Submitted:
23 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
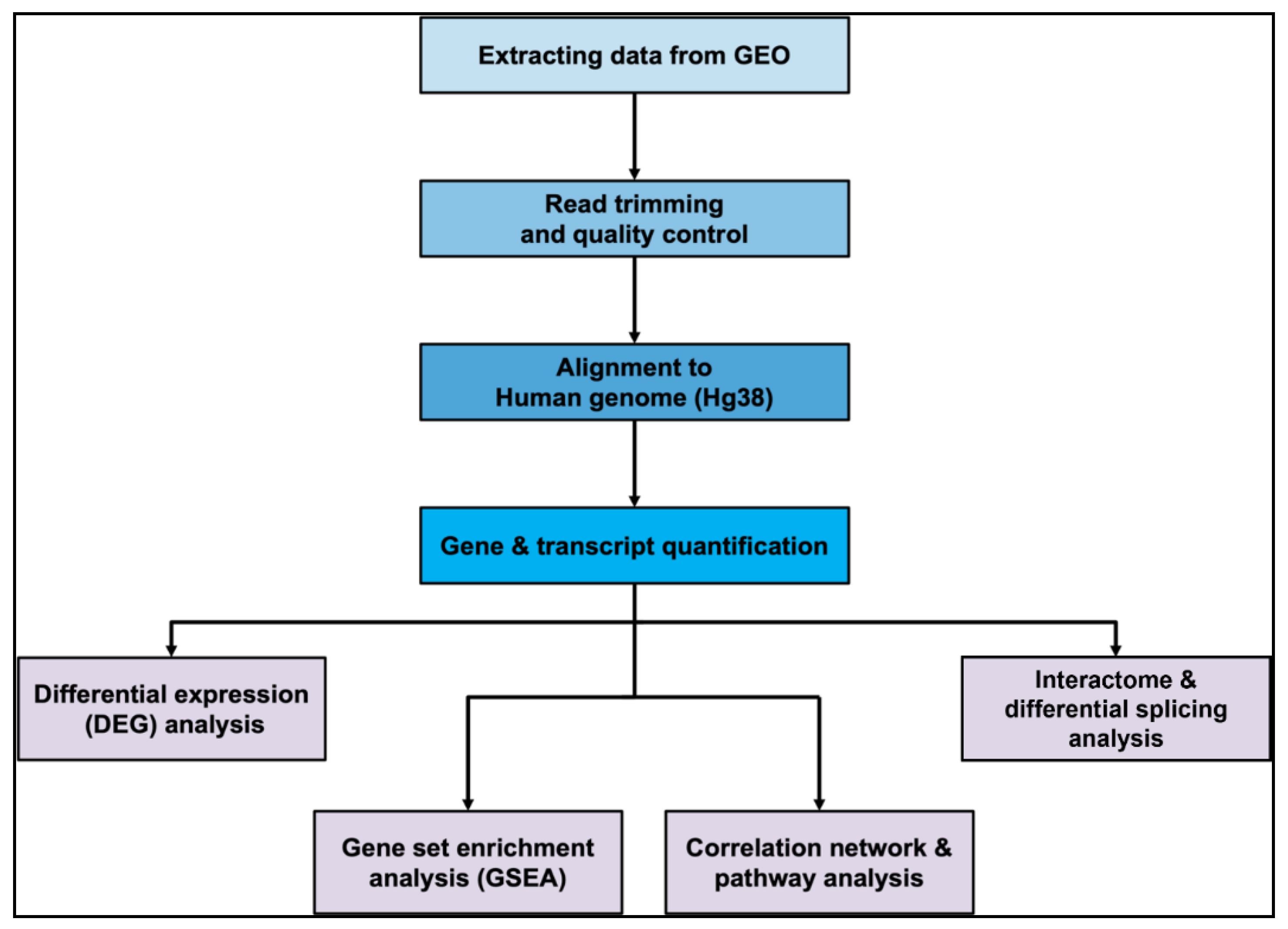
2. Materials and Methods
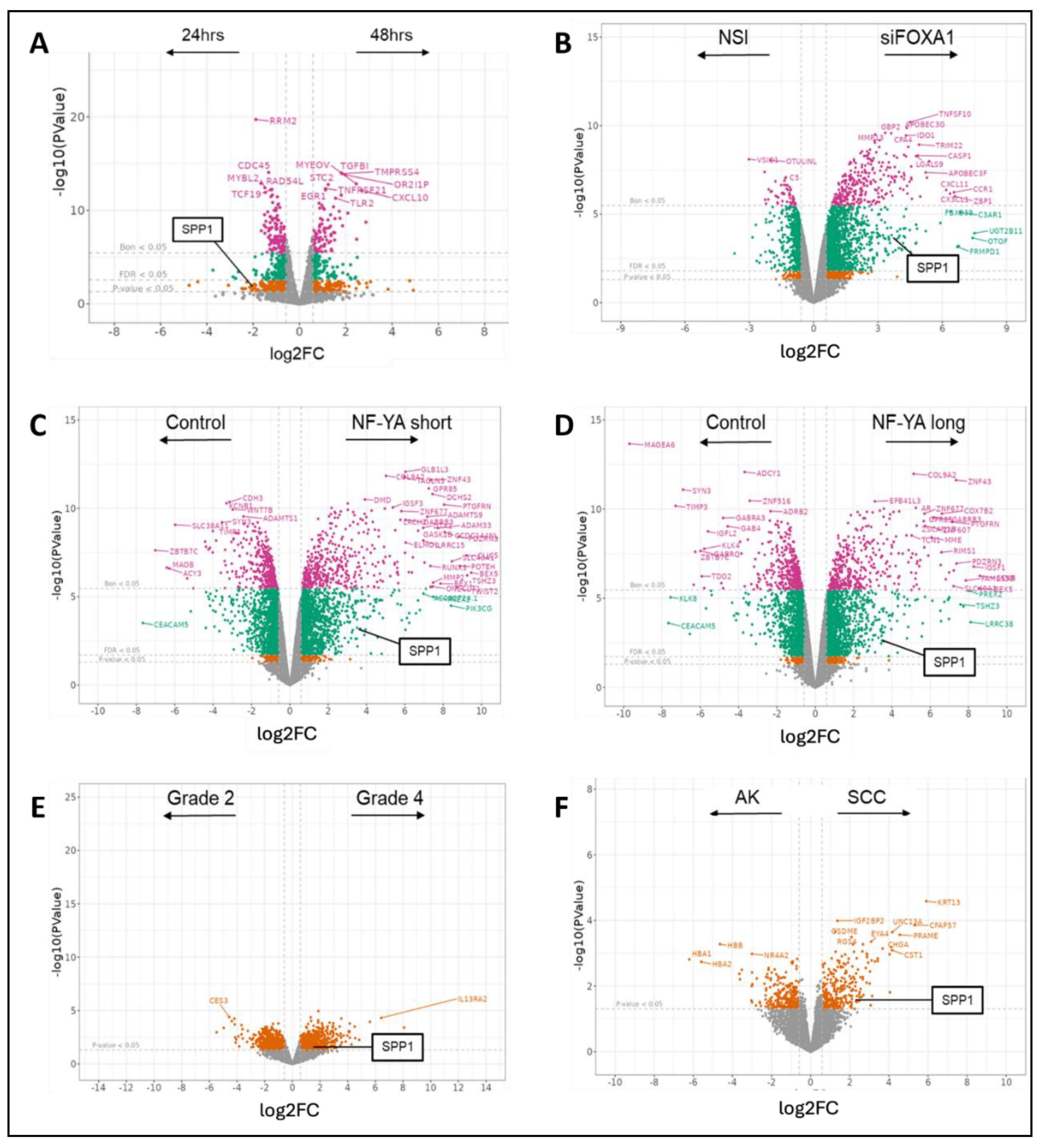
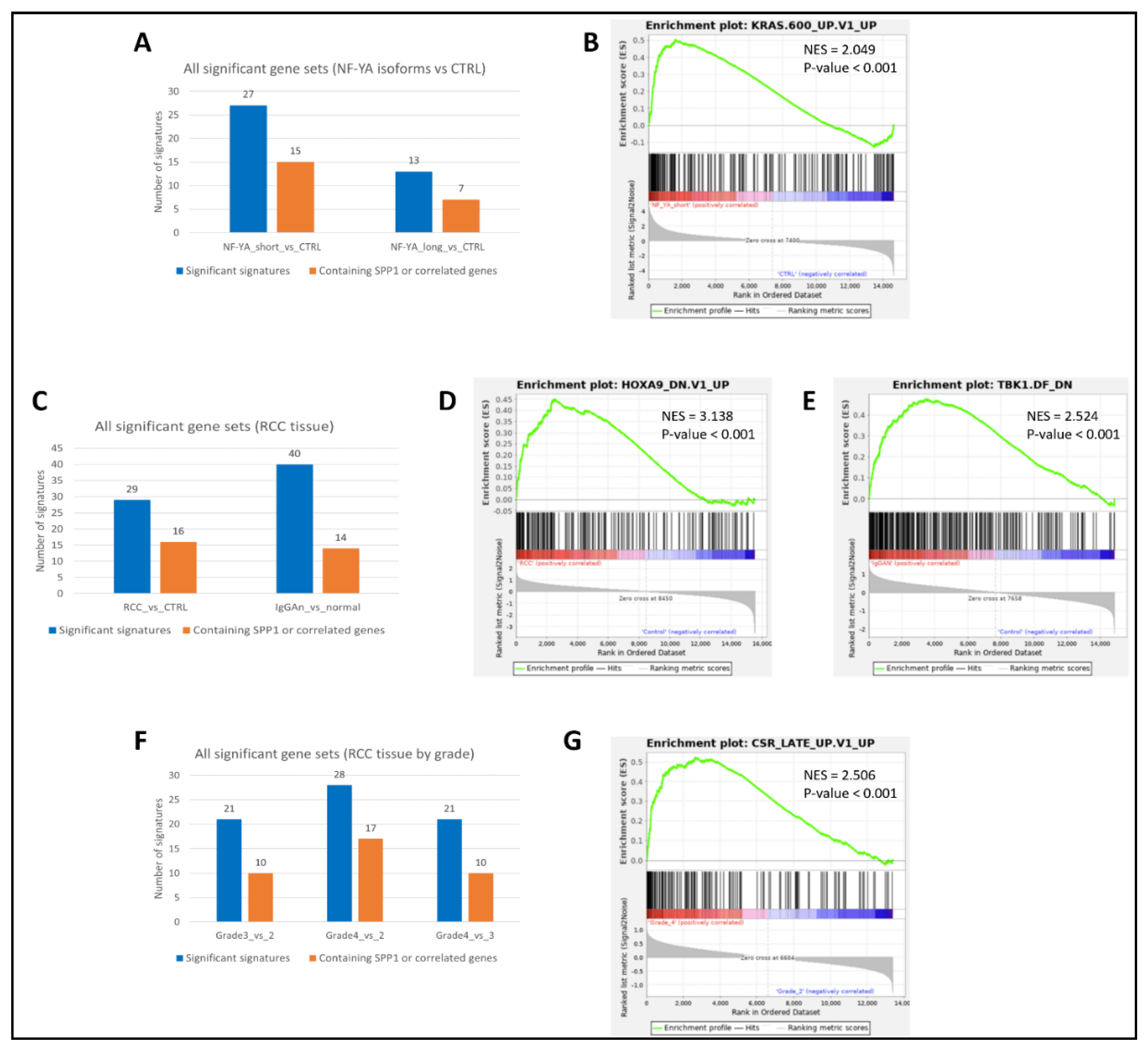
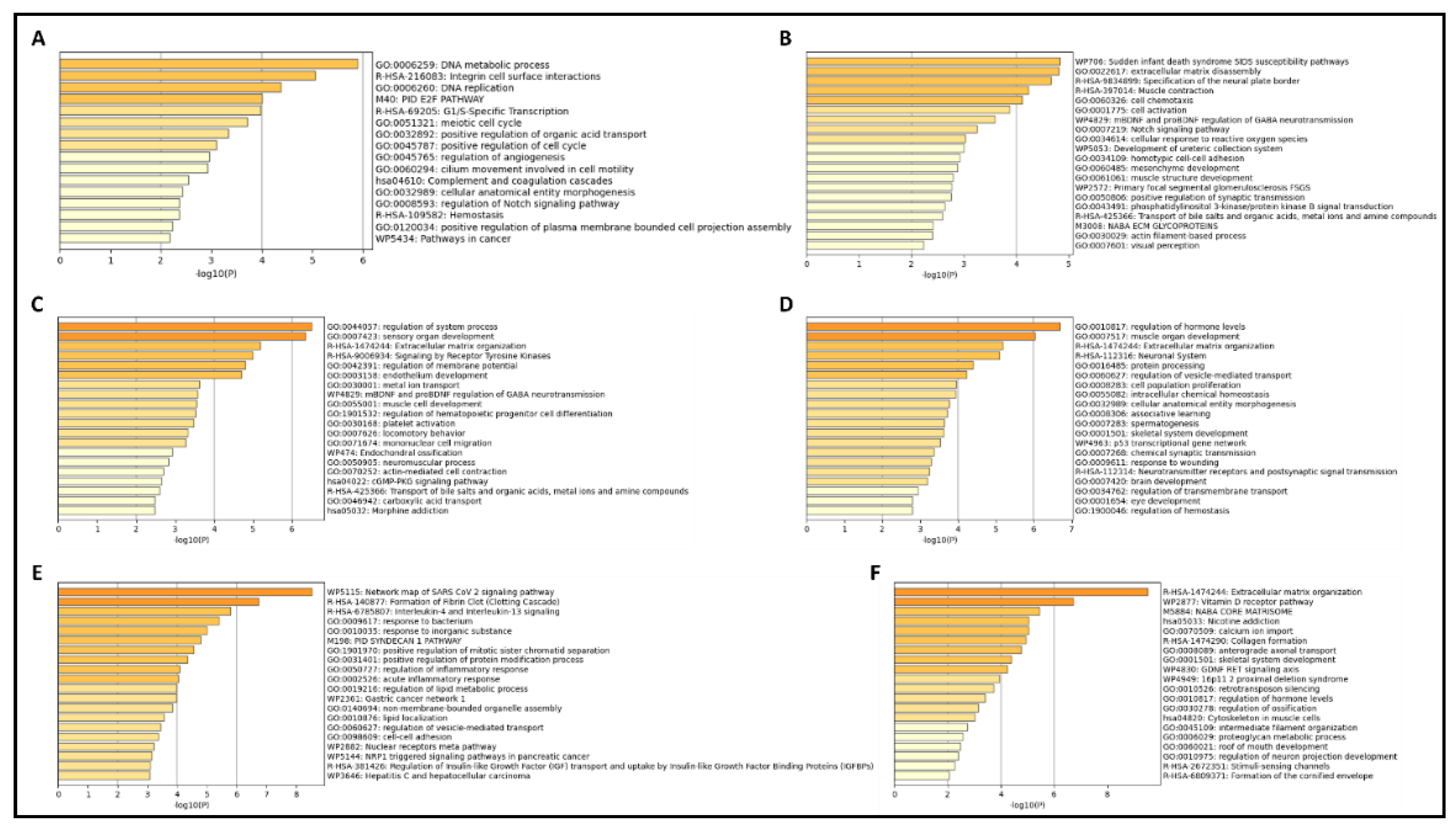
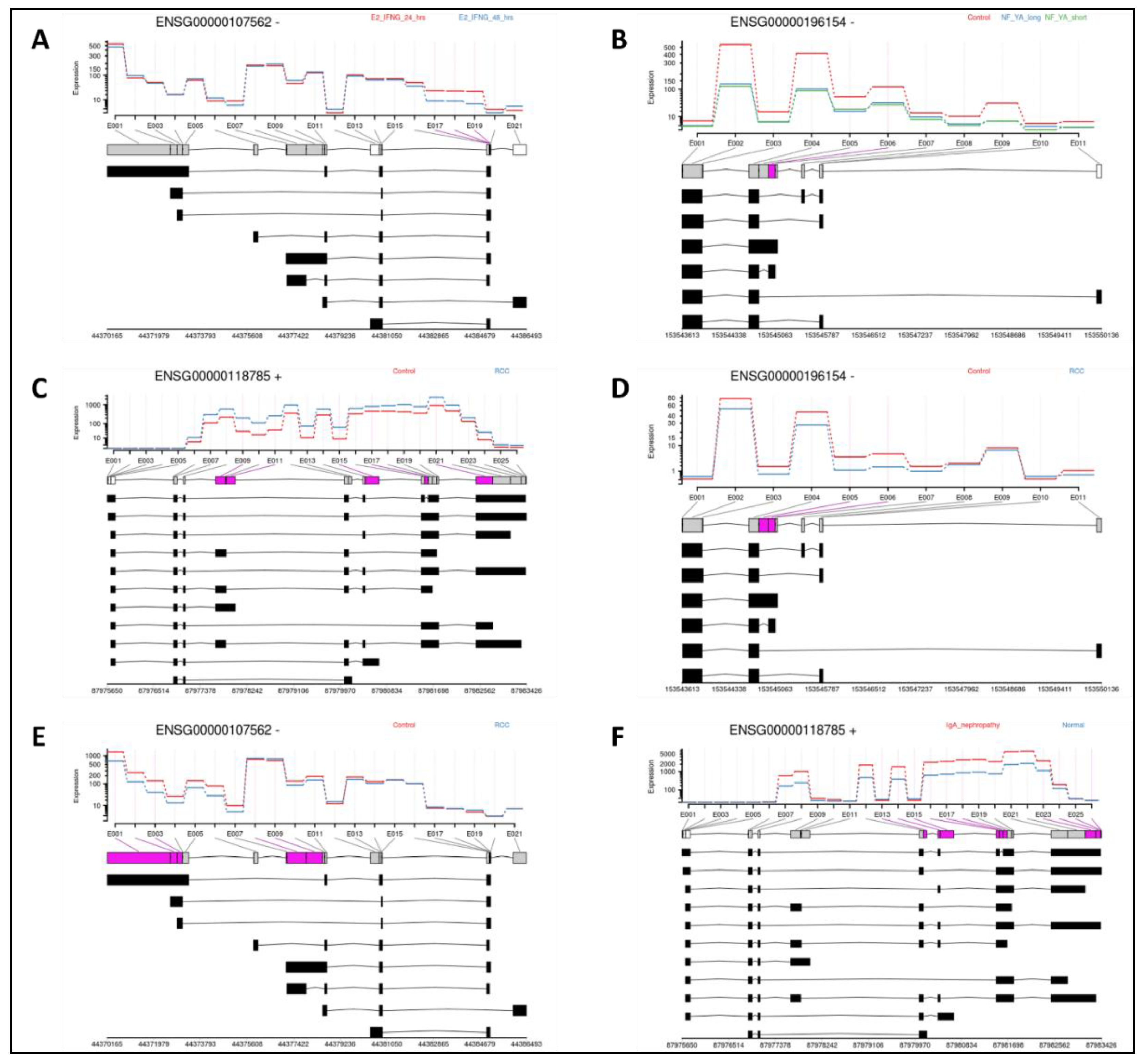
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. The Development and Causes of Cancer.
- Vicente-Dueñas, C., Romero-Camarero, I., Cobaleda, C., & Sánchez-García, I. (2013). Function of oncogenes in cancer development: a changing paradigm. The EMBO journal, 32(11), 1502–1513. [CrossRef]
- Zhao, H., Chen, Q., Alam, A., Cui, J., Suen, K. C., Soo, A. P., Eguchi, S., Gu, J., & Ma, D. (2018). The role of osteopontin in the progression of solid organ tumour. Cell death & disease, 9(3), 356. [CrossRef]
- Wei, R., Wong, J. P. C., & Kwok, H. F. (2017). Osteopontin -- a promising biomarker for cancer therapy. Journal of Cancer, 8(12), 2173–2183. [CrossRef]
- Rangaswami, H., Bulbule, A. & Kundu, G. C. Osteopontin: role in cell signaling and cancer progression. Trends Cell. Biol. 16, 79–87 (2006). [CrossRef]
- Tan, Y., Zhao, L., Yang, Y. G., & Liu, W. (2022). The Role of Osteopontin in Tumor Progression Through Tumor-Associated Macrophages. Frontiers in oncology, 12, 953283. [CrossRef]
- Butti, R., Nimma, R., Kundu, G., Bulbule, A., Kumar, T. V. S., Gunasekaran, V. P., Tomar, D., Kumar, D., Mane, A., Gill, S. S., Patil, T., Weber, G. F., & Kundu, G. C. (2021). Tumor-derived osteopontin drives the resident fibroblast to myofibroblast differentiation through Twist1 to promote breast cancer progression. Oncogene, 40(11), 2002–2017. [CrossRef]
- Wang, C., Ying, J., Nie, X. et al. Targeting angiogenesis for fracture nonunion treatment in inflammatory disease. Bone Res 9, 29 (2021). [CrossRef]
- Wein, M., Huelter-Hassler, D., Nelson, K. et al. Differential osteopontin expression in human osteoblasts derived from iliac crest and alveolar bone and its role in early stages of angiogenesis. J Bone Miner Metab 37, 105–117 (2019). [CrossRef]
- Wei, T., Bi, G., Bian, Y., Ruan, S., Yuan, G., Xie, H., Zhao, M., Shen, R., Zhu, Y., Wang, Q., Yang, Y., & Zhu, D. (2020). The Significance of Secreted Phosphoprotein 1 in Multiple Human Cancers. Frontiers in molecular biosciences, 7, 565383. [CrossRef]
- Shurin M. R. (2018). Osteopontin controls immunosuppression in the tumor microenvironment. The Journal of clinical investigation, 128(12), 5209–5212. [CrossRef]
- Hao, C., Lane, J., & Jiang, W. G. (2023). Osteopontin and Cancer: Insights into Its Role in Drug Resistance. Biomedicines, 11(1), 197. [CrossRef]
- Zeng, P., Zhang, X., Xiang, T., Ling, Z., Lin, C., & Diao, H. (2022). Secreted phosphoprotein 1 as a potential prognostic and immunotherapy biomarker in multiple human cancers. Bioengineered, 13(2), 3221–3239. [CrossRef]
- Weber, G. F., Lett, G. S., & Haubein, N. C. (2010). Osteopontin is a marker for cancer aggressiveness and patient survival. British journal of cancer, 103(6), 861–869. [CrossRef]
- Sanchez-Vega, F., Mina, M., Armenia, J., Chatila, W. K., Luna, A., La, K. C., Dimitriadoy, S., Liu, D. L., Kantheti, H. S., Saghafinia, S., Chakravarty, D., Daian, F., Gao, Q., Bailey, M. H., Liang, W. W., Foltz, S. M., Shmulevich, I., Ding, L., Heins, Z., Ochoa, A., … Schultz, N. (2018). Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell, 173(2), 321–337.e10. [CrossRef]
- Ergun A, Doran G, Costello JC, Paik HH, Collins JJ, Mathis D, Benoist C & ImmGen Consortium (2013). Differential splicing across immune system lineages. PNAS, 110(35), 14324-14329. https://doi/10.1073/pnas.1311839110.
- Zhang, Y., Qian, J., Gu, C., & Yang, Y. (2021). Alternative splicing and cancer: a systematic review. Signal transduction and targeted therapy, 6(1), 78. [CrossRef]
- Gimba ERP, Brum MCM, Nestal De Moraes G. (2019). Full-length osteopontin and its splice variants as modulators of chemoresistance and radioresistance (Review). Int J Oncol. 54(2):420-430. [CrossRef]
- Do, H. T. T., Shanak, S., Barghash, A., & Helms, V. (2023). Differential exon usage of developmental genes is associated with deregulated epigenetic marks. Scientific reports, 13(1), 12256. [CrossRef]
- SRA Toolkit Development Team. https://trace.ncbi.nlm.nih.gov/Traces/sra/sra.cgi?view=software.
- Bolger, A. M., Lohse, M., & Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics (Oxford, England), 30(15), 2114–2120. [CrossRef]
- Andrews, S. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Kim, D., Paggi, J. M., Park, C., Bennett, C., & Salzberg, S. L. (2019). Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nature biotechnology, 37(8), 907–915. [CrossRef]
- Liao, Y., Smyth, G. K., & Shi, W. (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics (Oxford, England), 30(7), 923–930. [CrossRef]
- Robinson, M. D., McCarthy, D. J., & Smyth, G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England), 26(1), 139–140. [CrossRef]
- Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L., Gillette, M. A., Paulovich, A., Pomeroy, S. L., Golub, T. R., Lander, E. S., & Mesirov, J. P. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America, 102(43), 15545–15550. [CrossRef]
- Liberzon, A., Birger, C., Thorvaldsdóttir, H., Ghandi, M., Mesirov, J. P., & Tamayo, P. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell systems, 1(6), 417–425. [CrossRef]
- Zhou, Y., Zhou, B., Pache, L., Chang, M., Khodabakhshi, A. H., Tanaseichuk, O., Benner, C., & Chanda, S. K. (2019). Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nature communications, 10(1), 1523. [CrossRef]
- Anders, S., Reyes, A., & Huber, W. (2012). Detecting differential usage of exons from RNA-seq data. Genome research, 22(10), 2008–2017. [CrossRef]
- Hermida-Prado, F., Xie, Y., Sherman, S., Nagy, Z., Russo, D., Akhshi, T., Chu, Z., Feit, A., Campisi, M., Chen, M., Nardone, A., Guarducci, C., Lim, K., Font-Tello, A., Lee, I., García-Pedrero, J., Cañadas, I., Agudo, J., Huang, Y., Sella, T., … Jeselsohn, R. (2023). Endocrine Therapy Synergizes with SMAC Mimetics to Potentiate Antigen Presentation and Tumor Regression in Hormone Receptor-Positive Breast Cancer. Cancer research, 83(19), 3284–3304. [CrossRef]
- Hölzen, L., Mitschke, J., Schönichen, C., Hess, M. E., Ehrenfeld, S., Boerries, M., Miething, C., Brummer, T., & Reinheckel, T. (2022). RNA interference screens discover proteases as synthetic lethal partners of PI3K inhibition in breast cancer cells. Theragnostics, 12(9), 4348–4373. [CrossRef]
- Saotome, M., Poduval, D. B., Nair, R., Cooper, M., & Takaku, M. (2022). GATA3 Truncation Mutants Alter EMT Related Gene Expression via Partial Motif Recognition in Luminal Breast Cancer Cells. Frontiers in genetics, 13, 820532. [CrossRef]
- Qiu, Z., Guo, W., Dong, B., Wang, Y., Deng, P., Wang, C., Liu, J., Zhang, Q., Grosschedl, R., Yu, Z., Deng, J., & Wu, Y. (2022). EBF1 promotes triple-negative breast cancer progression by surveillance of the HIF1α pathway. Proceedings of the National Academy of Sciences of the United States of America, 119(28), e2119518119. [CrossRef]
- Ma, S., Tang, T., Probst, G., Konradi, A., Jin, C., Li, F., Gutkind, J. S., Fu, X. D., & Guan, K. L. (2022). Transcriptional repression of estrogen receptor alpha by YAP reveals the Hippo pathway as therapeutic target for ER+ breast cancer. Nature communications, 13(1), 1061. [CrossRef]
- Han, H., Wang, Y., Curto, J., Gurrapu, S., Laudato, S., Rumandla, A., Chakraborty, G., Wang, X., Chen, H., Jiang, Y., Kumar, D., Caggiano, E. G., Capogiri, M., Zhang, B., Ji, Y., Maity, S. N., Hu, M., Bai, S., Aparicio, A. M., Efstathiou, E., … Giancotti, F. G. (2022). Mesenchymal and stem-like prostate cancer linked to therapy-induced lineage plasticity and metastasis. Cell reports, 39(1), 110595. [CrossRef]
- Basil, P., Robertson, M. J., Bingman, W. E., 3rd, Dash, A. K., Krause, W. C., Shafi, A. A., Piyarathna, B., Coarfa, C., & Weigel, N. L. (2022). Cistrome and transcriptome analysis identifies unique androgen receptor (AR) and AR-V7 splice variant chromatin binding and transcriptional activities. Scientific reports, 12(1), 5351. [CrossRef]
- Kumar, A., Kasikci, Y., Badredine, A., Azzag, K., Quintyn Ranty, M. L., Zaidi, F., Aragou, N., Mazerolles, C., Malavaud, B., Mendoza-Parra, M. A., Vandel, L., & Gronemeyer, H. (2021). Patient-matched analysis identifies deregulated networks in prostate cancer to guide personalized therapeutic intervention. American journal of cancer research, 11(11), 5299–5318.
- Del Giudice, M., Foster, J. G., Peirone, S., Rissone, A., Caizzi, L., Gaudino, F., Parlato, C., Anselmi, F., Arkell, R., Guarrera, S., Oliviero, S., Basso, G., Rajan, P., & Cereda, M. (2022). FOXA1 regulates alternative splicing in prostate cancer. Cell reports, 40(13), 111404. [CrossRef]
- Belluti, S., Semeghini, V., Rigillo, G., Ronzio, M., Benati, D., Torricelli, F., Reggiani Bonetti, L., Carnevale, G., Grisendi, G., Ciarrocchi, A., Dominici, M., Recchia, A., Dolfini, D., & Imbriano, C. (2021). Alternative splicing of NF-YA promotes prostate cancer aggressiveness and represents a new molecular marker for clinical stratification of patients. Journal of experimental & clinical cancer research: CR, 40(1), 362. [CrossRef]
- Sun, G., Chen, J., Liang, J., Yin, X., Zhang, M., Yao, J., He, N., Armstrong, C. M., Zheng, L., Zhang, X., Zhu, S., Sun, X., Yang, X., Zhao, W., Liao, B., Pan, X., Nie, L., Yang, L., Chen, Y., Zhao, J., … Zeng, H. (2021). Integrated exome and RNA sequencing of TFE3-translocation renal cell carcinoma. Nature communications, 12(1), 5262. Sun, G., Chen, J., Liang, J. et al. Integrated exome and RNA sequencing of TFE3-translocation renal cell carcinoma. Nat Commun 12, 5262 (2021). [CrossRef]
- Park, S., Yang, S. H., Jeong, C. W., Moon, K. C., Kim, D. K., Joo, K. W., Kim, Y. S., Lee, J. W., & Lee, H. (2020). RNA-Seq profiling of microdissected glomeruli identifies potential biomarkers for human IgA nephropathy. American journal of physiology. Renal physiology, 319(5), F809–F821. [CrossRef]
- Kajdasz, A., Majer, W., Kluzek, K., Sobkowiak, J., Milecki, T., Derebecka, N., Kwias, Z., Bluyssen, H. A. R., & Wesoly, J. (2021). Identification of RCC Subtype-Specific microRNAs-Meta-Analysis of High-Throughput RCC Tumor microRNA Expression Data. Cancers, 13(3), 548. [CrossRef]
- Yao, X., Hong, J. H., Nargund, A. M., Ng, M. S. W., Heng, H. L., Li, Z., Guan, P., Sugiura, M., Chu, P. L., Wang, L. C., Ye, X., Qu, J., Kwek, X. Y., Lim, J. C. T., Ooi, W. F., Koh, J., Wang, Z., Pan, Y. F., Ong, Y. S., Tan, K. Y., … Teh, B. T. (2023). PBRM1-deficient PBAF complexes target aberrant genomic loci to activate the NF-κB pathway in clear cell renal cell carcinoma. Nature cell biology, 25(5), 765–777. [CrossRef]
- Querfeld, C., Leung, S., Myskowski, P. L., Curran, S. A., Goldman, D. A., Heller, G., Wu, X., Kil, S. H., Sharma, S., Finn, K. J., Horwitz, S., Moskowitz, A., Mehrara, B., Rosen, S. T., Halpern, A. C., & Young, J. W. (2018). Primary T Cells from Cutaneous T-cell Lymphoma Skin Explants Display an Exhausted Immune Checkpoint Profile. Cancer immunology research, 6(8), 900–909. [CrossRef]
- Di Raimondo, C., Han, Z., Su, C., Wu, X., Qin, H., Sanchez, J. F., Yuan, Y. C., Martinez, X., Abdulla, F., Zain, J., Chen, C. W., Rosen, S. T., & Querfeld, C. (2021). Identification of a Distinct miRNA Regulatory Network in the Tumor Microenvironment of Transformed Mycosis Fungoides. Cancers, 13(22), 5854. [CrossRef]
- Park, D. E., Cheng, J., McGrath, J. P., Lim, M. Y., Cushman, C., Swanson, S. K., Tillgren, M. L., Paulo, J. A., Gokhale, P. C., Florens, L., Washburn, M. P., Trojer, P., & DeCaprio, J. A. (2020). Merkel cell polyomavirus activates LSD1-mediated blockade of non-canonical BAF to regulate transformation and tumorigenesis. Nature cell biology, 22(5), 603–615. [CrossRef]
- Chitsazzadeh, V., Coarfa, C., Drummond, J. A., Nguyen, T., Joseph, A., Chilukuri, S., Charpiot, E., Adelmann, C. H., Ching, G., Nguyen, T. N., Nicholas, C., Thomas, V. D., Migden, M., MacFarlane, D., Thompson, E., Shen, J., Takata, Y., McNiece, K., Polansky, M. A., Abbas, H. A., … Tsai, K. Y. (2016). Cross-species identification of genomic drivers of squamous cell carcinoma development across preneoplastic intermediates. Nature communications, 7, 12601. [CrossRef]
- Fu, D., Hu, Z., Xu, X., Dai, X., & Liu, Z. (2022). Key signal transduction pathways and crosstalk in cancer: Biological and therapeutic opportunities. Translational oncology, 26, 101510. [CrossRef]
| Tissue | Number of studies | Samples obtained |
|---|---|---|
| Breast | 5 | 78 |
| Prostate | 5 | 101 |
| Renal | 4 | 96 |
| Skin | 3 | 115 |
| Total | 17 | 390 |
| Study | Cell types | Samples | Comparisons |
|---|---|---|---|
| GSE213474 [30] | MCF7 cells supplemented with heat-inactivated charcoal stripped FBS (HD); and HD with estradiol (E2) | 30 | Stimulation with IFN-γ for 6, 12, 24 and 48 hours v/s control |
| GSE186196 [31] | MCF7 and MDA-MB231 cells | 18 | Treatment with BKM and DMSO v/s control |
| GSE195816 [32] | MDA157 and MDA436 cells | 12 | Treatment with EBF1 shRNA v/s control |
| GSE190381 [33] | T47D cells containing 3 types of mutations (GATA splice-site deletion, C321 frame-shift, A333 frame-shift) | 12 | Mutated v/s control |
| GSE181460 [34] | T47D cells | 6 | LATS1/2 knockout v/s control |
| Study | Cell types | Samples | Comparisons |
|---|---|---|---|
| GSE162294 [35] | LNCap (prostate adenocarcinoma) cells | 36 | Treatment with enzalutamide for 1, 3, 5, 7 and 14 days v/s control Treatment with shTP53 (oligo #1 and #2) v/s control Treatment with shBRCA1 (oligo #1 and #2) v/s control |
| GSE184676 [36] | LN95 (CPRC) cells | 28 | Treatment with R1881, exon7-targeted siRNA and AR-V7 exon-targeted siRNA v/s control |
| GSE133626 [37] | Prostate cancer tissue | 16 | Tumorous v/s control |
| GSE193127 [38] | VCaP and PC-3 cells | 12 | Transfected with FOXA1-targeting siRNA v/s non-targeting siRNA |
| GSE179990 [39] | PC-3 cells | 9 | Expressing NF-YA (short and long isoforms) v/s control |
| Study | Cell types | Samples | Comparisons |
|---|---|---|---|
| GSE167573 [40] | Renal cell carcinoma (RCC) tissue | 28 | Tumorous v/s control |
| GSE141295 [41] | Renal cell carcinoma (RCC) tissue | 24 | Affected by IgA nephropathy v/s control |
| GSE151419 [42] | Renal cell carcinoma (RCC) tissue | 24 | Tumor grade 2 v/s grade 3 and grade 4 |
| GSE222245 [43] | 786-O cells (wild-type and PBRM1 knockout) | 20 | Treatment with bortezomib (BTZ) for 9 hours v/s DMSO control Treatment with SMARC2/4 ATPase inhibitor (BRM014) for 24 hours v/s DMSO control |
| Study | Cell types | Samples | Comparisons |
|---|---|---|---|
| GSE113113 [44,45] | Cutaneous T-cell lymphoma (cuTCL) tissue | 47 | Tumor stage IA v/s IB, II and IVA2 |
| GSE124857 [46] | MKL-1, MKL-2, MS-1, WaGa, PeTa, BroLi and UISO cells | 42 | Treatment with GSK-LSD1 inhibitor for 3 days, or CPI-670242 antibody for 1 day v/s control |
| GSE84293 [47] | Cutaneous squamous cell carcinoma (cuSCC) tissue | 26 | Non-lesional (NS) v/s actinic keratosis (AK) and cutaneous squamous cell carcinoma (SCC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





