Submitted:
23 October 2024
Posted:
23 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
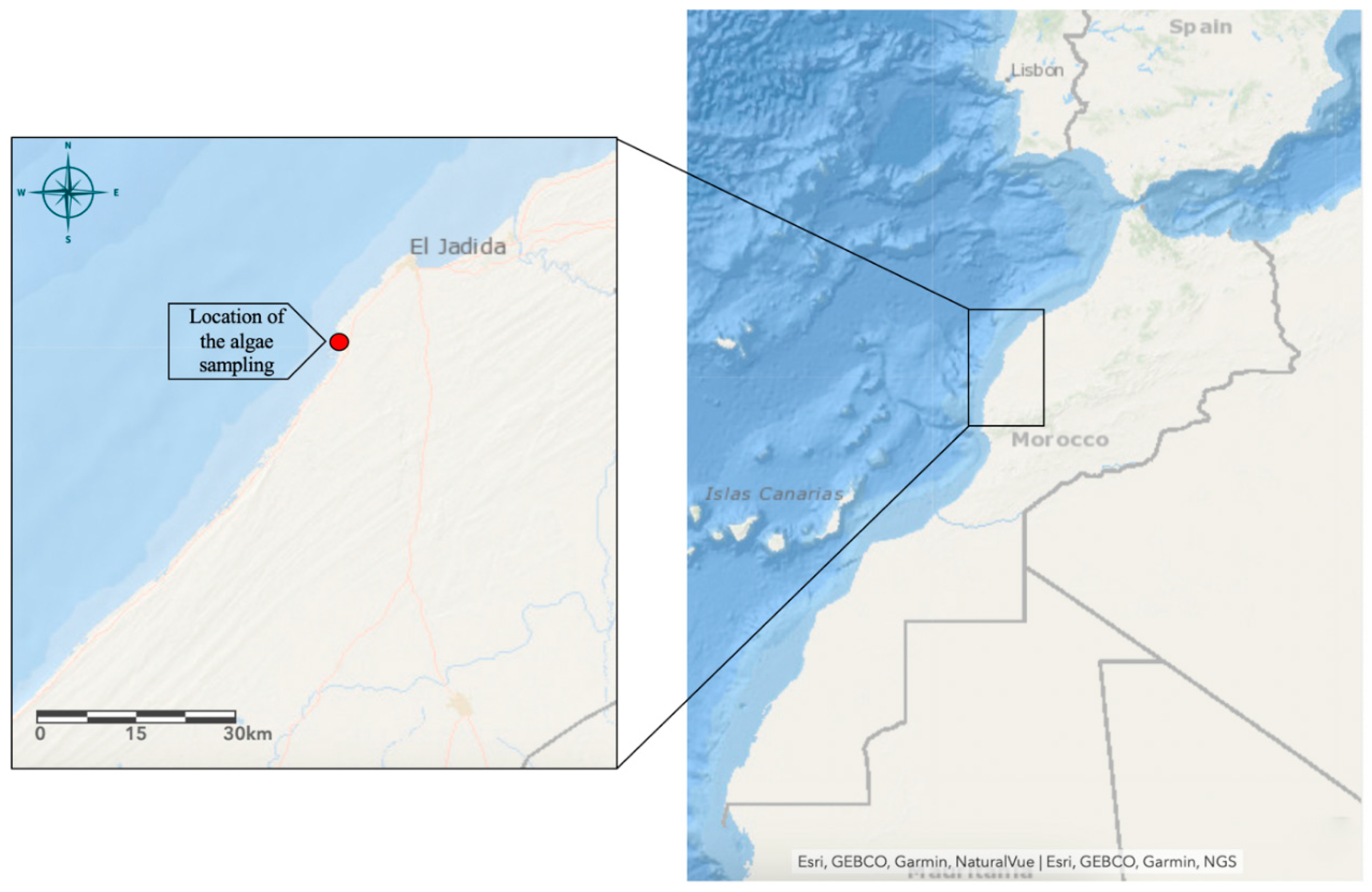
2.1. Algae Collection and Identification
2.2. Extraction of the Polysaccharide-Rich Osmundea pinnatifida (PSOP)
2.3. Chemical Characterization
2.4. Polysaccharides Spectroscopic Analysis
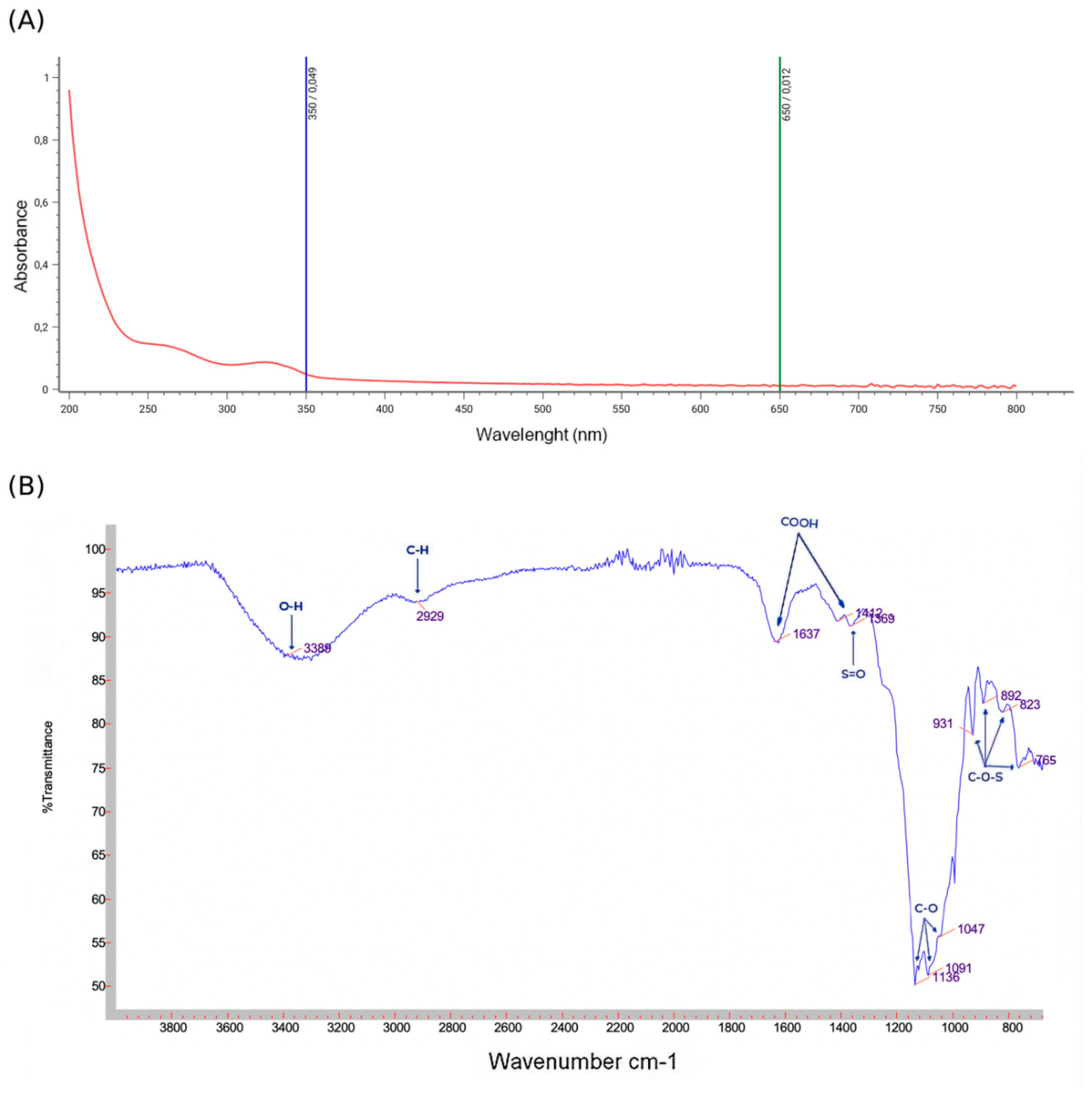
2.4.1. Ultraviolet Absorption Peak Detection
2.4.2. Fourier Transform Infrared Spectrometry Analysis
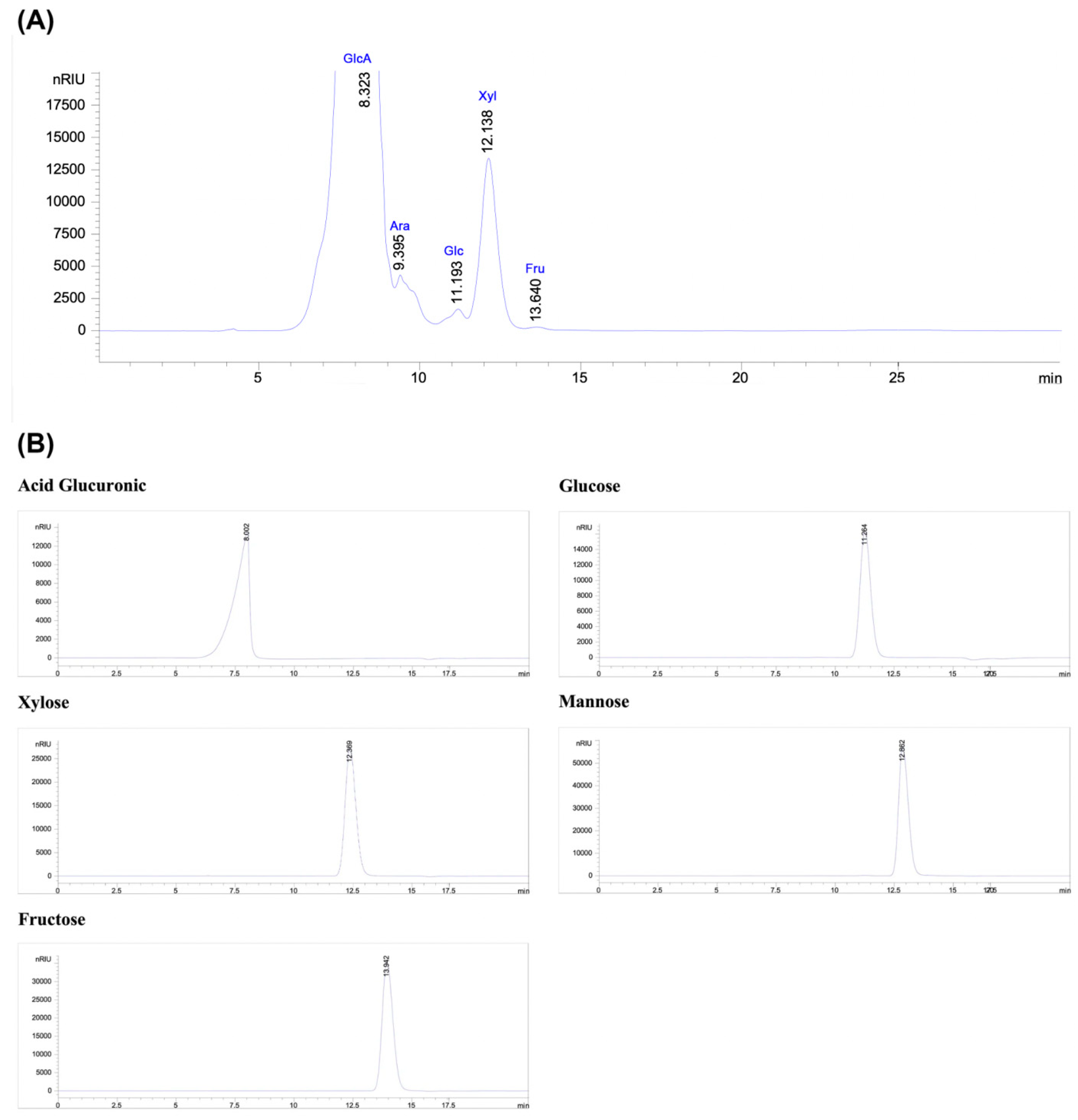
2.4.3. Monosaccharide Analysis by High-Performance Liquid Chromatography (HPLC-RID)
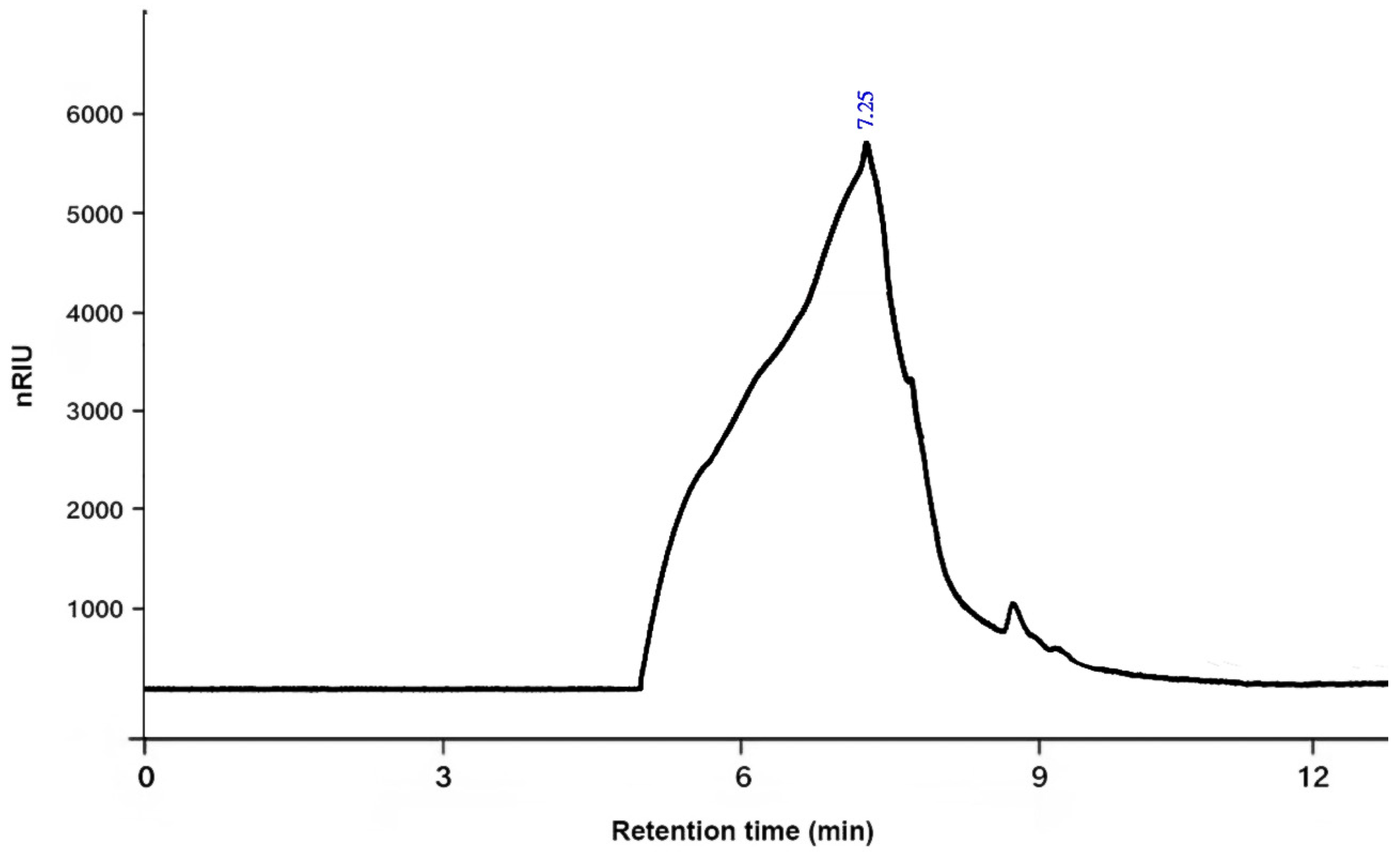
2.4.4. The Average Molecular Weight
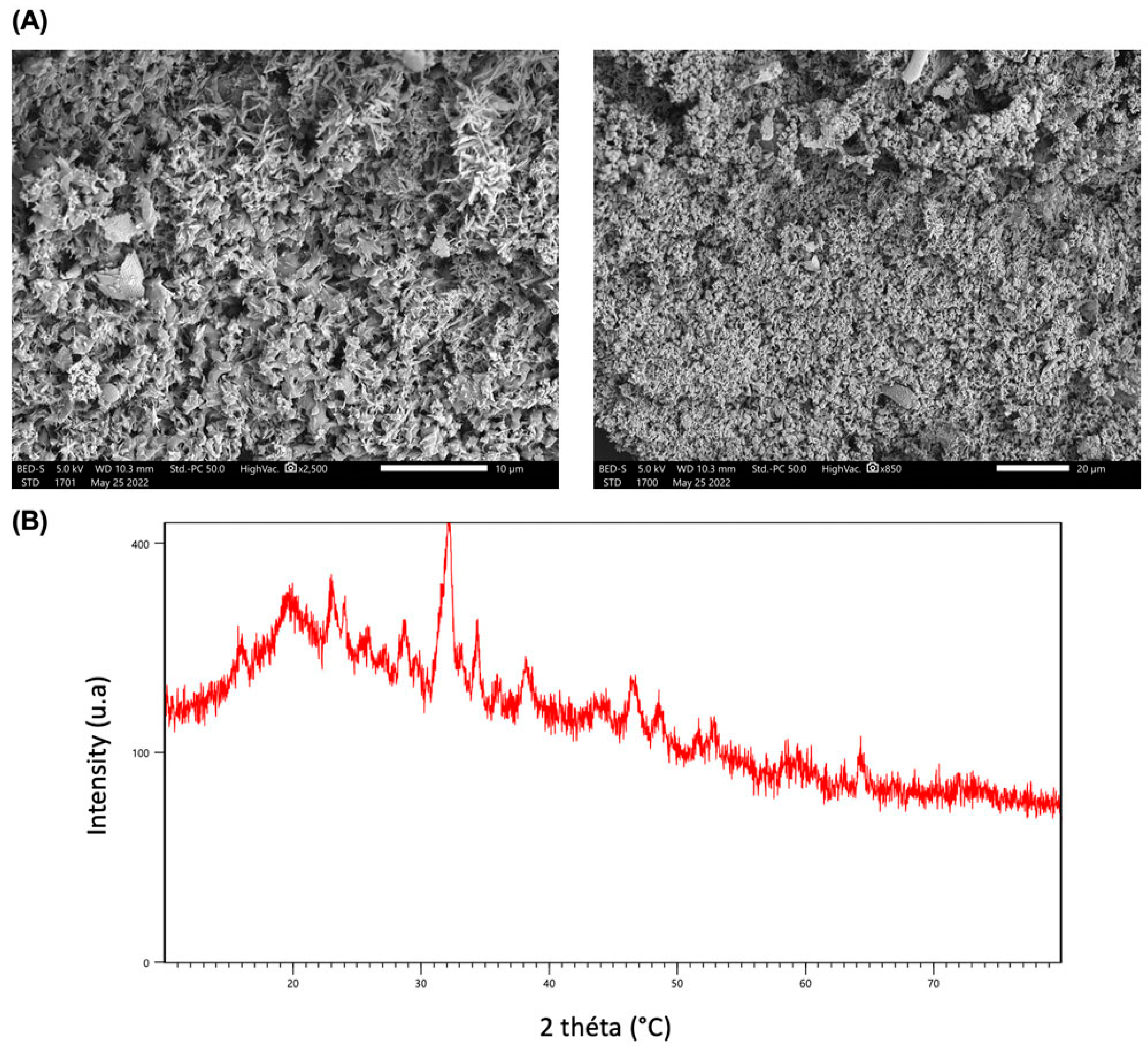
2.4.5. Scanning Electron Microscopy
2.4.6. X-Ray Diffraction
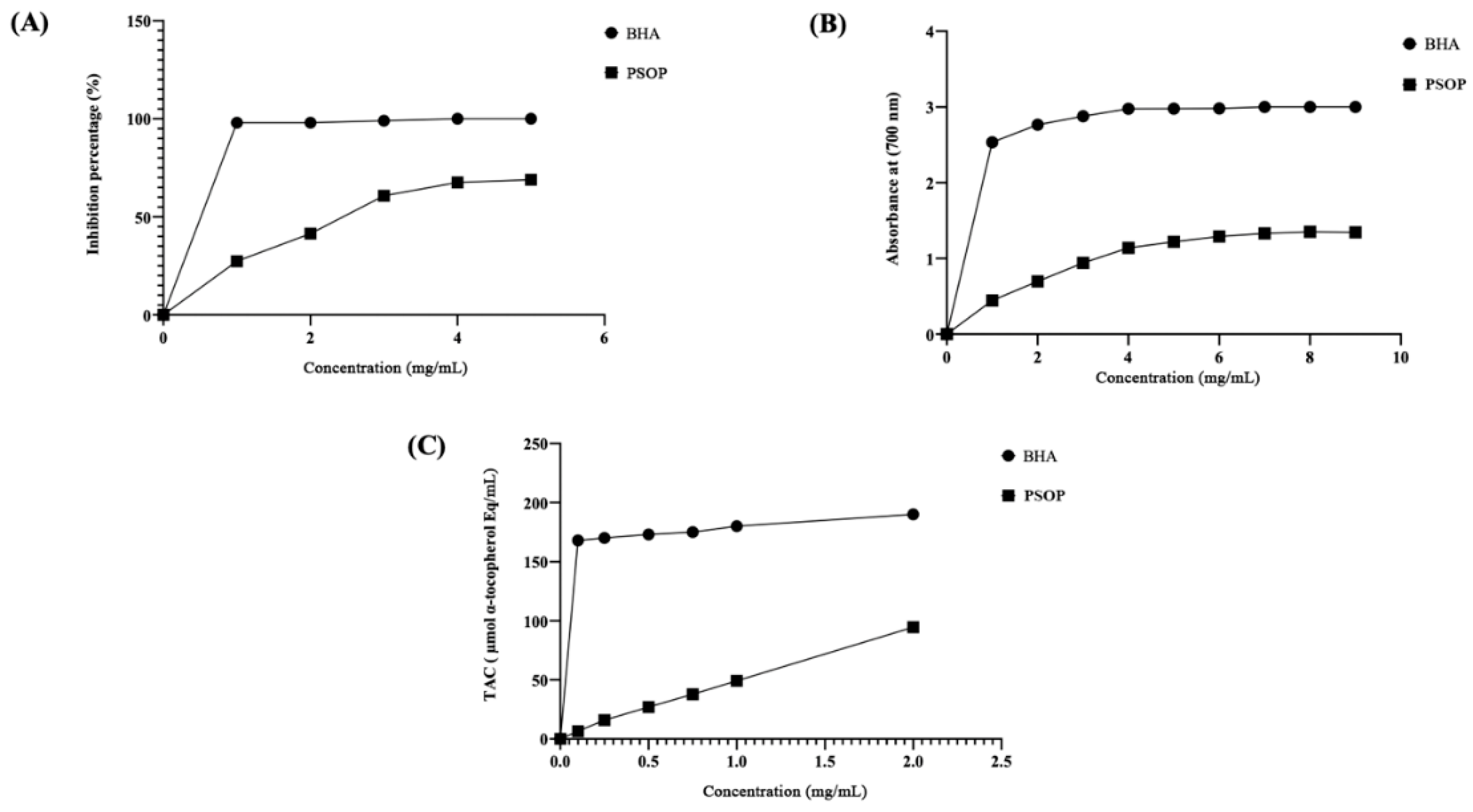
2.5. Antioxidant Activity of Polysaccharide
2.5.1. DPPH Free Radical Scavenging Assay
2.5.2. Total Antioxidant Activity
2.5.3. Reducing Power Assay
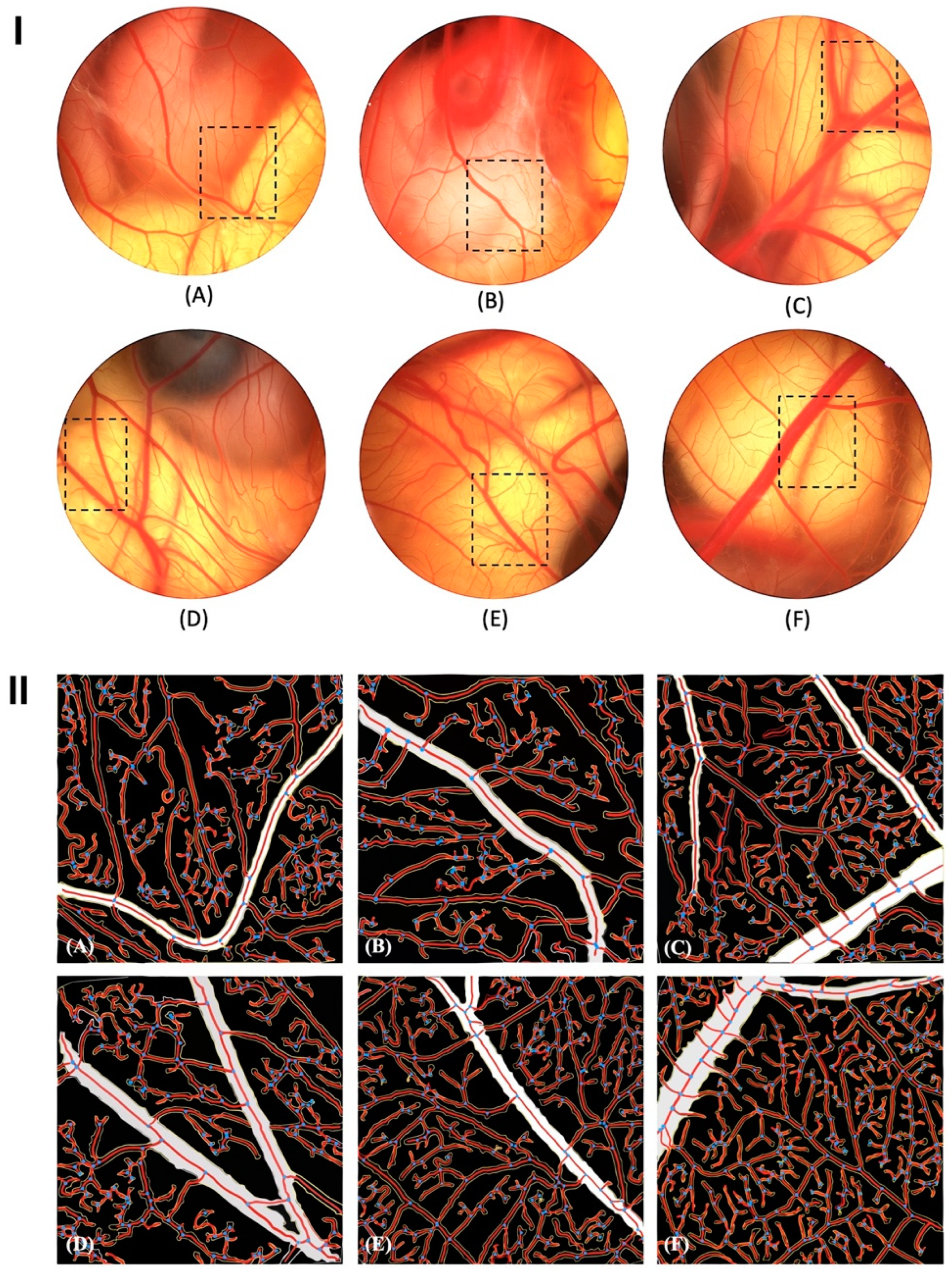
2.6. Chorioallantoic Membrane Assay
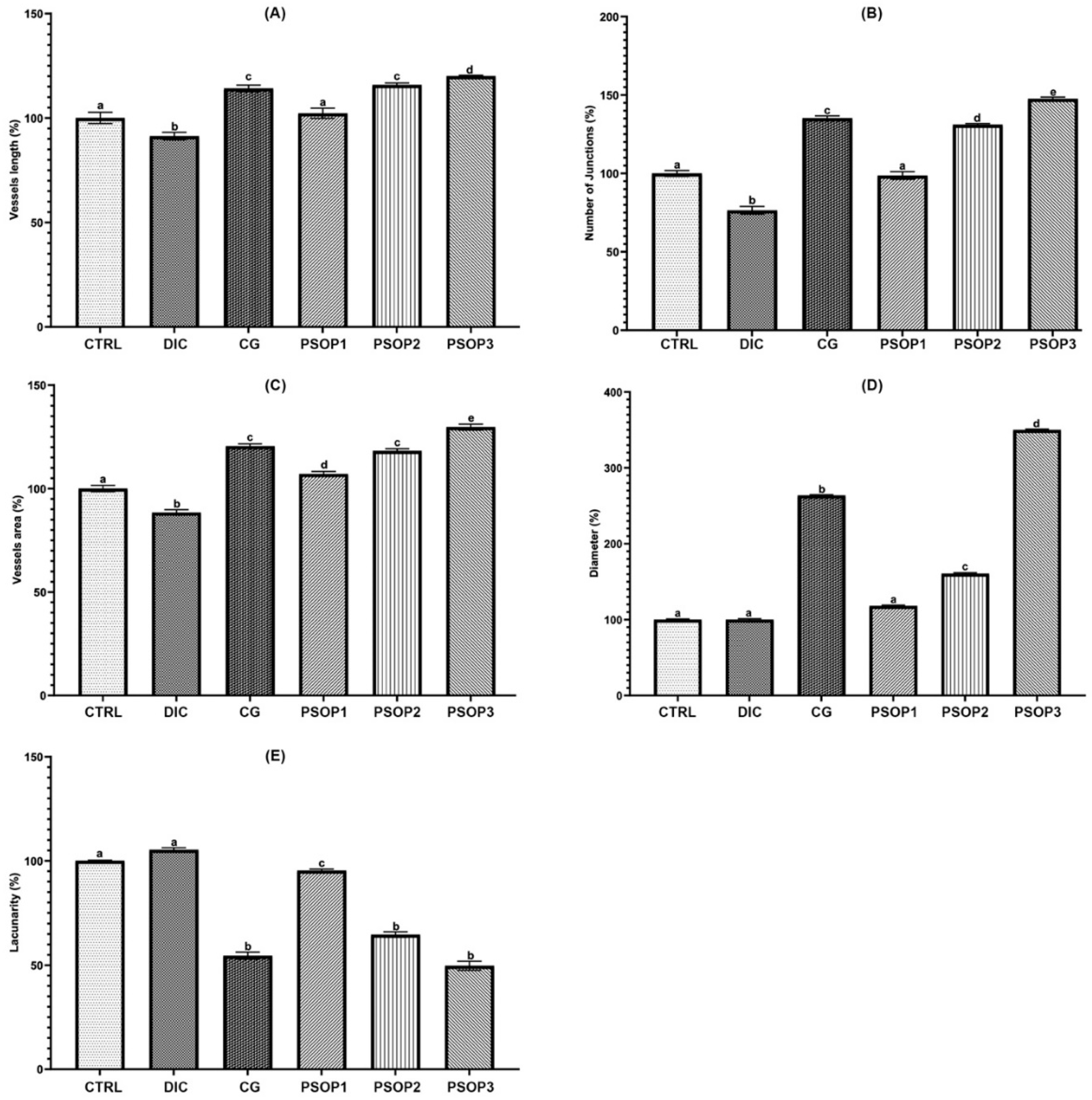
2.7. Quantitative Analysis of Vascular Network
2.8. In Vivo Experimental Study
2.8.1. Animals
2.8.2. Experimental Protocol
2.8.3. Measurement of Wound Area and Burn Contraction Rate
2.8.4. Determination of Hydroxyproline Content
2.8.5. Histological Examination
2.8.6. Statistical Analysis
2.9. Computational Modeling and Interactions Assay
3. Results and Discussion
3.1. Chemical Characterization of PSOP
3.2. Spectroscopic Analysis and Monosaccharide Composition
3.3. Antioxidant Activity of PSOP
3.4. Angiogenesis Stimulation Assays
3.5. Quantitative Analysis of Vascular Network
3.6. In Vivo Experimental Study
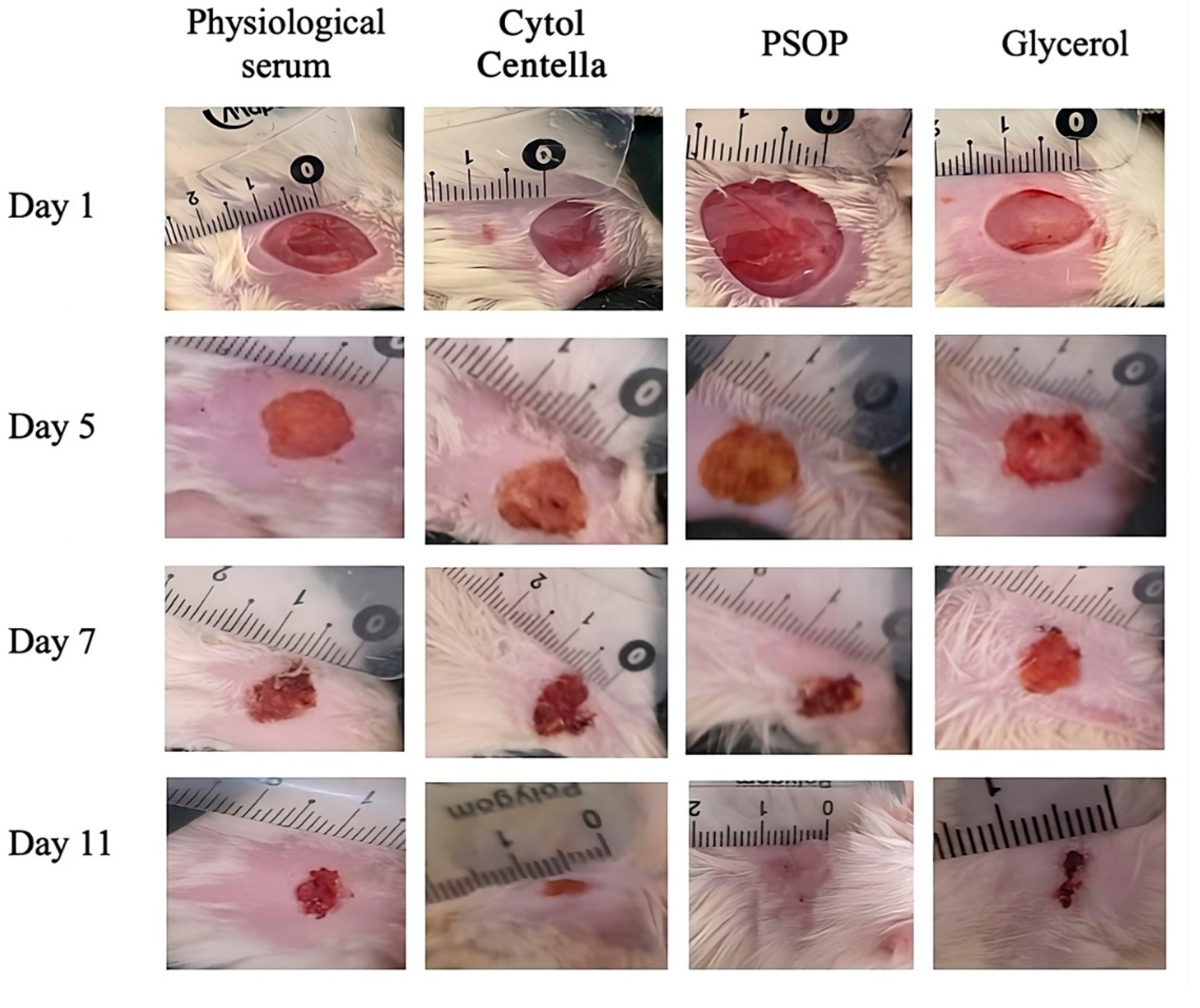
3.6.1. Morphological Evaluation
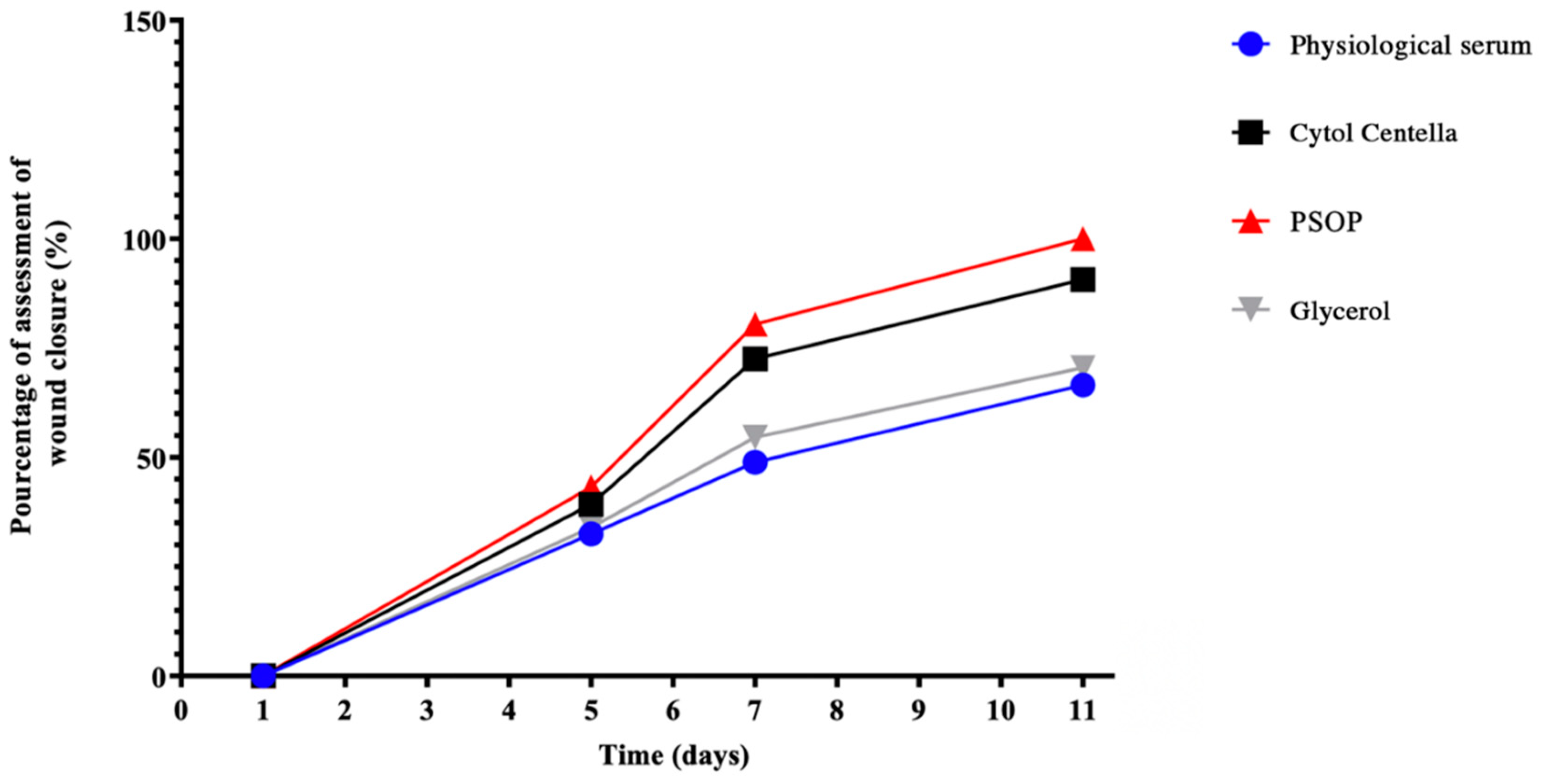
3.6.2. Assessment of Wound Area
| 5 days | 7 days | 11 days | |
| Physiological serum | 32.5 ± 1.24a | 48.9 ± 2.02a | 66.59 ± 1.59a |
| Cytol Centella | 39.3 ± 1.41b | 72.54 ± 2.85b | 90.67 ± 2.08b |
| PSOP | 43.22 ± 1.92c | 80.45 ± 3.01c | 100c |
| Glycerol | 34 ± 1.74d | 54.62 ± 2.07d | 70.49 ± 1.27d |
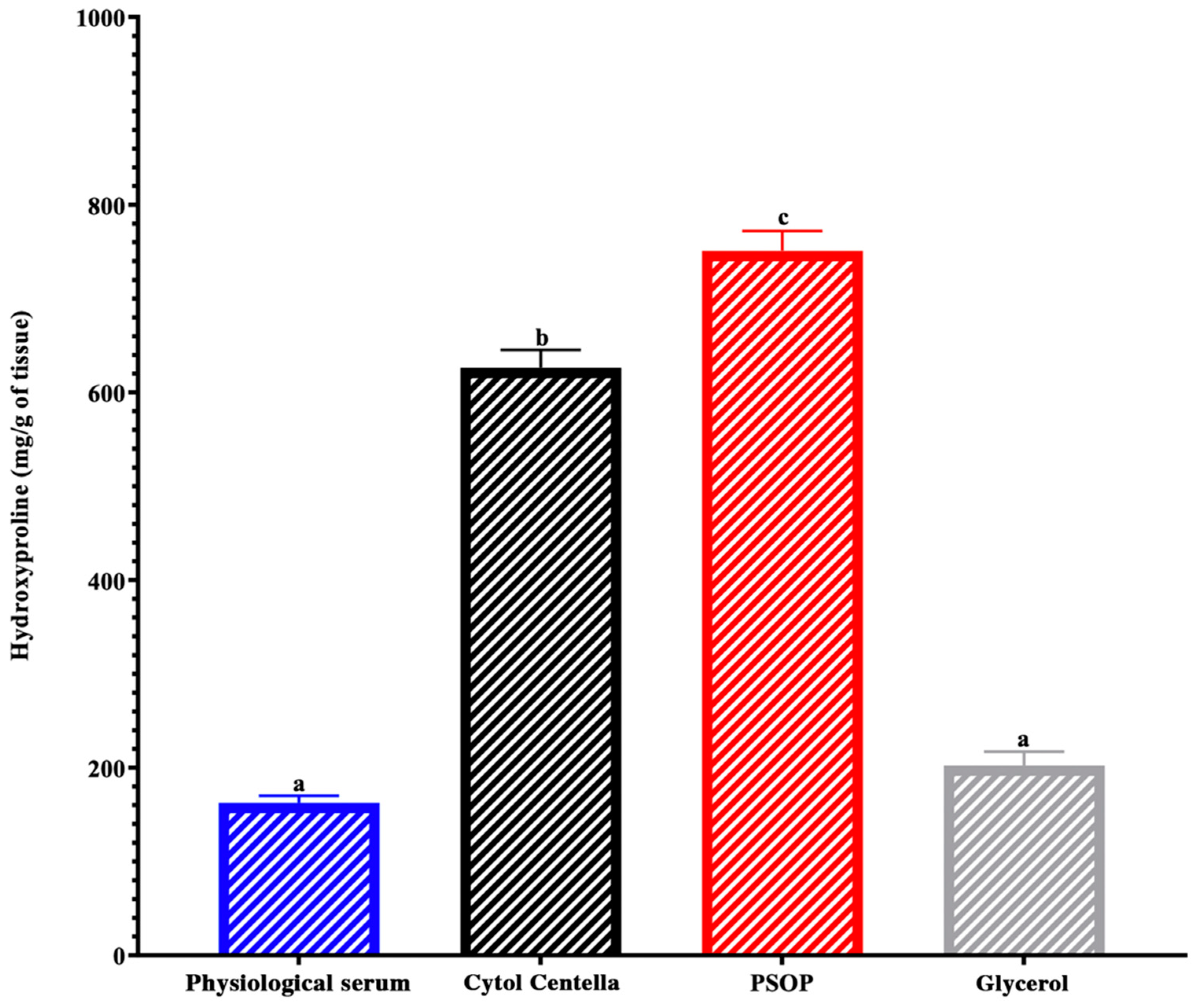
3.6.3. Hydroxyproline and Collagen Regeneration
3.6.4. Histological Evaluation
3.7. Computational Findings
4. Conclusions
Author Contributions
Funding
Ethical Statements
Acknowledgments
Conflicts of Interest
References
- Han, X.; Ju, L.S.; Irudayaraj, J. Oxygenated Wound Dressings for Hypoxia Mitigation and Enhanced Wound Healing. Mol. Pharmaceutics 2023, 20, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.P.; Maurizio, A.; Motta, A.; Podini, P.; Diprima, S.; Malpighi, C.; Brambilla, I.; Martins, L.; Badaloni, A.; Boselli, D.; et al. Structured Wound Angiogenesis Instructs Mesenchymal Barrier Compartments in the Regenerating Nerve. Neuron 2024, 112, 209–229.e11. [Google Scholar] [CrossRef] [PubMed]
- Mota, F.A.R.; Passos, M.L.C.; Santos, J.L.M.; Saraiva, M.L.M.F.S. Comparative Analysis of Electrochemical and Optical Sensors for Detection of Chronic Wounds Biomarkers: A Review. Biosensors and Bioelectronics 2024, 251, 116095. [Google Scholar] [CrossRef] [PubMed]
- Rathna, R.P.; Kulandhaivel, M. Advancements in Wound Healing: Integrating Biomolecules, Drug Delivery Carriers, and Targeted Therapeutics for Enhanced Tissue Repair. Arch Microbiol 2024, 206, 199. [Google Scholar] [CrossRef]
- Boujhoud, Z.; Feki, A.; Eleroui, M.; Lakhram, M.; Kraiem, M.; Dghim, A.; Zeroual, A.; Youlyouz Marfak, I.; Essayagh, S.; Hilali, S.; et al. The Anti-Angiogenic, Anti-Inflammatory and Anticoagulant Potential of a Polysaccharide Extracted from the Brown Alga Cystoseira Humilis. European Polymer Journal 2024, 220, 113461. [Google Scholar] [CrossRef]
- Peipei, L.; Qinghong, Z.; Yin, C.; Pengfei, H.; Junjie, Z. Structure and Anticoagulant Activity of a Galactoarabinan Sulfate Polysaccharide and Its Oligosaccharide from the Green Algae, Codium Fragile. International Journal of Biological Macromolecules 2024, 279, 135255. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Chondrus Crispus Treated with Ultrasound as a Polysaccharides Source with Improved Antitumoral Potential. Carbohydrate Polymers 2021, 273, 118588. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Yang, M.; Ding, J.; Huang, Y.; Zhu, Y.; Zhou, M.; Yan, B. Antiviral Activity of a Polysaccharide from Sargassum Fusiforme against Respiratory Syncytial Virus. International Journal of Biological Macromolecules 2024, 279, 135267. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.; Wu, J.; Zhao, J. Modifications of Polysaccharide-Based Biomaterials under Structure-Property Relationship for Biomedical Applications. Carbohydrate Polymers 2021, 266, 118097. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-Derived Polysaccharides and Their Therapeutic Potential in Wound Healing Application - A Review. International Journal of Biological Macromolecules 2023, 253, 127331. [Google Scholar] [CrossRef] [PubMed]
- Biancacci, C.; Abell, R.; McDougall, G.J.; Day, J.G.; Stanley, M.S. Annual Compositional Variation in Wild Osmundea Pinnatifida (Hudson) Stackhouse from the West Coast of Scotland. J Appl Phycol 2022, 34, 1661–1675. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical Composition of Red, Brown and Green Macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chemistry 2015, 183, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Pereira, L. Concise Review of Osmundea Pinnatifida (Hudson) Stackhouse. J Appl Phycol 2020, 32, 2761–2771. [Google Scholar] [CrossRef]
- Moreira, B.R.; Vega, J.; Sisa, A.D.A.; Bernal, J.S.B.; Abdala-Díaz, R.T.; Maraschin, M.; Figueroa, F.L.; Bonomi-Barufi, J. Antioxidant and Anti-Photoaging Properties of Red Marine Macroalgae: Screening of Bioactive Molecules for Cosmeceutical Applications. Algal Research 2022, 68, 102893. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of Enzyme- and Ultrasound-Assisted Extraction Methods on Biological Properties of Red, Brown, and Green Seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.; Chen, L. Chemical Composition and Bioactivities of a Water-Soluble Polysaccharide from the Endodermis of Shaddock. International Journal of Biological Macromolecules 2012, 51, 763–766. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Mazuelos, C.; Hermosin, B.; Saiz-Jimenez, C. Chemical Composition ofSpirulina and Eukaryotic Algae Food Products Marketed in Spain. J Appl Phycol 1993, 5, 425–435. [Google Scholar] [CrossRef]
- Lowry, OliverH. ; Rosebrough, NiraJ.; Farr, A.L.; Randall, RoseJ. PROTEIN MEASUREMENT WITH THE FOLIN PHENOL REAGENT. Journal of Biological Chemistry 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Llyod, A.G.; Tudball, N.; Dodgson, K.S. Infrared Studies on Sulphate Esters III. O-Sulphate Esters of Alcohols, Amino Alcohols and Hydroxylated Amino Acids. Biochimica et Biophysica Acta 1961, 52, 413–419. [Google Scholar] [CrossRef]
- He, R.; Zhao, Y.; Zhao, R.; Sun, P. Antioxidant and Antitumor Activities in Vitro of Polysaccharides from E. Sipunculoides. International Journal of Biological Macromolecules 2015, 78, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-J.; Zhang, J.-G.; Sun, Y.-H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.-J. Physicochemical Properties and Antioxidant Activities of Polysaccharides Sequentially Extracted from Peony Seed Dreg. International Journal of Biological Macromolecules 2016, 91, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Xie, M.-Y.; Nie, S.-P.; Shen, M.-Y.; Wang, Y.-X.; Li, C. Isolation, Chemical Composition and Antioxidant Activities of a Water-Soluble Polysaccharide from Cyclocarya Paliurus (Batal.) Iljinskaja. Food Chemistry 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and Characterization of Three Polysaccharides Extracted from Opuntia Ficus Indica Cladodes. International Journal of Biological Macromolecules 2016, 92, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a Heated Histidine-Glucose Model System. I: Investigation of the Antioxidant Role of Histidine and Isolation of Antioxidants by High-Performance Liquid Chromatography. J Amer Oil Chem Soc 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Analytical Biochemistry 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of Antioxidant and Antimicrobial Activities of Rumex Crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, Antiproliferative, and Antiangiogenesis Effects of Polyphenol-Rich Seaweed ( Sargassum Muticum ). BioMed Research International 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Institutional Animal Care and Use Committee Guidebook; Office of Laboratory Animal Welfare, 2002.
- Edwards, C.A.; O’Brien, W.D. Modified Assay for Determination of Hydroxyproline in a Tissue Hydrolyzate. Clinica Chimica Acta 1980, 104, 161–167. [Google Scholar] [CrossRef]
- Badraoui, R.; Allouche, M.; El Ouaer, D.; Siddiqui, A.J.; Ishak, S.; Hedfi, A.; Beyrem, H.; Pacioglu, O.; Rudayni, H.A.; Boufahja, F. Ecotoxicity of Chrysene and Phenanthrene on Meiobenthic Nematodes with a Case Study of Terschellingia Longicaudata: Taxonomics, Toxicokinetics, and Molecular Interactions Modelling. Environmental Pollution 2023, 316, 120459. [Google Scholar] [CrossRef]
- Chira, A.; Kadmi, Y.; Badraoui, R.; Aouadi, K.; Alhawday, F.; Boudaya, M.; Jamoussi, K.; Kallel, C.; El Feki, A.; Kadri, A.; et al. GC-MS/MS Analysis and Wound Repair Potential of Urtica Dioica Essential Oil: In Silico Modeling and In Vivo Study in Rats. Curr Pharm Biotechnol 2024. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.; Allouche, M.; Alotaibi, G.S.; Alwthery, N.S.; Al-Subaie, R.A.; Al-Hoshani, N.; Plavan, O.-A.; Selamoglu, Z.; Özdemir, S.; Plavan, G.; et al. Experimental and Computational Assessment of Antiparkinson Medication Effects on Meiofauna: Case Study of Benserazide and Trihexyphenidyl. Marine Pollution Bulletin 2024, 205, 116668. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, F.; Hamdaoui, L.; Saoudi, M.; Badraoui, R.; Rebai, T. Antioxidant and Antiproliferative Effects of Teucrium Polium Extract: Computational and in Vivo Study in Rats. Toxicology Mechanisms and Methods 2024, 34, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Kraiem, M.; Ben Hamouda, S.; Eleroui, M.; Ajala, M.; Feki, A.; Dghim, A.; Boujhoud, Z.; Bouhamed, M.; Badraoui, R.; Pujo, J.M.; et al. Anti-Inflammatory and Immunomodulatory Properties of a Crude Polysaccharide Derived from Green Seaweed Halimeda Tuna: Computational and Experimental Evidences. Marine Drugs 2024, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, N.; Dgachi, S.; Belgacem, S.; Ahmed, A.B.; Henry, N.; Loiseau, T.; Nasr, S.; Badraoui, R.; Naϊli, H. Design, Theoretical Study, Druggability, Pharmacokinetics and Properties Evolution of a New Organo-Bromocadmate Compound as Prospective Anticancer Agent. Journal of Molecular Structure 2023, 1274, 134439. [Google Scholar] [CrossRef]
- Arunkumar, K.; Sreena, K.S.; Moosa, M.; Mohan, G.; Raja, R. Cytotoxic Characterization of Optically Negative Codium Fragile Polysaccharide against HeLa and MCF Cell Lines. Bioactive Carbohydrates and Dietary Fibre 2023, 29, 100341. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Byankina Barabanova, A.O.; Glazunov, V.P.; Yakovleva, I.M.; Yermak, I.M. Seasonal Variations in a Polysaccharide Composition of Far Eastern Red Seaweed Ahnfeltiopsis Flabelliformis (Phyllophoraceae). J Appl Phycol 2018, 30, 535–545. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effect of Extraction Conditions on the Yield and Purity of Ulvan Extracted from Ulva Lactuca. Food Hydrocolloids 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Siddhanta, A.; Goswami, A.M.; Munisamy, S.; Mody, K.H.; Ramavat, B.K.; Mairh, O.P. Sulphated Galactans of Marine Red Alga Laurencia Spp. (Rhodomelaceae, Rhodophyta) from the West Coast of India. Indian Journal of Marine Sciences 2002, 31, 305–309. [Google Scholar]
- Hu, W.; Yu, A.; Bi, H.; Gong, Y.; Wang, H.; Kuang, H.; Wang, M. Recent Advances in Artemisia Argyi Levl. et Vant. Polysaccharides: Extractions, Purifications, Structural Characteristics, Pharmacological Activities, and Existing and Potential Applications. International Journal of Biological Macromolecules 2024, 279, 135250. [Google Scholar] [CrossRef]
- Yi, Y.; Xu, W.; Wang, H.-X.; Huang, F.; Wang, L.-M. Natural Polysaccharides Experience Physiochemical and Functional Changes during Preparation: A Review. Carbohydrate Polymers 2020, 234, 115896. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, Deproteinization and Comparison of Bioactive Polysaccharides. Trends in Food Science & Technology 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated Modification of Polysaccharides: Synthesis, Characterization and Bioactivities. Trends in Food Science & Technology 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Jose, G.M.; Raghavankutty, M.; Kurup, G.M. Sulfated Polysaccharides from Padina Tetrastromatica Induce Apoptosis in HeLa Cells through ROS Triggered Mitochondrial Pathway. Process Biochemistry 2018, 68, 197–204. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Zhang, J.; Kang, W. Isolation, Purification, Structural Analysis and Coagulatory Activity of Water-Soluble Polysaccharides from Ligustrum Lucidum Ait Flowers. Chemistry Central Journal 2017, 11, 98. [Google Scholar] [CrossRef]
- Eljoudi, S.; Feki, A.; Bkhairia, I.; Barkia, A.; Ben Amara, I.; Nasri, M.; Hajji, M. New Polysaccharides Extracted from Malcolmia Triloba: Structure Characterization, Biological Properties and Application to Beef Meat Preservation. Journal of Food Composition and Analysis 2022, 107, 104380. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R.; et al. Effects of Extraction Methods on the Physicochemical Characteristics and Biological Activities of Polysaccharides from Okra (Abelmoschus Esculentus). International Journal of Biological Macromolecules 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; S. Lakshmana, S.; Meivelu, M.; Jeganathan, A. Structural Characterization and Anticancer Activity of Extracellular Polysaccharides from Ascidian Symbiotic Bacterium Bacillus Thuringiensis. Carbohydrate Polymers 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, S.; Xiao, Z.; Zhou, C.; Hong, P.; Qian, Z.-J. Structural Characterization of Sulfated Polysaccharide Isolated From Red Algae (Gelidium Crinale) and Antioxidant and Anti-Inflammatory Effects in Macrophage Cells. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Martera, G.; Goñi, I.; Villanueva-Suárez, M.-J.; Redondo-Cuenca, A. Chemical Structure and Molecular Weight Influence the in Vitro Fermentability of Polysaccharide Extracts from the Edible Seaweeds Himathalia Elongata and Gigartina Pistillata. Food Hydrocolloids 2018, 83, 348–354. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian Red Seaweed Chondrus Canaliculatus: Structural Characteristics, Antioxidant Activity and in Vivo Hemato-Nephroprotective Properties on Maneb Induced Toxicity. International Journal of Biological Macromolecules 2019, 123, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Costa-Pinto, A.R.; Sousa, S.; Vasconcelos, M.W.; Pintado, M.M.; Pereira, L.; Rocha-Santos, T.A.P.; Costa, J.P. da; Silva, A.M.S.; Duarte, A.C.; et al. Sargassum Muticum and Osmundea Pinnatifida Enzymatic Extracts: Chemical, Structural, and Cytotoxic Characterization. Marine Drugs 2019, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; da Silva, A.C.R.; Noseda, M.D.; Fuly, A.L.; de Carvalho, M.M.; Fujii, M.T.; Sanchez, E.F.; Carneiro, J.; Duarte, M.E.R. Chemical Structure and Snake Antivenom Properties of Sulfated Agarans Obtained from Laurencia Dendroidea (Ceramiales, Rhodophyta). Carbohydrate Polymers 2019, 218, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hans, N.; Pattnaik, F.; Malik, A.; Naik, S. Comparison of Different Green Extraction Techniques and Their Influence on Chemical Characteristics of Sulfated Polysaccharide (Fucoidan) from Padina Tetrastromatica and Turbinaria Conoides. Algal Research 2023, 74, 103199. [Google Scholar] [CrossRef]
- Konstantin, B.; Anastasia, P.; Nikolay, I.; Daria, P. Seasonal Variations in the Chemical Composition of Arctic Brown Macroalgae. Algal Research 2023, 72, 103112. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of Monosaccharide Composition and Proportion on the Bioactivity of Polysaccharides: A Review. International Journal of Biological Macromolecules 2024, 254, 127955. [Google Scholar] [CrossRef]
- Lee, Q.; Xue, Z.; Luo, Y.; Lin, Y.; Lai, M.; Xu, H.; Liu, B.; Zheng, M.; Lv, F.; Zeng, F. Low Molecular Weight Polysaccharide of Tremella Fuciformis Exhibits Stronger Antioxidant and Immunomodulatory Activities than High Molecular Weight Polysaccharide. International Journal of Biological Macromolecules 2024, 136097. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ma, X.; Ren, H.; Fan, W.; Leng, F.; Yang, M.; Wang, X. Extraction, Purification, and Bioactivities Analyses of Polysaccharides from Glycyrrhiza Uralensis. Industrial Crops and Products 2018, 122, 596–608. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.-M.; Xu, S.-Y.; Liu, Y.; Cheong, K.-L. Physicochemical Characterization and Antioxidant Activity of Sulphated Polysaccharides Derived from Porphyra Haitanensis. International Journal of Biological Macromolecules 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Teng, C.; Li, S.; Xu, L.; Ma, K.; Lu, Y.; Feng, J.; Chai, Z.; Hu, X.; Zhou, W.; Li, Y. Structural Characterization, Physicochemical Properties and Hypoglycemic Activity of Sulfated Polysaccharides from Porphyra Yezoensis. Food Bioscience 2024, 62, 105163. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Tabarsa, M.; Abdollahi, M. Ultrasonic-Assisted Enzymatic Extraction of Sulfated Polysaccharide from Skipjack Tuna by-Products. Ultrasonics Sonochemistry 2023, 95, 106385. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Habibzadeh, S.; Munir, M.T.; Vahabi, H.; Bagheri, B.; Rabiee, N.; Zarrintaj, P.; Saeb, M.R. Crystalline Polysaccharides: A Review. Carbohydrate Polymers 2022, 275, 118624. [Google Scholar] [CrossRef] [PubMed]
- Marinho, G.S.; Sørensen, A.-D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant Content and Activity of the Seaweed Saccharina Latissima: A Seasonal Perspective. J Appl Phycol 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Hermund, D. Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; 2018; pp. 201–221 ISBN 978-0-12-813312-5.
- Hamzaoui, A.; Ghariani, M.; Sellem, I.; Hamdi, M.; Feki, A.; Jaballi, I.; Nasri, M.; Amara, I.B. Extraction, Characterization and Biological Properties of Polysaccharide Derived from Green Seaweed “Chaetomorpha Linum” and Its Potential Application in Tunisian Beef Sausages. International Journal of Biological Macromolecules 2020, 148, 1156–1168. [Google Scholar] [CrossRef]
- Ma, X.-T.; Sun, X.-Y.; Yu, K.; Gui, B.-S.; Gui, Q.; Ouyang, J.-M. Effect of Content of Sulfate Groups in Seaweed Polysaccharides on Antioxidant Activity and Repair Effect of Subcellular Organelles in Injured HK-2 Cells. Oxidative Medicine and Cellular Longevity 2017, 2017, e2542950. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, Antioxidant and Antibacterial Activities of Polysaccharides Extracted from Olive Leaves. International Journal of Biological Macromolecules 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Medicine and Cellular Longevity 2015, 2016, e5692852. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Najjaa, H.; Zouari, N.; Sebai, H.; Nasri, M. Sulfated Polysaccharides from the Viscera of Mustelus Shark: Characterization and Antioxidant, Anticoagulant and Anti-Proliferative Activities. Bioactive Carbohydrates and Dietary Fibre 2024, 31, 100399. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated Modification, Characterization and Bioactivities of an Acidic Polysaccharide Fraction from an Edible Mushroom Pleurotus Eous (Berk.) Sacc. Heliyon 2021, 7. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Burri, P.H.; Djonov, V. Chorioallantoic Membrane Capillary Bed: A Useful Target for Studying Angiogenesis and Anti-Angiogenesis in Vivo. Anat. Rec. 2001, 264, 317–324. [Google Scholar] [CrossRef]
- Muhammad, O.; Lama, R. The Effect of Diclofenac Sodium on Blood Vessel Formation. Medbiotech Journal 2018, 02. [Google Scholar] [CrossRef]
- Berndt, S.; Blacher, S.; Perrier d’Hauterive, S.; Thiry, M.; Tsampalas, M.; Cruz, A.; Péqueux, C.; Lorquet, S.; Munaut, C.; Noël, A.; et al. Chorionic Gonadotropin Stimulation of Angiogenesis and Pericyte Recruitment. The Journal of Clinical Endocrinology & Metabolism 2009, 94, 4567–4574. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Yu, R.; Zhong, J.; Zhou, Q.; Ren, W.; Liu, Z.; Bian, Y. Kaempferol Prevents Angiogenesis of Rat Intestinal Microvascular Endothelial Cells Induced by LPS and TNF-α via Inhibiting VEGF/Akt/P38 Signaling Pathways and Maintaining Gut-Vascular Barrier Integrity. Chemico-Biological Interactions 2022, 366, 110135. [Google Scholar] [CrossRef]
- Mauroux, A.; Gofflo, S.; Breugnot, J.; Malbouyres, M.; Atlas, Y.; Ardidie-Robouant, C.; Marchand, L.; Monnot, C.; Germain, S.; Bordes, S.; et al. Angiogenesis and Full Thickness Wound Repair in a Cell Sheet-Based Vascularized Skin Substitute. Acta Biomaterialia 2024, 187, 123–137. [Google Scholar] [CrossRef]
- Jozkowicz, A.; Cooke, J.P.; Guevara, I.; Huk, I.; Funovics, P.; Pachinger, O.; Weidinger, F.; Dulak, J. Genetic Augmentation of Nitric Oxide Synthase Increases the Vascular Generation of VEGF. Cardiovascular Research 2001, 51, 773–783. [Google Scholar] [CrossRef]
- Keykhaee, M.; Sorouri, F.; Rahimifard, M.; Baeeri, M.; Forumadi, A.; Firoozpour, L.; Khoobi, M. Polysaccharide-Based Hydrogel Enriched by Epidermal Growth Factor Peptide Fragment for Improving the Wound Healing Process. Heliyon 2023, 9, e22749. [Google Scholar] [CrossRef]
- Malektaj, H.; Nour, S.; Imani, R.; Siadati, M.H. Angiogenesis Induction as a Key Step in Cardiac Tissue Regeneration: From Angiogenic Agents to Biomaterials. International Journal of Pharmaceutics 2023, 643, 123233. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-Scavenging Materials for Skin Wound Healing: Advancements and Applications. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Zheng, Q.; Lu, H.; Huang, H.; Zhang, J.; Fang, Z.; Lin, L.; Ma, P. Recent Progress in the Efficacy of Algal Saccharides on Skin Repair. Algal Research 2024, 78, 103403. [Google Scholar] [CrossRef]
- Tan, S.T.; Dosan, R. Lessons From Epithelialization: The Reason Behind Moist Wound Environment. [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. Journal of Applied Polymer Science 2019, 136, 47738. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-Dependent Regulation of Collagen Metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Cen, Y. Burn Wound Healing Potential of a Polysaccharide from Sanguisorba Officinalis L. in Mice. International Journal of Biological Macromolecules 2018, 112, 862–867. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Zhang, M.; Xiao, Y.; Jin, J.; Qin, T.; Li, Y. Bovine Collagen Oligopeptides Accelerate Wound Healing by Promoting Fibroblast Migration via PI3K/Akt/mTOR Signaling Pathway. Journal of Functional Foods 2022, 90, 104981. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Chen, S.-H.; Chen, C.-H.; Chou, P.-Y.; Yang, C.-C.; Lin, F.-H. Polysaccharide Extracted from Bletilla Striata Promotes Proliferation and Migration of Human Tenocytes. Polymers 2020, 12, 2567. [Google Scholar] [CrossRef]
- Nie, X.; Li, J.; Cheng, Y.; Rangsinth, P.; Wu, X.; Zheng, C.; Shiu, P.H.-T.; Li, R.; Xu, N.; He, Y.; et al. Characterization of a Polysaccharide from Amauroderma Rugosum and Its Proangiogenic Activities in Vitro and in Vivo. International Journal of Biological Macromolecules 2024, 271, 132533. [Google Scholar] [CrossRef]
- Chouikhi, A.; Ktari, N.; Bardaa, S.; Hzami, A.; Ben Slima, S.; Trabelsi, I.; Asehraou, A.; Ben Salah, R. A Novel Probiotic Strain, Lactiplantibacillus Plantarum LC38, Isolated from Tunisian Camel Milk Promoting Wound Healing in Wistar Diabetic Rats. Arch Microbiol 2021, 204, 24. [Google Scholar] [CrossRef]
- Ben Saad, H.; Frikha, D.; Bouallegue, A.; Badraoui, R.; Mellouli, M.; Kallel, H.; Pujo, J.M.; Ben Amara, I. Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum Corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals 2023, 16, 1305. [Google Scholar] [CrossRef]
- Bédoui, I.; Nasr, H.B.; Ksouda, K.; Ayadi, W.; Louati, N.; Chamkha, M.; Choura, S.; Gargouri, J.; Hammami, S.; Affes, H.; et al. Phytochemical Composition, Bioavailability and Pharmacokinetics of Scorzonera Undulata Methanolic Extracts: Antioxidant, Anticancer, and Apoptotic Effects on MCF7 Cells. Pharmacognosy Magazine 2024, 20, 218–229. [Google Scholar] [CrossRef]
 : Ulceration
: Ulceration  : Inflammatory infiltrate
: Inflammatory infiltrate  : Fibers of collagen
: Fibers of collagen
 : Ulceration
: Ulceration  : Inflammatory infiltrate
: Inflammatory infiltrate  : Fibers of collagen
: Fibers of collagen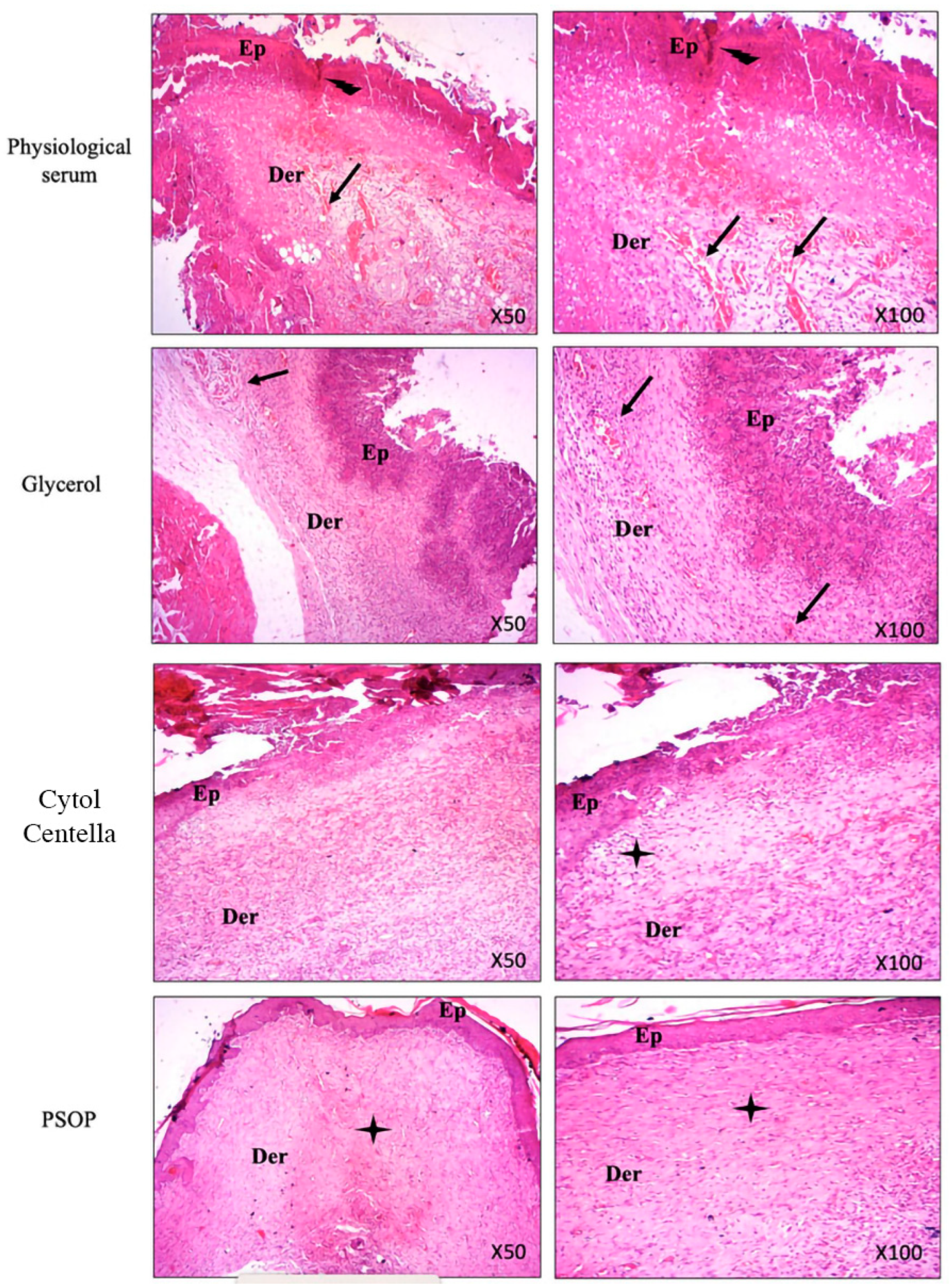
| Groups | Control | Diclofenac (50 μg/egg) |
Choriogonadotropin (30 μg/egg) |
PSOP (25 μg/egg) |
PSOP (50 μg/egg) |
PSOP (100 μg/egg) |
|---|---|---|---|---|---|---|
| Vessel Number (%) | 100a | 71.14 ± 3.97b | 168.36 ± 2.72c | 137.22 ± 3.7d | 200.66 ± 2.73e | 250.66 ± 3.49f |
| Compound No. | COX-2 (1cx2) |
VEGF (2c7w) |
|---|---|---|
| Binding Affinity (kcal × mol−1) | ||
| Arabinose | −5.7 | −4.5 |
| Fructose | −6.4 | −4.5 |
| Glucose | −6.3 | −4.6 |
| Glucoronic acid | −5.6 | −4.4 |
| Xylose | −6.1 | −4.1 |
| RMSD (lower – upper) | ||
| Arabinose | 0.0 - 30.13 | 0.0 - 31.90 |
| Fructose | 0.0 - 30.82 | 0.0 - 9.08 |
| Glucose | 0.0 - 22.70 | 0.0 - 9.14 |
| Glucoronic acid | 0.0 - 41.78 | 0.0 - 14.83 |
| Xylose | 0.0 - 9.86 | 0.0 - 30.91 |
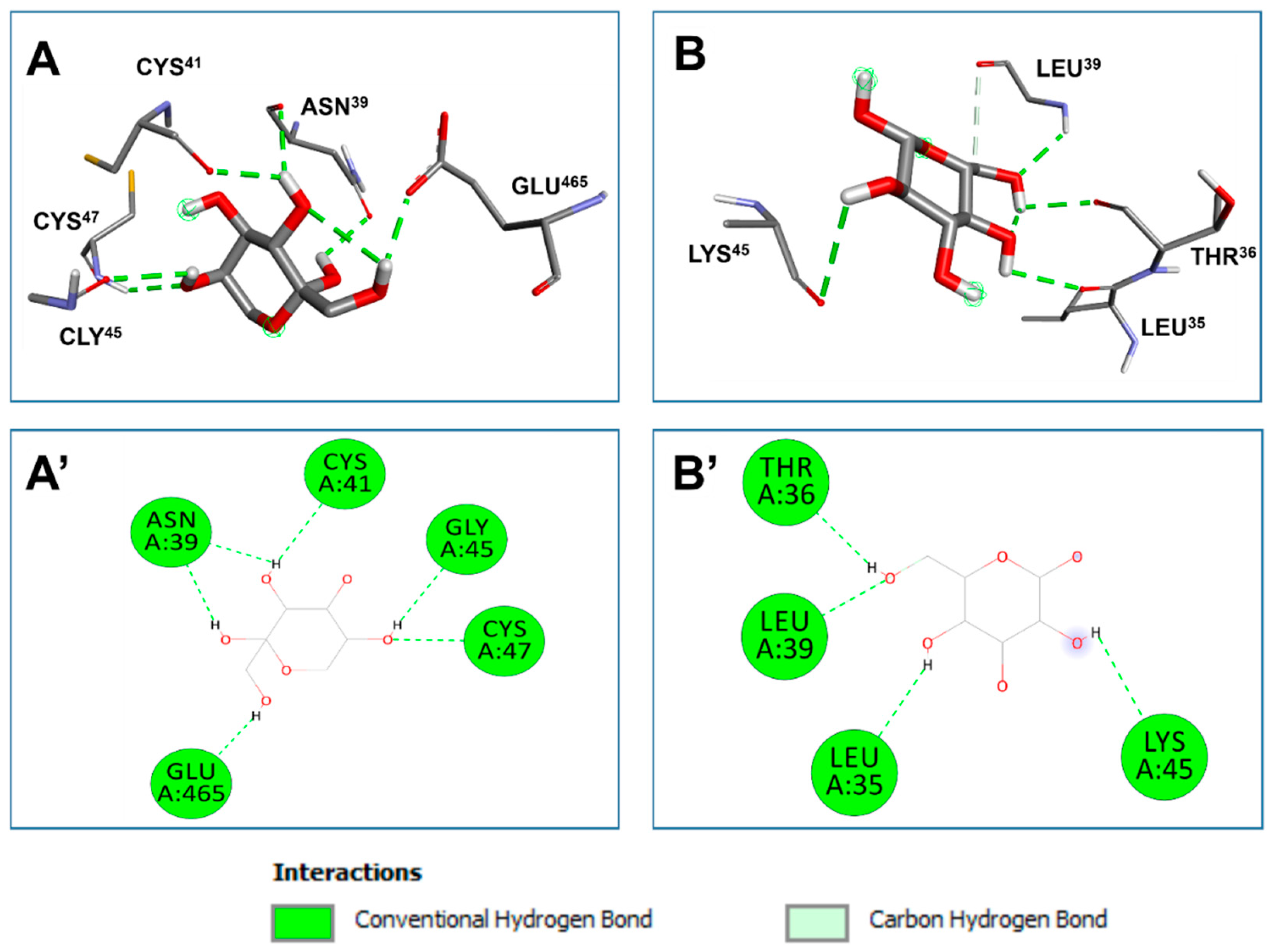
| Saccharide | No. Conventional H-Bonds |
Closest Interacting Residues |
||
|---|---|---|---|---|
| Interacting Residues | Closest residue (Distance, Å) | No. closest interacting residues | ||
| Cyclooxygenase 2 (COX-2) | ||||
| Fructose | 6 | CYS47, ASN39, GLU465, ASN39, CYS41, GLY45 | GLU465:OE1 (2.072) |
5 |
| Vascular Endothelial growth factor (VEGF) | ||||
| Glucose | 4 | LEU39, THR36, LEU35, LYS45, LEU39 | LEU39:HN (2.247) |
4 |
| Bold residues: amino acids interacting with conventional H-bond | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





