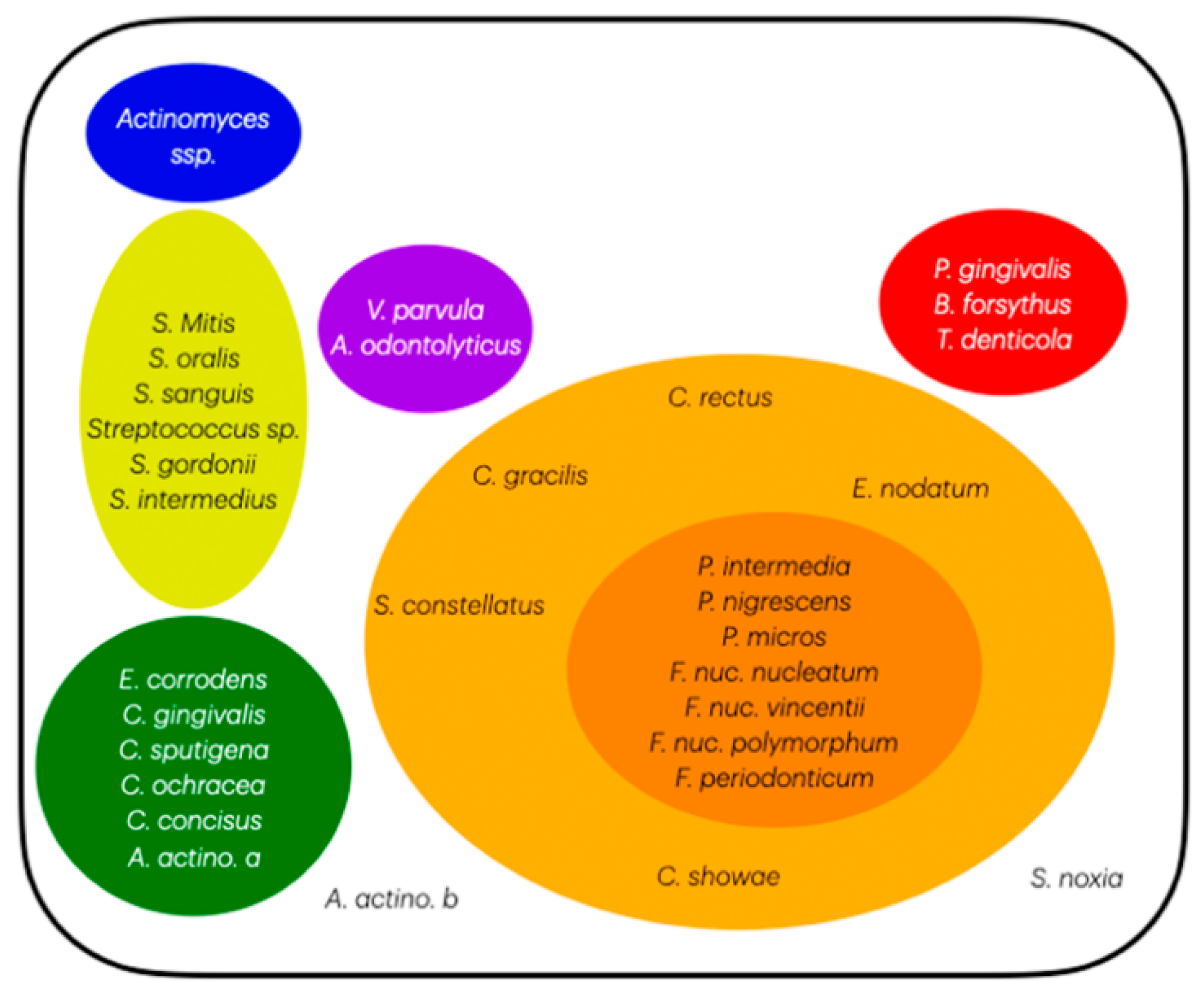
2.4. Socransky’s Complexes
2.5. GF-MoR’s Complexes Organization
3. Results
3.1. Clusters and the Seven Most Relevant Bacteria per Group
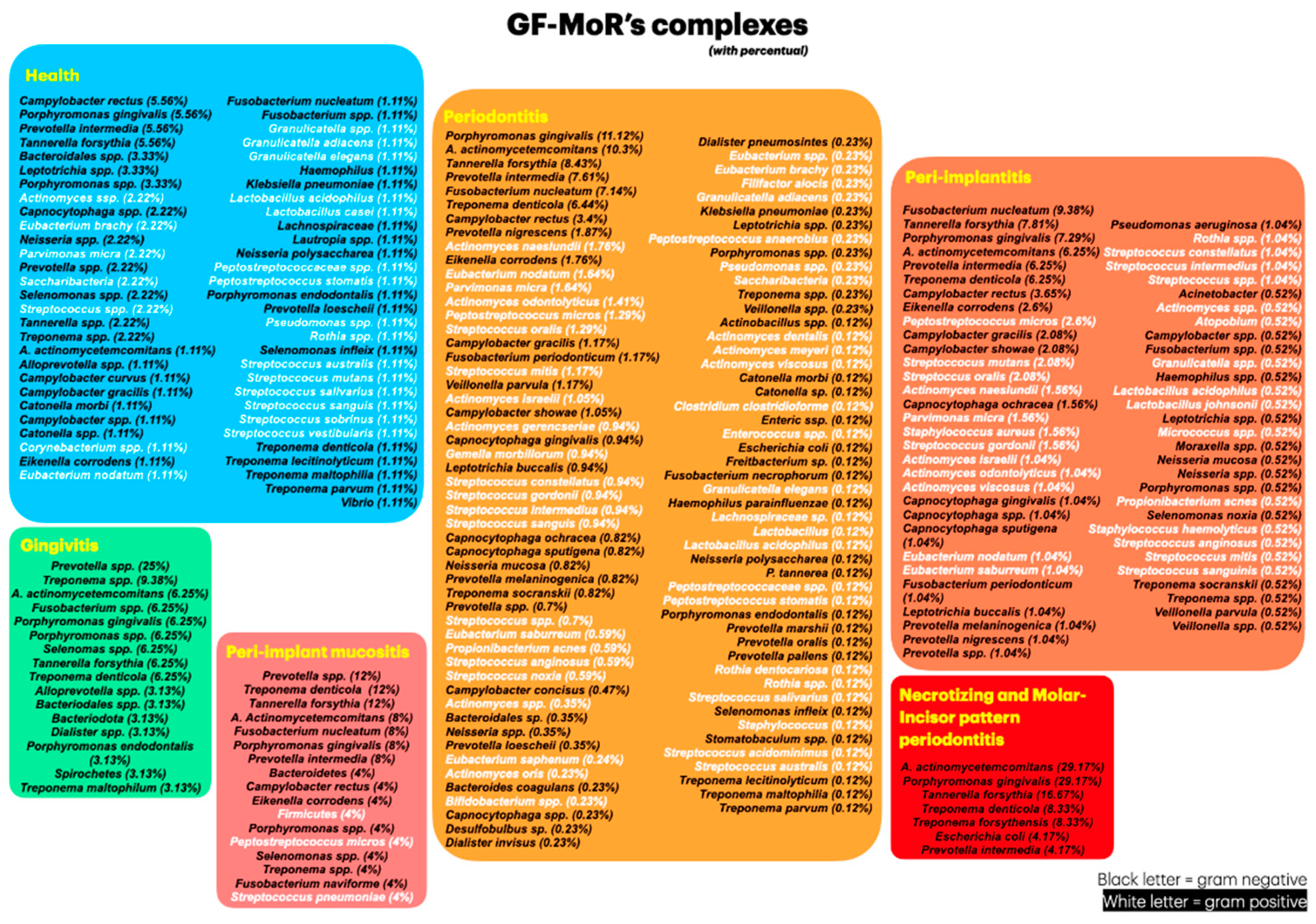
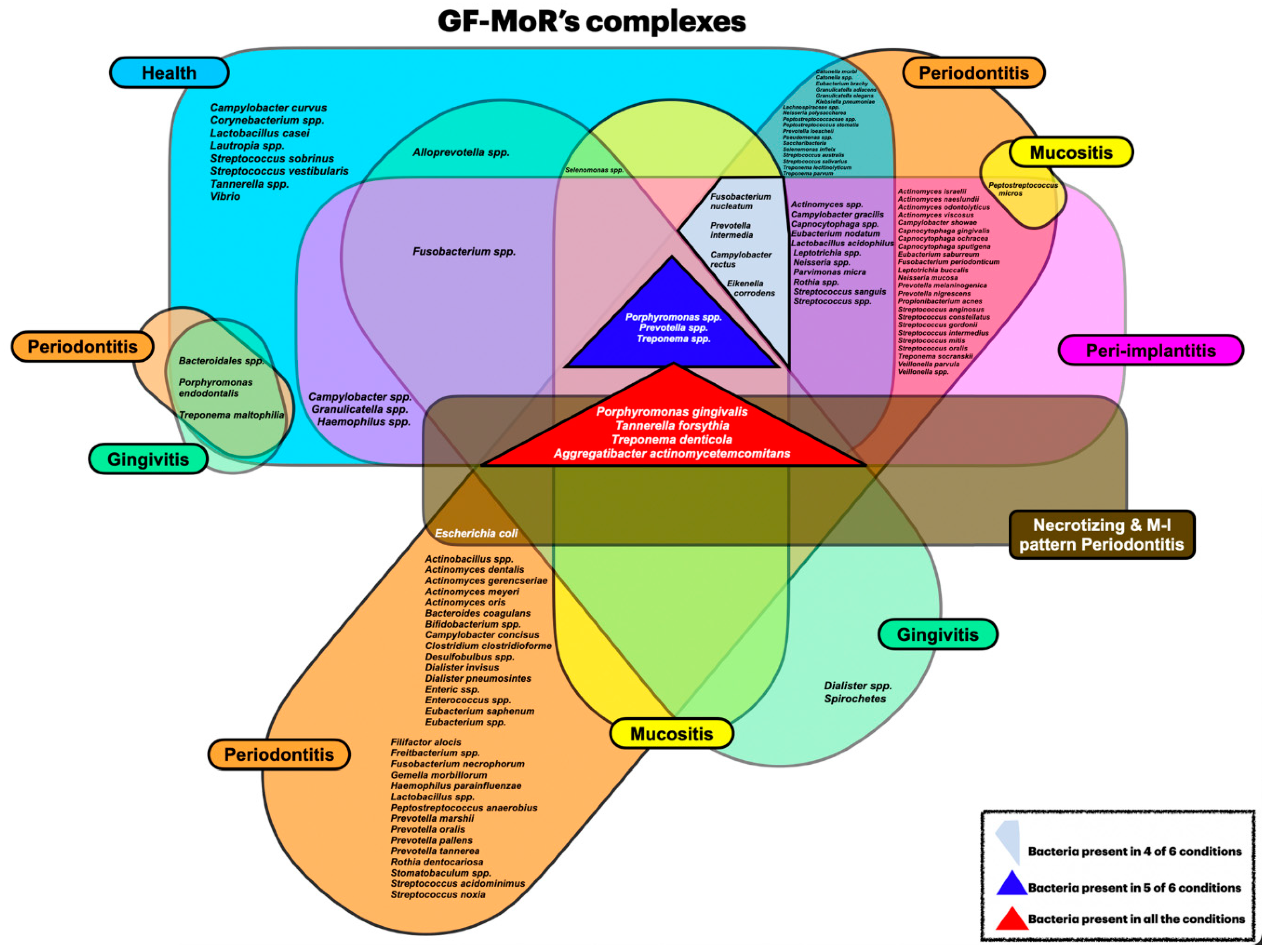
3.2. Periodontal and Peri-Implant Diseases and Conditions
3.3. Modifiers
3.4. Limitations of the Study
4. Conclusions
Funding
Authors’ contributions
Data Availability Statement
Conflicts of Interest
References
- Krom, B.P., Kidwai, S., Ten Cate, J.M. Candida and other fungal species: Forgotten players of healthy oral microbiota. J Dent Res, 2014, 93(5), 445-451. [CrossRef]
- Verma, D., Garg, P.K., Dubey, A.K. Insights into the human oral microbiome. Arch Microbiol, 2018, 200(4), 525-540. [CrossRef]
- Siddiqui, R., Badran, Z., Boghossian, A., Alharbi, A.M., Alfahemi, H., Khan, N.A. The increasing importance of the oral microbiome in periodontal health and disease. Future Sci OA, 2023, 9(8). [CrossRef]
- Fernandes, G.V.O., Akman, A.C., Hakki, S.S. Periodontal therapy for hopeless mandibular anterior teeth: a retrospective case report with a multidisciplinary approach and a 20-year follow-up. Int J Sci Dent, 2024, 66(1), 1-11. (https://periodicos.uff.br/ijosd/article/view/60226). [CrossRef]
- Abusleme, L., Dupuy, A.K., Dutzan, N., Silva, N., Burleson, J.A., Strausbaugh, L.D., Gamonal, J., Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J, 2013, 7(5), 1016–1025. [CrossRef]
- Socransky, S.S., Haffajee, A.D., Cugini, M.A., Smith, C., Kent Jr., R.L. Microbial complexes in subgingival plaque. J Clin Periodontol, 1998, 25(2), 134-144. [CrossRef]
- Yoshida, A., Ansai, T. Microbiological Diagnosis for Periodontal Diseases - A Clinician's Guide, Dr. Jane Manakil (Ed.), ISBN: 978-953-307-818-2, InTech., 2012.
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol, 2014, 35(1), 3-11. [CrossRef]
- Martins, B.G.S., Fernandes, J.C.H., Martins, A.G., Castilho, R.M., Fernandes, G.V.O. Surgical and non-surgical treatment perspectives for peri-implantitis: an overview of systematic reviews. Int J Oral Maxillofac Implants, 2022, 37(4), 660-676. [CrossRef]
- . Fernandes, G.V.O. Peri-implantitis matter: possibilities of treatment but without a strong predictability for solution. Env Dent J. 2021; 3(2).
- Romandini, M., Lima, C., Pedrinaci, I., Araoz, A., Soldini, M. C., Sanz, M. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study. Clin Oral Implants Res, 2020, 32(1), 112–122. [CrossRef]
- Assery, N.M., Jurado, C.A., Assery, M.K., Afrashtehfar, K.I. (2023). Peri-implantitis and systemic inflammation: A critical update. Saudi Dent J, 2023, 35(5), 443–450. [CrossRef]
- Abdulkareem, A.A., Al-Taweel, F.B., Al-Sharqi, A.J.B., Gul, S.S., Sha, A, Chapple, I.L.C. Current concepts in the pathogenesis of periodontitis: from symbiosis to dysbiosis. J Oral Microbiol, 2023, 15(1), 2197779. [CrossRef]
- Fernandes, G. V. O., Fernandes, J.C.H. Revisiting and Rethinking on Staging (Severity and Complexity) Periodontitis from the New Classification System: A Critical Review with Suggestions for Adjustments and a Proposal of a New Flowchart. Preprints 2024, 2024061763. [CrossRef]
- Fernandes, G.V.O., Fernandes, J.C.H. A New Artificial Intelligence (AI) Tool Used to Achieve a More Reliable and Precise Periodontal Risk Assessment, Diagnosis, and Prognosis (GF-PeDRA): A Pilot Study with 221 Patients. Preprints 2024, 2024081646. [CrossRef]
- Maurotto, M., Costa, L.G., Manso, M.C., Mosley, G.A., Fernandes, J.C.H., Fernandes, G.V.O., Castro, F. Correlation between Periodontitis and Gastritis Induced by Helicobacter Pylori: A Comprehensive Review. Microorganisms, 2024, 12, 1579. [CrossRef]
- Tavares, L.T.R., Saavedra-Silva, M., Marcos, J.F.L., Veiga, N.J., Castilho, R.M., Fernandes, G.V.O. Blood and salivary inflammatory biomarkers profile in patients with chronic kidney disease and periodontal disease: A systematic review. Diseases, 2022, 10, 12.
- Hajishengallis, G., Lamont, R.J. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol, 2012, 27(6), 409–419. [CrossRef]
- Bik, E.M., Long, C.D., Armitage, G.C., Loomer, P., Emerson, J., Mongodin, E.F., Nelson, K.E., Gill, S.R., Fraser-Liggett, C.M., Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J, 2010, 4, 962-974.
- Suzuki, N., Yoneda, M., Hirofuji, T. Mixed Red-Complex Bacterial Infection in Periodontitis. Int J Dent, 2013, 2013, 587279. [CrossRef]
- Silva, N., Abusleme, L., Bravo, D., Dutzan, N., García-Sesnich, J., Vernal, R., Hernández, M., Gamonal, J. Host response mechanisms in periodontal diseases. J Applied Oral Sci, 2015, 23(3), 329-355. [CrossRef]
- Bartold, P., Dyke, T. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin Periodontol, 2019, 46(1), 6-11. [CrossRef]
- Krishnamurthy, B., Arshad, M., Jacob, B, Kumar, L., Priya, D. Comparative expression of periodontal pathogens in pregnant women with periodontitis and diabetes in Indian population – A case-control study. IOSR J Dent Med Sci, 2014. [CrossRef]
- Inquimbert, C., Bourgeois, D., Bravo, M., Viennot, S., Tramini, P., Llodra, J.C., Molinari, N., Dussart, C., Giraudeau, N., Carrouel, F. The Oral Bacterial Microbiome of Interdental Surfaces in Adolescents According to Carious Risk. Microorganisms, 2019, 7(9), 319. [CrossRef]
- Tomás, I., Regueira-Iglesias, A., López, M.C., Arias-Bujanda, N., Novoa, L., Balsa-Castro, C., Tomás, M. Quantification by qPCR of Pathobionts in Chronic Periodontitis: Development of Predictive Models of Disease Severity at Site-Specific Level. Front Microbiol, 2017, 8, 1443. [CrossRef]
- Murakami, H. Investigation of bacteria species most involved in peri-implantitis. Open J Stomatol, 2023, 13(10), 353-366. [CrossRef]
- Haraszthy, V., Sreenivasan, P. (2017). Microbiological and clinical effects of an oral hygiene regimen. Contemp Clin Trials Communicat, 2017, 8, 85-89. [CrossRef]
- Mombelli, A., Décaillet, F. The characteristics of biofilms in peri-implant disease. J Clin Periodontol, 2011, 38(S11), 203-213. [CrossRef]
- Han, A., Tsoi, J., Rodrigues, F., Leprince, J., Palin, W. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int J Adhesion Adhesives, 2016, 69, 58-71.
- Fraga, R., Antunes, L., Fontes, K., Küchler, É., Iório, N., Antunes, L. Is antimicrobial photodynamic therapy effective for microbial load reduction in peri-implantitis treatment? A systematic review and meta-analysis. Photochem Photobiol, 2018, 94(4), 752-759. [CrossRef]
- Bamashmous, S., Kotsakis, G.A., Kerns, K.A., Leroux, B.G., Zenobia, C., Chen, D., Trivedi, H.M., McLean, J.S., Darveau, RP. Human variation in gingival inflammation. Proc Natl Acad Sci USA, 2021, 118(27), e2012578118. [CrossRef]
- Rakić, M., Grusovin, M., Canullo, L. The microbiologic profile associated with peri-implantitis in humans: a systematic review. Int J Oral Maxillofac Implants, 2016, 31(2), 359-368. [CrossRef]
- Grecchi, F., Di Girolamo, M., Curá, F., Candotto, V. A new system of implant abutment connection: how to improve a two piece implant system sealing. Oral Implantol, 2017, 10(3), 234-240. [CrossRef]
- Song, L., Jiang, J., Li, J., Zhou, C., Chen, Y., Lu, H., He, F. The characteristics of microbiome and cytokines in healthy implants and peri-implantitis of the same individuals. J Clin Med, 2022, 11(19), 5817. [CrossRef]
- Ito, T., Mori, G., Oda, Y., Hirano, T., Sasaki, H., Honma, S., Furuya, Y., Yajima, Y. Clinical evaluation of periodontal pathogen levels by real-time polymerase chain reaction in peri-implantitis patients. Int J Implant Dent, 2021, 7(1), 105. [CrossRef]
- Vega-Chin, A., Fuente, S., Gómez-Fernández, A., Ortiz-Acuña, L., Mora-González, A., Rodríguez-Masís, R., Ramírez, K. Gingival state and presence of red complex bacteria in 12-year-old schoolchildren. Odovtos Int J Dent Sci, 2022, 24(3), 482-496. [CrossRef]
- Wirth, R., Pap, B., Maróti, G., Vályi, P., Komlósi, L., Barta, N., Strang, O., Minárovits, J., Kovács, K.L. Toward personalized oral diagnosis: Distinct microbiome clusters in periodontitis biofilms. Front Cell Infect Microbiol, 2021, 11. [CrossRef]
- Bertelsen, R.J., Barrionuevo, A.M.P., Shigdel, R., Lie, S.A., Lin, H., Real, F.G., Ringel-Kulka, T., Åstrøm, A.N., Svanes, C. Association of oral bacteria with oral hygiene habits and self-reported gingival bleeding. J Clin Periodontol, 2022, 49(8), 768-781. [CrossRef]
- Deo, P.N., Deshmukh, R. Oral microbiome: Unveiling the fundamentals. Journal of oral and maxillofacial pathology. J Oral Maxillofac Pathol, 2019, 23(1), 122–128. [CrossRef]
- Groeger, S., Doman, E., Chakraborty, T., Meyle, J. Effects of porphyromonas gingivalis infection on human gingival epithelial barrier function in vitro. Eur J Oral Sci, 2010, 118(6), 582-589. [CrossRef]
- Lamont, R., Koo, H., Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol, 2018, 16(12), 745-759. [CrossRef]
- Rahimi, C., Rahimi, B., Padova, D., Rooholghodos, S.A., Bienek, D.R., Luo, X., Kaufman G., Raub, C.B. Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials. Biomicrofluidics, 2018, 12(5), 054106. [CrossRef]
- Ghensi, P., Manghi, P., Zolfo, M., Armanini, F., Pasolli, E., Bolzan, M., Bertelle, A., Dell’Acqua, F., Dellasega, E., Waldner, R., Tessarolo, F., Tomasi, C., Segata, N. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microb, 2020, 6. [CrossRef]
- Koyanagi, T., Sakamoto, M., Takeuchi, Y., Maruyama, N., Ohkuma, M., Izumi, Y. Comprehensive microbiological findings in peri-implantitis and periodontitis. J Clin Periodontol, 2013, 40(3), 218-226. [CrossRef]
- Güntsch, A., Erler, M., Preshaw, P.M., Sigusch, B.W., Klinger, G., Glockmann, E. Effect of smoking on crevicular polymorphonuclear neutrophil function in periodontally healthy subjects. J Periodontal Res, 2006, 41(3), 184-188. [CrossRef]
- Zappacosta, B., Martorana, G.E., Papini, S., Gervasoni, J., Iavarone, F., Fasanella, S., Giardina, B., De Sole, P., Persichilli, S. Morpho-functional modifications of human neutrophils induced by aqueous cigarette smoke extract: Comparison with chemiluminescence activity. Luminescence, 2011, 26(5), 331-335. [CrossRef]
- Graswinckel, J.E., van der Velden, U., van Winkelhoff, A.J., Hoek, F.J., Loos, B.G. Plasma antibody levels in periodontitis patients and controls. J Clin Periodontol, 2004, 31(7), 562-568. [CrossRef]
- Tebloeva, L.M., Revazova, Z.E., Fabrikant, K.G., Dmitrieva, L.A., Gurevich, K.G. Differences in immune response to porphyromonas gingivalis. J Contemp Dent Pract, 2014, 15(5):573-575. [CrossRef]
- Vlachojannis, C., Dye, B.A., Herrera-Abreu, M., Pikdöken, L., Lerche-Sehm, J., Pretzl, B., Celenti, R., Papapanou, P.N. Determinants of serum IGG responses to periodontal bacteria in a nationally representative sample of US adults. J Clin Periodontol, 2010, 37(8), 685-696. [CrossRef]
- Thorstensson, H., Dahlén, G., Hugoson, A. Some suspected periodontopathogens and serum antibody response in adult long-duration insulin-dependent diabetics. J Clin Periodontol, 1995, 22(6), 449-458. [CrossRef]
- Srimaneepong, V., Heboyan, A., Zafar, M.S., Khurshid, Z., Marya, A., Fernandes, G.V.O., Rokaya, D. Fixed Prosthetic Restorations and Periodontal Health: A Narrative Review. J Funct Biomater, 2022, 13, 15. [CrossRef]
- . Partouche, A.J.D., Castro, F., Baptista, A.S., Costa, L.G., Fernandes, J.C.H., Fernandes, G.V.O. Effects of Multibracket Orthodontic Treatment versus Clear Aligners on Periodontal Health: An Integrative Review. Dent J, 2022, 10, 177. [CrossRef]
- Alarcón-Sánchez, M.A., Heboyan, A., Fernandes, G.V.O., Castro-Alarcón, N., Romero-Castro, N.S. Potential Impact of Prosthetic Biomaterials on the Periodontium: A Comprehensive Review. Molecules, 2023, 28, 1075. [CrossRef]
- Ochôa, C., Castro, F., Bulhosa, J.F., Manso, C., Fernandes, J.C.H., Fernandes, G.V.O. Influence of the Probiotic L. reuteri in the Nonsurgical Treatment of Periodontal Disease: A Systematic Review. Microorganisms, 2023, 11, 1449. [CrossRef]
- Purpura, S., Fernandes, G.V.O., Oliveira, F.P., Castro, F.C. Effects of Melatonin in the Non-Surgical Treatment of Periodontitis: A Systematic Review. Appl Sci, 2022, 12, 11698. [CrossRef]
| CODE | Study (Title, Authors, Journal, Year of publication, doi) |
|---|---|
| 7 | Effects of a stabilized stannous fluoride dentifrice on clinical, immunomodulatory, and microbial outcomes in a human experimental gingivitis model. |
| Fine N, Barbour A, Kaura K, Kerns KA, Chen D, Trivedi HM, Gomez J, Sabharwal A, McLean JS, Darveau RP, Glogauer M. J Periodontol. 2024;95(5):421-431. doi: 10.1002/JPER.22-0710 | |
| 8 | Omega-3 nanoemulgel in prevention of radiation-induced oral mucositis and its associated effect on microbiome: a randomized clinical trial. |
| Morsy BM, El Domiaty S, Meheissen MAM, Heikal LA, Meheissen MA, Aly NM. BMC Oral Health. 2023;23(1):612. doi: 10.1186/s12903-023-03276-5 | |
| 10 | Effect of scaling and root planing with and without minocycline HCl microspheres on periodontal pathogens and clinical outcomes: A randomized clinical trial. |
| Arnett MC, Chanthavisouk P, Costalonga M, Blue CM, Evans MD, Paulson DR. J Periodontol. 2023;94(9):1133-1145. doi: 10.1002/JPER.23-0002 | |
| 11 | Effect of laser-assisted reconstructive surgical therapy of peri-implantitis on protein biomarkers and bacterial load. |
| Di Gianfilippo R, Wang CW, Xie Y, Kinney J, Sugai J, Giannobile WV, Wang HL. Clin Oral Implants Res. 2023;34(4):393-403. doi: 10.1111/clr.14059 | |
| 12 | A Placebo-Controlled Trial to Evaluate Two Locally Delivered Antibiotic Gels (Piperacillin Plus Tazobactam vs. Doxycycline) in Stage III-IV Periodontitis Patients. |
| Ilyes I, Rusu D, Rădulescu V, Vela O, Boariu MI, Roman A, Surlin P, Kardaras G, Boia S, Chinnici S, Jentsch HFR, Stratul SI. Medicina (Kaunas). 2023;59(2):303. doi: 10.3390/medicina59020303 | |
| 15 | Clinical Evaluation of Diode Laser-Assisted Surgical Periodontal Therapy: A Randomized Split-Mouth Clinical Trial and Bacteriological Study. |
| Doğan ŞB, Akça G. Photobiomodul Photomed Laser Surg. 2022;40(9):646-655. doi: 10.1089/photob.2022.0035 | |
| 17 | Different scaling and root planing strategies in Turkish patients with aggressive periodontitis: A randomized controlled clinical trial. |
| Mamaklıoğlu D, Karched M, Kuru L, Kuru B, Asikainen S, Doğan B. Int J Dent Hyg. 2022;20(2):347-363. doi: 10.1111/idh.12592 | |
| 19 | Chemomechanical preparation influences the microbial community and the levels of LPS, LTA and cytokines in combined endodontic-periodontal lesions: A clinical study. |
| Gomes BPFA, Berber VB, Marinho ACS, Louzada LM, Arruda-Vasconcelos R, Passini MRZ, Lopes EM, Pecorari VGA, Chen T, Paster BJ. J Periodontal Res. 2022;57(2):341-356. doi: 10.1111/jre.12964 | |
| 21 | The microbiome of dental and peri-implant subgingival plaque during peri-implant mucositis therapy: A randomized clinical trial. |
| Philip J, Buijs MJ, Pappalardo VY, Crielaard W, Brandt BW, Zaura E. J Clin Periodontol. 2022;49(1):28-38. doi: 10.1111/jcpe.13566 | |
| 25 | Evaluation of different materials used for sealing of implant abutment access channel and the peri-implant sulcus microbiota: A 6-month, randomized controlled trial. |
| Rubino CV, Katz BG, Langlois K, Wang HH, Carrion JA, Walker SG, Collier JL, Iacono VJ, Myneni SR. Clin Oral Implants Res. 2021;32(8):941-950. doi: 10.1111/clr.13787 | |
| 28 | Effectiveness of single versus multiple sessions of photodynamic therapy as adjunct to scaling and root planing on periodontopathogenic bacteria in patients with periodontitis. |
| Muzaheed, Acharya S, Hakami AR, Allemailem KS, Alqahtani K, Al Saffan A, Aldakheel FM, Divakar DD. Photodiagnosis Photodyn Ther. 2020;32:102035. doi: 10.1016/j.pdpdt.2020.102035 | |
| 29 | Effects on clinical outcomes of adjunctive moxifloxacin versus amoxicillin plus metronidazole in periodontitis patients harboring Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythia: exploratory analyses from a clinical trial. |
| Ardila CM, Hernández-Casas C, Bedoya-García JA. Quint Int. 2021;52(1):20-29. doi: 10.3290/j.qi.a44927 | |
| 34 | Clinical and microbiological effect of frequent subgingival air polishing on periodontal conditions: a split-mouth randomized controlled trial. |
| Sekino S, Ogawa T, Murakashi E, Ito H, Numabe Y. Odontology. 2020;108(4):688-696. doi: 10.1007/s10266-020-00493-0 | |
| 39 | Clinical and Microbiological Evaluation of Surgical and Nonsurgical Treatment of Aggressive Periodontitis. |
| Cirino CCDS, Vale HFD, Casati MZ, Sallum EA, Casarin RCV, Sallum AW. Braz Dent J. 2019;30(6):577-586. doi: 10.1590/0103-6440201902930 | |
| 41 | Clinical and microbiological outcomes of photodynamic and systemic antimicrobial therapy in smokers with peri-implant inflammation. |
| Deeb MA, Alsahhaf A, Mubaraki SA, Alhamoudi N, Al-Aali KA, Abduljabbar T. Photodiagnosis Photodyn Ther. 2020;29:101587. doi: 10.1016/j.pdpdt.2019.101587 | |
| 43 | Short-term effects of hyaluronic acid on the subgingival microbiome in peri-implantitis: A randomized controlled clinical trial. |
| Soriano-Lerma A, Magán-Fernández A, Gijón J, Sánchez-Fernández E, Soriano M, García-Salcedo JA, Mesa F. J Periodontol. 2020;91(6):734-745. doi: 10.1002/JPER.19-0184 | |
| 46 | Microbiological dynamics of red complex bacteria following full-mouth air polishing in periodontally healthy subjects-a randomized clinical pilot study. |
| Reinhardt B, Klocke A, Neering SH, Selbach S, Peters U, Flemmig TF, Beikler T. Clin Oral Investig. 2019;23(10):3905-3914. doi: 10.1007/s00784-019-02821-3 | |
| 47 | Periodontal condition in Japanese coronary heart disease patients: A comparison between coronary and non-coronary heart diseases. |
| Aoyama N, Kobayashi N, Hanatani T, Ashigaki N, Yoshida A, Shiheido Y, Sato H, Takamura C, Yoshikawa S, Matsuo K, Izumi Y, Isobe M. J Periodontal Res. 2019;54(3):259-265. doi: 10.1111/jre.12626 | |
| 48 | Impact of dental cement on the peri-implant biofilm-microbial comparison of two different cements in an in vivo observational study. |
| Korsch M, Marten SM, Walther W, Vital M, Pieper DH, Dötsch A. Clin Implant Dent Relat Res. 2018;20(5):806-813. doi: 10.1111/cid.12650 | |
| 50 | The Effects of Antimicrobial Peptide Nal-P-113 on Inhibiting Periodontal Pathogens and Improving Periodontal Status. |
| Wang H, Ai L, Zhang Y, Cheng J, Yu H, Li C, Zhang D, Pan Y, Lin L. Biomed Res Int. 2018;2018:1805793. doi: 10.1155/2018/1805793 | |
| 51 | What is the influence of tonsillectomy on the level of periodontal pathogens on the tongue dorsum and in periodontal pockets. |
| Diener VN, Gay A, Soyka MB, Attin T, Schmidlin PR, Sahrmann P. BMC Oral Health. 2018;18(1):62. doi: 10.1186/s12903-018-0521-7 | |
| 52 | Diamond burs versus curettes in root planing: a randomized clinical trial. |
| Türktekin F, Buduneli N, Lappin DF, Türk T, Buduneli E. Aust Dent J. 2018;63(2):242-252. doi: 10.1111/adj.12602 | |
| 53 | Diversity analysis of subgingival microbial bacteria in peri-implantitis in Uygur population. |
| Gao X, Zhou J, Sun X, Li X, Zhou Y. Medicine (Baltimore). 2018;97(5):e9774. doi: 10.1097/MD.0000000000009774 | |
| 54 | Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. |
| Galofré M, Palao D, Vicario M, Nart J, Violant D. J Periodontal Res. 2018;53(3):378-390. doi: 10.1111/jre.12523 | |
| 56 | The effects of Lactobacillus reuteri probiotics combined with azithromycin on peri-implantitis: A randomized placebo-controlled study. |
| Tada H, Masaki C, Tsuka S, Mukaibo T, Kondo Y, Hosokawa R. J Prosthodont Res. 2018;62(1):89-96. doi: 10.1016/j.jpor.2017.06.006 | |
| 59 | Combined application of Er:YAG and Nd:YAG lasers in treatment of chronic periodontitis. A split-mouth, single-blind, randomized controlled trial. |
| Sağlam M, Köseoğlu S, Taşdemir I, Erbak Yılmaz H, Savran L, Sütçü R. J Periodontal Res. 2017;52(5):853-862. doi: 10.1111/jre.12454 | |
| 61 | Bacterial colonization of the peri-implant sulcus in dentate patients: a prospective observational study. |
| Stokman MA, van Winkelhoff AJ, Vissink A, Spijkervet FK, Raghoebar GM. Clin Oral Investig. 2017;21(2):717-724. doi: 10.1007/s00784-016-1941-x | |
| 63 | Impact of implant-abutment connection on osteoimmunological and microbiological parameters in short implants: a randomized controlled clinical trial. |
| Öztürk VÖ, Emingil G, Bostanci N, Belibasakis GN. Clin Oral Implants Res. 2017;28(9):e111-e120. doi: 10.1111/clr.12937 | |
| 66 | Influence of a triclosan toothpaste on periodontopathic bacteria and periodontitis progression in cardiovascular patients: a randomized controlled trial. |
| Seymour GJ, Palmer JE, Leishman SJ, Do HL, Westerman B, Carle AD, Faddy MJ, West MJ, Cullinan MP. J Periodontal Res. 2017;52(1):61-73. doi: 10.1111/jre.12369 | |
| 68 | Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: A split-mouth RCT. |
| Corrêa MG, Oliveira DH, Saraceni CH, Ribeiro FV, Pimentel SP, Cirano FR, Casarin RC. Lasers Surg Med. 2016;48(10):944-950. doi: 10.1002/lsm.22449 | |
| 69 | Subgingivally applied minocycline microgranules in subjects with chronic periodontitis: A randomized clinical and microbiological trial. |
| Chiappe VB, Gómez MV, Rodríguez C, Fresolone M, Romanelli HJ. Acta Odontol Latinoam. 2015;28(2):122-31. doi: 10.1590/S1852-48342015000200005 | |
| 70 | Clinical and Microbiologic Evaluation of Scaling and Root Planing per Quadrant and One-Stage Full-Mouth Disinfection Associated With Azithromycin or Chlorhexidine: A Clinical Randomized Controlled Trial. |
| Fonseca DC, Cortelli JR, Cortelli SC, Miranda Cota LO, Machado Costa LC, Moreira Castro MV, Oliveira Azevedo AM, Costa FO. J Periodontol. 2015;86(12):1340-51. doi: 10.1902/jop.2015.150227 | |
| 73 | Alcohol Consumption and Periodontitis: Quantification of Periodontal Pathogens and Cytokines. |
| Lages EJ, Costa FO, Cortelli SC, Cortelli JR, Cota LO, Cyrino RM, Lages EM, Nobre-Franco GC, Brito JA, Gomez RS. J Periodontol. 2015;86(9):1058-68. doi: 10.1902/jop.2015.150087 | |
| 78 | Microbiologic Observations After Four Treatment Strategies Among Patients With Periodontitis Maintaining a High Standard of Oral Hygiene: Secondary Analysis of a Randomized Controlled Clinical Trial. |
| Preus HR, Dahlen G, Gjermo P, Baelum V. J Periodontol. 2015;86(7):856-65. doi: 10.1902/jop.2015.140620 | |
| 84 | The effect of metronidazole on the presence of P. gingivalis and T. forsythia at 3 and 12 months after different periodontal treatment strategies evaluated in a randomized, clinical trial. |
| Preus HR, Gjermo P, Scheie AA, Baelum V. Acta Odontol Scand. 2015;73(4):258-66. doi: 10.3109/00016357.2014.920106 | |
| 88 | Clinical, microbial, and immune responses observed in patients with diabetes after treatment for gingivitis: a three-month randomized clinical trial. |
| Raslan SA, Cortelli JR, Costa FO, Aquino DR, Franco GC, Cota LO, Gargioni-Filho A, Cortelli SC. J Periodontol. 2015;86(4):516-26. doi: 10.1902/jop.2014.140197 | |
| 89 | Adjunctive moxifloxacin in the treatment of generalized aggressive periodontitis patients: clinical and microbiological results of a randomized, triple-blind and placebo-controlled clinical trial. |
| Ardila CM, Martelo-Cadavid JF, Boderth-Acosta G, Ariza-Garcés AA, Guzmán IC. J Clin Periodontol. 2015;42(2):160-8. doi: 10.1111/jcpe.12345 | |
| 92 | The association of clinical and microbiologic parameters with histologic observations in relatively healthy peri-implant conditions- a preliminary short-term in vivo study. |
| van Brakel R, Meijer GJ, de Putter C, Verhoeven JW, Jansen J, Cune MS. Int J Prosthodont. 2014;27(6):573-6. doi: 10.11607/ijp.3922 | |
| 93 | Pilot study on the clinical and microbiological effect of subgingival glycine powder air polishing using a cannula-like jet. |
| Kargas K, Tsalikis L, Sakellari D, Menexes G, Konstantinidis A. Int J Dent Hyg. 2015;13(3):161-9. doi: 10.1111/idh.12104 | |
| 94 | Microbial signature profiles of periodontally healthy and diseased patients. |
| Lourenço TG, Heller D, Silva-Boghossian CM, Cotton SL, Paster BJ, Colombo AP. J Clin Periodontol. 2014;41(11):1027-36. doi: 10.1111/jcpe.12302 | |
| 95 | Impact of baseline microbiological status on clinical outcomes in generalized aggressive periodontitis patients treated with or without adjunctive amoxicillin and metronidazole: an exploratory analysis from a randomized controlled clinical trial. |
| Guerrero A, Nibali L, Lambertenghi R, Ready D, Suvan J, Griffiths GS, Wilson M, Tonetti MS. J Clin Periodontol. 2014;41(11):1080-9. doi: 10.1111/jcpe.12299 | |
| 96 | Clinical and microbiological effects of systemic azithromycin in adjunct to nonsurgical periodontal therapy in treatment of Aggregatibacter actinomycetemcomitans associated periodontitis: a randomized placebo-controlled clinical trial. |
| Martande SS, Pradeep AR, Singh SP, Kumari M, Naik SB, Suke DK, Singh P. J Investig Clin Dent. 2016;7(1):72-80. doi: 10.1111/jicd.12115 | |
| 98 | Metronidazole and amoxicillin as adjuncts to scaling and root planing for the treatment of type 2 diabetic subjects with periodontitis: 1-year outcomes of a randomized placebo-controlled clinical trial. |
| Miranda TS, Feres M, Perez-Chaparro PJ, Faveri M, Figueiredo LC, Tamashiro NS, Bastos MF, Duarte PM. J Clin Periodontol. 2014;41(9):890-9. doi: 10.1111/jcpe.12282 | |
| 99 | Internal bacterial colonization of implants: association with peri-implant bone loss. |
| Jervøe-Storm PM, Jepsen S, Jöhren P, Mericske-Stern R, Enkling N. Clin Oral Implants Res. 2015;26(8):957-963. doi: 10.1111/clr.12421 | |
| 101 | Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: a secondary analysis of microbiological results from a randomized clinical trial. |
| Soares GM, Mendes JA, Silva MP, Faveri M, Teles R, Socransky SS, Wang X, Figueiredo LC, Feres M. J Clin Periodontol. 2014;41(4):366-76. doi: 10.1111/jcpe.12217 | |
| 103 | Clinical and microbiological effects of levofloxacin in the treatment of chronic periodontitis: a randomized, placebo-controlled clinical trial. |
| Pradeep AR, Singh SP, Martande SS, Naik SB, N P, Kalra N, Suke DK. J Investig Clin Dent. 2015;6(3):170-8. doi: 10.1111/jicd.12091 | |
| 104 | Photodynamic therapy during supportive periodontal care: clinical, microbiologic, immunoinflammatory, and patient-centered performance in a split-mouth randomized clinical trial. |
| Kolbe MF, Ribeiro FV, Luchesi VH, Casarin RC, Sallum EA, Nociti FH Jr, Ambrosano GM, Cirano FR, Pimentel SP, Casati MZ. J Periodontol. 2014;85(8):e277-86. doi: 10.1902/jop.2014.130559 | |
| 107 | Oral prophylaxis and its effects on halitosis-associated and inflammatory parameters in patients with chronic periodontitis. |
| Guentsch A, Pfister W, Cachovan G, Raschke G, Kuepper H, Schaefer O, Eick S. Int J Dent Hyg. 2014;12(3):199-207. doi: 10.1111/idh.12063 | |
| 109 | Oral hygiene reinforcement in the simplified periodontal treatment of 1 hour. |
| Apatzidou DA, Zygogianni P, Sakellari D, Konstantinidis A. J Clin Periodontol. 2014;41(2):149-56. doi: 10.1111/jcpe.12200 | |
| 110 | Photodynamic therapy to treat periimplantitis. |
| Bombeccari GP, Guzzi G, Gualini F, Gualini S, Santoro F, Spadari F. Implant Dent. 2013;22(6):631-8. doi: 10.1097/01.id.0000433592.18679.91 | |
| 112 | Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. |
| Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. J Clin Periodontol. 2013;40(11):1025-35. doi: 10.1111/jcpe.12155 | |
| 113 | The evaluation of enamel matrix derivative on subgingival microbial environment in non-surgical periodontal therapy. |
| Wyganowska-Świątkowska M, Szkaradkiewicz AK, Karpiński TM, Marcinkowski JT. Ann Agric Environ Med. 2013;20(3):431-5. | |
| 115 | Immunological and microbiological findings after the application of two periodontal surgical techniques: a randomized, controlled clinical trial. |
| Kyriazis T, Gkrizioti S, Tsalikis L, Sakellari D, Deligianidis A, Konstantinidis A. J Clin Periodontol. 2013;40(11):1036-42. doi: 10.1111/jcpe.12149 | |
| 116 | Effects of 2 bracket and ligation types on plaque retention: a quantitative microbiologic analysis with real-time polymerase chain reaction. |
| Baka ZM, Basciftci FA, Arslan U. Am J Orthod Dentofacial Orthop. 2013;144(2):260-7. doi: 10.1016/j.ajodo.2013.03.022 | |
| 118 | LPS-induced inflammatory response after therapy of aggressive periodontitis. |
| Shaddox LM, Gonçalves PF, Vovk A, Allin N, Huang H, Hou W, Aukhil I, Wallet SM. J Dent Res. 2013;92(8):702-8. doi: 10.1177/0022034513495242 | |
| 119 | Porphyromonas gingivalis, Treponema denticola and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: a case-control study. |
| Chaparro A, Blanlot C, Ramírez V, Sanz A, Quintero A, Inostroza C, Bittner M, Navarro M, Illanes SE. J Periodontal Res. 2013;48(6):802-9. doi: 10.1111/jre.12074 | |
| 120 | Effect of periodontal therapy on the subgingival microbiota over a 2-year monitoring period. I. Overall effect and kinetics of change. |
| Socransky SS, Haffajee AD, Teles R, Wennstrom JL, Lindhe J, Bogren A, Hasturk H, van Dyke T, Wang X, Goodson JM. J Clin Periodontol. 2013;40(8):771-80. doi: 10.1111/jcpe.12117 | |
| 122 | A randomized controlled clinical trial on the clinical and microbiological efficacy of systemic satranidazole in the treatment of chronic periodontitis. |
| Pradeep AR, Priyanka N, Kalra N, Naik SB. J Int Acad Periodontol. 2013 Apr;15(2):43-50. | |
| 125 | Clinical and microbiological effects of systemic antimicrobials combined to an anti-infective mechanical debridement for the management of aggressive periodontitis: a 12-month randomized controlled trial. |
| Silva-Senem MX, Heller D, Varela VM, Torres MC, Feres-Filho EJ, Colombo AP. J Clin Periodontol. 2013;40(3):242-51. doi: 10.1111/jcpe.12052 | |
| 126 | Periodontal status and bacteremia with oral viridans streptococci and coagulase negative staphylococci in allogeneic hematopoietic stem cell transplantation recipients: a prospective observational study. |
| Raber-Durlacher JE, Laheij AM, Epstein JB, Epstein M, Geerligs GM, Wolffe GN, Blijlevens NM, Donnelly JP. Support Care Cancer. 2013;21(6):1621-7. doi: 10.1007/s00520-012-1706-2 | |
| 127 | Implant decontamination during surgical peri-implantitis treatment: a randomized, double-blind, placebo-controlled trial. |
| de Waal YC, Raghoebar GM, Huddleston Slater JJ, Meijer HJ, Winkel EG, van Winkelhoff AJ. J Clin Periodontol. 2013;40(2):186-95. doi: 10.1111/jcpe.12034 | |
| 129 | Boric acid irrigation as an adjunct to mechanical periodontal therapy in patients with chronic periodontitis: a randomized clinical trial. |
| Sağlam M, Arslan U, Buket Bozkurt Ş, Hakki SS. J Periodontol. 2013;84(9):1297-308. doi: 10.1902/jop.2012.120467 | |
| 130 | Hyaluronic Acid as an adjunct after scaling and root planing: a prospective randomized clinical trial. |
| Eick S, Renatus A, Heinicke M, Pfister W, Stratul SI, Jentsch H. J Periodontol. 2013;84(7):941-9. doi: 10.1902/jop.2012.120269 | |
| 132 | Influence of IL-6 haplotypes on clinical and inflammatory response in aggressive periodontitis. |
| Nibali L, Pelekos G, D'Aiuto F, Chaudhary N, Habeeb R, Ready D, Parkar M, Donos N. Clin Oral Investig. 2013;17(4):1235-42. doi: 10.1007/s00784-012-0804-3 | |
| 135 | Er:YAG laser in the treatment of periodontal sites with recurring chronic inflammation: a 12-month randomized, controlled clinical trial. |
| Krohn-Dale I, Bøe OE, Enersen M, Leknes KN. J Clin Periodontol. 2012;39(8):745-52. doi: 10.1111/j.1600-051X.2012.01912.x | |
| 136 | Effects of systemic sitafloxacin on periodontal infection control in elderly patients. |
| Nakajima T, Okui T, Miyauchi S, Honda T, Shimada Y, Ito H, Akazawa K, Yamazaki K. Gerodontology. 2012;29(2):e1024-32. doi: 10.1111/j.1741-2358.2011.00605.x | |
| 137 | Effect of azithromycin, as an adjunct to nonsurgical periodontal treatment, on microbiological parameters and gingival crevicular fluid biomarkers in generalized aggressive periodontitis. |
| Emingil G, Han B, Ozdemir G, Tervahartiala T, Vural C, Atilla G, Baylas H, Sorsa T. J Periodontal Res. 2012;47(6):729-39. doi: 10.1111/j.1600-0765.2012.01488.x | |
| 138 | Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. |
| Hallström H, Persson GR, Lindgren S, Olofsson M, Renvert S. J Clin Periodontol. 2012;39(6):574-81. doi: 10.1111/j.1600-051X.2012.01884.x | |
| 139 | Clinical and microbiological effects of ozone nano-bubble water irrigation as an adjunct to mechanical subgingival debridement in periodontitis patients in a randomized controlled trial. |
| Hayakumo S, Arakawa S, Mano Y, Izumi Y. Clin Oral Investig. 2013;17(2):379-88. doi: 10.1007/s00784-012-0711-7 | |
| 140 | Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: a 6-month clinical trial. |
| Euzebio Alves VT, de Andrade AK, Toaliar JM, Conde MC, Zezell DM, Cai S, Pannuti CM, De Micheli G. Clin Oral Investig. 2013;17(1):87-95. doi: 10.1007/s00784-012-0703-7 | |
| 142 | Connective tissue graft plus resin-modified glass ionomer restoration for the treatment of gingival recession associated with non-carious cervical lesions: microbiological and immunological results. |
| Santamaria MP, Casati MZ, Nociti FH Jr, Sallum AW, Sallum EA, Aukhil I, Wallet SM, Shaddox LM. Clin Oral Investig. 2013;17(1):67-77. doi: 10.1007/s00784-012-0690-8 | |
| 143 | Effects of oil drops containing Lactobacillus salivarius WB21 on periodontal health and oral microbiota producing volatile sulfur compounds. |
| Suzuki N, Tanabe K, Takeshita T, Yoneda M, Iwamoto T, Oshiro S, Yamashita Y, Hirofuji T. J Breath Res. 2012;6(1):017106. doi: 10.1088/1752-7155/6/1/017106 | |
| 144 | A randomized clinical trial on the clinical and microbiological efficacy of a xanthan gel with chlorhexidine for subgingival use. |
| Matesanz P, Herrera D, Echeverría A, O'Connor A, González I, Sanz M. Clin Oral Investig. 2013;17(1):55-66. doi: 10.1007/s00784-012-0685-5 | |
| 145 | Azithromycin as an adjunctive treatment of generalized severe chronic periodontitis: clinical, microbiologic, and biochemical parameters. |
| Han B, Emingil G, Özdemir G, Tervahartiala T, Vural C, Atilla G, Baylas H, Sorsa T. J Periodontol. 2012;83(12):1480-91. doi: 10.1902/jop.2012.110519 | |
| 146 | The combination of amoxicillin and metronidazole improves clinical and microbiologic results of one-stage, full-mouth, ultrasonic debridement in aggressive periodontitis treatment. |
| Casarin RC, Peloso Ribeiro ED, Sallum EA, Nociti FH Jr, Gonçalves RB, Casati MZ. J Periodontol. 2012;83(8):988-98. doi: 10.1902/jop.2012.110513 | |
| 148 | Efficacy of locally-delivered doxycycline microspheres in chronic localized periodontitis and on Porphyromonas gingivalis. |
| Rao SK, Setty S, Acharya AB, Thakur SL. J Investig Clin Dent. 2012;3(2):128-34. doi: 10.1111/j.2041-1626.2011.00110.x | |
| 149 | Effect of a self-etching adhesive containing an antibacterial monomer on clinical periodontal parameters and subgingival microbiologic composition in orthodontic patients. |
| Amasyali M, Enhos S, Uysal T, Saygun I, Kilic A, Bedir O. Am J Orthod Dentofacial Orthop. 2011;140(4):e147-53. doi: 10.1016/j.ajodo.2011.02.022 | |
| 150 | Microbiologic findings 1 year after partial- and full-mouth scaling in the treatment of moderate chronic periodontitis. |
| Knöfler GU, Purschwitz RE, Eick S, Pfister W, Roedel M, Jentsch HF. Quintessence Int. 2011;42(9):e107-17. | |
| 153 | Clinical and microbiologic results 12 months after scaling and root planing with different irrigation solutions in patients with moderate chronic periodontitis: a pilot randomized trial. |
| Krück C, Eick S, Knöfler GU, Purschwitz RE, Jentsch HF. J Periodontol. 2012;83(3):312-20. doi: 10.1902/jop.2011.110044 | |
| 157 | Microbiologic results after non-surgical erbium-doped:yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: a randomized clinical trial. |
| Persson GR, Roos-Jansåker AM, Lindahl C, Renvert S. J Periodontol. 2011;82(9):1267-78. doi: 10.1902/jop.2011.100660 | |
| 158 | Bacterial adhesion and colonization differences between zirconium oxide and titanium alloys: an in vivo human study. |
| Salihoglu U, Boynuegri D, Engin D, Duman AN, Gokalp P, Balos K. Int J Oral Maxillofac Implants. 2011;26(1):101-7. | |
| 159 | Impact of systemic antimicrobials combined with anti-infective mechanical debridement on the microbiota of generalized aggressive periodontitis: a 6-month RCT. |
| Heller D, Varela VM, Silva-Senem MX, Torres MC, Feres-Filho EJ, Colombo AP. J Clin Periodontol. 2011;38(4):355-64. doi: 10.1111/j.1600-051X.2011.01707.x | |
| 161 | Comparison of gingival crevicular fluid sampling methods in patients with severe chronic periodontitis. |
| Guentsch A, Kramesberger M, Sroka A, Pfister W, Potempa J, Eick S. J Periodontol. 2011;82(7):1051-60. doi: 10.1902/jop.2011.100565 | |
| 162 | Early bacterial colonization and soft tissue health around zirconia and titanium abutments: an in vivo study in man. |
| van Brakel R, Cune MS, van Winkelhoff AJ, de Putter C, Verhoeven JW, van der Reijden W. Clin Oral Implants Res. 2011;22(6):571-7. doi: 10.1111/j.1600-0501.2010.02005.x | |
| 164 | Efficacy and safety of adjunctive local moxifloxacin delivery in the treatment of periodontitis. |
| Flemmig TF, Petersilka G, Völp A, Gravemeier M, Zilly M, Mross D, Prior K, Yamamoto J, Beikler T. J Periodontol. 2011;82(1):96-105. doi: 10.1902/jop.2010.100124 | |
| 167 | Nd:YAG (1064 nm) laser for the treatment of chronic periodontitis: a pilot study. |
| Jensen J, Lulic M, Heitz-Mayfield LJ, Joss A, Lang NP. J Investig Clin Dent. 2010;1(1):16-22. doi: 10.1111/j.2041-1626.2010.00009.x | |
| 172 | Mechanical non-surgical treatment of peri-implantitis: a single-blinded randomized longitudinal clinical study. II. Microbiological results. |
| Persson GR, Samuelsson E, Lindahl C, Renvert S. J Clin Periodontol. 2010;37(6):563-73. doi: 10.1111/j.1600-051X.2010.01561.x | |
| 173 | The recolonization hypothesis in a full-mouth or multiple-session treatment protocol: a blinded, randomized clinical trial. |
| Zijnge V, Meijer HF, Lie MA, Tromp JA, Degener JE, Harmsen HJ, Abbas F. J Clin Periodontol. 2010;37(6):518-25. doi: 10.1111/j.1600-051X.2010.01562.x | |
| 175 | Clinical and microbiologic follow-up evaluations after non-surgical periodontal treatment with erbium:YAG laser and scaling and root planing. |
| Lopes BM, Theodoro LH, Melo RF, Thompson GM, Marcantonio RA. J Periodontol. 2010;81(5):682-91. doi: 10.1902/jop.2010.090300 | |
| 176 | Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum-infected periodontitis patients. |
| Sigusch BW, Engelbrecht M, Völpel A, Holletschke A, Pfister W, Schütze J. J Periodontol. 2010;81(7):975-81. doi: 10.1902/jop.2010.090246 | |
| 178 | Efficacy of amoxicillin and metronidazole combination for the management of generalized aggressive periodontitis. |
| Yek EC, Cintan S, Topcuoglu N, Kulekci G, Issever H, Kantarci A. J Periodontol. 2010;81(7):964-74. doi: 10.1902/jop.2010.090522 | |
| 179 | Microbiologic testing and outcomes of full-mouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis. |
| Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. J Periodontol. 2010;81(1):15-23. doi: 10.1902/jop.2009.090390 | |
| 180 | Photodynamic therapy of persistent pockets in maintenance patients-a clinical study. |
| Rühling A, Fanghänel J, Houshmand M, Kuhr A, Meisel P, Schwahn C, Kocher T. Clin Oral Investig. 2010;14(6):637-44. doi: 10.1007/s00784-009-0347-4 | |
| 183 | One-stage full-mouth versus partial-mouth scaling and root planing during the effective half-life of systemically administered azithromycin. |
| Yashima A, Gomi K, Maeda N, Arai T. J Periodontol. 2009;80(9):1406-13. doi: 10.1902/jop.2009.090067 | |
| 184 | Clinical and microbiological benefits of strict supragingival plaque control as part of the active phase of periodontal therapy. |
| Feres M, Gursky LC, Faveri M, Tsuzuki CO, Figueiredo LC. J Clin Periodontol. 2009;36(10):857-67. doi: 10.1111/j.1600-051X.2009.01471.x | |
| 185 | Quantification of periodontal pathogens by paper point sampling from the coronal and apical aspect of periodontal lesions by real-time PCR. |
| Jervøe-Storm PM, AlAhdab H, Koltzscher M, Fimmers R, Jepsen S. Clin Oral Investig. 2010;14(5):533-41. doi: 10.1007/s00784-009-0333-x | |
| 186 | Full-mouth ultrasonic debridement associated with amoxicillin and metronidazole in the treatment of severe chronic periodontitis. |
| Ribeiro Edel P, Bittencourt S, Zanin IC, Bovi Ambrosano GM, Sallum EA, Nociti FH, Gonçalves RB, Casati MZ. J Periodontol. 2009;80(8):1254-64. doi: 10.1902/jop.2009.080403 | |
| 187 | Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: a double-blinded, placebo-controlled, randomized clinical trial. |
| Mayanagi G, Kimura M, Nakaya S, Hirata H, Sakamoto M, Benno Y, Shimauchi H. J Clin Periodontol. 2009;36(6):506-13. doi: 10.1111/j.1600-051X.2009.01392.x | |
| 188 | Local application of tetracycline solution with a microbrush: an alternative treatment for persistent periodontitis. |
| Bosco JMD, Lopes BMV, Bosco AF, Spolidorio DMP, Marcantonio RAC. Quint Int. 2009;40(1):29-40. | |
| 190 | Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. |
| Guentsch A, Jentsch H, Pfister W, Hoffmann T, Eick S. J Periodontol. 2008;79(10):1894-903. doi: 10.1902/jop.2008.070493 | |
| 191 | Periodontal bacterial profiles in pregnant women: response to treatment and associations with birth outcomes in the obstetrics and periodontal therapy (OPT) study. |
| Novak MJ, Novak KF, Hodges JS, Kirakodu S, Govindaswami M, Diangelis A, Buchanan W, Papapanou PN, Michalowicz BS. J Periodontol. 2008;79(10):1870-9. doi: 10.1902/jop.2008.070554 | |
| 193 | Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. |
| Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rössler R, Sculean A. J Periodontol. 2008;79(9):1638-44. doi: 10.1902/jop.2008.070652 | |
| 194 | Nutritional intervention in patients with periodontal disease: clinical, immunological and microbiological variables during 12 months. |
| Jenzsch A, Eick S, Rassoul F, Purschwitz R, Jentsch H. Br J Nutr. 2009;101(6):879-85. doi: 10.1017/S0007114508047776 | |
| 195 | Periodontal debridement as a therapeutic approach for severe chronic periodontitis: a clinical, microbiological and immunological study. |
| Del Peloso Ribeiro E, Bittencourt S, Sallum EA, Nociti FH Jr, Gonçalves RB, Casati MZ. J Clin Periodontol. 2008;35(9):789-98. doi: 10.1111/j.1600-051X.2008.01292.x | |
| 197 | Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. |
| Chondros P, Nikolidakis D, Christodoulides N, Rössler R, Gutknecht N, Sculean A. Lasers Med Sci. 2009;24(5):681-8. doi: 10.1007/s10103-008-0565-z | |
| 198 | Microbial changes in patients with acute periodontal abscess after treatment detected by PadoTest. |
| Eguchi T, Koshy G, Umeda M, Iwanami T, Suga J, Nomura Y, Kawanami M, Ishikawa I. Oral Dis. 2008;14(2):180-4. doi: 10.1111/j.1601-0825.2007.01370.x | |
| 199 | Clinical and microbiological analysis of subjects treated with Brånemark or AstraTech implants: a 7-year follow-up study. |
| Renvert S, Lindahl C, Renvert H, Persson GR. Clin Oral Implants Res. 2008;19(4):342-7. doi: 10.1111/j.1600-0501.2007.01476.x | |
| 202 | Subantimicrobial dose doxycycline effects on osteopenic bone loss: microbiologic results. |
| Walker C, Puumala S, Golub LM, Stoner JA, Reinhardt RA, Lee HM, Payne JB. J Periodontol. 2007;78(8):1590-601. doi: 10.1902/jop.2007.070015 | |
| 203 | Minocycline HCl microspheres reduce red-complex bacteria in periodontal disease therapy. |
| Goodson JM, Gunsolley JC, Grossi SG, Bland PS, Otomo-Corgel J, Doherty F, Comiskey J. J Periodontol. 2007;78(8):1568-79. doi: 10.1902/jop.2007.060488 | |
| 204 | Microbiological findings after periodontal therapy using curettes, Er:YAG laser, sonic, and ultrasonic scalers. |
| Derdilopoulou FV, Nonhoff J, Neumann K, Kielbassa AM. J Clin Periodontol. 2007;34(7):588-98. doi: 10.1111/j.1600-051X.2007.01093.x | |
| 205 | Effects of full-mouth scaling and root planing in conjunction with systemically administered azithromycin. |
| Gomi K, Yashima A, Nagano T, Kanazashi M, Maeda N, Arai T. J Periodontol. 2007;78(3):422-9. doi: 10.1902/jop.2007.060247 | |
| 206 | Microbiological outcomes of quadrant versus full-mouth root planing as monitored by real-time PCR. |
| Jervøe-Storm PM, AlAhdab H, Semaan E, Fimmers R, Jepsen S. J Clin Periodontol. 2007;34(2):156-63. doi: 10.1111/j.1600-051X.2006.01035.x | |
| 207 | Periodontal healing after non-surgical therapy with a new ultrasonic device: a randomized controlled clinical trial. |
| Christgau M, Männer T, Beuer S, Hiller KA, Schmalz G. J Clin Periodontol. 2007;34(2):137-47. doi: 10.1111/j.1600-051X.2006.01031.x | |
| 209 | Periodontal healing after non-surgical therapy with a modified sonic scaler: a controlled clinical trial. |
| Christgau M, Männer T, Beuer S, Hiller KA, Schmalz G. J Clin Periodontol. 2006;33(10):749-58. doi: 10.1111/j.1600-051X.2006.00981.x | |
| 210 | Effects of metronidazole plus amoxicillin as the only therapy on the microbiological and clinical parameters of untreated chronic periodontitis. |
| López NJ, Socransky SS, Da Silva I, Japlit MR, Haffajee AD. J Clin Periodontol. 2006;33(9):648-60. doi: 10.1111/j.1600-051X.2006.00957.x | |
| 211 | Short-term clinical and microbiologic effects of pocket debridement with an Er:YAG laser during periodontal maintenance. |
| Tomasi C, Schander K, Dahlén G, Wennström JL. J Periodontol. 2006;77(1):111-8. doi: 10.1902/jop.2006.77.1.111 | |
| 212 | Local oxygen therapy for treating acute necrotizing periodontal disease in smokers. |
| Gaggl AJ, Rainer H, Grund E, Chiari FM. J Periodontol. 2006;77(1):31-8. doi: 10.1902/jop.2006.77.1.31 | |
| 213 | Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. |
| Xajigeorgiou C, Sakellari D, Slini T, Baka A, Konstantinidis A. J Clin Periodontol. 2006;33(4):254-64. doi: 10.1111/j.1600-051X.2006.00905.x | |
| 216 | Dynamics of initial subgingival colonization of 'pristine' peri-implant pockets. |
| Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A. Clin Oral Implants Res. 2006;17(1):25-37. doi: 10.1111/j.1600-0501.2005.01194.x | |
| 217 | Supportive periodontal therapy using mechanical instrumentation or 2% minocycline gel: a 12 month randomized, controlled, single masked pilot study. |
| McColl E, Patel K, Dahlen G, Tonetti M, Graziani F, Suvan J, Laurell L. J Clin Periodontol. 2006;33(2):141-50. doi: 10.1111/j.1600-051X.2005.00879.x | |
| 218 | Microbial colonization patterns predict the outcomes of surgical treatment of intrabony defects. |
| Heitz-Mayfield L, Tonetti MS, Cortellini P, Lang NP; European Research Group on Periodontology (ERGOPERIO). J Clin Periodontol. 2006;33(1):62-8. doi: 10.1111/j.1600-051X.2005.00872.x | |
| 227 | Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. |
| Rodrigues RM, Gonçalves C, Souto R, Feres-Filho EJ, Uzeda M, Colombo AP. J Clin Periodontol. 2004;31(6):420-7. doi: 10.1111/j.1600-051X.2004.00493.x | |
| 230 | Quadrant root planing versus same-day full-mouth root planing. II. Microbiological findings. |
| Apatzidou DA, Riggio MP, Kinane DF. J Clin Periodontol. 2004;31(2):141-8. doi: 10.1111/j.0303-6979.2004.00462.x | |
| 231 | Debridement and local application of tetracycline-loaded fibres in the management of persistent periodontitis: results after 12 months. |
| Aimetti M, Romano F, Torta I, Cirillo D, Caposio P, Romagnoli R. J Clin Periodontol. 2004;31(3):166-72. doi: 10.1111/j.0303-6979.2004.00457.x | |
| 232 | Relationship between periodontal pocket sulfide levels and subgingival species. |
| Torresyap G, Haffajee AD, Uzel NG, Socransky SS. J Clin Periodontol. 2003;30(11):1003-10. doi: 10.1034/j.1600-051x.2003.00377.x | |
| 235 | The effect of a triclosan-containing dentifrice on the progression of periodontal disease in an adult population. |
| Cullinan MP, Westerman B, Hamlet SM, Palmer JE, Faddy MJ, Seymour GJ. J Clin Periodontol. 2003;30(5):414-9. doi: 10.1034/j.1600-051x.2003.20030.x | |
| 237 | A split-mouth study on periodontal and microbial parameters in children with complete unilateral cleft lip and palate. |
| Quirynen M, Dewinter G, Avontroodt P, Heidbüchel K, Verdonck A, Carels C. J Clin Periodontol. 2003;30(1):49-56. doi: 10.1034/j.1600-051x.2003.300108.x | |
| 244 | Guided tissue regeneration in intrabony defects using an experimental bioresorbable polydioxanon (PDS) membrane. A 24-month split-mouth study. |
| Christgau M, Bader N, Felden A, Gradl J, Wenzel A, Schmalz G. J Clin Periodontol. 2002;29(8):710-23. doi: 10.1034/j.1600-051x.2002.290808.x | |
| 245 | Initial effect of controlled release chlorhexidine on subgingival microorganisms. |
| Daneshmand N, Jorgensen MG, Nowzari H, Morrison JL, Slots J. J Periodontal Res. 2002;37(5):375-9. doi: 10.1034/j.1600-0765.2002.01003.x | |
| 246 | Local antimicrobial therapy after initial periodontal treatment. |
| Salvi GE, Mombelli A, Mayfield L, Rutar A, Suvan J, Garrett S, Lang NP. J Clin Periodontol. 2002;29(6):540-50. doi: 10.1034/j.1600-051x.2002.290611.x | |
| 250 | Adjunctive effects to non-surgical periodontal therapy of systemic metronidazole and amoxycillin alone and combined. A placebo controlled study. |
| Rooney J, Wade WG, Sprague SV, Newcombe RG, Addy M. J Clin Periodontol. 2002;29(4):342-50. doi: 10.1034/j.1600-051x.2002.290410.x | |
| 256 | One-stage full-mouth disinfection. Long-term microbiological results analyzed by checkerboard DNA-DNA hybridization. |
| De Soete M, Mongardini C, Peuwels M, Haffajee A, Socransky S, van Steenberghe D, Quirynen M. J Periodontol. 2001;72(3):374-82. doi: 10.1902/jop.2001.72.3.374 | |
| 259 | A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. |
| Sigusch B, Beier M, Klinger G, Pfister W, Glockmann E. J Periodontol. 2001;72(3):275-83. doi: 10.1902/jop.2001.72.3.275 | |
| 260 | Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double-blind placebo-controlled study. |
| Winkel EG, Van Winkelhoff AJ, Timmerman MF, Van der Velden U, Van der Weijden GA. J Clin Periodontol. 2001;28(4):296-305. doi: 10.1034/j.1600-051x.2001.028004296.x | |
| 262 | Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. |
| Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. J Periodontol. 2000;71(4):521-32. doi: 10.1902/jop.2000.71.4.521 | |
| 263 | Systemic doxycycline administration in the treatment of periodontal infections (II). Effect on antibiotic resistance of subgingival species. |
| Feres M, Haffajee AD, Goncalves C, Allard KA, Som S, Smith C, Goodson JM, Socransky SS. J Clin Periodontol. 1999;26(12):784-92. doi: 10.1111/j.1600-051x.1999.tb02521.x | |
| 264 | Systemic doxycycline administration in the treatment of periodontal infections (I). Effect on the subgingival microbiota. |
| Feres M, Haffajee AD, Goncalves C, Allard KA, Som S, Smith C, Goodson JM, Socransky SS. J Clin Periodontol. 1999;26(12):775-83. doi: 10.1111/j.1600-051x.1999.tb02520.x | |
| 265 | Repeated local metronidazole-therapy as adjunct to scaling and root planing in maintenance patients. |
| Riep B, Purucker P, Bernimoulin JP. J Clin Periodontol. 1999;26(11):710-5. doi: 10.1034/j.1600-051x.1999.t01-2-261101.x | |
| 266 | Clonal infection with Actinobacillus actinomycetemcomitans following periodontal therapy. |
| Ehmke B, Schmidt H, Beikler T, Kopp C, Karch H, Klaiber B, Flemmig TF. J Dent Res. 1999;78(9):1518-24. doi: 10.1177/00220345990780090601 | |
| 267 | Longitudinal effect of non-surgical treatment and systemic metronidazole for 1 week in smokers and non-smokers with refractory periodontitis: a 5-year study. |
| Söder B, Nedlich U, Jin LJ. J Periodontol. 1999;70(7):761-71. doi: 10.1902/jop.1999.70.7.761 | |
| 268 | Clinical and microbiological effects of initial periodontal therapy in conjunction with amoxicillin and clavulanic acid in patients with adult periodontitis. A randomised double-blind, placebo-controlled study. |
| Winkel EG, van Winkelhoff AJ, Barendregt DS, van der Weijden GA, Timmerman MF, van der Velden U. J Clin Periodontol. 1999;26(7):461-8. doi: 10.1034/j.1600-051x.1999.260708.x | |
| 269 | A 15-month evaluation of the effects of repeated subgingival minocycline in chronic adult periodontitis. |
| van Steenberghe D, Rosling B, Söder PO, Landry RG, van der Velden U, Timmerman MF, McCarthy EF, Vandenhoven G, Wouters C, Wilson M, Matthews J, Newman HN. J Periodontol. 1999;70(6):657-67. doi: 10.1902/jop.1999.70.6.657 | |
| 270 | One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. II. Long-term impact on microbial load. |
| Quirynen M, Mongardini C, Pauwels M, Bollen CM, Van Eldere J, van Steenberghe D. J Periodontol. 1999;70(6):646-56. doi: 10.1902/jop.1999.70.6.646 | |
| 271 | Microbiological and clinical effects of a 1% chlorhexidine-gel in untreated periodontal pockets from adult periodontitis patients. |
| Piccolomini R, Di Bonaventura G, Catamo G, Tumini V, Di Placido G, D'Ercole S, Perfetti G, Paolantonio M. New Microbiol. 1999;22(2):111-6. | |
| 273 | The prevalence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Bacteroides forsythus in humans 1 year after 4 randomized treatment modalities. |
| Shiloah J, Patters MR, Dean JW 3rd, Bland P, Toledo G. J Periodontol. 1998;69(12):1364-72. doi: 10.1902/jop.1998.69.12.1364 | |
| 274 | Clinical and microbiological effects of adjunctive antibiotics in treatment of localized juvenile periodontitis. A controlled clinical trial. |
| Tinoco EM, Beldi MI, Campedelli F, Lana M, Loureiro CA, Bellini HT, Rams TE, Tinoco NM, Gjermo P, Preus HR. J Periodontol. 1998;69(12):1355-63. doi: 10.1902/jop.1998.69.12.1355 | |
| 276 | Additional clinical and microbiological effects of amoxicillin and metronidazole after initial periodontal therapy. |
| Winkel EG, van Winkelhoff AJ, van der Velden U. J Clin Periodontol. 1998;25(11 Pt 1):857-64. doi: 10.1111/j.1600-051x.1998.tb02382.x | |
| 277 | Local metronidazole application in maintenance patients. Clinical and microbiological evaluation. |
| Rudhart A, Purucker P, Kage A, Hopfenmüller W, Bernimoulin JP. J Periodontol. 1998;69(10):1148-54. doi: 10.1902/jop.1998.69.10.1148 | |
| 278 | Effects of topical metronidazole and tetracycline in treatment of adult periodontitis. |
| Lie T, Bruun G, Böe OE. J Periodontol. 1998;69(7):819-27. doi: 10.1902/jop.1998.69.7.819 | |
| 280 | Treatment of periodontal pockets with a diode laser. |
| Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W. Lasers Surg Med. 1998;22(5):302-11. doi: 10.1002/(sici)1096-9101(1998)22:5<302::aid-lsm7>3.0.co;2-t | |
| 281 | The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease. A prospective, controlled clinical trial. |
| Berglundh T, Krok L, Liljenberg B, Westfelt E, Serino G, Lindhe J. J Clin Periodontol. 1998;25(5):354-62. doi: 10.1111/j.1600-051x.1998.tb02455.x | |
| 282 | The effect of a 1-stage full-mouth disinfection on oral malodor and microbial colonization of the tongue in periodontitis. A pilot study. |
| Quirynen M, Mongardini C, van Steenberghe D. J Periodontol. 1998;69(3):374-82. doi: 10.1902/jop.1998.69.3.374 | |
| 283 | The effect of a one-stage full-mouth disinfection on different intra-oral niches. Clinical and microbiological observations. |
| Bollen CM, Mongardini C, Papaioannou W, Van Steenberghe D, Quirynen M. J Clin Periodontol. 1998;25(1):56-66. doi: 10.1111/j.1600-051x.1998.tb02364.x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





