3.7 Elemental Analysis
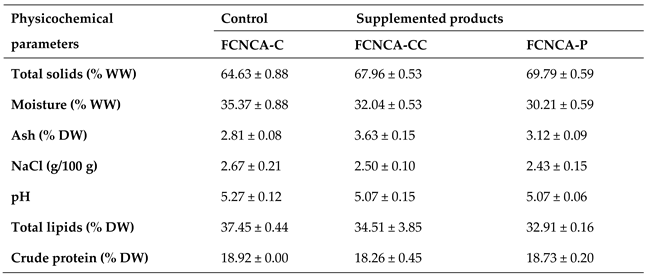
The concentrations of minerals and trace elements for seaweed and the FCNCAs are presented in
Table 2, and the
p-values of supplemented products in supplementary material (
Table S3).
For the FCNCAs, calcium (Ca) ranged from 0.81 to 1.77 g·kg
–1, showing a significant difference between FCNCA-C and FCNCA-CC (
p = 0.0495). The results also show
that C. crispus has higher levels of Ca (4.29 g·kg
–1) than
Porphyra sp. (1.77 g·kg
–1,), and this ends up having a decisive impact on the paste of FCNCA-CC (1.07 g·kg
–1). As Boukid et al. reported, plant-based products have lower calcium content when compared to dairy products. However, the values determined in this study are higher than those observed for cashew cheese brie (0.41 g·kg
–1) without any type of supplementation [
19].
Potassium (K) content in FCNCAs ranged from 2.59 to 3.79 g·kg
–1, showing significant differences between FCNCA-C and FCNCA-CC (
p=0.0495) reflecting the higher K content found in
C. crispus. When compared to fermented cashew cheese brie (1.76 g·kg
–1) [
19], all FCNCAs levels are higher, especially when supplemented with
C. crispus (3.79 g·kg
–1).
Magnesium (Mg) ranges from 3.13 to 3.51 g·kg
–1, showing a significant difference between FCNCA-C and FCNCA-CC (
p = 0.0495), with
C. crispus exhibiting a positive effect when added to the paste, which is by the obtained results for seaweeds, namely 4.26 for
Porphyra sp. and 6.82 for
C. crispus. Magnesium (Mg) deficiency is common in humans [
36], playing an important role in the pathogenesis of ischemic heart disease, congestive heart failure, cardiac arrhythmias, and hypertension, among others [
37], and as such is a critical mineral in the human body [
38].
Sodium (Na) ranged from 6.19 to 9.53 g·kg–1 and showed significant differences between all samples (p = 0.0495) with supplemented products presenting higher values than control, which are directly related to the contents determined for seaweeds, namely, 31.81 g·kg–1 for C. crispus and 22.40 g·kg–1 for Porphyra sp. The values obtained for control (6.19 g·kg–1) are in agreement with values reported by Chen et al. for cashew cheese brie (6.26 g·kg–1).
Phosphorus (P) ranged from 6.64 to 7.98 g·kg–1 and showed significant differences between FCNCA-C and FCNCA-CC (p = 0.0495), since the P levels measured in C. crispus are very low (1.70 g·kg–1).
Regarding trace elements, iron (Fe) levels range from 48.21 to 60.14 mg·kg–1, showing significant differences between FCNCA-C and FCNCA-CC, and FCNCA-CC and FCNCA-P (p = 0.0495). The high Fe content found in C. crispus (123.95 mg·kg–1) significantly impacted the supplemented cheese, contrary to Porphyra sp. (79.96 mg·kg–1). However, all values obtained for FCNCAs are higher than the value reported by Chen et al. for cashew cheese brie (17 mg·kg–1).
Iodine (I) ranged from – 0.07 to 1.26 mg·kg–1 and showed significant differences between FCNCA-C and FCNCA-CC, and FCNCA-CC and FCNCA-P (p = 0.0495), which can be attributed to the higher iodine content of C. crispus (29.33 mg·kg–1). The results for FCNCA-C and FCNCA-P are below the detection limit, and the low percentage (2%) of Porphyra sp. seaweed used for supplementation can justify this.
In a recent study carried out by Clegg et al. [
7], of 109 cheese alternatives available in the UK, none of them were fortified with iodine, an essential nutrient required to produce thyroid hormones, which play a crucial role in growth mechanisms and the development of tissues [
7,
39].
Manganese (Mn) ranged from 14.88 to 15.84 mg·kg–1 and did not show significant differences among analyzed samples (p > 0.05).
Selenium (Se) ranges from 2.05 to 2.54 mg·kg
–1, showing a significant difference between FCNCA-C and FCNCA-CC (
p = 0.0495). Although
Porphyra sp. content is higher (2.49 mg·kg
–1) than that of
C. crispus (0.84 mg·kg
–1), its addition to the paste had no enrichment effect, contrary to
C. crispus. Selenium (Se) is an important oligoelement playing a crucial role in the antioxidant defense system due to its requirement by the Se-dependent GSH-Px, which is involved in cellular antioxidant protection [
40].
Zinc (Zn) ranged from 40.13 to 65.76 mg·kg–1, showing significant differences between FCNCA-C and FCNCA-CC, and FCNCA-CC and FCNCA-P (p = 0.0495), with FCNCA-CC presenting the best results, which are in agreement with the content found in C. crispus (66.24 mg·kg–1).
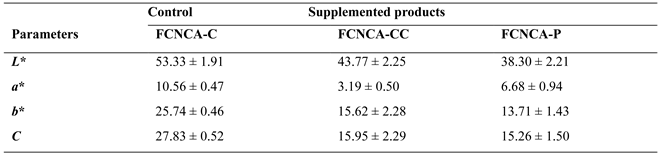
3.8 Color
The color of FCNCAs was significantly influenced by adding seaweeds (
Table 3). The
p-values are presented in supplementary material (
Table S4). Lightness (
L*) is significantly different for FCNCA-C
vs. FCNCA-CC and FCNCA-C
vs. FCNCA-P (
p = 0.0002), and FCNCA-CC
vs. FCNCA-P (
p = 0.0012). Fermented cashew nut cheese alternative with
Porphyra sp. (FCNCA-P) recorded the lowest value (38.30) for
L*, followed by FCNCA-CC (43.77), being the highest for FCNCA-C (53.33),
i.e., the pigments from red seaweeds affected the lightness of FCNCAs, specially
Porphyra sp..
The redness to greenness (
a*) shows significant differences among all the samples (
p=0.0002). Fermented cashew nut cheese alternative with
C. crispus (FCNCA-CC) exhibits the lowest value (3.19), followed by FCNCA-P (6.68) with the highest value for FCNCA-C (10.56). Seaweeds of the division Rhodophyta are a valuable source of chlorophyll
a and
d, phycobilins (allophycocyanin (APC), R-phycoerythrin (R-PE), and R-phycocyanin (R-PC)), carotenoids (
α-, and
β-carotene) and xanthophylls (lutein) which influenced the color of FCNCAs [
41,
42].
C. crispus has a lower value of total pigment content (0.52 mg/g), with
β-carotene (73.76%) as the main pigment, followed by chlorophyll
a (26.17%), and lutein (0.061%) [
43]. However, in dried form (DF) and after hydration treatment (HT),
β-carotene is not detectable in
C. crispus. At the same time, phycoerythrin (∆λ=10) decreases from 528.3 ± 69.0 mg/Kg dry matter (DF) to 525.6 ± 74.2 mg/Kg dry matter (HT); phycocyanin (∆λ=40), increases from 149.2 ± 22.8 mg/Kg dry matter (DF) to 232.5 ± 27.0 mg/Kg dry matter (HT); and lutein from not detected (DF) to 1.80 ± 0.17 mg/Kg dry matter (HT) [
44]. In the case of
Porphyra spp., besides phycobiliproteins such as phycoerythrin (PE) and phycocyanin (PC) pigments, chlorophylls
a and
d are also present, as well as several carotenoids (
β-carotene,
α-carotene, lutein, zeaxanthin, violaxanthin, and fucoxanthin) [
45]. In the present case, the pigments of both seaweeds affect the redness to greenness (
a*) parameter, being the values for the FCNCAs closer to the greenish direction. Due to the high fat content and the lower polarity of chlorophylls (compared to phycobiliproteins), it is possible that there was better solubilization of chlorophylls in the cashew paste.
Yellowness to blueness (b*) is positive in all FCNCAs, therefore in the yellow range of color values, but significant differences were found between FCNCA-C and FCNCA-CC, between FCNCA-C and FCNCA-P (p=0.0002), and between FCNCA-CC and FCNCA-P (p=0.0494). Fermented cashew nut cheese alternative with Porphyra sp. (FCNCA-P) presents the lowest value (13.71), followed by FCNCA-CC (15.62), and the highest by FCNCA-C (25.74). These values also result from the presence of the various seaweed pigments referred to, contributing to less yellowness.
Chroma (C*) values, measuring color purity and intensity, reveal significant differences between FCNCA-C and FCNCA-CC and between FCNCA-C and FCNCA-P (p=0.0002), with the lowest value being found in FCNCA-P (15.26), followed by FCNCA-CC (15.95), and the highest values determined in FCNCA-C (27.83), reflecting the effect of the various pigments present in the seaweeds.
Overall, FCNCA-C presents the highest value in all parameters. In general, FCNCAs exhibited mean levels of
L* (≤53), with component
b* predominant over component
a*, suggesting that the degree of lightness and yellowness mostly contributed to the color features of the FCNCAs, as well reported by other authors [
46,
47].
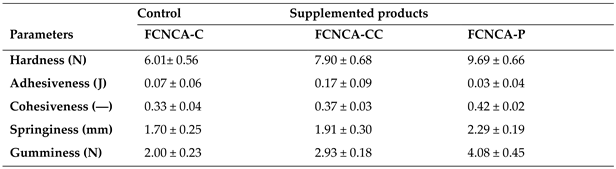
3.9 Texture Profile Analysis (TPA)
The textural properties of the FCNCAs are reported in
Table 4 and
p-values in supplementary material (
Table S5). TPA results are difficult to discuss as there is little or no data from other studies.
The hardness of the FCNCAs ranged from 6.01 to 9.69 N, showing statistical differences between FCNCA-C and FCNCA-CC (
p=0.0065), between FCNCA-C and FCNCA-P, and between FCNCA-CC and FCNCA-P (
p=0.0039). Hardness can be defined as the force require to attain a given deformation [
48]. Increasing the moisture content has the opposite effect on hardness [
49], and in fact, there is an inverse relationship between the hardness and moisture content of the FCNCAs.
An inverse relationship with lipid content can also be observed which can explain hardness differences. The lowest total lipids resulted in FCNCAs with higher hardness. A possible explanation for this might be that the fat in these alternative cheeses is mainly unsaturated. In fact, a study conducted by Devi and Khatkar concluded that saturated fatty acids contribute to dough hardness and fats rich in unsaturated fatty acids such as sunflower oil produced the softest cookie dough.
Adhesiveness ranged from 0.03 to 0.17 J but did not exhibit significant differences between samples (
p > 0.05). The slightly higher value attributed to FCNCA-CC (0.17 J) can be due to the carrageen’s polysaccharides in
C. crispus [
51,
52].
Adhesiveness can be defined as the work necessary to overcome the attractive forces between the surface of the food and the surface of other materials with which the food comes into contact [
48]. The positive low adhesiveness values show that FCNCAs are not very sticky or adhesive.
Cohesiveness values range from 0.33 to 0.42, showing significant differences between FCNCA-C and FCNCA-P (
p = 0.0035), and between FCNCA-CC and FCNCA-P (
p = 0.0240). Cohesiveness indicates the strength of the internal bonds making up the body of the product [
53]. The mean cohesiveness values for FCNCA-P indicate that the structure is not easily disintegrated [
54]. The fibre content can also justify the highest cohesiveness values for supplemented products. The reported insoluble dietary fibre content of Spanish seaweeds for
C. crispus (12.04%), and for
Porphyra sp. (19.22%) [
55], is directly related to the values found in the present study. Other factors contributing to these differences may be the moisture and lipids contents.
Springiness (elasticity) is defined as the distance recovered by the sample during the time that elapsed between the end of the first compression and the start of the second one [
48,
56]. Springiness results revealed that the minimum (1.70 mm) was found in FCNCA-C, while the maximum value (2.29 mm) is for FCNCA-P. Significant differences between FCNCA-C and FCNCA-P (
p = 0.0103), and between FCNCA-CC and FCNCA-P (
p = 0.0245) were found.
Grasso et al. reported values for different commercial plant-based block-style al- ternatives to cheese, ranging from 0.32 to 0.57 (unitless).
Gumminess values ranged from 2.00 to 4.08 N, showing significant differences between all samples (
p = 0.0039). Gumminess is the denseness that persists through chewing, or the energy needed to disintegrate a semisolid food until it is ready for swallowing [
56]. Since supplemented products present higher values, more chews are required before swallowing. There is also a clear correlation between gumminess and hardness, cohesiveness, and springiness, as well as an inverse correlation with total lipids content and moisture.
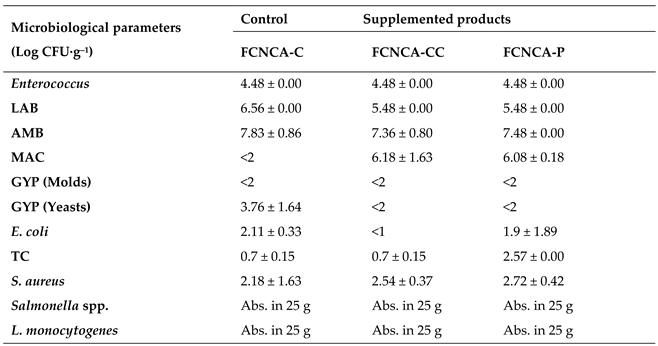
3.Microbial Load
The microbiological evaluation of FCNCAs is presented in
Table 5, and the
p-values are in supplementary material (
Table S6). The mean counts of
Enterococcus exceed 4.0 log CFU·g
–1 for all samples and do not show significant differences between them (
p > 0.005).
Lactic Acid Bacteria (LAB) showed a significant difference for FCNCA-C
vs. FCNCA-CC and FCNCA-CC
vs. FCNCA-P (
p=0.0139). The LAB predominated over other microbiological groups, achieving the same counts for FCNCA-CC and FCNCA-P (5.48 CFU·g
–1), and slightly exceeding by 1.0 log CFU·g
–1 for FCNCA-C. In all cases, the values obtained were higher than the limits referred by Saraiva et al. [
15]. These elevated counts can be related to the addition of Advanced Acidophilus Plus (Solgar®), which contained a large number of
Lactobacillus acidophilus and
Bifidobacterium lactis. In fact, Lactobacillus is a genus with important applications in food fermentation, as it is also capable of producing lactic acid due to the metabolism of sugars [
58]. Apart from that, LAB is responsible for producing substances that improve flavor, texture, nutritional value, shelf-life and safety of foods [
59].
Aerobic mesophilic bacteria (AMB) are the most represented group, ranging from 7.36 to 7.83 log CFU·g
–The statistical analyses revealed significant differences among all FCNCAs (
p < 0.05). Usually, cashew nut or cashew nut products present a higher AMB activity. For example, Muniz et al. reported 4.0 to 7.0 log CFU·g
–1 for cashew nut, whereas Göçer and Koptagel reported 8.84 ± 0.26 log CFU·g
–1 for a cashew nut-based beverage fermented with kefir. According to Saraiva et al. [
15], all FCNCAs are rated as unsatisfactory (>107 CFU·g
–1) (see supplementary material,
Table S7).
Counts on marine agar achieved similar values among FCNCA-CC (6.18 log CFU·g–1) and FCNCA-P (6.08 log CFU·g–1). Fermented cashew nut cheese alternative control (FCNCA-C) does not show microbiological activity (<2 log CFU·g–1). A plausible justification for this is that the values for the supplemented products are due to seaweeds.
Molds were deemed satisfactory, presenting the same values for all samples (<2 log CFU·g
–1), and did not show significant differences between them (
p>0.05). Some authors suggest that contamination of cashew nuts by molds can occur early in the field or during a prolonged storage time [
62], which was not the case. Simultaneously, the previous blanching of cashew nuts in hot water helps destroy microorganisms such as bacteria, yeasts, and molds [
63].
Concerning yeasts, FCNCA-CC, and FCNCA-P register <2 log CFU·g
–1, whereas FCNCA-C exceeded 3.0 log units, showing statistical differences for FCNCA-C
vs. FCNCA-CC and FCNCA-C
vs. FCNCA -P (
p = 0.0139). The results indicated that seaweeds could inhibit yeast’s activity, which is unsurprising since some demonstrate anti-fungal capacity against yeast strains [
64].
Results of Escherichia coli ranged from <1 log CFU·g–1 to 2.11 log CFU·g–1, showing statistical differences for FCNCA-C vs. FCNCA-CC and FCNCA-CC vs. FCNCA-P (p = 0.0126). Mendes et al. demonstrate that ethyl acetate and diethyl ester extracts of C. crispus from the wild and from an integrated multi-trophic aquaculture system possess antimicrobial activity against the growth of bacteria such as E. coli and others.
Total coliforms (TC) ranged from 0.7 to 2.57 log CFU·g–1, revealing differences for FCNCA-C vs. FCNCA-P and FCNCA-CC vs. FCNCA-P (p = 0.0126). The FCNCA-P shows a high content of total coliforms.
Counts of coagulase-positive
S. aureus reached similar counts between samples, ranging from 2.18 to 2.72 log CFU·g
–The statistical analyses revealed significant differences for FCNCA-C
vs. FCNCA-P (
p = 0.0194). The low values for
S. aureus were considered satisfactory from a safety point of view since the production of toxins only occurs at higher counts (>104) [
14].
Salmonella spp. was absent through the plating count (not present per 25 g). In fact, a US surveillance study shows that the prevalence of
Salmonella (95% of confidence interval) in cashew nuts was minimal (0.55%),
i.e., it occurred in just 4 of 733 samples [
66].
Listeria monocytogenes was also absent through the plating count (not present per 25 g). Eglezos reported that «there are no data available on the prevalence of
L. monocytogenes in cooked edible nut kernels or any foodborne illness lined to the presence of
L. monocytogenes in this kind of product». On the other hand, LAB, which is one of the best represented groups in the FCNCAs (5.48-6.56 log CFU·g
–1), has shown bactericidal and bacteriostatic properties against foodborne pathogens such as
Salmonella spp. and
L. monocytogenes [
19].
3.Flash Profile Methodology
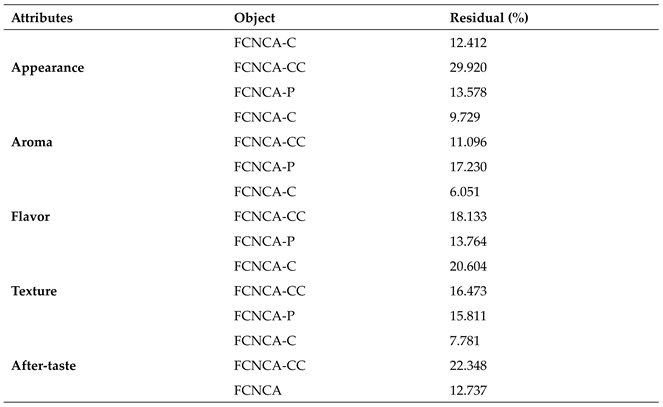
Results obtained in the second session of the Flash Profile from the eight panellists ranking for the FCNCAs (FCNCA-C, FCNCA-CC, and FCNCA-P) per attribute, according to the final list of terms defined, were analyzed by Generalized Procruste Analysis (GPA).
FCNCA-C showed the most consensual rankings as it presents the lowest residual variance for all attributes, except for texture, for which it presents the highest residual variance (20.6%) (
Table 6).
Fermented cashew nut cheese alternative with C. crispus (FCNCA-CC) had the highest residual variances for appearance (29.9%), flavor (18.1%), and after-taste (22.3%). In comparison, FCNCA-P had the highest residual variance for aroma (17.2%), showing that the supplementation of seaweeds, an ingredient that is not very common, influences the sensory characteristics of products and consequently affects the consensus.
Residual variances values for each panellist calculated by GPA reveals the panellists with higher residual variance values: a higher percentage for appearance for panellist 7 (14.1%), aroma for panellist 8 (10.2%), flavor for panellist 1 (15.0%), texture for panellist 4 (22.2%), and after-taste for panellist 1 (13.4%), indicating that these panellists were further from the consensus (see supplementary material,
Table S8).
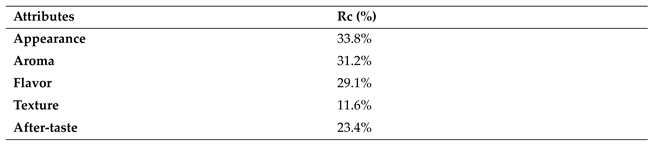
The values of consensus index (Rc) (
Table 7) were as follows: appearance (33.8%), aroma (31.2%), flavor (29.1%), texture (11.6%), and after-taste (23.4%). All attributes show an inadequate consensus in the performance of the panellists, particularly on texture and after-taste.
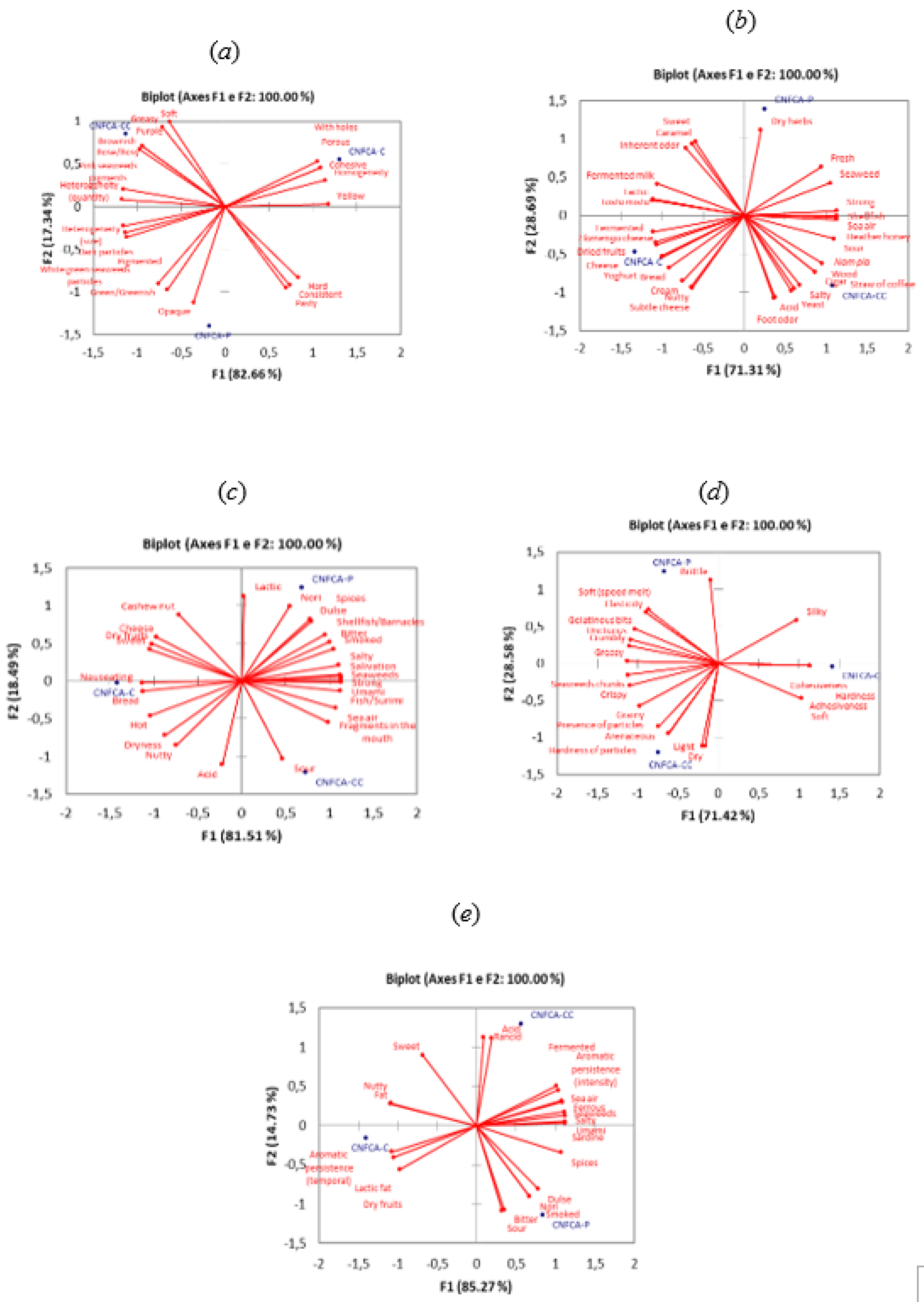
Figure 3 shows the coordinates of the objects (FCNCAs) after GPA analysis and the correlations between the attributes (appearance, aroma, flavor, texture, and after-taste), and the dimensions F1 (first axis) and F2 (second axis).
For the appearance attribute (a), the FCNCA-C is perceived as cohesive, porous, and with holes, whereas FCNCA-CC is brownish, greasy, purple, rose/rosy, and soft, and FCNCA-P is opaque. Although there is a discrepancy between the terms used to describe FCNCA-C, the terms porous and with holes are in line with TPA findings, with FCNCA-C showing the lowest values for hardness (6.01 N) and cohesiveness (0.33). FCNCA-CC is described by terms such as purple, rose/rosy and pink seaweeds, corresponding with its value (3.19) for the color parameter a*. The opacity related to FCNCA-P concords with results for lightness (L*) and chroma (C*) since it shows the lowest values for this parameter, namely 38.30, and 15.20, respectively.
Regarding aroma (b), FCNCA-C was mainly characterized by cheese, dried fruits, Flamengo cheese, fermented, and yogurt, whereas FCNCA-CC was characterized by cigar, nam pla, salty, straw of coffee, and wood, and FCNCA-P by dry herbs.
Terms such as cheese, Flamengo cheese, fermented or even yogurt suggest a similitude between FCNCA-C and dairy cheese, due to the addition of L. acidophilus LA-5®. In fact, FCNCA-C is the sample that shows the higher count of LAB (6.56). The aroma of dried fruits attributed to FCNCA-C is desirable since dried fruit is at the product's base. Lima et al. (2012) reported an analog term, nutty, to a cashew nut butter made with different grades of kernel quality.
In red seaweeds, the formation of apocarotenoids such as
β-ionone contributes significantly for the aroma of algae and marine environments [
69], as suggested by terms such seaweeds, shellfish and sea air. The fishy aroma of
nam pla may arise from compounds such as 1-octen-3-one, from various aldehydes, for example, when combined, hexanal, (2E,4E)-decadienal and (2E,4E)-heptadienal, heptanalmcan create a strong and penetrating fishy odor, often associated with seaweeds [
70]. Furthermore, dry herbs described for FCNCA-P can be associated with a green odor related to esters [
71].
The results for flavor (c) show that FCNCA-C was mainly characterized by bread and nauseating; FCNCA-CC by sour; and FCNCA-P by Dulse, Nori, and spices.
It can be inferred that some of the panellists, despite being trained, were confused about the flavor of seaweeds, since FCNCA-P was described as Dulse (
Palmaria palmata) and Nori (
Porphyra sp.); which is quite understandable, as sometimes aromas and flavours can be very complex and difficult to distinguish from each other’s. Another term attributed to FCNCA-P, ‘spices’, is related to carboxylic acids [
71].
Regarding texture (d), FCNCA-C was characterized mainly by cohesiveness, hardness and adhesiveness, while FCNCA-CC by terms such as arenaceous, dry, hardness of particles, and light, and FCNCA-P by brittle. The cohesiveness described for FCNCA-C agrees with the value obtained in this study (0.33).
Dry and brittle terms described for FCNCA-CC and FCNCA-P also comply with the founded hardness values, namely 7.90 and 9.69 N, respectively. Particles hardness for FCNCA-CC can be attributed to the texture of
C. crispus, which is generally firm, or cartilaginous-like [
72]. On the other hand, the size of the particles (2.87 mm) may also have influenced the sensation described.
For after-taste (e), FCNCA-C is characterized by several terms, such as aromatic persistence (temporal), lactic fat, and dry fruits. In contrast, FCNCA-CC by acidic and rancid, and FCNCA-P by bitter, Dulse, Nori, smoked, and sour.
During the fermentation, lactic acid is produced through sugar metabolism, which is responsible for the increasing sourness as the sweetness reduces [
58]. In general, the supplementation of food products with seaweeds compromises their sensory attributes due to the appearance of off-flavors [
73]. For example, rancidity, as described for FCNCA-CC. This rancidity can be attributed to aldehydes, including heptanal and octanal, which contribute to undesirable rancid odour and flavour during spoilage of fat and fatty foods [
74,
75]. Also, aldehydes such as heptanal and 2-octenal are associated with ‘smoked’ and ‘sour’ [
75], respectively, both attributed to FCNCA-P.
In summary, seaweeds have a decisive role in various attributes, especially aroma, flavour, and after-taste, which changed the descriptions of all the attributes, making it possible to distinguish FCNCAs clearly.