1. Introduction
The Phosphorous is a highly reactive element, therefore can not be found as such on Earth. However, it is essential for living organisms, both vegetal and animal, since it is present in nucleic acids and in energetic compounds of cells metabolism such as ATP, in bones, teeth and in phospholipidic membranes.
Although broadly distributed on Earth’s crust as apatites and phosphorites, minerals containing the phosphate group and commonly known as phosphate rocks, phosphorous can be found rarely in a concentrated form. Moreover, rich mineral deposits of such phosphate rocks are located in a small number of states such as Morocco, China, Syria, Algeria and Unites States [
1]. Therefore, geopolitical consequences adversely affected the market and are likely to influence the availability of fossil phosphorous sources even more in the future. Notably, huge price variations have already occurred in the past [
2]. This unbalanced situation might be anticipated to become worse due to emergent Africa and Asia economies promising to rise further the phosphorous demand. First generation biofuels also contribute significantly to the consumption of phosphorous fertilizers [
3].
The massive extraction of phosphate rocks, in particular for fertilizers production but also for pesticides, insecticides and flame retardants manufacture, is having a strong impact on future availability of this element. Indeed, phosphate rocks reserves are largely limited due to the geological timescales needed for natural cycling [
4]. It has been estimated that 220 Mt/year of phosphorous resources are mined and somehow enter the economy [
5,
6].
As a consequence, both phosphorous and phosphate rocks have recently been added to the critical raw materials [
7] list by the European Union due to their economic importance and potential future shortages [
8]. In addition, the new periodic table of elements depicting element scarcity, issued by the European Chemical Society in 2019, considers phosphorous as an element of limited availability with potential future risks to supply [
9].
In the USA phosphorus has also been considered in 2011 an element of crucial importance to US security due to potential supply bottlenecks [
10].
Therefore, the need to design new procedures in order to recycle, when possible, phosphorous compounds of industrial importance, based on circular economy principles, strongly emerges today. In particular, great efforts should be directed towards a sustainable phosphorous management in order to decrease phosphate rock consumption, minimising wastes that can no longer be reused, at the same time setting reasonable targets to lower P demand.
Some technologies have already been designed, or are under development to increase their efficiency, to recover phosphorous from various waste, in particular for reuse as fertilizer. For example, phosphate recovery from livestock waste has been investigated through precipitation induced by various chemicals, electrocoagulation and biomass pyrolysis producing biochar [
11]. Plant response to increasing addition of expired fire extinguisher powder as a potential source of mineral nutrients has also been investigated [
12].
On one hand, the importance of recovering phosphorus resources is strengthened by the need to meet the increasing global demand for fertilizers due to the continuous population growth. On the other hand, phosphorous becomes a pollutant when extensively introduced into soil and water ecosystems causing eutrophication. In order to address these issues, some regulations begin to be issued. For example, it will be mandatory in the future to recover phosphorous from waste of cattle farming in Germany [
13], Switzerland [
14] and Austria [
15].
It is worth noting that the technical feasibility of the phosphorous recover as pure phosphoric acid from low concentration sewage sludge ashes from sewage sludge incinerators has been demonstrated in a new plant in Hambourg [
16,
17]. In addition, calcium sulphate, useful as construction material, and acidic metal salt solutions reusable for precipitation in sewage treatment plants, are also recovered [
18].
2. Composition and Manufacture of ABC Powders for Portable Fire Extinguishers
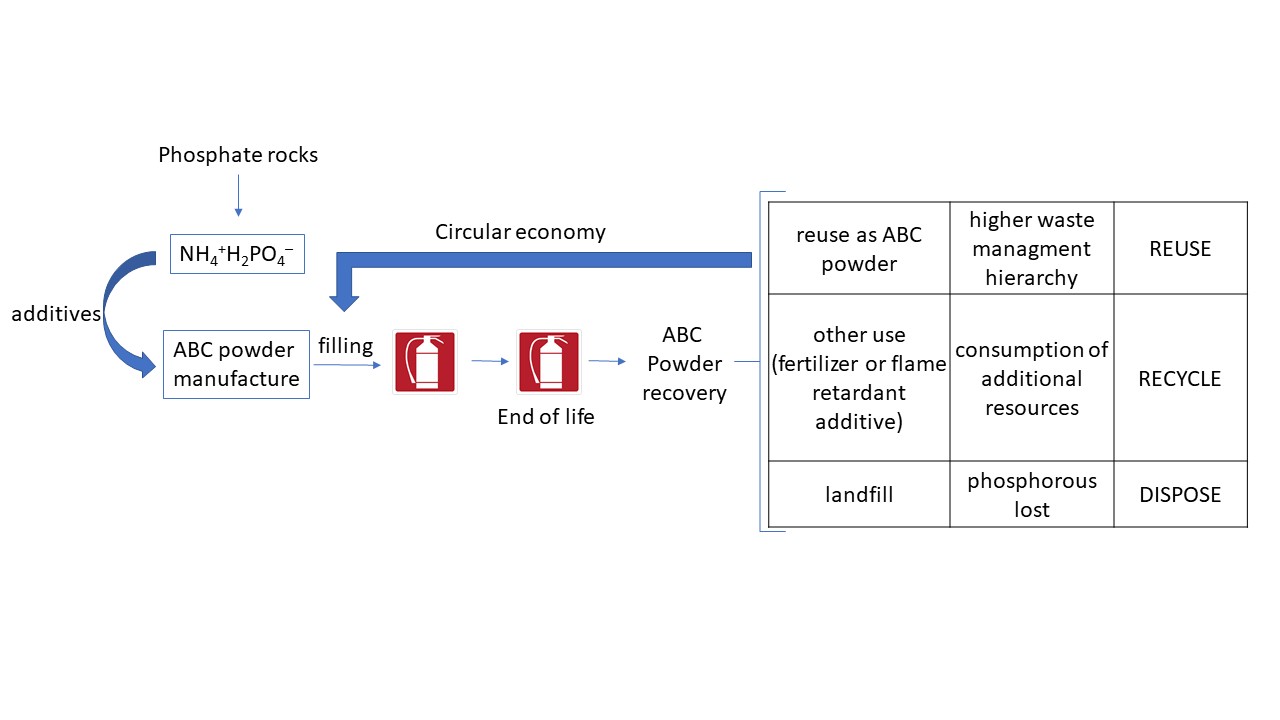
A major field of use of phosphate compounds is the manufacture of powders for portable fire extinguishers. These latter are widespread due to their versatility, high extinguishing power and limited cost. In particular, portable fire extinguishers using ABC powder are the most prevalent ones because suitable to extinguish fires generated by solids (class A), liquids (class B) and gases (class C). These fire extinguishers are devoted both to industrial as well as commercial and residential fires. It is anticipated that their production will increase significantly in the coming years due to increasing investments of nations in new facilities in accordance with fire protection laws.
Although the real composition of commercial powders is not always easily available, their main ingredient of ABC powders is ammonium dihydrogenphosphate NH4+H2PO4–, often named mono-ammonium phosphate (MAP), typically in the 20-90% range of the mixture. The final formulation includes other salts such as ammonium sulphate to absorb moisture and additives, mainly silicon derivatives as anticaking agent to avoid the formation of agglomerates that would hamper the efficiency during powder release in case of fire. Indeed, the powder need to remain free-flowing in order to be able to cover with a uniform layer the burned material at the same time separating it from oxygen, thus quenching the combustion chemical reaction.
The MAP is produced by reacting phosphoric acid, mainly produced by treating phosphate rocks with concentrated sulfuric acid, and dry ammonia in the right proportions. Some byproducts of the process and calcium sulphate need to be separated. Following a different approach, the phosphate group is reduced to elemental phosphorous P
4 at 1500 °C with an electrothermic process in the presence of coal and silica [
19]. In the next step, high purity phosphoric acid is generated through combustion, followed by hydration. Although this approach represents not more than 2-3% of the world phosphate rocks consumption, it is of strategic importance to generate a variety of specialists organophosphorous compounds.
When heated, MAP is broken down into phosphoric acid and ammonia at the same time absorbing some heat produced by fire and creating a barrier between the combustible material and the comburent [
20].
Fire extinguishers undergo a series of checks and inspections and a precise activity of maintenance to keep the devices in optimal conditions. For example, in Italy the standard UNI 9994-1:2013 states the powder should be replaced each 36 months and then considered as a waste with EER (CER) code 160509.
The standard, originated from the clear need to maintain over time the fire extinguisher efficiency, seems to be too cautious because it can be reasonably expected that, after staying 36 months at room temperature in the closed fire extinguisher, the powder would not be subjected to changes which justify the replacement. Indeed, it is known that MAP and other salt and additives such as silicones used in ABC powders are stable at the usual temperatures these devices are exposed to [
21].
The legislation may be different in other countries as regards to the lifetime but, as far as we are concerned, the powder always remains a waste to be disposed of with disposal costs borne by the waste producer.
Without considering the economic aspect, the disposal procedure clearly consumes additional resources to properly dispose of a waste that could probably be reused as such.
Indeed, both ABC powder composition and conditions of stay in the fire extinguisher, a closed pressure vessel at room temperature, reasonably allow the assumption that the powder composition along with the fluidization properties would not change even after a few years.
Therefore, the possibility of re-introduce the powder in the device, once the efficiency and sealing properties of other components during standard checking procedures are verified, might be considered in a circular economy perspective aiming to save and reuse critical raw materials.
3. Materials and Experimental Section
All reagents were used as received from chemical reagent companies without any further purification. The tests reported below were carried out in triplicate.
3.1. Ammonium Quantification
Three different samples of FUREX 710 fire extinguishing powders (batch 470124, manufactured in 2007 and subjected to analytical tests in October 2022, CALDIC Deutschland Chemie B.V., Am Karlshof 10, 40231 Dusseldorf, Germany) acc. to EN 615 were analyzed in this study. The ammonium ion was determined with the “salicylate method” and phosphorous determination with Inductively Coupled Plasma (ICP) technique. The sample concentration can be established by ICP spectroscopy units via calibration with verified ICP spectroscopy reference standards.
The “salicylate method” is a variation of the indophenol method where the harmful phenol was replaced by salicylic acid. At the same time, the formation of harmful
ortho-chlorophenol during the analysis could be avoided. Briefly, the sample was treated with 0.05 M sodium hypochlorite solution in the presence of 1 M sodium hydroxide solution to give a pH in the 9.7-11.5 range, and salicylic acid (5 wt %), sodium citrate (5 wt %) and sodium nitroprusside [Na
2Fe(CN)
5NO]. The monochloramine initially obtained further reacts with salicylate producing the blue adduct which was measured with UV-Vis absorption spectrum at 694 ± 5 nm. UV-vis spectroscopy is based on the electronic transition of molecules that absorb light [
22].
The sodium nitroprusside facilitates the formation of the coloured adduct. Ammonium chloride of known purity, dried at 110 °C for 2 h, was used to prepare solutions in the range of 0.01 to 140 mg/L to be used as a reference for calibration of the instrument.
3.2. Sieve Analysis
The granulometric distribution was determined with nest of sieves with a nominal diameter of 0.2 μm and aperture sizes of 0.125 μm, 0.063 μm and 0.04 μm. The powder (20 g ± 0.02 g) was charged into the top sieve. After shaking for 10 minutes (± 0.2 min), the quantity of the powder retained on each sieve as well as in the collecting pan was measured. The test can only be considered valid when all the weights of powders taken by sieves equals the initial amount to within ± 2 %. All tests were carried out at 20 °C (± 5 °C).
3.3. Moisture Content
The powder (20 g ± 0.001) is accurately weighed into the Petri dish and stored uncovered for 48 h (± 2 h) at a temperature of 20 ° C (± 3 °C) in the dessicator. The weight loss is calculated from the difference in weight after the storage in the dessicator and expressed as a percentage of the original sample loss.
4. Results and Discussion
In order to give a second life to the ABC powders, research efforts have been devoted to their use as fertilizer or flame-retardant additive [
23].
However, additional treatments are needed to generate a suitable material for the new use from end-of-life powders. At first the powder was subjected to a sieving procedure to remove any metal parts and plastic debris. After homogenization, the material is washed with organic solvents to remove silicon oil and any residual dye or contaminant.
Although the procedure might provide a new use of expired ABC powders, additional energy and raw materials such as organic solvents are consumed, thus limiting the efficiency of the process both from the economic and circular economy point of view [
24].
A polyethylene composite obtained by mold compression with untreated ABC powder has also been investigated for the flame resistance properties [
25].
However, considering potential risks of future supply of phosphorous materials, and the waste resources consumed to allow a second different use, undoubtedly, the best option in terms of sustainable phosphorous management would be to reuse expired ABC powders as such, namely to refill other fire extinguishers.
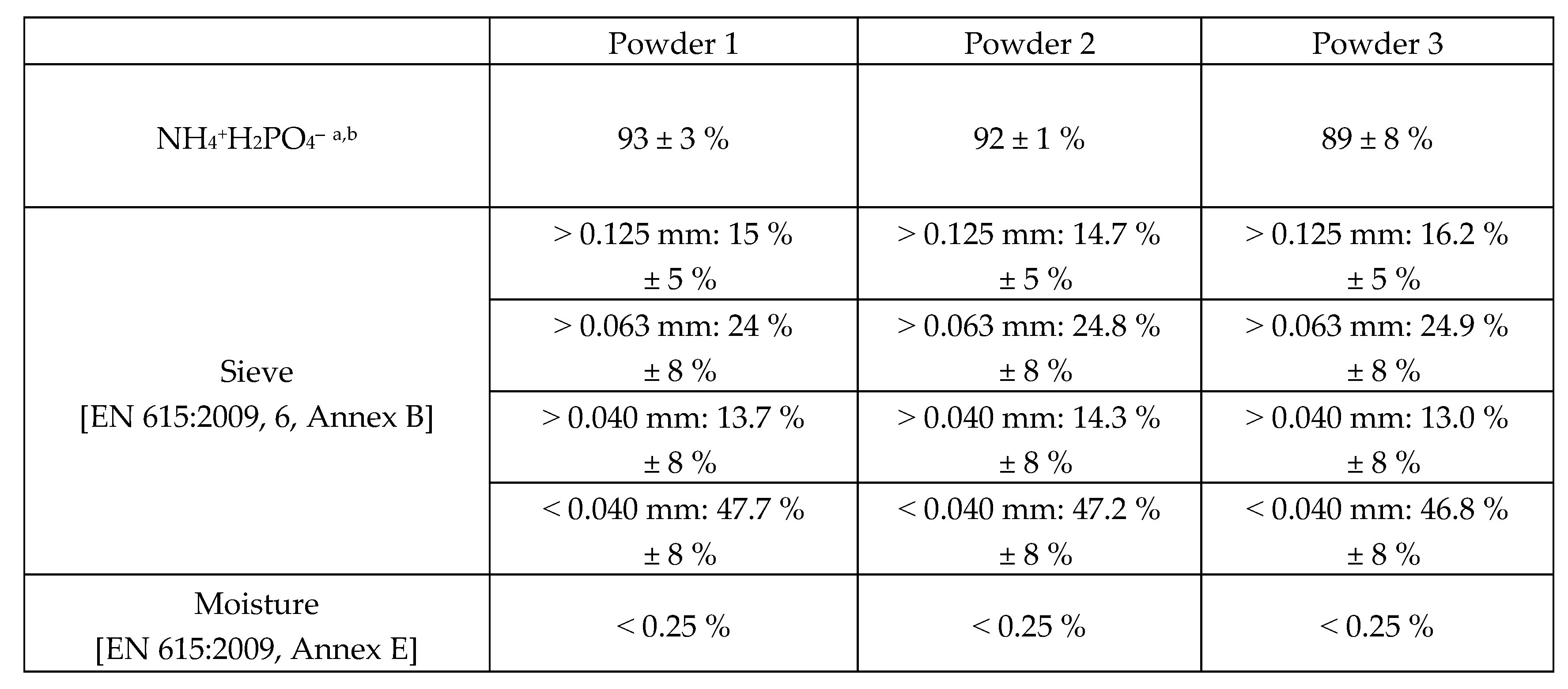
To investigate the feasibility of this approach, three different commercial batches of 15 years old ABC powders, kept in their original shipping bag, were tested as regards to ammonium ion assay, phosphorous content, moisture content and granulometry.
The experimental data collected through the analytical tests performed by a certified laboratory seem to provide promising result in favor of the idea to recycle the ABC powders as such, thus saving all the energy and raw materials used for their manufacture.
The results reported in
Figure 1 confirmed that the properties of the powders nicely match the rules of the technical sheet in force today. Moreover, all data did not show any significant difference and the three powders seems to be undistinguishable.
Therefore, it does not appear to have occurred alterations suggesting the material could not be used as extinguishing powder even after such a long storage time.
These results suggest the possibility to modify the current legislation allowing to recycle the expired ABC powder as such to fill other fire extinguishers. In addition, the duration of the powder might also be prolonged.
In summary, the results presented herein suggest a new standard for ABC powders management in order to save phosphorous resources.
Although the use of MAP in the fire protection field is not the main phosphate consumer on a quantitative basis, a significant contribution in terms of phosphate rocks savings would easily be obtained through the reuse of ABC powders as such. It has recently been estimated that around 100.000 t/year of ABC powders are disposed of as industrial waste [12,[
26].
The phosphorous recycle and reuse is of even higher importance considering how the phosphorous biogeochemical cycle is slow [
27] and how the continuous massive extraction of phosphate rocks has generated the actual unbalanced situation of phosphate rocks supply and risks for future commercialization. For example, Europe seems to be almost totally dependent from imports. Therefore, it would be of high strategic interest to set procedures to reduce the consumption of phosphate resources.
A more sustainable management of phosphate resources can be reached through the implementation of phosphorous recovery processes from waste cattle farming and municipal waste treatment basins.
However, the recovery of ABC powders during the scheduled revision, instead of the waste disposal actual procedure, would also greatly contribute to the sustainable phosphorous management without affecting the safety of fire extinguishers.
Although there are companies dedicated to collect ABC powder waste, quite often no options to recycle are available nearby the site where the waste has been generated, and the shipping is expensive. As a result, this kind of waste build up is not uncommon.
It is also worth noting that the fast-rising market of lithium iron phosphate for batteries will heavily contribute in the near future to increase further the phosphate consumption [
28]. Moreover, the phosphoric acids fuel cells market is also expected to increase [
29].
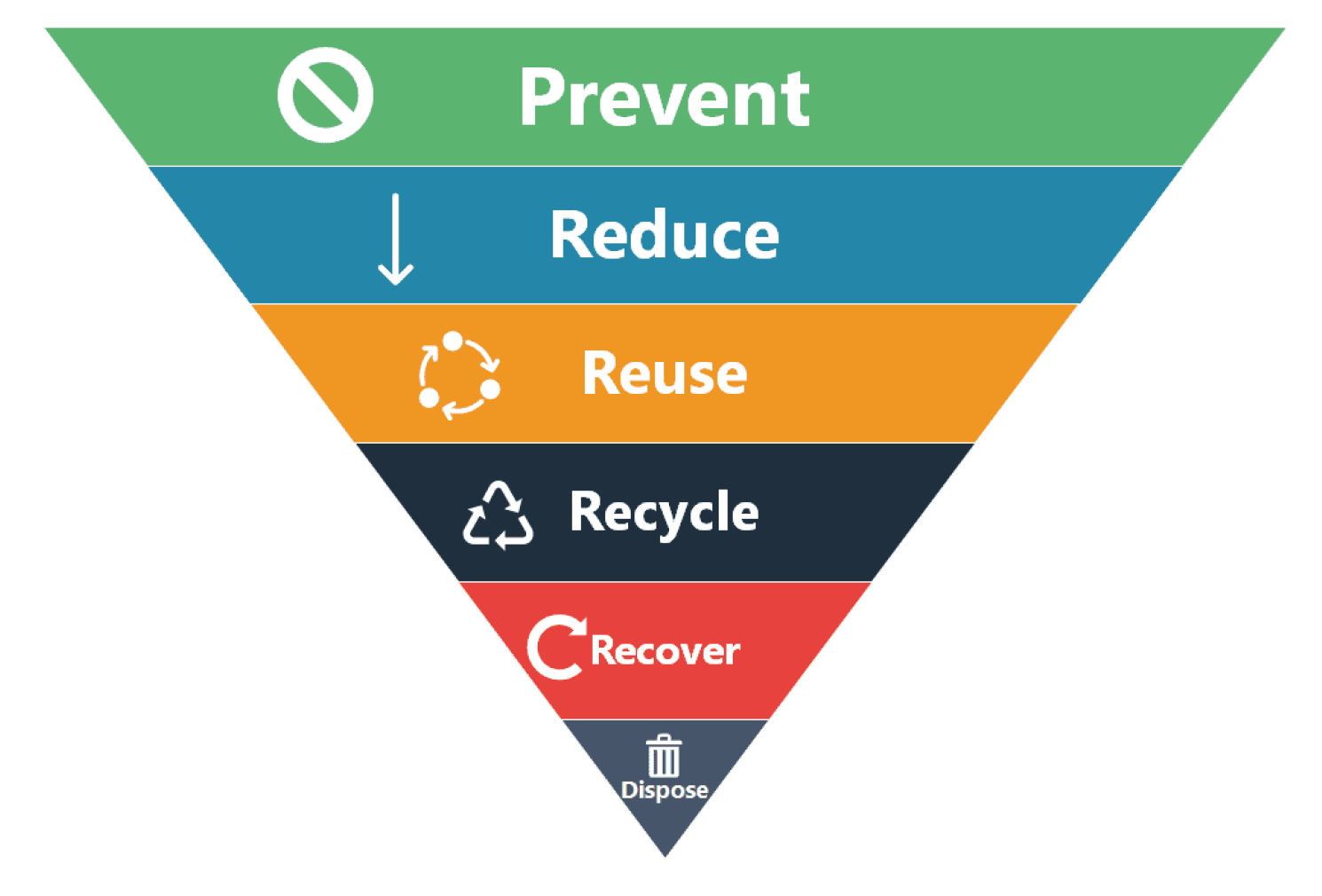
The direct reuse of ABC powders does not require special treatment plants. Only an analytical and/or a visual check from a skilled operator would be required. Thus, the reuse of expired ABC material, therefore using the waste as a resource at the same time diverting from disposal, would ensure a significantly higher hierarchy of sustainability allowing to preserve phosphorous for the future life on Earth (
Figure 2).
Finally, the mining of huge amounts of phosphate rocks is not devoid of drawbacks related to the water quality, radionuclide emissions and toxic metals pollution [
30]. Therefore, any reduction of phosphate rocks mining due to reuse of phosphate resources would also be beneficial for the preservation of Earth.
Author Contributions
Conceptualization, Domenico C. M. Albanese; methodology, M. Annatelli; formal analysis, Domenico C. M. Albanese; investigation, Domenico C. M. Albanese; resources, M. Annatelli; data curation, Domenico C. M. Albanese; writing—original draft preparation, Domenico C: M. Albanese; writing—review and editing, Domenico C. M. Albanese; visualization, Domenico C. M. Albanese; supervision, M. Annatelli; project administration, M. Annatelli; funding acquisition, M. Annatelli. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Walan, P.; Davidsson, S.; Johansson, S.; Höök, M. Phosphate rock production and depletion: Regional disaggregated modeling and global implications. Resources, Conservation and Recycling, 2014, vol. 93, n. 12, 178-187.
- See for example: https://www.indexmundi.com/commodities/?commodity=rock-phosphate&months=120. Accessed on October 11th, 2024.
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer N.; Lercher, J.; Awad, D.; Brück, T. The potential of biofuels from first to fourth generation. PLoS Biol. 2013, 21: e3002063. [CrossRef]
- Hein, L.; Leemans, R. The impact of first-generation biofuels on the depletion of global phosphorous reserve. AMBIO 2012, 41, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resources, Conservation and Recycling 2014, 93, 32–49. [Google Scholar] [CrossRef]
-
https://www.statista.com/statistics/681617/phosphate-rock-production-by-country/#statisticContainer. Accessed on October 11st, 2024.
- Critical raw materials are materials that are essential to the economy and whose supply may be at risk.
- Critical Raw Materials. Available online: URL (accessed on August 17th, 2024) https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-specific-interest/critical-raw-materials_en.
- The Periodic Table and us. Available online: URL (accessed on August 17th, 2024) https://www.euchems.eu/the-periodic-table-and-us/.
- Drury, M. Global futures and government towns: Phosphates and the production of Western Sahara as a space of contention. Arab World Geographer 2013, 16, pp. 101-124.
- a) Inventory of phosphorus “recovery and/or recycling” facilities operating or under construction at or downstream of wastewater treatment installations. Available online: URL (accessed on August 17th, 2024) https://phosphorusplatform.eu/images/download/Kabbe_P-recovecy_tech_implementation%20Table_2021_07.pdf; b) Zangarini, S.; Pepè Sciarria, T.; Tambone, F.; Adani, F. Phosphorus removal from livestock effluents: recent technologies and new perspectives on low cost strategies. Environ Sci Pollut Res 2020, 27, 5730–5743. [CrossRef]
- Gelsomino, A. ; Petrovicovà, B,; Panuccio M. R. Exhausted fire-extinguishing powders: A potential source of mineral nutrients for reuse and valorisation in compost enrichment for soilless cultivation Sci. Total Environ. 2024, 906, 167633–167646. [Google Scholar]
- The German Sewage Sludge Ordinance. Available online: URL (accessed on August 19th, 2024) https://ptc-parforce.de/en/german-abfklaerv/.
- a) Phosphor recycling. Available online: URL (accessed on August 19th, 2024) https://www.bafu.admin.ch/bafu/en/home/topics/waste/info-specialists/waste-policy-and-measures/phosphorrecycling.html.
- Austria Abfallverbrennungsverordnung 2024 – AVV 2024, CELEX 32010L0075, published in the Austrian Official Journal, 13th May 2024 (see section 4) https://www.bmk.gv.at/themen/klima_umwelt/abfall/recht/vo/abfallverbrennung.html (accessed on October21st, 2024).
- Phosphor recycling Hambourg. Available online: URL (accessed on August 17th, 2024) http://www.phosphorrecycling-hh.de/recycling/recycling.html.
- Ruscheweyh, R.; Lebek, M.; Rak, A. Phosphorrecycling nach dem TetraPhos-Verfahren. Wasser und Abfall 2021, 23, 69–73. [Google Scholar] [CrossRef]
- Lebek, M. World Intellectual Property Organization Patent WO2024/149781 A1.
- Montchamp, J.-L. Phosphinate Chemistry in the 21st Century: A Viable Alternative to the Use of Phosphorus Trichloride in Organophosphorus Synthesis Acc. Chem. Res. 2014, 47, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Romero, J.; Ortiz, E. High-temperature behaviour of ammonium dihydrogenphosphate. Journal of Physics: Conf. Series 2017, 935, 012050. [Google Scholar]
- Guerrant, G.O.; Brown, D.E. Thermal Decomposition of High-Analysis Fertilizers Based on Ammonium Phosphate J. Agr. Food Chem. 1965, 13, 493–497. [Google Scholar] [CrossRef]
- Akash, M. S. H.; Rehman, K. Ultraviolet-Visible (UV-VIS). In Spectroscopy Essentials of Pharmaceutical Analysis, Springer Nature Singapore: Singapore, 2020; pp. 2956.
- Extinguisher powder reused in fertilisers and fire retardants. Available online: URL (accessed on August 17th, 2024) https://cordis.europa.eu/article/id/241182-extinguisher-powder-reused-in-fertilisers-and-fire-retardants.
- Keijer, T.; Bakker, V.; Slootweg, J.C. Nat. Chem. 2019, 11, 190–195
.
- Ortega, Z.; Paz, R.; Montejo, A.; Suárez, L. Mechanical and fire characterization of composite material made of polyethylene matrix and dry chemical powder obtained from end-of-life extinguishers. Fire and materials 2021, 45, 215–224 doiorg/101002/fam2926. [Google Scholar] [CrossRef]
- EESPP, 2017. European Sustainable Phosphorus Platform. https://www.phosphorusplatform.eu (Last accessed 30 June 2023).
- Schipanski, M. E.; Bennett, E. M., The Phosphorus Cycle. In Fundamentals of Ecosystem Science, 2nd ed.; Weathers, K.C.; Strayer, D.L.; Likens, G.E., Eds.; Elsevier Inc., 2021; Chapter 9 - pp 189-213.
- Yao, H.; Zhang, Y.; Yang, G.; Fu, L.; Li, Y.; Zhou, L.; Geng, S.; Xiang, Y.; She, Z. W. Recycling of Spent Lithium Iron Phosphate Cathodes: Challenges and Progress. ACS Appl. Mater. Interfaces 2024. [CrossRef] [PubMed]
- Qasem, N. A. A., Abdulrahman, G. A. Q., A Recent Comprehensive Review of Fuel Cells: History, Types, and Applications Int. J. Energy Res. 2024, Volume 2024, Article ID 7271748, 36 pages. [CrossRef]
- G. Reta, Dong, X.; Li, Z.; Su, B. Hu, X.; Bo, H.; Yu, D.; Wan, H.; Liu, J.; Li, Y.; Xu, G.; Wang, K.; Xu, S. Environmental impact of phosphate mining and beneficiation: reviewInt. J. Hydro. 2018, 2, 424‒431.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).