Submitted:
24 October 2024
Posted:
25 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microarray Expression Data Acquisition, Processing and Exploratory Analysis
2.1.1. Classical Approaches
2.1.2. Comparative Analysis of Shapley Value (CASh) Approach
2.2. Gene Set Enrichment Analysis and Functional Annotation
3. Results
3.1. Datasets and Samples Analyzed
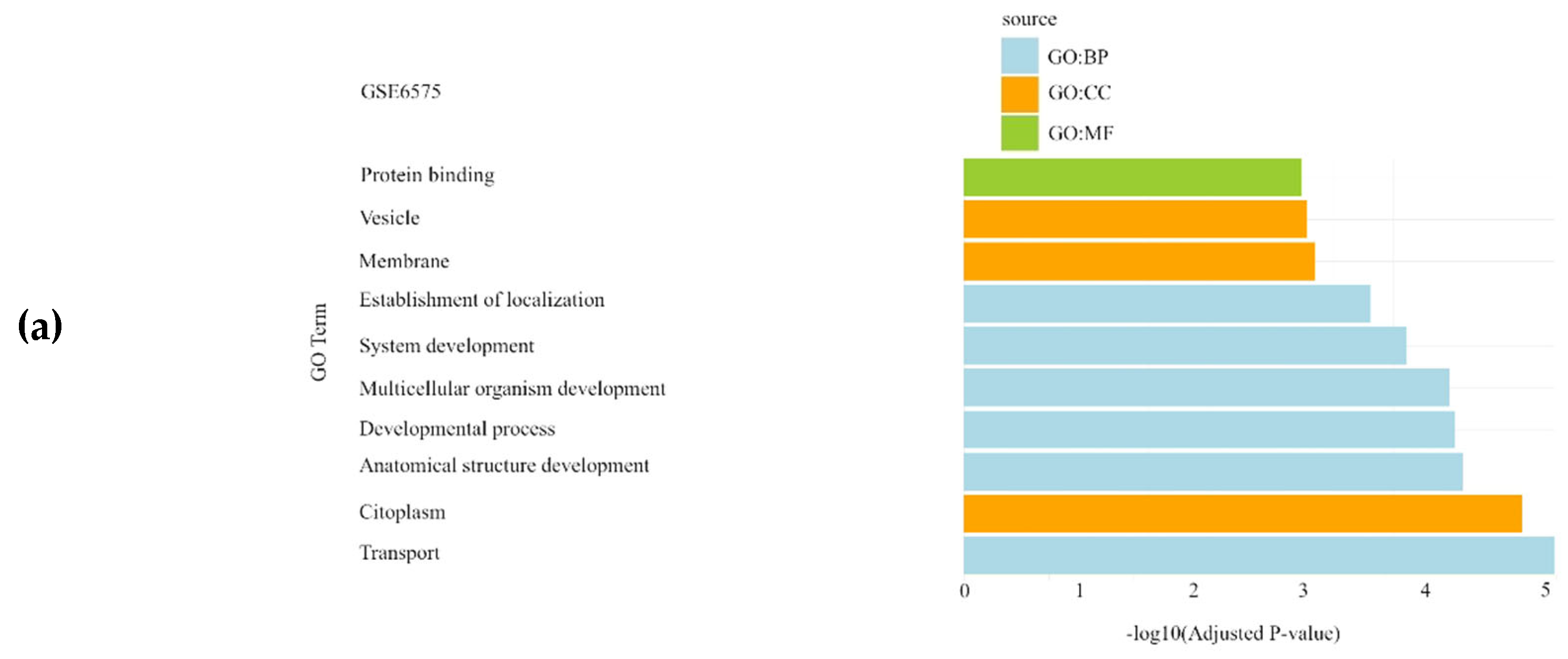
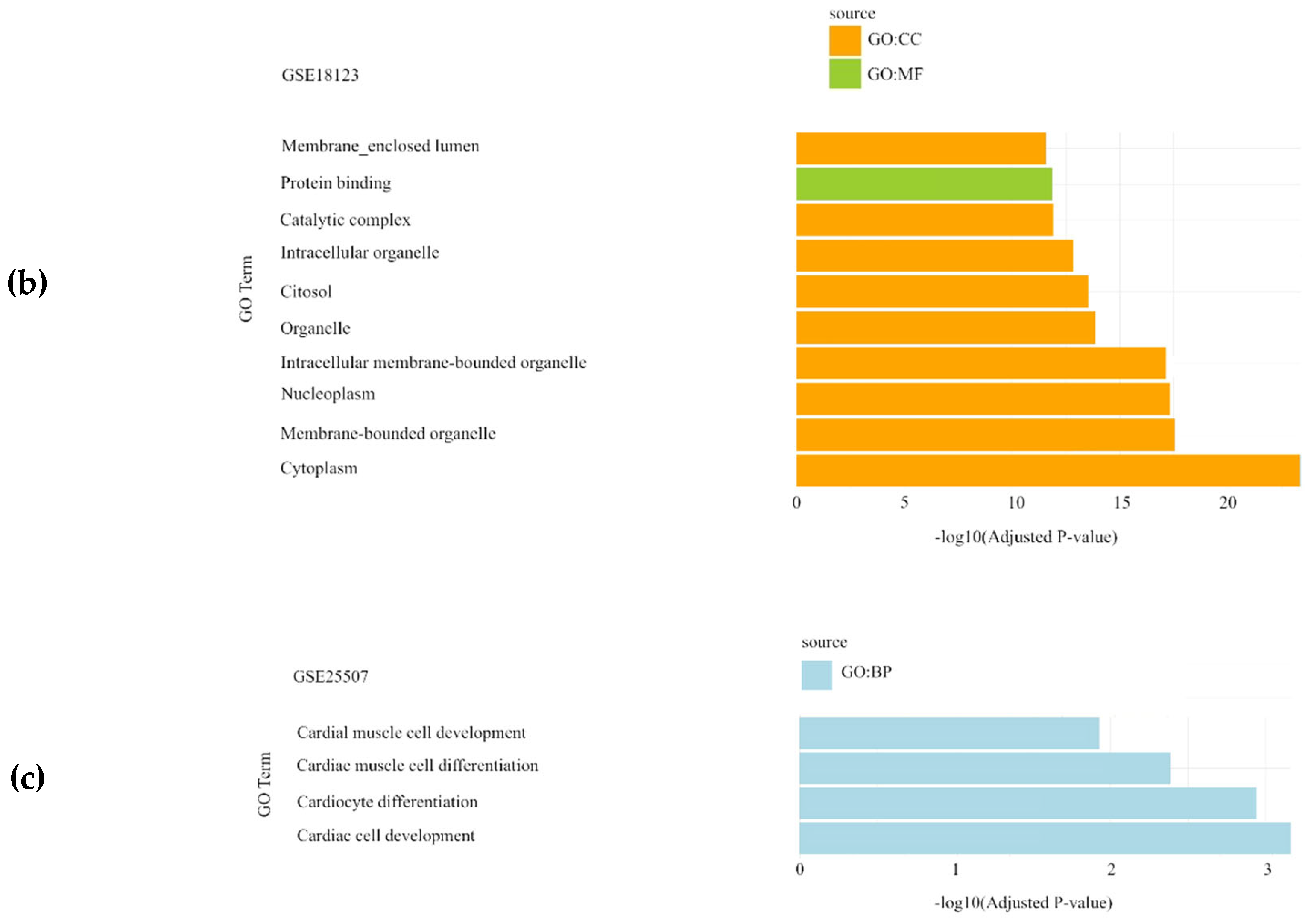
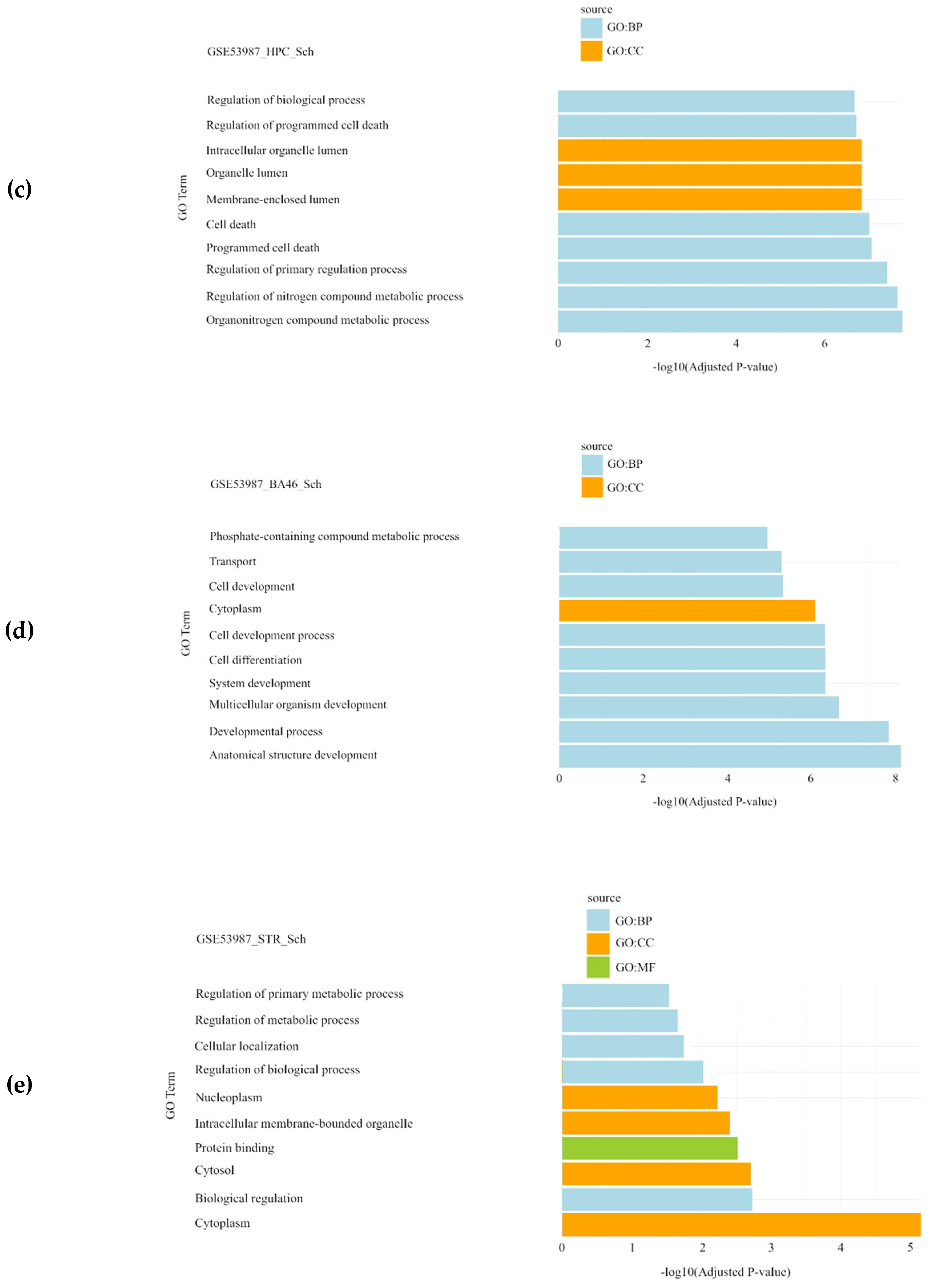
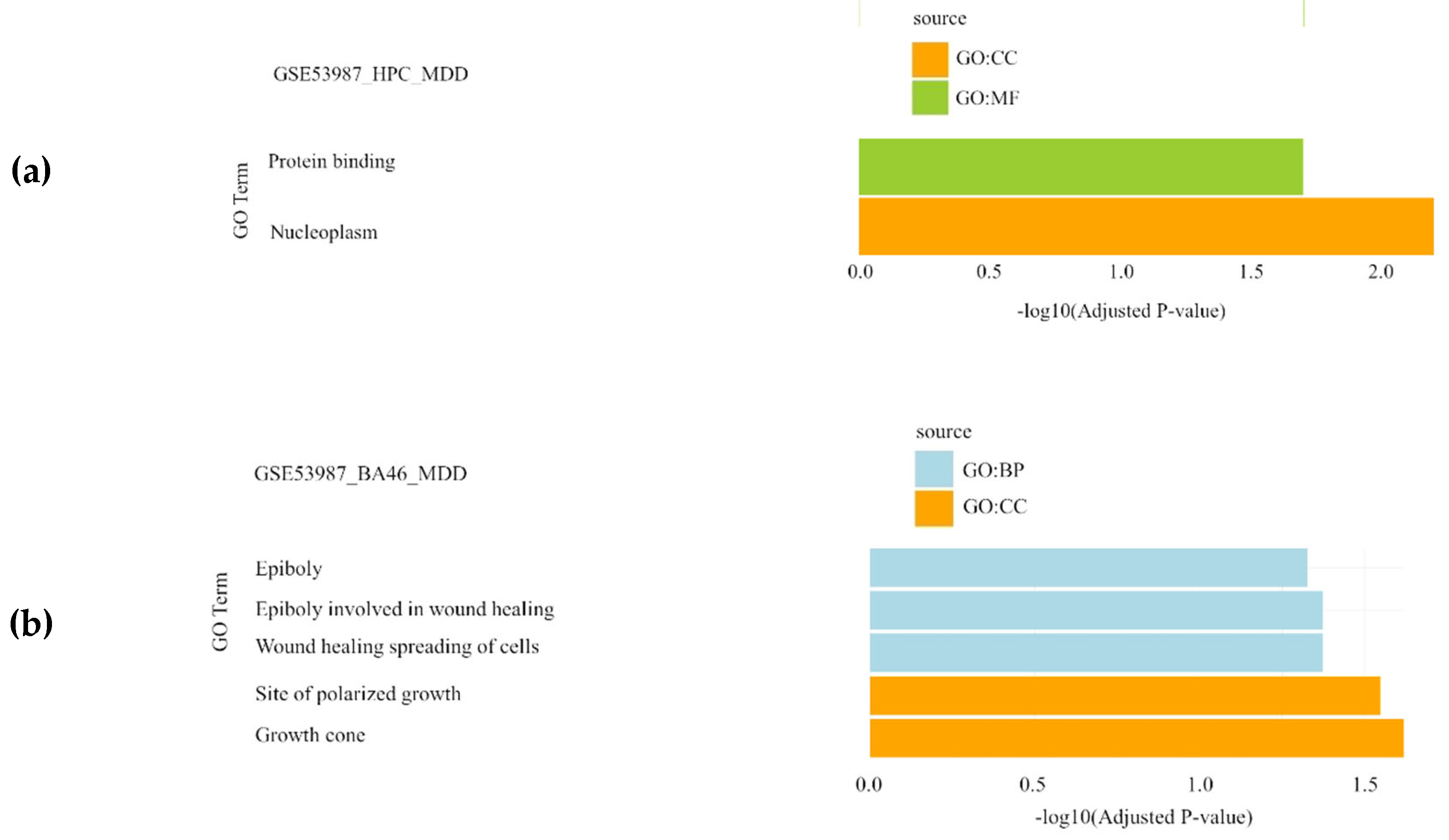
3.2. Functional Enrichment Analysis of the Differentially Expressed Genes
4. Discussion
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldinger, F.; Schulze, T. G. Environmental factors, life events, and trauma in the course of bipolar disorder. Psychiatry Clin. Neurosci. 2017, 71, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Alter, M. D.; Kharkar, R.; Ramsey, K. E.; Craig, D. W.; Melmed, R. D.; Grebe, T. A.; Bay, R. C.; Ober-Reynolds, S.; Kirwan, J.; Jones, J. J.; Turner, J. B.; Hen, R.; Stephan, D. A. Autism and increased paternal age related changes in global levels of gene expression regulation. PloS One 2011, 6, e16715. [Google Scholar] [CrossRef]
- Arberas, C.; Ruggieri, V. Autismo: aspectos genéticos y biológicos. Medicina (B Aires).
- Åstrand, M.; Mostad, P.; Rudemo, M. Empirical Bayes models for multiple probe type microarrays at the probe level. BMC Bioinformatics 2008, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Balhara, Y. P.; Verma, R. Schizophrenia and suicide. East Asian Arch. Psychiatry 2012, 22, 126–133. [Google Scholar]
- Barnett, A. H.; Mackin, P.; Chaudhry, I.; Farooqi, A.; Gadsby, R.; Heald, A.; Hill, J.; Millar, H.; Peveler, R.; Rees, A.; Singh, V.; Taylor, D.; Vora, J.; Jones, P. B. Minimising metabolic and cardiovascular risk in schizophrenia: Diabetes, obesity and dyslipidaemia. J. Psychopharmacol. 2007, 21, 357–373. [Google Scholar] [CrossRef]
- Bauer, M.; Rush, A. J.; Ricken, R.; Pilhatsch, M.; Adli, M. Algorithms For Treatment of Major Depressive Disorder: Efficacy and Cost-Effectiveness. Pharmacopsychiatry 2019, 52, 117–125. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling The False Discovery Rate—A Practical And Powerful Approach To Multiple Testing. J. R. Stat. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bettencourt, C.; Skene, N.; Bandres-Ciga, S.; Anderson, E.; Winchester, L. M.; Foote, I. F.; Schwartzentruber, J.; Botia, J. A.; Nalls, M.; Singleton, A.; Schilder, B. M.; Humphrey, J.; Marzi, S. J.; Toomey, C. E.; Kleifat, A. A.; Harshfield, E. L.; Garfield, V.; Sandor, C.; Keat, S.; Tamburin, S.; Frigerio, C. S.; Lourida, I.; the Deep Dementia Phenotyping (DEMON) Network; Ranson, J. M.; Llewellyn, D. J. Artificial intelligence for dementia genetics and omics. Alzheimers Dement. 2023, 19, 5905–5921. [Google Scholar] [CrossRef]
- Beyer, J. L.; Weisler, R. H. Suicide Behaviors in Bipolar Disorder: A Review and Update for the Clinician. Psychiat. Clin. North Am. 2016, 39, 111–123. [Google Scholar] [CrossRef]
- Bobilev, A. M.; Perez, J. M.; Tamminga, C. A. Molecular alterations in the medial temporal lobe in schizophrenia. Schizophr. Res. 2020, 217, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Bolstad, B.; Collin, F.; Brettschneider, J.; Simpson, K.; Cope, L.; Irizarry, R.; Speed, T. P. Quality Assessment of Affymetrix GeneChip Data. En Bioinformatics and computational biology solutions using R and bioconductor 2005, 33–47. [Google Scholar] [CrossRef]
- Bolstad, B. M.; Irizarry, R. A.; Åstrand, M.; Speed, T. P. A Comparison of Normalization Methods for High Density Oligonucleotide Array Data Based on Variance and Bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef]
- Breitling, R.; Herzyk, P. Rank-based methods as a non-parametric alternative of the T-statistic for the analysis of biological microarray data. J. Bioinform. Comput. Biol. 2005, 3, 1171–1189. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P. A.; Venter, D.; Robins-Browne, R.; Curtis, N. Chips with everything: DNA microarrays in infectious diseases. Lancet Infect. Dis. 2004, 4, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Carlborg, A.; Winnerbäck, K.; Jönsson, E. G.; Jokinen, J.; Nordström, P. Suicide in schizophrenia. Expert Review of Neurotherapeutics 2010, 10, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Castro-Martínez, J.A.; Vargas, E.; Díaz-Beltrán, L.; Esteban, F.J. Comparative Analysis of Shapley values enhances transcriptomics insights across some common uterine pathologies. Genes 2024, 15, 723. [Google Scholar] [CrossRef] [PubMed]
- Cattane, N.; Minelli, A.; Milanesi, E.; Maj, C.; Bignotti, S.; Bortolomasi, M.; Chiavetto, L. B.; Gennarelli, M. Altered Gene Expression in Schizophrenia: Findings from Transcriptional Signatures in Fibroblasts and Blood. PLoS ONE 2015, 10, e0116686. [Google Scholar] [CrossRef]
- Cesari, G.; Algaba, E.; Moretti, S.; Nepomuceno, J. A. An application of the Shapley value to the analysis of co-expression networks. Appl. Netw. Sci. 2018, 3, 1. [Google Scholar] [CrossRef]
- Cheng, W.; van der Meer, D.; Parker, N.; Hindley, G.; O’Connell, K. S.; Wang, Y.; Shadrin, A. A.; Alnæs, D.; Bahrami, S.; Lin, A.; Karadag, N.; Holen, B.; Fernandez-Cabello, S.; Fan, C. C.; Dale, A. M.; Djurovic, S.; Westlye, L. T.; Frei, O.; Smeland, O. B.; Andreassen, O. A. Shared genetic architecture between schizophrenia and subcortical brain volumes implicates early neurodevelopmental processes and brain development in childhood. Mol. Psychiatry 2022, 27, 5167–5176. [Google Scholar] [CrossRef]
- Clemente, A. S.; Diniz, B. S.; Nicolato, R.; Kapczinski, F. P.; Soares, J. C.; Firmo, J. O.; Castro-Costa, É. Bipolar disorder prevalence: A systematic review and meta-analysis of the literature. Rev. Bras. Psiquiatr. 2015, 37, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Copland, J. A.; Davies, P. J.; Shipley, G. L.; Wood, C. G.; Luxon, B. A.; Urban, R. J. The use of DNA microarrays to assess clinical samples: The transition from bedside to bench to bedside. Recent Prog. Horm. Res. 2003, 58, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Cordero, F.; Botta, M.; Calogero, R. A. Microarray data analysis and mining approaches. Brief. Funct. Genomic. Proteomic. 2007, 6, 265–281. [Google Scholar] [CrossRef]
- Dissanayake, C.; Searles, J.; Barbaro, J.; Sadka, N.; Lawson, L. P. Cognitive and behavioral differences in toddlers with autism spectrum disorder from multiplex and simplex families. Autism Res. 2019, 12, 682–693. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut–Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef] [PubMed]
- Ducray, F.; Honnorat, J.; Lachuer, J. DNA microarray technology: Principles and applications to the study of neurological disorders. Revue Neurologique 2007, 163, 409–420. [Google Scholar] [CrossRef]
- Esteban, F. J.; Wall, D. P. Using game theory to detect genes involved in Autism Spectrum Disorder. TOP 2011, 19, 121–129. [Google Scholar] [CrossRef]
- Evans-Lacko, S.; Courtin, E.; Fiorillo, A.; Knapp, M.; Luciano, M.; Park, A.L.; Brunn, M.; Byford, S.; Chevreul, K.; Forsman, A. K.; Gulacsi, L.; Haro, J. M.; Kennelly, B.; Knappe, S.; Lai, T.; Lasalvia, A.; Miret, M.; O’Sullivan, C.; Obradors-Tarragó, C.; Rüsch, N.; Sartorius, N.; Švab, V.; van Weeghel, J.; Van Audenhove, C.; Wahlbeck, K.; Zlati, A.; McDaid, D.; Thornicroft, G.; ROAMER Consortium. The state of the art in European research on reducing social exclusion and stigma related to mental health: A systematic mapping of the literature. Eur. Psychiatr. 2014, 29, 381–389. [Google Scholar] [CrossRef]
- Fagiolini, A.; Coluccia, A.; Maina, G.; Forgione, R. N.; Goracci, A.; Cuomo, A.; Young, A. H. Diagnosis, Epidemiology and Management of Mixed States in Bipolar Disorder. CNS Drugs 2015, 29, 725–740. [Google Scholar] [CrossRef]
- Figueroa-Hall, L. K.; Paulus, M. P.; Savitz, J. Toll-Like Receptor Signaling in Depression. Psychoneuroendocrinology 2020, 121, 104843. [Google Scholar] [CrossRef]
- Filatova, E. V.; Shadrina, M. I.; Slominsky, P. A. Major Depression: One Brain, One Disease, One Set of Intertwined Processes. Cells 2021, 10, 1283. [Google Scholar] [CrossRef]
- Fuglewicz, A. J.; Piotrowski, P.; Stodolak, A. Relationship between toxoplasmosis and schizophrenia: A review. Adv. Clin. Exp. Med. 2017, 26, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Gadad, B. S.; Jha, M. K.; Czysz, A.; Furman, J. L.; Mayes, T. L.; Emslie, M. P.; Trivedi, M. H. Peripheral Biomarkers of Major Depression and Antidepressant Treatment Response: Current Knowledge and Future Outlooks. J. Affect. Disord. 2018, 233, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.; Mishra, A.; Soni, M. R.; Kumar, P.; Sadagopan, M.; Kanthi, A. V.; Patric, I. R. P.; George, S.; Sridharan, A.; Thyagarajan, T. C.; Aswathy, S. L.; Vidya, H. K.; Chinnappa, S. M.; Nayanala, S.; Prakash, M. B.; Raghavendrachar, V. G.; Parulekar, M.; Gowda, V. K.; Nampoothiri, S.; Menon, R. M.; Pachat, D.; Udani, V.; Naik, N.; Kamate, M.; Devi, A. R. R.; Kunju, P. A. M.; Nair, M.; Hedge, A. U.; Kumar, M. P.; Sundaram, S.; Tilak, P.; Puri, R. D.; Shah, K.; Sheth, J.; Hasan, Q.; Sheth, F.; Agrawal, P.; Katragadda, S.; Veeramachaneni, V.; Chandru, V.; Hariharam, R.; Mannan, A. U. Multi-gene testing in neurological disorders showed an improved diagnostic yield: Data from over 1000 Indian patients. J. Neurol. 2019, 266, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M. J.; Zhang, P.; Hadjimichael, E.; Walker, R. L.; Chen, C.; Liu, S.; Won, H.; van Bakel, H.; Varghese, M.; Wang, Y.; Shieh, A. W.; Haney, J.; Parhami, S.; Belmont, J.; Kim, M.; Moran Losada, P.; Khan, Z.; Mleczko, J.; Xia, Y.; Dai, R.; Wang, D.; Yang, Y. T.; Xu, M.; Fish, K.; Hof, P. R.; Warrell, J.; Fiztgerald, D.; White, K.; Jaffe, A. E.; Psychencode Consortium; Peters, M. A.; Gerstein, M.; Liu, C.; Iakoucheva, L. M.; Pinto, D.; Geschwind, D. H. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018, 362, eaat8127. [Google Scholar] [CrossRef]
- Germann, M.; Brederoo, S. G. , Sommer, I. E. C. Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders. Curr. Opin. Psychiatry 2021, 34, 222–227. [Google Scholar] [CrossRef]
- Gómez Maquet, Y. , Ángel, J. D., Cañizares, C., Lattig, M. C., Agudelo, D. M., Arenas, Á., Ferro, E. The role of stressful life events appraisal in major depressive disorder. Rev. Colomb. Psiquiatr. (Engl. Ed.), 2020, 49, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, R. , Pannucci, J. A., Kuske, C. R.; Brettin, T. Statistical analysis of microarray data: A Bayesian approach. Biostatistics 2003, 4, 597–620. [Google Scholar] [CrossRef]
- Greenberg, P. E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S. H.; Kessler, R. C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). PharmacoEconomics 2021, 39, 653–665. [Google Scholar] [CrossRef]
- Gregg, J. P.; Lit, L.; Baron, C. A.; Hertz-Picciotto, I.; Walker, W.; Davis, R. A.; Croen, L. A.; Ozonoff, S.; Hansen, R.; Pessah, I. N.; Sharp, F. R. Gene expression changes in children with autism. Genomics 2008, 91, 22–29. [Google Scholar] [CrossRef]
- Gulayín, M. E. Burden in family caregivers of people with schizophrenia: A literature review. Vertex Rev. Argent. Psiquiatr. 2022, XXXIII, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J. A. Prevalence and correlates of major depressive disorder: A systematic review. Rev. Bras. Psiquiatr. 2020, 42, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Häfner, H.; an der Heiden, W. Epidemiology of schizophrenia. Can. J. Psychiatry 1997, 42, 139–151. [Google Scholar] [CrossRef]
- Hagi, K.; Nosaka, T.; Dickinson, D.; Lindenmayer, J. P.; Lee, J.; Friedman, J.; Boyer, L.; Han, M.; Abdul-Rashid, N. A.; Correll, C. U. Association Between Cardiovascular Risk Factors and Cognitive Impairment in People With Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Harder, A.; Nguyen, T.D. , Pasman, J. A., Mosing, M. A., Hägg, S., Lu, Y. Genetics of age-at-onset in major depression. Transl. Psychiatry 2022, 12, 124. [Google Scholar] [CrossRef]
- Heckers, S. , Konradi, C. Hippocampal neurons in schizophrenia. J. Neural Transm. (Vienna) 2002, 109, 891–905. [Google Scholar] [CrossRef]
- Heidari, A. , Rostam-Abadi, Y., Rezaei, N. The immune system and autism spectrum disorder: Association and therapeutic challenges. Acta Neurobiol. Exp. 2021, 81, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; King, B. H. Autism Spectrum Disorder: A Review. JAMA 2023, 329, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx) 2022.
- Irizarry, R. A.; Bolstad, B. M.; Collin, F.; Cope, L. M.; Hobbs, B.; Speed, T. P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003, 31, e15. [Google Scholar] [CrossRef]
- Iwamoto, K.; Kakiuchi, C.; Bundo, M.; Ikeda, K.; Kato, T. Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Mol. Psychiatry 2004, 9, 406–416. [Google Scholar] [CrossRef]
- Janoutová, J.; Janácková, P.; Serý, O.; Zeman, T.; Ambroz, P.; Kovalová, M.; Varechová, K.; Hosák, L.; Jirík, V.; Janout, V. Epidemiology and risk factors of schizophrenia. Neuro Endocrinol. Lett. 2016, 37, 1–8. [Google Scholar]
- Jeffery, I. B.; Higgins, D. G.; Culhane, A. C. Comparison and evaluation of methods for generating differentially expressed gene lists from microarray data. BMC Bioinformatics 2006, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R. S.; Sommer, I. E. The neurobiology and treatment of first-episode schizophrenia. Mol. Psychiatry 2015, 20, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J. B.; Gregory, A. C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; Pollard, E. L.; Roux, S.; Sadowsky, M. J.; Lipson, K. S.; Sullivan, M. B.; Caporaso, J. G.; Krajmalnik-Brown, R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Katz, I. R.; Rogers, M. P.; Lew, R.; Thwin, S. S.; Doros, G.; Ahearn, E.; Ostacher, M. J.; DeLisi, L. E.; Smith, E. G.; Ringer, R. J.; Ferguson, R.; Hoffman, B.; Kaufman, J. S.; Paik, J. M.; Conrad, C. H.; Holmberg, E. F.; Boney, T. Y.; Huang, G. D.; Liang, M. H.; Li+ plus Investigators. Lithium Treatment in the Prevention of Repeat Suicide-Related Outcomes in Veterans With Major Depression or Bipolar Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 24–32. [Google Scholar] [CrossRef]
- Kealy, J.; Greene, C.; Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 2020, 726, 133664. [Google Scholar] [CrossRef] [PubMed]
- Kendall, K. M.; Van Assche, E.; Andlauer, T. F. M.; Choi, K. W.; Luykx, J. J.; Schulte, E. C.; Lu, Y. The genetic basis of major depression. Psychol. Med. 2021, 51, 2217–2230. [Google Scholar] [CrossRef]
- Kennedy, S. H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar] [CrossRef]
- Keshavarz, K.; Hedayati, A.; Rezaei, M.; Goudarzi, Z.; Moghimi, E.; Rezaee, M.; Lotfi, F. Economic burden of major depressive disorder: A case study in Southern Iran. BMC Psychiatry 2022, 22, 577. [Google Scholar] [CrossRef]
- Khan, Z. U.; Martin-Montañez, E.; Muly, E. C. Schizophrenia: Causes and treatments. Curr. Pharm. Des. 2013, 19, 6451–6461. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. G:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Kong, S. W.; Collins, C. D.; Shimizu-Motohashi, Y.; Holm, I. A.; Campbell, M. G.; Lee, I.H.; Brewster, S. J.; Hanson, E.; Harris, H. K.; Lowe, K. R.; Saada, A.; Mora, A.; Madison, K.; Hundley, R.; Egan, J.; McCarthy, J.; Eran, A.; Galdzicki, M.; Rappaport, L.; Kunkel, L. M.; Kohane, I. S. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PloS One 2012, 7, e49475. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Zhu, Y.; Huhn, M.; Schneider-Thoma, J.; Bighelli, I. , Chaimani, A., Leucht, S. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: A network meta-analysis. Eur. Neuropsychopharmacol. 2018, 28, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Krokidis, M. G. , Vlamos, P. Transcriptomics in amyotrophic lateral sclerosis. Front. Biosci. (Elite Ed.) 2018, 10, 103–121. [Google Scholar] [CrossRef]
- Lanz, T. A. , Reinhart, V., Sheehan, M. J., Rizzo, S. J. S., Bove, S. E., James, L. C., Volfson, D., Lewis, D. A., Kleiman, R. J. Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: A comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl. Psychiatry 2019, 9, 151. [Google Scholar] [CrossRef]
- Legati, A. , Giacopuzzi, E., Spinazzi, M., Lek, M. Editorial: Application of Omics Approaches to the Diagnosis of Genetic Neurological Disorders. Front. Neurol. 2021, 12, 712010. [Google Scholar] [CrossRef]
- Li, X. , Mu, F., Liu, D., Zhu, J., Yue, S., Liu, M., Liu, Y., Wang, J. Predictors of suicidal ideation, suicide attempt and suicide death among people with major depressive disorder: A systematic review and meta-analysis of cohort studies. J. Affect. Disord. 2022, 302, 332–351. [Google Scholar] [CrossRef] [PubMed]
- Lord, C. , Elsabbagh, M., Baird, G., Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G. S. , Gessler, D., Outhred, T. The use of lithium for the treatment of bipolar disorder: Recommendations from clinical practice guidelines. J. Affect. Disord. 2017, 217, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Martin, C. R. , Osadchiy, V., Kalani, A., Mayer, E. A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Matigian, N. , Windus, L., Smith, H., Filippich, C., Pantelis, C., McGrath, J., Mowry, B., Hayward, N. Expression profiling in monozygotic twins discordant for bipolar disorder reveals dysregulation of the WNT signalling pathway. Mol. Psychiatry 2007, 12, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Matta, S. M. , Hill-Yardin, E. L., Crack, P. J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Maycox, P. R. , Kelly, F., Taylor, A., Bates, S., Reid, J., Logendra, R., Barnes, M. R., Larminie, C., Jones, N., Lennon, M., Davies, C., Hagan, J. J., Scorer, C. A., Angelinetta, C., Akbar, M. T., Hirsch, S., Mortimer, A. M., Barnes, T. R. E., de Belleroche, J. Analysis of gene expression in two large schizophrenia cohorts identifies multiple changes associated with nerve terminal function. Mol. Psychiatry 2009, 14, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J. , Saha, S., Chant, D., Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, A. , Van de Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar] [CrossRef]
- Miller, J. N. , Black, D. W. Bipolar Disorder and Suicide: A Review. Curr. Psychiatry Rep. 2020, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Morera-Fumero, A. L. , Abreu-Gonzalez, P. Role of Melatonin in Schizophrenia. Int. J. Mol. Sci. 2013, 14, 9037–9050. [Google Scholar] [CrossRef]
- Moretti, S. Statistical analysis of the Shapley value for microarray games. Comput. Oper. Res. 2010, 37, 1413–1418. [Google Scholar] [CrossRef]
- Moretti, S. , Fragnelli, V., Patrone, F., Bonassi, S. Using coalitional games on biological networks to measure centrality and power of genes. Bioinformatics 2010, 26, 2721–2730. [Google Scholar] [CrossRef]
- Moretti, S. , Patrone, F. Transversality of the Shapley value. TOP 2008, 16, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S. , van Leeuwen, D., Gmuender, H., Bonassi, S., van Delft, J., Kleinjans, J., Patrone, F., Merlo, D. F. Combining Shapley value and statistics to the analysis of gene expression data in children exposed to air pollution. BMC Bioinformatics 2008, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Mouridsen, S. E. , Rich, B., Isager, T. Diseases of the circulatory system among adult people diagnosed with infantile autism as children: A longitudinal case control study. Res. Dev. Disabil. 2016, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ormstad, H. , Bryn, V., Saugstad, O. D., Skjeldal, O., Maes, M. Role of the Immune System in Autism Spectrum Disorders (ASD). CNS Neurol. Disord. Drug Targets 2018, 17, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Perlick, D. A. , Rosenheck, R. A., Kaczynski, R., Swartz, M. S., Canive, J. M., Lieberman, J. A. Impact of antipsychotic medication on family burden in schizophrenia: Longitudinal results of CATIE trial. Schizophr. Res. 2010, 116, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Pino, O. , Guilera, G., Gómez-Benito, J., Najas-García, A., Rufián, S., Rojo, E. Neurodevelopment or neurodegeneration: Review of theories of schizophrenia. Actas Esp. Psiquiatri. 2014, 42, 185–195. [Google Scholar]
- Pollard, K. S. , Dudoit, S., van der Laan, M. J. Multiple Testing Procedures: The multtest Package and Applications to Genomics. En R. Gentleman, V. J. Carey, W. Huber, R. A. Irizarry, S. Dudoit (Eds.), Bioinformatics and Computational Biology Solutions Using R and Bioconductor 2005, 249-271. Springer. [CrossRef]
- Qiu, S. , Qiu, Y., Li, Y., Cong, X. Genetics of autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Transl. Psychiatry 2022, 12, 249. [Google Scholar] [CrossRef]
- Rai, G. , Rai, R., Saeidian, A. H., Rai, M. Microarray to deep sequencing: Transcriptome and miRNA profiling to elucidate molecular pathways in systemic lupus erythematosus. Immunol. Res. 2016, 64, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U. , Kolberg, L., Kuzmin, I., Arak, T., Adler, P., Peterson, H., Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Ribé, J. M. , Salamero, M., Pérez-Testor, C., Mercadal, J., Aguilera, C., Cleris, M. Quality of life in family caregivers of schizophrenia patients in Spain: Caregiver characteristics, caregiving burden, family functioning, and social and professional support. Int. J. Psychiatry Clin. Pract. 2018, 22, 25–33. [Google Scholar] [CrossRef]
- Robinson-Agramonte, M. de L. A., Noris García, E., Fraga Guerra, J., Vega Hurtado, Y., Antonucci, N., Semprún-Hernández, N., Schultz, S., Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef] [PubMed]
- Ruzzo, E. K. , Pérez-Cano, L., Jung, J. Y., Wang, L. K., Kashef-Haghighi, D., Hartl, C., Singh, C., Xu, J., Hoekstra, J. N., Leventhal, O., Leppä, V. M., Gandal, M. J., Paskov, K., Stockham, N., Polioudakis, D., Lowe, J. K., Prober, D. A., Geschwind, D. H., Wall, D. P. Inherited and De Novo Genetic Risk for Autism Impacts Shared Networks. Cell 2019, 178, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M. M. , Lockstone, H. E., Huffaker, S. J., Wayland, M. T., Webster, M. J., Bahn, S. Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol. Psychiatry 2006, 11, 965–978. [Google Scholar] [CrossRef]
- Saha, S. , Chant, D., Welham, J., McGrath, J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005, 2, e141. [Google Scholar] [CrossRef] [PubMed]
- Saunders, E. F. H. , Ramsden, C. E., Sherazy, M. S., Gelenberg, A. J., Davis, J. M., Rapoport, S. I. Omega-3 and Omega-6 Polyunsaturated Fatty Acids in Bipolar Disorder. J. Clin. Psychiatry 2016, 77, e1301–e1308. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S. , Natarajan, J. Microarray Data Analysis and Mining Tools. Bioinformation 2011, 6, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Serra, G. , De Crescenzo, F., Maisto, F., Galante, J. R., Iannoni, M. E., Trasolini, M., Maglio, G., Tondo, L., Baldessarini, R. J., Vicari, S. Suicidal behavior in juvenile bipolar disorder and major depressive disorder patients: Systematic review and meta-analysis. J. Affect. Disord. 2022, 311, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Shai, R. M. Microarray tools for deciphering complex diseases. Front. Biosci. 2006, 11, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. R. , Gonda, X., Tarazi, F. I. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol. Therapeut. 2018, 190, 91–104. [Google Scholar] [CrossRef]
- Sharon, G. , Sampson, T. R., Geschwind, D. H., Mazmanian, S. K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Sher, L. , Kahn, R. S. Suicide in Schizophrenia: An Educational Overview. Medicina (Kaunas) 2019, 55, 361. [Google Scholar] [CrossRef]
- Silverman, J. L. , Yang, M., Lord, C., Crawley, J. N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Smith, D. J. , Whitham, E. A., Ghaemi, S. N. Bipolar disorder. Handb. Clin. Neurol. 2012, 106, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. C. , Osborn, G. G., Dwamena, F. C., D’Mello, D., Freilich, L., Laird-Fick, H. S. Major Depression and Related Disorders. En Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting 2019, McGraw-Hill Education. accessmedicine.mhmedical.com/content.aspx? 1163. [Google Scholar]
- Srikantha, P. , Mohajeri, M. H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. International Journal of Molecular Sciences 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Stilo, S. A. , Murray, R. M. Non-Genetic Factors in Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Suda, K. , Matsuda, K. How Microbes Affect Depression: Underlying Mechanisms via the Gut–Brain Axis and the Modulating Role of Probiotics. Int. J. Mol. Sci. 2022, 23, 1172. [Google Scholar] [CrossRef] [PubMed]
- Sun, M. W. , Moretti, S., Paskov, K. M., Stockham, N. T., Varma, M., Chrisman, B. S., Washington, P. Y., Jung, J. Y., Wall, D. P. Game theoretic centrality: A novel approach to prioritize disease candidate genes by combining biological networks with the Shapley value. BMC Bioinformatics 2020, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Tondo, L. , Vázquez, G. H., Baldessarini, R. J. Depression and Mania in Bipolar Disorder. Current Neuropharmacology 2017, 15, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V. , Pardiñas, A. F., Qi, T., Panagiotaropoulou, G., Awasthi, S., Bigdeli, T. B., Bryois, J., Chen, C.Y., Dennison, C. A., Hall, L. S., Lam, M., Watanabe, K., Frei, O., Ge, T., Harwood, J. C., Koopmans, F., Magnusson, S., Richards, A. L., Sidorenko, J., … Schizophrenia Working Group of the Psychiatric Genomics Consortium. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Tseng, C.E. J. , McDougle, C. J., Hooker, J. M., Zürcher, N. R. Epigenetics of Autism Spectrum Disorder: Histone Deacetylases. Biol. Psychiatry 2022, 91, 922–933. [Google Scholar] [CrossRef]
- Valdés-Tovar, M. , Rodríguez-Ramírez, A. M., Rodríguez-Cárdenas, L., Sotelo-Ramírez, C. E., Camarena, B., Sanabrais-Jiménez, M. A., Solís-Chagoyán, H., Argueta, J., López-Riquelme, G. O. Insights into myelin dysfunction in schizophrenia and bipolar disorder. World J. Psychiatry 2022, 12, 264–285. [Google Scholar] [CrossRef]
- Vilain, J. , Galliot, A.M., Durand-Roger, J., Leboyer, M., Llorca, P.M., Schürhoff, F., Szöke, A. Environmental risk factors for schizophrenia: A review. L’Encephale 2013, 39, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Villani, E. R. , Marzetti, E. Molecular Signals and Genetic Regulations of Neurological Disorders. Int. J. Mol. Sci. 2023, 24, 5902. [Google Scholar] [CrossRef] [PubMed]
- Von Hausswolff-Juhlin, Y. , Bjartveit, M., Lindström, E., Jones, P. Schizophrenia and physical health problems. Acta Psychiatr. Scand. 2009, 119, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang Q, Wei J, Pan Y, Xu S. An efficient empirical Bayes method for genomewide association studies. J. Anim. Breed. Genet. 2016, 133, 253–63. [Google Scholar] [CrossRef] [PubMed]
- Ward, K. Microarray technology in obstetrics and gynecology: A guide for clinicians. Am. J. Obstet. Gynecol. 2006, 195, 364–372. [Google Scholar] [CrossRef]
- Wilkinson, L. ggplot2: Elegant Graphics for Data Analysis by H. WICKHAM. Biometrics 2011, 67, 678–679. [Google Scholar] [CrossRef]
- World Health Organization. Schizophrenia. 2022.
- Xu, J. , Mao, C., Hou, Y., Luo, Y., Binder, J. L., Zhou, Y., Bekris, L. M., Shin, J., Hu, M., Wang, F., Eng, C., Oprea, T. I., Flanagan, M. E., Pieper, A. A., Cummings, J., Leverenz, J. B., Cheng, F. Interpretable deep learning translation of GWAS and multi-omics findings to identify pathobiology and drug repurposing in Alzheimer’s disease. Cell Rep. 2022, 41, 111717. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y. , Walsh, W. J., McGinnis, W. R., Praticò, D. Altered vascular phenotype in autism: Correlation with oxidative stress. Arch. Neurol. 2006, 63, 1161–1164. [Google Scholar] [CrossRef]
- Zhou, X. , Liu, L., Lan, X., Cohen, D., Zhang, Y., Ravindran, A. V., Yuan, S., Zheng, P., Coghill, D., Yang, L., Hetrick, S. E., Jiang, X., Benoliel, J.J., Cipriani, A., Xie, P. Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents. Mol. Psychiatry 2019, 24, 1478–1488. [Google Scholar] [CrossRef]
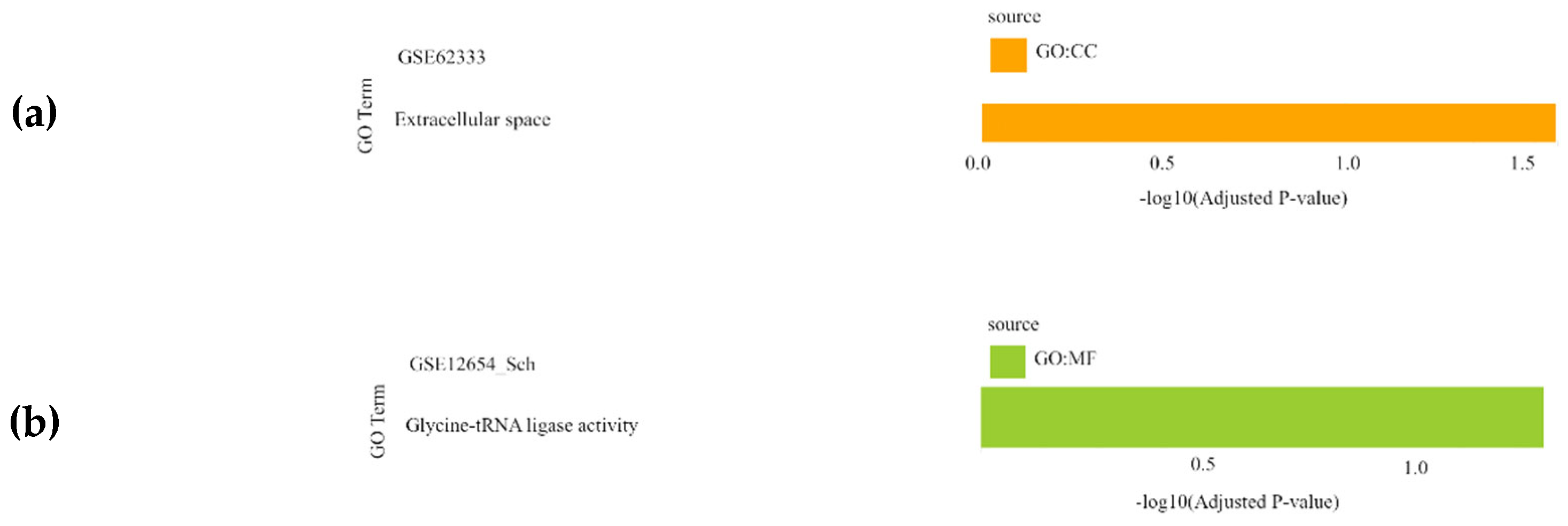

| Phenotype group | Dataset ID | No. of samples | Description of samples |
|---|---|---|---|
| GSE6575 | 25 | Whole blood autism (n=14) vs. controls (n=11) | |
| Autism | GSE18123 | 23 | Whole blood autism (n=13) vs. controls (n=10) |
| GSE25507 | 26 | Peripheral blood lymphocytes (n=12) vs. controls (n=14) | |
| Schizophrenia | GSE17612 | 30 | Brain tissue (n=17) vs. controls (n=13) |
| GSE62333 | 25 | Skin fibroblasts (n=11) vs. controls (n=14) | |
| Bipolar disorder | GSE5389 | 17 | Brain tissue (n=7) vs. controls (n=10) |
| GSE7036 | 6 | Lymphoblastoid cell lines (n=3) vs. controls (n=3) | |
| Miscellanea (SCH, BD, MDD) | GSE12654 | 38 | Brain tissue (n=24) vs. controls (n=14) |
| GSE53987 | 186 | Brain tissue (n=135) vs. controls (n=51) |
| Dataset ID | Welch’s t-test |
EBayes FDR<0.01 | EBayes FDR<0.05 | CASh 0.05 FDR<0.05 |
CASh 0.01 | CASh 0.05 | |
|---|---|---|---|---|---|---|---|
| GSE6575 GSE18123 GSE25507 |
0 | 0 | 0 | 0 | 204 (87 ↑, 117 ↓) | 930 (324 ↑, 606 ↓) | |
| 947 | 205 | 2973 | 45 (12 ↑, 33 ↓) | 879 (467 ↑, 412 ↓) | 1862 (1027 ↑, 835 ↓) | ||
| 0 | 0 | 0 | 0 | 28 (10 ↑, 18 ↓) | 141 (41 ↑, 100 ↓) | ||
| GSE17612 GSE62333 |
0 | 0 | 0 | 0 | 1 (1 ↑, 0 ↓) | 11 (8 ↑, 3 ↓) | |
| 5 | 0 | 5 | 0 | 68 (33 ↑, 35 ↓) | 164 (95 ↑, 69 ↓) | ||
| GSE5389 | 1 | 0 | 2 | 0 | 40 (24 ↑, 16 ↓) | 162 (103 ↑, 59 ↓) | |
| GSE7036 | 0 | 0 | 0 | 0 | 8 (4 ↑, 4 ↓) | 35 (12 ↑, 23 ↓) | |
| GSE12654_SCH | 0 | 0 | 0 | 0 | 2 (2 ↑, 0 ↓) | 8 (4 ↑, 4 ↓) | |
| GSE12654_BD | 0 | 0 | 0 | 0 | 0 | 8 (6 ↑, 2 ↓) | |
| GSE12654_MDD | 0 | 0 | 0 | 0 | 0 | 0 | |
| GSE53987_HPC_SCH | 283 | 2 | 1393 | 4 (0 ↑, 4 ↓) | 794 (595 ↑, 199 ↓) | 655 (357 ↑, 298 ↓) | |
| GSE53987_HPC_BD | 0 | 0 | 0 | 0 | 41 (14 ↑, 27 ↓) | 152 (48 ↑, 104 ↓) | |
| GSE53987_HPC_MDD | 0 | 0 | 0 | 0 | 47 (14 ↑, 33 ↓) | 163 (41 ↑, 122 ↓) | |
| GSE53987_PFC_SCH | 0 | 0 | 32 | 0 | 182 (106 ↑, 76 ↓) | 354 (179 ↑, 175 ↓) | |
| GSE53987_PFC_BD | 0 | 0 | 0 | 0 | 157 (54 ↑, 103 ↓) | 394 (141 ↑, 253 ↓) | |
| GSE53987_PFC_MDD | 0 | 0 | 0 | 0 | 61 (11 ↑, 50 ↓) | 175 (34 ↑, 141 ↓) | |
| GSE53987_STR_SCH | 0 | 0 | 1 | 0 | 81 (36 ↑, 45 ↓) | 258 (139 ↑, 119 ↓) | |
| GSE53987_STR_BD | 0 | 0 | 0 | 0 | 42 (10 ↑, 32 ↓) | 77 (33 ↑, 44 ↓) | |
| GSE53987_STR_MDD | 0 | 0 | 0 | 0 | 19 (8 ↑, 11 ↓) | 32 (12 ↑, 30 ↓) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





