Submitted:
22 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
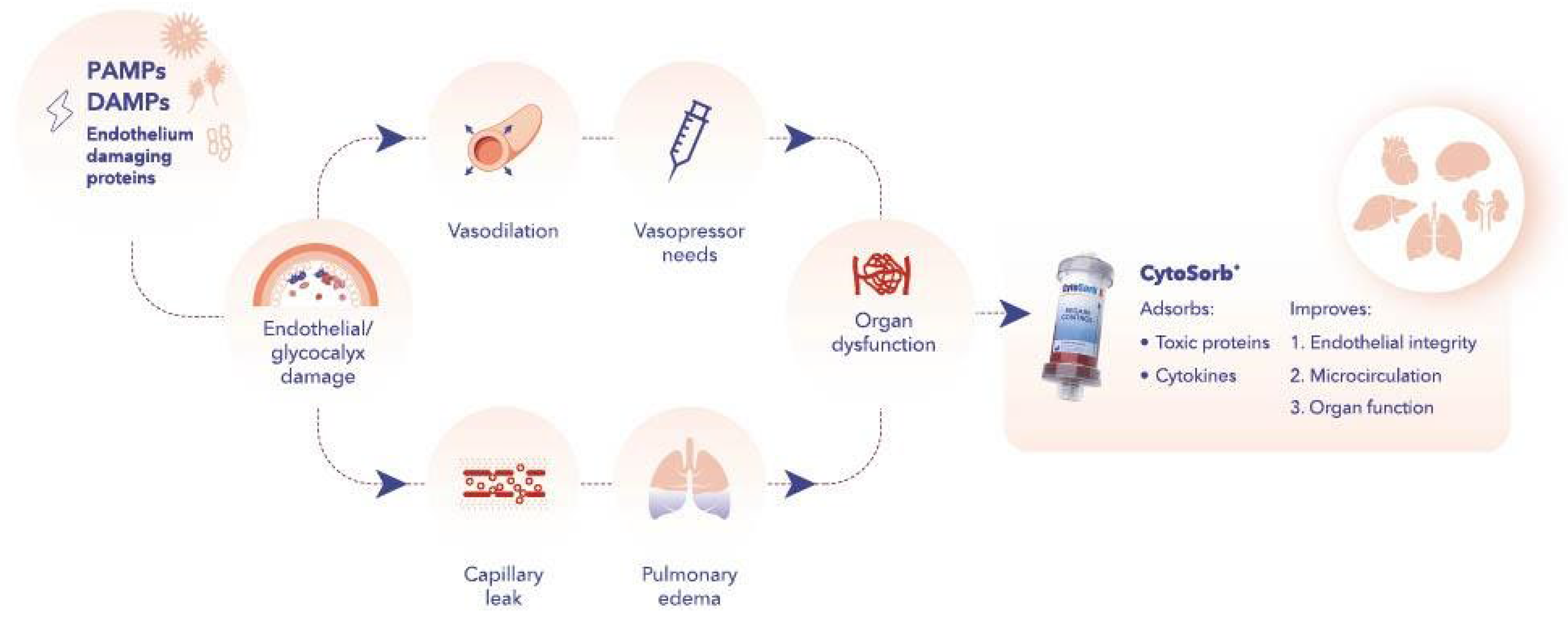
2. The Endothelium and the Glycocalyx
3. Endothelium Protection with HA
4. Other Potential Inflammatory Markers that Could Damage the Glycocalyx
5. Clinical Implications
6. Limitations and Future Perspectives
7. Conclusions
Funding
Competing Interests Statement (even if there are none)
References
- Singer, M., et al., The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA, 2016. 315(8): p. 801-10.10.1001/jama.2016.0287. [CrossRef]
- Ronco, C. and R. Bellomo, History and Development of Sorbents and Requirements for Sorbent Materials. Contrib Nephrol, 2023. 200: p. 2-7.10.1159/000529569. [CrossRef]
- Kellum, J.A., et al., Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med, 2007. 167(15): p. 1655-63.10.1001/archinte.167.15.1655. [CrossRef]
- Peng, Z.Y., M.J. Carter, and J.A. Kellum, Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit Care Med, 2008. 36(5): p. 1573-7.10.1097/CCM.0b013e318170b9a7. [CrossRef]
- Song, M., et al., Cytokine removal with a novel adsorbent polymer. Blood Purif, 2004. 22(5): p. 428-34.10.1159/000080235. [CrossRef]
- Jansen, A., et al., CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit Care, 2023. 27(1): p. 117.10.1186/s13054-023-04391-z. [CrossRef]
- Dilken, O., et al., Successful Reduction of Creatine Kinase and Myoglobin Levels in Severe Rhabdomyolysis Using Extracorporeal Blood Purification (CytoSorb(R)). Blood Purif, 2020. 49(6): p. 743-747.10.1159/000505899. [CrossRef]
- Zuccari, S., et al., Changes in Cytokines, Haemodynamics and Microcirculation in Patients with Sepsis/Septic Shock Undergoing Continuous Renal Replacement Therapy and Blood Purification with CytoSorb. Blood Purif, 2020. 49(1-2): p. 107-113.10.1159/000502540. [CrossRef]
- Bottari, G., et al., Impact of CytoSorb and CKRT on hemodynamics in pediatric patients with septic shock: the PedCyto study. Front Pediatr, 2023. 11: p. 1259384.10.3389/fped.2023.1259384. [CrossRef]
- Bottari, G., et al., Hemoperfusion with Cytosorb in pediatric patients with septic shock: A retrospective observational study. Int J Artif Organs, 2020. 43(9): p. 587-593.10.1177/0391398820902469. [CrossRef]
- Friesecke, S., et al., Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs, 2017. 20(3): p. 252-259.10.1007/s10047-017-0967-4. [CrossRef]
- Hawchar, F., et al., Hemoadsorption in the critically ill-Final results of the International CytoSorb Registry. PLoS One, 2022. 17(10): p. e0274315.10.1371/journal.pone.0274315. [CrossRef]
- Hawchar, F., et al., Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J Crit Care, 2019. 49: p. 172-178.10.1016/j.jcrc.2018.11.003. [CrossRef]
- Kogelmann, K., et al., First Evaluation of a New Dynamic Scoring System Intended to Support Prescription of Adjuvant CytoSorb Hemoadsorption Therapy in Patients with Septic Shock. J Clin Med, 2021. 10(13): p. 2939.10.3390/jcm10132939. [CrossRef]
- Kogelmann, K., et al., Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care, 2017. 21(1): p. 74.10.1186/s13054-017-1662-9. [CrossRef]
- Rugg, C., et al., Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective 'Genetic' Matched Analysis. Biomedicines, 2020. 8(12): p. 539.10.3390/biomedicines8120539. [CrossRef]
- Brouwer, W.P., S. Duran, and C. Ince, Improved Survival beyond 28 Days up to 1 Year after CytoSorb Treatment for Refractory Septic Shock: A Propensity-Weighted Retrospective Survival Analysis. Blood Purif, 2021. 50(4-5): p. 539-545.10.1159/000512309. [CrossRef]
- Brouwer, W.P., et al., Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care, 2019. 23(1): p. 317.10.1186/s13054-019-2588-1. [CrossRef]
- Heymann, M., R. Schorer, and A. Putzu, The Effect of CytoSorb on Inflammatory Markers in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit Care Med, 2023. 51(12): p. 1659-73.10.1097/CCM.0000000000006007. [CrossRef]
- Becker, S., et al., Efficacy of CytoSorb(R): a systematic review and meta-analysis. Crit Care, 2023. 27(1): p. 215.https://doi.org/10.1159/000528706. [CrossRef]
- Vincent, J.L., C. Ince, and P. Pickkers, Endothelial dysfunction: a therapeutic target in bacterial sepsis? Expert Opin Ther Targets, 2021. 25(9): p. 733-748.10.1080/14728222.2021.1988928. [CrossRef]
- Jacob, M., et al., The endothelial glycocalyx affords compatibility of Starling's principle and high cardiac interstitial albumin levels. Cardiovasc Res, 2007. 73(3): p. 575-86.10.1016/j.cardiores.2006.11.021. [CrossRef]
- Reitsma, S., et al., Endothelial glycocalyx structure in the intact carotid artery: a two-photon laser scanning microscopy study. J Vasc Res, 2011. 48(4): p. 297-306.10.1159/000322176. [CrossRef]
- Villalba, N., S. Baby, and S.Y. Yuan, The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front Cell Dev Biol, 2021. 9: p. 711003.10.3389/fcell.2021.711003. [CrossRef]
- Joffre, J., et al., Endothelial Responses in Sepsis. Am J Respir Crit Care Med, 2020. 202(3): p. 361-370.10.1164/rccm.201910-1911TR. [CrossRef]
- Evans, L., et al., Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med, 2021. 47(11): p. 1181-1247.10.1007/s00134-021-06506-y. [CrossRef]
- Kusza, K., et al., Ringer's lactate solution enhances the inflammatory response during fluid resuscitation of experimentally induced haemorrhagic shock in rats. Arch Med Sci, 2018. 14(3): p. 655-670.10.5114/aoms.2017.69771. [CrossRef]
- Ince, C., Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care, 2015. 19 Suppl 3(Suppl 3): p. S8.10.1186/cc14726. [CrossRef]
- Zarbock, A., et al., Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol, 2023. 19(6): p. 401-417.10.1038/s41581-023-00683-3. [CrossRef]
- Saemann, L., et al., Cytokine Adsorber Use during DCD Heart Perfusion Counteracts Coronary Microvascular Dysfunction. Antioxidants (Basel), 2022. 11(11): p. 2280.10.3390/antiox11112280. [CrossRef]
- Denzinger, M., et al., Bioassay for Endothelial Damage Mediators Retrieved by Hemoadsorption. Sci Rep, 2019. 9(1): p. 14522.10.1038/s41598-019-50517-1. [CrossRef]
- Piskovatska, V., et al., Proteins Adsorbed during Intraoperative Hemoadsorption and Their In Vitro Effects on Endothelium. Healthcare (Basel), 2023. 11(3): p. 310.10.3390/healthcare11030310. [CrossRef]
- David, S., et al., Effect of extracorporeal cytokine removal on vascular barrier function in a septic shock patient. J Intensive Care, 2017. 5: p. 12.10.1186/s40560-017-0208-1. [CrossRef]
- Drost, C.C., et al., Interleukin-6 drives endothelial glycocalyx damage in COVID-19 and bacterial sepsis. Angiogenesis, 2024.10.1007/s10456-024-09916-w. [CrossRef]
- Nylen, E.S., et al., Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med, 1998. 26(6): p. 1001-6.10.1097/00003246-199806000-00015. [CrossRef]
- Brabenec, L., et al., Targeting Procalcitonin Protects Vascular Barrier Integrity. Am J Respir Crit Care Med, 2022. 206(4): p. 488-500.10.1164/rccm.202201-0054OC. [CrossRef]
- Mehta, Y., et al., Modulating the Inflammatory Response With Hemadsorption (CytoSorb) in Patients Undergoing Major Aortic Surgery. J Cardiothorac Vasc Anesth, 2021. 35(2): p. 673-675.10.1053/j.jvca.2020.06.028. [CrossRef]
- Akil, A., et al., Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac Cardiovasc Surg, 2021. 69(3): p. 246-251.10.1055/s-0040-1708479. [CrossRef]
- Öveges, N., et al., Early cytokine adsorption in septic shock (ACESS-trial): results of a proof concept, pilot study, in ISICEM. 2018, Crit Care: Brussels. p. P113.
- Cyrille, N.B., P.A. Villablanca, and H. Ramakrishna, Soluble urokinase plasminogen activation receptor--An emerging new biomarker of cardiovascular disease and critical illness. Ann Card Anaesth, 2016. 19(2): p. 214-6.10.4103/0971-9784.179588. [CrossRef]
- Vicka, V., et al., Kinetics of SuPAR hemoadsorption in critical COVID-19 patients on renal replacement therapy. BMC Nephrol, 2022. 23(1): p. 371.10.1186/s12882-022-03003-2. [CrossRef]
- Roca, N., et al., Relationship between soluble urokinase-type plasminogen activator receptor and serum biomarkers of endothelial activation in patients with idiopathic nephrotic syndrome. Clin Kidney J, 2021. 14(2): p. 543-549.10.1093/ckj/sfz173. [CrossRef]
- Nusshag, C., et al., suPAR links a dysregulated immune response to tissue inflammation and sepsis-induced acute kidney injury. JCI Insight, 2023. 8(7): p. e165740.10.1172/jci.insight.165740. [CrossRef]
- Schenk, H., et al., Removal of focal segmental glomerulosclerosis (FSGS) factor suPAR using CytoSorb. J Clin Apher, 2017. 32(6): p. 444-452.10.1002/jca.21538. [CrossRef]
- Rumpret, M., et al., Inhibitory pattern recognition receptors. J Exp Med, 2022. 219(1).10.1084/jem.20211463. [CrossRef]
- Li, L. and Y.Q. Lu, The Regulatory Role of High-Mobility Group Protein 1 in Sepsis-Related Immunity. Front Immunol, 2020. 11: p. 601815.10.3389/fimmu.2020.601815. [CrossRef]
- Yang, R., et al., HMGB1 and Extracellular Histones Significantly Contribute to Systemic Inflammation and Multiple Organ Failure in Acute Liver Failure. Mediators Inflamm, 2017. 2017: p. 5928078.10.1155/2017/5928078. [CrossRef]
- Shen, X. and W.Q. Li, High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol, 2015. 21(5): p. 1424-35.10.3748/wjg.v21.i5.1424. [CrossRef]
- Abrams, S.T., et al., Circulating Histones Are Mediators of Trauma-associated Lung Injury. American Journal of Respiratory and Critical Care Medicine, 2013. 187(2): p. 160-169.10.1164/rccm.201206-1037OC. [CrossRef]
- Shaw, R.J., et al., Circulating histones play a central role in COVID-19-associated coagulopathy and mortality. Haematologica, 2021. 106(9): p. 2493-2498.10.3324/haematol.2021.278492. [CrossRef]
- Gruda, M.C., et al., Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb(R) sorbent porous polymer beads. PLoS One, 2018. 13(1): p. e0191676.10.1371/journal.pone.0191676. [CrossRef]
- Weber, B., et al., Effects of Circulating HMGB-1 and Histones on Cardiomyocytes-Hemadsorption of These DAMPs as Therapeutic Strategy after Multiple Trauma. J Clin Med, 2020. 9(5): p. 1421.10.3390/jcm9051421. [CrossRef]
- Malbrain, M.L., et al., Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther, 2014. 46(5): p. 361-80.10.5603/AIT.2014.0060. [CrossRef]
- Russell, J.A., Vasopressor therapy in critically ill patients with shock. Intensive Care Med, 2019. 45(11): p. 1503-1517.10.1007/s00134-019-05801-z. [CrossRef]
- Duran, S., et al., Sublingual Microcirculatory Evaluation of Extracorporeal Hemoadsorption with CytoSorb(R) in Abdominal Sepsis: A Case Report. Blood Purif, 2022. 51(7): p. 634-638.10.1159/000518903. [CrossRef]
- Duranteau, J., et al., The future of intensive care: the study of the microcirculation will help to guide our therapies. Crit Care, 2023. 27(1): p. 190.10.1186/s13054-023-04474-x. [CrossRef]
- Traeger, K., et al., Cytokine Reduction in the Setting of an ARDS-Associated Inflammatory Response with Multiple Organ Failure. Case Rep Crit Care, 2016. 2016: p. 9852073.10.1155/2016/9852073. [CrossRef]
- Hayanga, J.W.A., et al., Extracorporeal hemoadsorption in critically ill COVID-19 patients on VV ECMO: the CytoSorb therapy in COVID-19 (CTC) registry. Crit Care, 2023. 27(1): p. 243.10.1186/s13054-023-04517-3. [CrossRef]
- Kogelmann, K., et al., Impact of CytoSorb Hemoadsorption Therapy on Fluid Balance in Patients with Septic Shock. J Clin Med, 2024. 13(1): p. 294.10.3390/jcm13010294. [CrossRef]
- Szigetvary, C.E., et al., Hemoadsorption as Adjuvant Therapy in Acute Respiratory Distress Syndrome (ARDS): A Systematic Review and Meta-Analysis. Biomedicines, 2023. 11(11): p. 3068.10.3390/biomedicines11113068. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




