Submitted:
24 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Classification of Hypersensitivity Reactions to Foods Focused on Non-IgE Mediated Mechanisms
2.1. Type I Hypersensitivity Reactions to Foods Involving Associated non-IgE-Mediated Mechanisms
2.2. Type II Hypersensitivity Reactions via Antibody-Mediated Cytotoxicity
2.3. Type III Hypersensitivity Reactions via Immune Complexes
2.4. Type IV Hypersensitivity Reactions via Cell-Mediated Mechanisms
2.4.1. Type IVa via T1 Immune Responses
2.4.2. Type IVb via T2 Immune Responses
2.4.3. Type IVc via T3 Immune Responses
2.5. Type V Tissue-Driven Hypersensitivity Reactions via Epithelial Barrier Impairment
2.6. Type VI Tissue-Driven Hypersensitivity Reactions via Metabolic-Induced Immune Dysregulation
2.7. Type VII Hypersensitivity Reactions via Direct Cellular and Inflammatory Responses to Chemical Substances
3. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Jutel, M.; Agache, I.; Zemelka-Wiacek, M.; Akdis, M.; Chivato, T.; Del Giacco, S.; Gajdanowicz, P.; Gracia, I.E.; Klimek, L.; Lauerma, A. , et al. Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper. Allergy 2023, 78, 2851–2874. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.N.; Xiang, L. Interpretation of 2023 EAACI guidelines on the diagnosis of IgE-mediated food allergy. Zhonghua Yu Fang Yi Xue Za Zhi 2024, 58, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Riggioni, C.; Agache, I.; Akdis, C.A.; Akdis, M.; Alvarez-Perea, A.; Alvaro-Lozano, M.; Ballmer-Weber, B.; Barni, S.; Beyer, K. , et al. EAACI guidelines on the diagnosis of IgE-mediated food allergy. Allergy 2023, 78, 3057–3076. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.G.; Hourihane, J.O.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; van Cauwenberge, P. , et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Jutel, M.; Papadopoulos, N.; Pfaar, O.; Akdis, C. Provocative proposal for a revised nomenclature for allergy and other hypersensitivity diseases. Allergy 2018, 73, 1939–1940. [Google Scholar] [CrossRef]
- Schmid-Grendelmeier, P.; Simon, D.; Simon, H.U.; Akdis, C.A.; Wüthrich, B. Epidemiology, clinical features, and immunology of the "intrinsic" (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis). Allergy 2001, 56, 841–849. [Google Scholar] [CrossRef]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J. , et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Meyer, R.; Chebar Lozinsky, A.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; Nowak-Wegrzyn, A.; Shah, N.; Venter, C. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy 2020, 75, 14–32. [Google Scholar] [CrossRef]
- Pat, Y.; Yazici, D.; D'Avino, P.; Li, M.; Ardicli, S.; Ardicli, O.; Mitamura, Y.; Akdis, M.; Dhir, R.; Nadeau, K.; et al. Recent advances in the epithelial barrier theory. Int Immunol 2024, 36, 211–222. [Google Scholar] [CrossRef]
- Popescu, F.D. Molecular biomarkers for grass pollen immunotherapy. World J Methodol 2014, 4, 26–45. [Google Scholar] [CrossRef]
- Riggioni, C.; Oton, T.; Carmona, L.; Du Toit, G.; Skypala, I.; Santos, A.F. Immunotherapy and biologics in the management of IgE-mediated food allergy: Systematic review and meta-analyses of efficacy and safety. Allergy 2024, 79, 2097–2127. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Suwanpradid, J.; Kim, I.H.; Staats, H.F.; Haniffa, M.; MacLeod, A.S.; Abraham, S.N. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science 2018, 362, eaao0666. [Google Scholar] [CrossRef]
- Lin, E.V.; Suresh, R.V.; Dispenza, M.C. Bruton's tyrosine kinase inhibition for the treatment of allergic disorders. Ann Allergy Asthma Immunol 2024, 133, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol 2008, 8, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Jeebhay, M.F.; Moscato, G.; Bang, B.E.; Folletti, I.; Lipińska-Ojrzanowska, A.; Lopata, A.L.; Pala, G.; Quirce, S.; Raulf, M.; Sastre, J.; et al. Food processing and occupational respiratory allergy - An EAACI position paper. Allergy 2019, 74, 1852–1871. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J Methodol 2015, 5, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Benedé, S.; Garrido-Arandia, M.; Martín-Pedraza, L.; Bueno, C.; Díaz-Perales, A.; Villalba, M. Multifactorial modulation of food-induced anaphylaxis. Front Immunol 2017, 8, 552. [Google Scholar] [CrossRef]
- Mortz, C.G.; Eller, E.; Garvik, O.S.; Kjaer, H.F.; Zuberbier, T.; Bindslev-Jensen, C. Challenge-verified thresholds for allergens mandatory for labeling: How little is too much for the most sensitive patient? Allergy 2024, 79, 1306–1316. [Google Scholar] [CrossRef]
- Spolidoro, G.C.I.; Ali, M.M.; Amera, Y.T.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Prevalence estimates of eight big food allergies in Europe: Updated systematic review and meta-analysis. Allergy 2023, 78, 2361–2417. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2024. Updated May 2024. Available from: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf (Accessed 8 September 2024).
- Chiang, V.; Mak, H.W.F.; Yeung, M.H.Y.; Kan, A.K.C.; Au, E.Y.L.; Li, P.H. Epidemiology, outcomes, and disproportionate burden of food-dependent exercise-induced anaphylaxis from the Hong Kong Multidisciplinary Anaphylaxis Management Initiative (HK-MAMI). J Allergy Clin Immunol Glob 2023, 2, 100127. [Google Scholar] [CrossRef]
- Kulthanan, K.; Ungprasert, P.; Jirapongsananuruk, O.; Rujitharanawong, C.; Munprom, K.; Trakanwittayarak, S.; Pochanapan, O.; Panjapakkul, W.; Maurer, M. Food-Dependent Exercise-Induced Wheals/Angioedema, Anaphylaxis, or Both: A Systematic Review of Phenotypes. J Allergy Clin Immunol Pract 2023, 11, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.; Popescu, F.D.; Vassilopoulou, E.; Smolinska, S. Allergenic Biomarkers in the Molecular Diagnosis of IgE-Mediated Wheat Allergy. Int J Mol Sci 2024, 25, 8210. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Villella, V.; Asero, R. Food-dependent exercise-induced allergic reactions in Lipid Transfer Protein (LTP) hypersensitive subjects: new data and a critical reappraisal. Eur Ann Allergy Clin Immunol 2024. [Google Scholar] [CrossRef]
- Chong, T.; Olivieri, B.; Skypala, I.J. Food-triggered anaphylaxis in adults. Curr Opin Allergy Clin Immunol 2024, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K. Food-dependent exercise-induced anaphylaxis: The need for better understanding and management of the disease. Allergy Asthma Immunol Res 2022, 14, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cano, R.; San Bartolome, C.; Casas-Saucedo, R.; Araujo, G.; Gelis, S.; Ruano-Zaragoza, M.; Roca-Ferrer, J.; Palomares, F.; Martin, M.; Bartra, J. , et al. Immune-mediated mechanisms in cofactor-dependent food allergy and anaphylaxis: Effect of cofactors in basophils and mast cells. Front Immunol 2021, 11, 623071. [Google Scholar] [CrossRef]
- Scherf, K.A.; Lindenau, A.C.; Valentini, L.; Collado, M.C.; García-Mantrana, I.; Christensen, M.; Tomsitz, D.; Kugler, C.; Biedermann, T.; Brockow, K. Cofactors of wheat-dependent exercise-induced anaphylaxis do not increase highly individual gliadin absorption in healthy volunteers. Clin Transl Allergy 2019, 9, 19. [Google Scholar] [CrossRef]
- Muñoz-Cano, R.; Pascal, M.; Bartra, J.; Picado, C.; Valero, A.; Kim, D.K.; Brooks, S.; Ombrello, M.; Metcalfe, D.D.; Rivera, J.; Olivera, A. Distinct transcriptome profiles differentiate nonsteroidal anti-inflammatory drug-dependent from nonsteroidal anti-inflammatory drug-independent food-induced anaphylaxis. J Allergy Clin Immunol 2016, 137, 137–146. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. Opioid toxicity: histamine, hypersensitivity, and MRGPRX2. Arch Toxicol 2023, 97, 359–375. [Google Scholar] [CrossRef]
- Kauppila, G.R.; Eagen, K.V. Opioid Use Disorder from Poppy Seed Tea Use: A Case Report. Am J Case Rep 2023, 24, e938675. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kuczyńska, R.; Żbikowska-Gotz, M.; Bartuzi, Z.; Krogulska, A. Anaphylaxis in an 8-Year-Old Boy Following the Consumption of Poppy Seed. J Investig Allergol Clin Immunol 2020, 30, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Podzhilkova, A.; Nagl, C.; Hummel, K.; Bindslev-Jensen, C.; Eller, E.; Mortz, C.G.; Bublin, M.; Hoffmann-Sommergruber, K. Poppy Seed Allergy: Molecular Diagnosis and Cross-Reactivity With Tree Nuts. J Allergy Clin Immunol Pract 2024, 12, 2144–2154.e11. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borges, M.; Fernández-Caldas, E.; Capriles-Hulett, A.; Caballero-Fonseca, F. Mite-induced inflammation: More than allergy. Allergy Rhinol (Providence) 2012, 3, e25–e29. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borges, M.; Suárez Chacón, R.; Capriles-Hulett, A.; Caballero-Fonseca, F.; Fernández-Caldas, E. Anaphylaxis from ingestion of mites: pancake anaphylaxis. J Allergy Clin Immunol 2013, 131, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Enrique, E.; Knulst, A.C.; Mari, A.; Muraro, A.; Ollert, M.; Poulsen, L.K. , et al. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy 2015, 70, 1079–1090. [Google Scholar] [CrossRef]
- Darsow, U.; Gelincik, A.; Jappe, U.; Platts-Mills, T.A.; Ünal, D.; Biedermann, T. Algorithms in allergy: An algorithm for alpha-Gal syndrome diagnosis and treatment, 2024 update. Allergy 2024. [Google Scholar] [CrossRef]
- Popescu, F.D.; Cristea, O.M.; Ionica, F.E.; Vieru, M. Drug allergies due to IgE sensitization to α-Gal. Farmacia 2019, 67, 43–49. [Google Scholar] [CrossRef]
- Ikemoto, C.; Tamagawa-Mineoka, R.; Masuda, K.; Iida, S.; Inomata, N.; Katoh, N. Immediate-onset anaphylaxis of Bacillus subtilis-fermented soybeans (natto). J Dermatol 2014, 41, 857–858. [Google Scholar] [CrossRef]
- Inomata, N.; Chin, K.; Aihara, M. Anaphylaxis caused by ingesting jellyfish in a subject with fermented soybean allergy: possibility of epicutaneous sensitization to poly-gamma-glutamic acid by jellyfish stings. J Dermatol 2014, 41, 752–753. [Google Scholar] [CrossRef]
- Lopata AL, Jeebhay MF. Airborne seafood allergens as a cause of occupational allergy and asthma. Curr Allergy Asthma Rep. 2013, 13, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.K.; Seternes, O.M.; Larsen, M.; Kishimur, H.; Rudenskaya, G.N.; Bang, B. Purified sardine and king crab trypsin display individual differences in PAR-2-, NF-κB-, and IL-8 signaling. Toxicol. Environ. Chem. 2011, 93, 1991–2011. [Google Scholar] [CrossRef]
- Pougnet, R.; Loddé, B.; Lucas, D.; Jégaden, D.; Bell, S.; Dewitte, J.D. A case of occupational asthma from metabisulphite in a fisherman. Int. Marit. Health 2010, 62, 180–184. [Google Scholar] [PubMed]
- Smit, L.A.; Wouters, I.M.; Hobo, M.M.; Eduard, W.; Doekes, G.; Heederik, D. Agricultural seed dust as a potential cause of organic dust toxic syndrome. Occup. Environ. Med. 2006, 63, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Amaro, C.; Goossens, A. Immunological occupational contact urticaria and contact dermatitis from proteins: a review. Contact Dermatitis 2008, 58, 67–75. [Google Scholar] [CrossRef]
- Brancaccio, R.R.; Alvarez, M.S. Contact allergy to food. Dermatol. Ther. 2004, 17, 302–313. [Google Scholar] [CrossRef]
- Giménez-Arnau, A.M.; Pesqué, D.; Maibach, H.I. Contact Urticaria Syndrome: a comprehensive review. Curr. Dermatol. Rep. 2022, 11, 194–201. [Google Scholar] [CrossRef]
- Walter, A.; Seegräber, M.; Wollenberg, A. Food-related contact dermatitis, contact urticaria, and atopy patch test with food. Clin. Rev. Allergy Immunol. 2019, 56, 19–31. [Google Scholar] [CrossRef]
- Ashbaugh, A.G.; Abel, M.K.; Murase, J.E. Protein causes of urticaria and dermatitis. Immunol. Allergy Clin. North Am. 2021, 41, 481–491. [Google Scholar] [CrossRef]
- Vethachalam, S. Persaud, Y. Contact Urticaria. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK549890/.
- Bajwa, S.F.; Mohammed, R.H. Type II hypersensitivity reaction. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. Available from: www.ncbi.nlm.nih.gov/books/NBK563264.
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Vashisht, R.; Zito, P.M. Immediate hypersensitivity reactions (Archived). 2023 May 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PubMed]
- Husebye, E.S.; Anderson, M.S.; Kämpe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 2018, 378, 1132–1141. [Google Scholar] [CrossRef]
- Lampasona, V.; Passerini, L.; Barzaghi, F.; Lombardoni, C.; Bazzigaluppi, E.; Brigatti, C.; Bacchetta, R.; Bosi, E. Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PLoS One 2013, 8, e78664. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, K.H.; Jeon, B.; Ochs, H.D.; Lee, J.S.; Gee, H.Y.; Seo, S.; Geum, D.; Piccirillo, C.A.; Eisenhut, M.; van der Vliet, H.J.; Lee, J.M.; Kronbichler, A.; Ko, Y.; Shin, J.I. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome: a systematic review. Autoimmun. Rev. 2020, 19, 102526. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, T.R.; Linane, A.; Moes, N.; Anover, S.; Mateo, V.; Rieux-Laucat, F.; Hermine, O.; Vijay, S.; Gambineri, E.; Cerf-Bensussan, N.; Fischer, A.; et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology 2007, 132, 1705–1717. [Google Scholar] [CrossRef]
- Verbsky, J.W.; Chatila, T.A. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr. Opin. Pediatr. 2013, 25, 708–714. [Google Scholar] [CrossRef]
- Altintas, A.; Pasa, S.; Cil, T.; Bayan, K.; Gokalp, D.; Ayyildiz, O. Thyroid and celiac diseases autoantibodies in patients with adult chronic idiopathic thrombocytopenic purpura. Platelets 2008, 19, 252–257. [Google Scholar] [CrossRef]
- Fisgin, T.; Yarali, N.; Duru, F.; Usta, B.; Kara, A. Hematologic manifestation of childhood celiac disease. Acta Haematol. 2004, 111, 211–214. [Google Scholar] [CrossRef]
- Guarina, A.; Marinoni, M.; Lassandro, G.; Saracco, P.; Perrotta, S.; Facchini, E.; Notarangelo, L.D.; Russo, G.; Giordano, P.; Romano, F.; et al. Association of immune thrombocytopenia and celiac disease in children: a retrospective case control study. Turk. J. Haematol. 2021, 38, 175–180. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Aeschlimann, P.; Sanders, D.S.; Mäki, M.; Kaukinen, K.; Grünewald, R.A.; Bandmann, O.; Woodroofe, N.; Haddock, G.; Aeschlimann, D.P. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology 2013, 80, 1740–1745. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Davies-Jones, G.A.; Sanders, D.S.; Grünewald, R.A. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry 2003, 74, 1221–1224. [Google Scholar] [CrossRef]
- Lauret, E.; Rodrigo, L. Celiac disease and autoimmune-associated conditions. Biomed Res Int. 2013, 2013, 127589. [Google Scholar] [CrossRef]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.K.; Shakkottai, V.G. Overview of cerebellar ataxia in adults. In: Hurtig, H.I., ed. UpToDate. Wolters Kluwer. Updated August 5, 2024. Available online: www.uptodate.com/contents/overview-of-cerebellar-ataxia-in-adults (accessed on 8 September 2024).
- Brenner, S.; Goldberg, I. Drug-induced pemphigus. Clin Dermatol. 2011, 29, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.; Ruocco, V.; Ruocco, E.; Russo, A.; Tur, E.; Luongo, V.; Lombardi, M.L. In vitro tannin acantholysis. Int J Dermatol. 2000, 39, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Halil Yavuz, I.; Ozaydın Yavuz, G. The role of pentraxin 3 in pemphigus vulgaris. Postepy Dermatol Alergol. 2020, 37, 503–507. [Google Scholar] [CrossRef]
- Hertl, M.; Sitaru, C. Pathogenesis, clinical manifestations, and diagnosis of pemphigus. In: Zone, J.J., ed. UpToDate. Wolters Kluwer. Updated January 25, 2024. Available online: www.uptodate.com/contents/pathogenesis-clinical-manifestations-and-diagnosis-of-pemphigus (accessed on 8 September 2024).
- Ruocco, V.; Brenner, S.; Ruocco, E. Pemphigus and diet: does a link exist? Int J Dermatol. 2001, 40, 161–163. [Google Scholar] [CrossRef]
- Usman, N.; Annamaraju, P. Type III Hypersensitivity Reaction. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available online: www.ncbi.nlm.nih.gov/books/NBK559122/.
- Costabel, U.; Miyazaki, Y.; Pardo, A.; Koschel, D.; Bonella, F.; Spagnolo, P.; Guzman, J.; Ryerson, C.J.; Selman, M. Hypersensitivity pneumonitis. Nat Rev Dis Primers 2020, 6, 65. [Google Scholar] [CrossRef]
- King, T.E. Hypersensitivity pneumonitis (extrinsic allergic alveolitis): Epidemiology, causes, and pathogenesis. In: Flaherty, K.R., Ed. UpToDate. Wolters Kluwer. Updated August 01, 2023. Available online: www.uptodate.com/contents/hypersensitivity-pneumonitis-extrinsic-allergic-alveolitis-epidemiology-causes-and-pathogenesis (Accessed 8 September 2024).
- Kongsupon, N.; Walters, G.I.; Sadhra, S.S. Occupational causes of hypersensitivity pneumonitis: a systematic review and compendium. Occup Med 2021, 71, 255–259. [Google Scholar] [CrossRef]
- Quirce, S.; Vandenplas, O.; Campo, P.; Cruz, M.J.; de Blay, F.; Koschel, D.; Moscato, G.; Pala, G.; Raulf, M.; Sastre, J.; Siracusa, A.; Tarlo, S.M.; Walusiak-Skorupa, J.; Cormier, Y. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy 2016, 71, 765–779. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A.; King, T.E., Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med 2012, 186, 314–324. [Google Scholar] [CrossRef]
- Arasi, S.; Mastrorilli, C.; Pecoraro, L.; Giovannini, M.; Mori, F.; Barni, S.; Caminiti, L.; Castagnoli, R.; Liotti, L.; Saretta, F. , et al. Heiner Syndrome and Milk Hypersensitivity: An Updated Overview on the Current Evidence. Nutrients 2021, 13, 1710. [Google Scholar] [CrossRef]
- Cox, A.L.; Sicherer, S.H. Classification of adverse food reactions. J Food Allergy 2020, 2, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, M.; Jung, J.H.; Kim, S.Y.; Kim, Y.H.; Hahn, S.M.; Kim, S.; Lee, M.J.; Shim, H.S.; Sohn, M.H.; Kim, K.W.; Kim, M.J. Children with Heiner Syndrome: A Single-Center Experience. Children 2021, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Aquino, M.; Rosner, G. Systemic Contact Dermatitis. Clin Rev Allergy Immunol 2019, 56, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Killig, C.; Werfel, T. Contact reactions to food. Curr Allergy Asthma Rep 2008, 8, 209–214. [Google Scholar] [CrossRef]
- Rozas-Muñoz, E.; Lepoittevin, J.P.; Pujol, R.M.; Giménez-Arnau, A. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr 2012, 103, 456–477. [Google Scholar] [CrossRef]
- Vergara, C.; Mauro, M.; Belloni Fortina, A.; Giulioni, E.; Larese Filon, F. Occupational Contact Dermatitis in Food Handlers: A 26-Year Retrospective Multicentre Study in North-East Italy (Triveneto Group on Research on Contact Dermatitis). Dermatitis 2024. [Google Scholar] [CrossRef]
- Yamaguchi, H.L.; Yamaguchi, Y.; Peeva, E. Role of Innate Immunity in Allergic Contact Dermatitis: An Update. Int J Mol Sci. 2023, 24, 12975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leonard, A.; Guttman-Yassky, E. The Unique Molecular Signatures of Contact Dermatitis and Implications for Treatment. Clin Rev Allergy Immunol. 2019, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brites, G.S.; Ferreira, I.; Sebastião, A.I.; Silva, A.; Carrascal, M.; Neves, B.M.; Cruz, M.T. Allergic contact dermatitis: From pathophysiology to development of new preventive strategies. Pharmacol Res. 2020, 162, 105282. [Google Scholar] [CrossRef]
- Scheinman, P.L.; Vocanson, M.; Thyssen, J.P.; Johansen, J.D.; Nixon, R.L.; Dear, K.; Botto, N.C.; Morot, J.; Goldminz, A.M. Contact dermatitis. Nat Rev Dis Primers. 2021, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Chipinda, I.; Hettick, J.M.; Siegel, P.D. Haptenation: chemical reactivity and protein binding. J Allergy (Cairo) 2011, 2011, 839682. [Google Scholar] [CrossRef] [PubMed]
- Lepoittevin, J.P.; Berl, V.; Giménez-Arnau, E. Alpha-methylene-gamma-butyrolactones: versatile skin bioactive natural products. Chem Rec 2009, 9, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Benezra, C. Quantitative structure-activity relationships for skin sensitization potential of urushiol analogues. Contact Dermatitis 1993, 29, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sukakul, T.; Bruze, M.; Svedman, C. Fragrance Contact Allergy—A Review Focusing on Patch Testing. Acta Derm Venereol 2024, 104, adv40332. [Google Scholar] [CrossRef]
- Azeem, M.; Kader, H.; Kerstan, A.; Hetta, H.F.; Serfling, E.; Goebeler, M.; Muhammad, K. Intricate Relationship Between Adaptive and Innate Immune System in Allergic Contact Dermatitis. Yale J Biol Med 2020, 93, 699–709. [Google Scholar]
- Sakamoto, E.; Katahira, Y.; Mizoguchi, I.; Watanabe, A.; Furusaka, Y.; Sekine, A.; Yamagishi, M.; Sonoda, J.; Miyakawa, S.; Inoue, S. , et al. Chemical- and Drug-Induced Allergic, Inflammatory, and Autoimmune Diseases Via Haptenation. Biology 2023, 12, 123. [Google Scholar] [CrossRef]
- Aleksic, M.; Rajagopal, R.; de-Ávila, R.; Spriggs, S.; Gilmour, N. The skin sensitization adverse outcome pathway: exploring the role of mechanistic understanding for higher tier risk assessment. Crit Rev Toxicol 2024, 54, 69–91. [Google Scholar] [CrossRef]
- Rodrigues Neves, C.; Gibbs, S. Progress on Reconstructed Human Skin Models for Allergy Research and Identifying Contact Sensitizers. Curr Top Microbiol Immunol 2021, 430, 103–129. [Google Scholar] [CrossRef]
- Dhingra, N.; Shemer, A.; Correa da Rosa, J.; Rozenblit, M.; Fuentes-Duculan, J.; Gittler, J.K.; Finney, R.; Czarnowicki, T.; Zheng, X.; Xu, H.; et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J Allergy Clin Immunol 2014, 134, 362–372. [Google Scholar] [CrossRef]
- Alcain, J.; Infante Cruz, A.D.P.; Barrientos, G.; Vanzulli, S.; Salamone, G.; Vermeulen, M. Mechanisms of unconventional CD8 Tc2 lymphocyte induction in allergic contact dermatitis: Role of H3/H4 histamine receptors. Front Immunol 2022, 13, 999852. [Google Scholar] [CrossRef]
- Afvari, S.; Zippin, J.H. Type I hypersensitivity in photoallergic contact dermatitis. JAAD Case Rep 2023, 44, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Scheman, A.; Gupta, S. Photoallergic contact dermatitis from diallyl disulfide. Contact Dermatitis 2001, 45, 179. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.; Issa, C.J.; Nedorost, S.T.; Lio, P.A. Is food-triggered atopic dermatitis a form of systemic contact dermatitis? Clin Rev Allergy Immunol 2024, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gil-Pallares, P.; Alvarez-Garcia, O.; González-Moure, C.; Castro-Murga, M.; Monteagudo-Sánchez, B. Fixed food eruption caused by Maja squinado (European spider crab) in a child. Contact Dermatitis 2020, 83, 510–512. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K. The prevalence of tree nut allergy: A systematic review. Curr Allergy Asthma Rep 2015, 15, 54. [Google Scholar] [CrossRef]
- Sharma, L.; Agarwal, R.; Chopra, A.; Mitra, B. A cross-sectional observational study of clinical spectrum and prevalence of fixed food eruption in a tertiary care hospital. Indian Dermatol Online J 2020, 11, 361–366. [Google Scholar] [CrossRef]
- Volz, T.; Berner, D.; Weigert, C.; Röcken, M.; Biedermann, T. Fixed food eruption caused by asparagus. J Allergy Clin Immunol 2005, 116, 1390–1392. [Google Scholar] [CrossRef]
- Lania, G.; Nanayakkara, M.; Maglio, M.; Auricchio, R.; Porpora, M.; Conte, M.; De Matteis, M.A.; Rizzo, R.; Luini, A.; Discepolo, V.; et al. Constitutive alterations in vesicular trafficking increase the sensitivity of cells from celiac disease patients to gliadin. Commun Biol 2019, 2, 190. [Google Scholar] [CrossRef]
- Maiuri, L.; Villella, V.R.; Raia, V.; Kroemer, G. The gliadin-CFTR connection: New perspectives for the treatment of celiac disease. Ital J Pediatr 2019, 45, 40. [Google Scholar] [CrossRef]
- Rizzi, A.; Di Gioacchino, M.; Gammeri, L.; Inchingolo, R.; Chini, R.; Santilli, F.; Nucera, E.; Gangemi, S. The emerging role of innate lymphoid cells (ILCs) and alarmins in celiac disease: An update on pathophysiological insights, potential use as disease biomarkers, and therapeutic implications. Cells 2023, 12, 1910. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Galipeau, H.J.; Agardh, D. Celiac disease: A review of current concepts in pathogenesis, prevention, and novel therapies. Front Pediatr 2018, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Vargas, J.; Green, P.H.R.; Bhagat, G. Innate lymphoid cells and celiac disease: Current perspective. Cell Mol Gastroenterol Hepatol 2021, 11, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Bijelić, B.; Matić, I.Z.; Besu, I.; Janković, L.; Juranić, Z.; Marušić, S.; Andrejević, S. Celiac disease-specific and inflammatory bowel disease-related antibodies in patients with recurrent aphthous stomatitis. Immunobiology 2019, 224, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Camarca, A.; Rotondi Aufiero, V.; Mazzarella, G. Role of regulatory T cells and their potential therapeutic applications in celiac disease. Int J Mol Sci 2023, 24, 14434. [Google Scholar] [CrossRef]
- Cellier, C.; Patey, N.; Mauvieux, L.; Jabri, B.; Delabesse, E.; Cervoni, J.P.; Burtin, M.L.; Guy-Grand, D.; Bouhnik, Y.; Modigliani, R.; Barbier, J.P.; Macintyre, E.; Brousse, N.; Cerf-Bensussan, N. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 1998, 114, 471–481. [Google Scholar] [CrossRef]
- Halttunen, T.; Mäki, M. Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology 1999, 116, 566–572. [Google Scholar] [CrossRef]
- Husby, S.; Murray, J.A.; Katzka, D.A. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: Expert review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef]
- Görög, A.; Antiga, E.; Caproni, M.; Cianchini, G.; De, D.; Dmochowski, M.; Dolinsek, J.; Drenovska, K.; Feliciani, C.; Hervonen, K.; et al. S2k guidelines (consensus statement) for diagnosis and therapy of dermatitis herpetiformis initiated by the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol 2021, 35, 1251–1277. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4‒JAK‒STAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J Clin Med 2020, 9, 3741. [Google Scholar] [CrossRef]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front Immunol 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Kogame, T.; Egawa, G.; Kabashima, K. Exploring the role of Janus kinase (JAK) in atopic dermatitis: a review of molecular mechanisms and therapeutic strategies. Immunol Med 2023, 46, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.R.; Seminario-Vidal, L.; Abe, B.; Ghobadi, C.; Sims, J.T. Cytokines and Epidermal Lipid Abnormalities in Atopic Dermatitis: A Systematic Review. Cells 2023, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Wasserer, S.; Jargosch, M.; Mayer, K.E.; Eigemann, J.; Raunegger, T.; Aydin, G.; Eyerich, S.; Biedermann, T.; Eyerich, K.; Lauffer, F. Characterization of High and Low IFNG-Expressing Subgroups in Atopic Dermatitis. Int J Mol Sci 2024, 25, 6158. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; D'Onghia, M.; Lazzeri, L.; Rubegni, G.; Cinotti, E. Blocking the IL-4/IL-13 Axis versus the JAK/STAT Pathway in Atopic Dermatitis: How Can We Choose? J Pers Med 2024, 14, 775. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, K. Atopic dermatitis endotypes: knowledge for personalized medicine. Curr Opin Allergy Clin Immunol 2022, 22, 153–159. [Google Scholar] [CrossRef]
- Sanyal, R.D.; Pavel, A.B.; Glickman, J.; Chan, T.C.; Zheng, X.; Zhang, N.; Cueto, I.; Peng, X.; Estrada, Y.; Fuentes-Duculan, J.; et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol 2019, 122, 99–110.e6. [Google Scholar] [CrossRef]
- Zhou, L.; Leonard, A.; Pavel, A.B.; Malik, K.; Raja, A.; Glickman, J.; Estrada, Y.D.; Peng, X.; Del Duca, E.; Sanz-Cabanillas, J.; et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2019, 144, 144–156. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Johnson, S.H.; DeLain, L.P.; Haedt, M.J.; Noll, A.L.; Sandbo, N.; Jarjour, N.N. Matrix Metalloproteinase-9-Dependent Release of IL-1β by Human Eosinophils. Mediators Inflamm. 2019, 7479107. [Google Scholar] [CrossRef]
- Packi, K.; Matysiak, J.; Klimczak, S.; Matuszewska, E.; Bręborowicz, A.; Pietkiewicz, D.; Matysiak, J. Analysis of the Serum Profile of Cytokines Involved in the T-Helper Cell Type 17 Immune Response Pathway in Atopic Children with Food Allergy. Int. J. Environ. Res. Public Health 2022, 19, 7877. [Google Scholar] [CrossRef]
- Park, C.O.; Kim, S.M.; Lee, K.H.; Bieber, T. Biomarkers for Phenotype-Endotype Relationship in Atopic Dermatitis: A Critical Review. EBioMedicine 2024, 103, 105121. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.; Guttman-Yassky, E. Biomarkers in Atopic Dermatitis—A Review on Behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 Induce Innate IL-17 Production from γδ T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Kido-Nakahara, M.; Onozuka, D.; Izuhara, K.; Saeki, H.; Nunomura, S.; Takenaka, M.; Matsumoto, M.; Kataoka, Y.; Fujimoto, R.; Kaneko, S.; et al. Exploring Patient Background and Biomarkers Associated with the Development of Dupilumab-Associated Conjunctivitis and Blepharitis. Allergol. Int. 2024, 73, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Akdis, C.A.; Akdis, M.; Bachert, C.; Bieber, T.; Canonica, G.W.; Guttman-Yassky, E.; Metz, M.; Mullol, J.; Palomares, O.; Renz, H.; Ständer, S.; Zuberbier, T.; Maurer, M. Type 2 Chronic Inflammatory Diseases: Targets, Therapies and Unmet Needs. Nat Rev Drug Discov 2023, 22, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Gonsalves, N.; Abonia, J.P.; Alexander, J.A.; Arva, N.C.; Atkins, D.; Attwood, S.E.; Auth, M.K.H.; Bailey, D.D.; Biederman, L.; et al. International Consensus Recommendations for Eosinophilic Gastrointestinal Disease Nomenclature. Clin Gastroenterol Hepatol 2022, 20, 2474–2484.e3. [Google Scholar] [CrossRef]
- Li, K.; Ruan, G.; Liu, S.; Xu, T.; Guan, K.; Li, J.; Li, J. Eosinophilic Gastroenteritis: Pathogenesis, Diagnosis, and Treatment. Chin Med J (Engl) 2023, 136, 899–909. [Google Scholar] [CrossRef]
- Neely, J.L.; Reimers, A.; Taylor, S.; Garg, S.; Masuda, M.Y.; Schroeder, S.; Wright, B.L.; Doyle, A.D. GATA-3 and T-bet as Diagnostic Markers of Non-Esophageal Eosinophilic Gastrointestinal Disease. Allergy 2022, 77, 1042–1044. [Google Scholar] [CrossRef]
- Greuter, T.; Straumann, A.; Fernandez-Marrero, Y.; Germic, N.; Hosseini, A.; Chanwangpong, A.; Yousefi, S.; Simon, D.; Collins, M.H.; Bussmann, C.; et al. A Multicenter Long-Term Cohort Study of Eosinophilic Esophagitis Variants and Their Progression to Eosinophilic Esophagitis Over Time. Clin Transl Gastroenterol 2024, 15, e00664. [Google Scholar] [CrossRef]
- Sorge, A.; Aldinio, G.; Marinoni, B.; Visaggi, P.; Penagini, R.; Maniero, D.; Ghisa, M.; Marabotto, E.; de Bortoli, N.; Pasta, A.; Dipace, V.; Calabrese, F.; Vecchi, M.; Savarino, E.V.; Coletta, M. Distribution of Esophageal Inflammation in Patients with Eosinophilic Esophagitis and Its Impact on Diagnosis and Outcome. Dig Liver Dis 2024, 56, S1590–S865800967. [Google Scholar] [CrossRef]
- Underwood, B.; Troutman, T.D.; Schwartz, J.T. Breaking Down the Complex Pathophysiology of Eosinophilic Esophagitis. Ann Allergy Asthma Immunol 2023, 130, 28–39. [Google Scholar] [CrossRef]
- Alsohaibani, F.I.; Peedikayil, M.C.; Alzahrani, M.A.; Azzam, N.A.; Almadi, M.A.; Dellon, E.S.; Al-Hussaini, A.A. Eosinophilic Esophagitis: Current Concepts in Diagnosis and Management. Saudi J Gastroenterol 2024, 30, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Shuker, M.; Brown-Whitehorn, T.; Hunter, H.; Venter, C.; Spergel, J.M. Food Avoidance Strategies in Eosinophilic Oesophagitis. Clin Exp Allergy 2019, 49, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Ridolo, E.; Barone, A.; Ottoni, M.; Peveri, S.; Montagni, M.; Nicoletta, F. The New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis: Biological Drugs. Int J Mol Sci 2024, 25, 1702. [Google Scholar] [CrossRef]
- Rossi, C.M.; Santacroce, G.; Lenti, M.V.; di Sabatino, A. Eosinophilic Esophagitis in the Era of Biologics. Expert Rev Gastroenterol Hepatol 2024, 18, 271–281. [Google Scholar] [CrossRef]
- Wilson, B.E.; Sacta, M.A.; Wright, B.L.; Spergel, J.; Wolfset, N. The Relationship Between Eosinophilic Esophagitis and Immunotherapy. Immunol Allergy Clin North Am 2024, 44, 281–291. [Google Scholar] [CrossRef]
- Franciosi, J.P.; Mougey, E.B.; Dellon, E.S.; Gutierrez-Junquera, C.; Fernandez-Fernandez, S.; Venkatesh, R.D.; Gupta, S.K. Proton Pump Inhibitor Therapy for Eosinophilic Esophagitis: History, Mechanisms, Efficacy, and Future Directions. J Asthma Allergy 2022, 15, 281–302. [Google Scholar] [CrossRef]
- Rochman, Y.; Kotliar, M.; Ben-Baruch Morgenstern, N.; Barski, A.; Wen, T.; Rothenberg, M.E. TSLP Shapes the Pathogenic Responses of Memory CD4+ T Cells in Eosinophilic Esophagitis. Sci Signal 2023, 16, eadg6360. [Google Scholar] [CrossRef]
- Greuter, T.; Straumann, A.; Fernandez-Marrero, Y.; Germic, N.; Hosseini, A.; Yousefi, S.; Simon, D.; Collins, M.H.; Bussmann, C.; Chehade, M.; et al. Characterization of Eosinophilic Esophagitis Variants by Clinical, Histological, and Molecular Analyses: A Cross-Sectional Multi-Center Study. Allergy 2022, 77, 2520–2533. [Google Scholar] [CrossRef]
- Odiase, E.; Zhang, X.; Chang, Y.; Nelson, M.; Balaji, U.; Gu, J.; Zhang, Q.; Pan, Z.; Spechler, S.J.; Souza, R.F. In Esophageal Squamous Cells From Eosinophilic Esophagitis Patients, Th2 Cytokines Increase Eotaxin-3 Secretion Through Effects on Intracellular Calcium and a Non-Gastric Proton Pump. Gastroenterology 2021, 160, 2072–2088.e6. [Google Scholar] [CrossRef]
- Kaneko, T.; Iwamura, C.; Kiuchi, M.; Kurosugi, A.; Onoue, M.; Matsumura, T.; Chiba, T.; Nakayama, T.; Kato, N.; Hirahara, K. Amphiregulin-Producing TH2 Cells Facilitate Esophageal Fibrosis of Eosinophilic Esophagitis. J Allergy Clin Immunol Glob 2024, 3, 100287. [Google Scholar] [CrossRef]
- Shoda, T.; Wen, T.; Caldwell, J.M.; Ben-Baruch Morgenstern, N.; Osswald, G.A.; Rochman, M.; Mack, L.E.; Felton, J.M.; Abonia, J.P.; et al. Loss of Endothelial TSPAN12 Promotes Fibrostenotic Eosinophilic Esophagitis via Endothelial Cell-Fibroblast Crosstalk. Gastroenterology 2022, 162, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.M.; Collins, M.H.; Stucke, E.M.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Rothenberg, M.E. Histologic Eosinophilic Gastritis Is a Systemic Disorder Associated with Blood and Extragastric Eosinophilia, TH2 Immunity, and a Unique Gastric Transcriptome. J Allergy Clin Immunol 2014, 134, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Shoda, T.; Ishimura, N.; Ohta, S.; Ono, J.; Azuma, Y.; Okimoto, E.; Izuhara, K.; Nomura, I.; Matsumoto, K.; Kinoshita, Y. Serum Biomarkers for the Diagnosis of Eosinophilic Esophagitis and Eosinophilic Gastroenteritis. Intern Med 2017, 56, 2819–2825. [Google Scholar] [CrossRef]
- Marasco, G.; Visaggi, P.; Vassallo, M.; Fiocca, M.; Cremon, C.; Barbaro, M.R.; De Bortoli, N.; Bellini, M.; Stanghellini, V.; Savarino, E.V.; Barbara, G. Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases. Int J Mol Sci 2023, 24, 15165. [Google Scholar] [CrossRef]
- Sunkara, T.; Rawla, P.; Yarlagadda, K.S.; Gaduputi, V. Eosinophilic Gastroenteritis: Diagnosis and Clinical Perspectives. Clin Exp Gastroenterol 2019, 12, 239–253. [Google Scholar] [CrossRef]
- Troncone, R.; Discepolo, V. Colon in Food Allergy. J Pediatr Gastroenterol Nutr 2009, 48 Suppl 2, S89–91. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.; Herbert, D.R.; Brombacher, F.; Lopata, A.L. Differential Requirements for Interleukin (IL)-4 and IL-13 in Protein Contact Dermatitis Induced by Anisakis. Allergy 2009, 64, 1309–1318. [Google Scholar] [CrossRef]
- Ogasawara, A.; Yuki, T.; Takai, T.; Yokozeki, K.; Katagiri, A.; Takahashi, Y.; Yokozeki, H.; Basketter, D.; Sakaguchi, H. Epicutaneous Challenge with Protease Allergen Requires Its Protease Activity to Recall TH2 and TH17/TH22 Responses in Mice Pre-sensitized via Distant Skin. J Immunotoxicol 2021, 18, 118–126. [Google Scholar] [CrossRef]
- Ogasawara, A.; Yuki, T.; Katagiri, A.; Lai, Y.T.; Takahashi, Y.; Basketter, D.; Sakaguchi, H. Proteolytic Activity Accelerates the TH17/TH22 Recall Response to an Epicutaneous Protein Allergen-Induced TH2 Response. J Immunotoxicol 2022, 19, 27–33. [Google Scholar] [CrossRef]
- Nowak, D.; Gomułka, K.; Dziemieszonek, P.; Panaszek, B. Systemowe Kontaktowe Zapalenie Skóry [Systemic Contact Dermatitis]. Postepy Hig Med Dosw 2016, 70, 124–134. [Google Scholar] [CrossRef]
- Scott, J.F.; Hammond, M.I.; Nedorost, S.T. Food Avoidance Diets for Dermatitis. Curr Allergy Asthma Rep 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Burden, A.D.; Wilkinson, S.M.; Beck, M.H.; Chalmers, R.J. Garlic-Induced Systemic Contact Dermatitis. Contact Dermatitis 1994, 30, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.K.; Zug, K.A. Systemic Contact Dermatitis to Raw Cashew Nuts in a Pesto Sauce. Am J Contact Dermat 1998, 9, 51–54. [Google Scholar] [PubMed]
- Kothari, R.; Kishore, K.; Sandhu, S.; Bhatnagar, A.; Pal, R.; Chand, S. Systemic Contact Dermatitis to Spices: Report of a Rare Case. Contact Dermatitis 2022, 86, 323–325. [Google Scholar] [CrossRef]
- Paulsen, E. Systemic Allergic Dermatitis Caused by Sesquiterpene Lactones. Contact Dermatitis 2017, 76, 1–10. [Google Scholar] [CrossRef]
- de Groot, A.C. Myroxylon Pereirae Resin (Balsam of Peru) - A Critical Review of the Literature and Assessment of the Significance of Positive Patch Test Reactions and the Usefulness of Restrictive Diets. Contact Dermatitis 2019, 80, 335–353. [Google Scholar] [CrossRef]
- Dooms-Goossens, A.; Dubelloy, R.; Degreef, H. Contact and Systemic Contact-Type Dermatitis to Spices. Dermatol Clin 1990, 8, 89–93. [Google Scholar] [CrossRef]
- Scheman, A.; Rakowski, E.M.; Chou, V.; Chhatriwala, A.; Ross, J.; Jacob, S.E. Balsam of Peru: Past and Future. Dermatitis 2013, 24, 153–160. [Google Scholar] [CrossRef]
- Ahuja, K.; Issa, C.J.; Nedorost, S.T.; Lio, P.A. Is Food-Triggered Atopic Dermatitis a Form of Systemic Contact Dermatitis? Clin Rev Allergy Immunol 2024, 66, 1–13. [Google Scholar] [CrossRef]
- Bhatia, J.; Sarin, A.; Wollina, U.; Lotti, T.; Navarini, A.A.; Mueller, S.M.; Grabbe, S.; Saloga, J.; Rokni, G.R.; Goldust, M. Review of Biologics in Allergic Contact Dermatitis. Contact Dermatitis 2020, 83, 179–181. [Google Scholar] [CrossRef]
- Gimenez-Rivera, V.A.; Patel, H.; Dupuy, F.P.; Allakhverdi, Z.; Bouchard, C.; Madrenas, J.; Bissonnette, R.; Piccirillo, C.A.; Jack, C. NOD2 Agonism Counter-Regulates Human Type 2 T Cell Functions in Peripheral Blood Mononuclear Cell Cultures: Implications for Atopic Dermatitis. Biomolecules 2023, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Mikajiri, R.; Fukunaga, A.; Miyoshi, M.; Maeshige, N.; Washio, K.; Masaki, T.; Nishigori, C.; Yamamoto, I.; Toda, A.; Takahashi, M.; Asahara, S.I.; Kido, Y.; Usami, M. Dietary Intervention for Control of Clinical Symptom in Patients with Systemic Metal Allergy: A Single Center Randomized Controlled Clinical Study. Kobe J Med Sci 2024, 69, E129–E143. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, L.; Arena, A.; Arena, E.; Zambito, M.; Ingrassia, A.; Valenti, G.; Loschiavo, G.; D'Angelo, A.; Saitta, S. Systemic Nickel Allergy Syndrome: Epidemiological Data from Four Italian Allergy Units. Int J Immunopathol Pharmacol 2014, 27, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Veien, N.K. Systemic Contact Dermatitis. Int J Dermatol 2011, 50, 1445–1456. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D. ; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; Nebbia, C.S.; et al. Update of the Risk Assessment of Nickel in Food and Drinking Water. EFSA J 2020, 18, e06268. [Google Scholar] [CrossRef]
- Roach, K.; Roberts, J. A Comprehensive Summary of Disease Variants Implicated in Metal Allergy. J Toxicol Environ Health B Crit Rev 2022, 25, 279–341. [Google Scholar] [CrossRef]
- Sharma, A.D. Low Chromate Diet in Dermatology. Indian J Dermatol 2009, 54, 293–295. [Google Scholar] [CrossRef]
- Tramontana, M.; Bianchi, L.; Hansel, K.; Agostinelli, D.; Stingeni, L. Nickel Allergy: Epidemiology, Pathomechanism, Clinical Patterns, Treatment and Prevention Programs. Endocr Metab Immune Disord Drug Targets 2020, 20, 992–1002. [Google Scholar] [CrossRef]
- Castagnoli, R.; Lougaris, V.; Giardino, G.; Volpi, S.; Leonardi, L.; La Torre, F.; Federici, S.; Corrente, S.; Cinicola, B.L.; Soresina, A.; et al. Immunology Task Force of the Italian Society of Pediatric Allergy and Immunology (SIAIP). Inborn Errors of Immunity with Atopic Phenotypes: A Practical Guide for Allergists. World Allergy Organ J 2021, 14, 100513. [Google Scholar] [CrossRef]
- Diaz-Cabrera, N.M.; Bauman, B.M.; Iro, M.A.; Dabbah-Krancher, G.; Molho-Pessach, V.; Zlotogorski, A.; Shamriz, O.; Dinur-Schejter, Y.; Sharon, T.D.; Stepensky, P.; et al. Management of Atopy with Dupilumab and Omalizumab in CADINS Disease. J Clin Immunol 2024, 44, 48. [Google Scholar] [CrossRef]
- Vaseghi-Shanjani, M.; Smith, K.L.; Sara, R.J.; Modi, B.P.; Branch, A.; Sharma, M.; Lu, H.Y.; James, E.L.; Hildebrand, K.J.; Biggs, C.M.; Turvey, S.E. Inborn Errors of Immunity Manifesting as Atopic Disorders. J Allergy Clin Immunol 2021, 148, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Su, H.C. Insights into the Pathogenesis of Allergic Disease from Dedicator of Cytokinesis 8 Deficiency. Curr Opin Immunol 2023, 80, 102277. [Google Scholar] [CrossRef] [PubMed]
- Barbieux, C.; Bonnet des Claustres, M.; de la Brassinne, M.; Bricteux, G.; Bagot, M.; Bourrat, E.; Hovnanian, A. Duality of Netherton Syndrome Manifestations and Response to Ixekizumab. J Am Acad Dermatol 2021, 84, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Hannula-Jouppi, K.; Laasanen, S.L.; Heikkilä, H.; Tuomiranta, M.; Tuomi, M.L.; Hilvo, S.; Kluger, N.; Kivirikko, S.; Hovnanian, A.; Mäkinen-Kiljunen, S.; et al. IgE Allergen Component-Based Profiling and Atopic Manifestations in Patients with Netherton Syndrome. J Allergy Clin Immunol 2014, 134, 985–988. [Google Scholar] [CrossRef]
- Paluel-Marmont, C.; Bellon, N.; Barbet, P.; Leclerc-Mercier, S.; Hadj-Rabia, S.; Dupont, C.; Bodemer, C. Eosinophilic Esophagitis and Colonic Mucosal Eosinophilia in Netherton Syndrome. J Allergy Clin Immunol 2017, 139, 2003–2005.e1. [Google Scholar] [CrossRef]
- Prodinger, C.; Yerlett, N.; MacDonald, C.; Chottianchaiwat, S.; Goh, L.; Du Toit, G.; Mellerio, J.E.; Petrof, G.; Martinez, A.E. Prevalence of and Risk Factors for Nutritional Deficiency and Food Allergy in a Cohort of 21 Patients with Netherton Syndrome. Pediatr Allergy Immunol 2023, 34, e13914. [Google Scholar] [CrossRef]
- Stuvel, K.; Heeringa, J.J.; Dalm, V.A.S.H.; Meijers, R.W.J.; van Hoffen, E.; Gerritsen, S.A.M.; van Zelm, M.C.; Pasmans, S.G.M.A. Comel-Netherton Syndrome: A Local Skin Barrier Defect in the Absence of an Underlying Systemic Immunodeficiency. Allergy 2020, 75, 1710–1720. [Google Scholar] [CrossRef]
- Abonia, J.P.; Wen, T.; Stucke, E.M.; Grotjan, T.; Griffith, M.S.; Kemme, K.A.; Collins, M.H.; Putnam, P.E.; Franciosi, J.P.; von Tiehl, K.F.; Tinkle, B.T.; Marsolo, K.A.; Martin, L.J.; Ware, S.M.; Rothenberg, M.E. High Prevalence of Eosinophilic Esophagitis in Patients with Inherited Connective Tissue Disorders. J Allergy Clin Immunol 2013, 132, 378–386. [Google Scholar] [CrossRef]
- O'Shea, K.M.; Aceves, S.S.; Dellon, E.S.; Gupta, S.K.; Spergel, J.M.; Furuta, G.T.; Rothenberg, M.E. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 333–345. [Google Scholar] [CrossRef]
- Lyons, J.J.; Liu, Y.; Ma, C.A.; Yu, X.; O'Connell, M.P.; Lawrence, M.G.; Zhang, Y.; Karpe, K.; Zhao, M.; Siegel, A.M.; et al. ERBIN Deficiency Links STAT3 and TGF-β Pathway Defects with Atopy in Humans. J Exp Med 2017, 214, 669–680. [Google Scholar] [CrossRef]
- Chaudhary, F.; Agrawal, D.K. Ethnic and Racial Disparities in Clinical Manifestations of Atopic Dermatitis. Arch Intern Med Res 2024, 7, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Savva, M.; Papadopoulos, N.G.; Gregoriou, S.; Katsarou, S.; Papapostolou, N.; Makris, M.; Xepapadaki, P. Recent Advancements in the Atopic Dermatitis Mechanism. Front Biosci 2024, 29, 84. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Guttman-Yassky, E.; Pawlikowski, J.; Ghorayeb, E.G.; Ota, T.; Lebwohl, M.G. Interleukin-1α Inhibitor Bermekimab in Patients with Atopic Dermatitis: Randomized and Nonrandomized Studies. Arch Dermatol Res 2024, 316, 589. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous Sensitization in the Development of Food Allergy: What is the Evidence and How Can This Be Prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef]
- Graham, F. , Eigenmann PA. Atopic dermatitis and its relation to food allergy. Curr Opin Allergy Clin Immunol. 2020, 20, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Hervé, P.L.; Dioszeghy, V.; Matthews, K.; Bee, K.J.; Campbell, D.E.; Sampson, H.A. Recent Advances in Epicutaneous Immunotherapy and Potential Applications in Food Allergy. Front Allergy 2023, 4, 1290003. [Google Scholar] [CrossRef]
- Singh, A.M.; Anvari, S.; Hauk, P.; Lio, P.; Nanda, A.; Sidbury, R.; Schneider, L. Atopic Dermatitis and Food Allergy: Best Practices and Knowledge Gaps—A Work Group Report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract 2022, 10, 697–706. [Google Scholar] [CrossRef]
- Dubin, C.; Del Duca, E.; Guttman-Yassky, E. The IL-4, IL-13 and IL-31 Pathways in Atopic Dermatitis. Expert Rev Clin Immunol 2021, 17, 835–852. [Google Scholar] [CrossRef]
- Huang IH, Chung WH, Wu PC, Chen CB. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agache, I.; Song, Y.; Posso, M.; Alonso-Coello, P.; Rocha, C.; Solà, I.; Beltran, J.; Akdis, C.A.; Akdis, M.; Brockow, K.; et al. Efficacy and safety of dupilumab for moderate-to-severe atopic dermatitis: A systematic review for the EAACI biologicals guidelines. Allergy 2021, 76, 45–58. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A.; Akdis, M.; Brockow, K.; Chivato, T.; Del Giacco, S.; Eiwegger, T.; Eyerich, K.; Giménez-Arnau, A.; Gutermuth, J.; et al. EAACI Biologicals Guidelines—dupilumab for children and adults with moderate-to-severe atopic dermatitis. Allergy 2021, 76, 988–1009. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Welchowski, T.; Herrmann, N.; Brauer, J.; Traidl-Hoffmann, C.; Havenith, R.; Müller, S.; Rhyner, C.; Dreher, A.; Schmid, M.; Bieber, T. ; CK-CARE study group. IL-13, periostin and dipeptidyl-peptidase-4 reveal endotype-phenotype associations in atopic dermatitis. Allergy 2023, 17 January. [CrossRef]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and immunotherapy of inflammatory skin diseases. Immune Netw 2022, 22, e7. [Google Scholar] [CrossRef] [PubMed]
- de Wijs, L.E.M.; Nguyen, N.T.; Kunkeler, A.C.M.; Nijsten, T.; Damman, J.; Hijnen, D.J. Clinical and histopathological characterization of paradoxical head and neck erythema in patients with atopic dermatitis treated with dupilumab: A case series. Br J Dermatol 2020, 183, 745–749. [Google Scholar] [CrossRef]
- Glatz, M.; Bossard, P.P.; Hoetzenecker, W.; Schmid-Grendelmeier, P. The role of Malassezia spp. in atopic dermatitis. J Clin Med 2015, 4, 1217–1228. [Google Scholar] [CrossRef]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol Int 2022, 71, 14–24. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.; Aggelidis, X.; Augustin, M.; Balan, R.G.; Bangert, C.; Beck, L.; Bieber, T.; Bernstein, J.A.; Bertolin Colilla, M.; et al. A concept for integrated care pathways for atopic dermatitis—A GA2LEN ADCARE initiative. Clin Transl Allergy 2023, 13, e12299. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Calatroni, A.; Zaramela, L.S.; LeBeau, P.K.; Dyjack, N.; Brar, K.; David, G.; Johnson, K.; Leung, S.; Ramirez-Gama, M.; et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med 2019, 11, eaav2685. [Google Scholar] [CrossRef]
- Bousquet, J.; Melén, E.; Haahtela, T.; Koppelman, G.H.; Togias, A.; Valenta, R.; Akdis, C.A.; Czarlewski, W.; Rothenberg, M.; Valiulis, A.; et al. Rhinitis associated with asthma is distinct from rhinitis alone: The ARIA-MeDALL hypothesis. Allergy 2023, 78, 1169–1203. [Google Scholar] [CrossRef]
- Davis, K.L.; Claudio-Etienne, E.; Frischmeyer-Guerrerio, P.A. Atopic dermatitis and food allergy: More than sensitization. Mucosal Immunol, 2024; S1933-021900059-X. [Google Scholar] [CrossRef]
- Sernicola, A.; Amore, E.; Rizzuto, G.; Rallo, A.; Greco, M.E.; Battilotti, C.; Svara, F.; Azzella, G.; Nisticò, S.P.; Dattola, A.; Chello, C.; Pellacani, G.; Grieco, T. Dupilumab as Therapeutic Option in Polysensitized Atopic Dermatitis Patients Suffering from Food Allergy. Nutrients 2024, 16, 2797. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Caubet, J.C.; Boguniewicz, M.; Eigenmann, P.A. Evaluation of food allergy in patients with atopic dermatitis. J Allergy Clin Immunol Pract 2013, 1, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Breuer, K.; Heratizadeh, A.; Wulf, A.; Baumann, U.; Constien, A.; Tetau, D.; Kapp, A.; Werfel, T. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy 2004, 34, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Manam, S.; Tsakok, T.; Till, S.; Flohr, C. The association between atopic dermatitis and food allergy in adults. Curr Opin Allergy Clin Immunol 2014, 14, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Papapostolou, N.; Xepapadaki, P.; Gregoriou, S.; Makris, M. Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn. J Clin Med 2022, 11, 4232. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Breuer, K. Role of food allergy in atopic dermatitis. Curr Opin Allergy Clin Immunol 2004, 4, 379–385. [Google Scholar] [CrossRef]
- Dębińska, A.; Sozańska, B. Epicutaneous sensitization and food allergy: preventive strategies targeting skin barrier repair—facts and challenges. Nutrients 2023, 15, 1070. [Google Scholar] [CrossRef]
- Werfel, T.; Ballmer-Weber, B.; Eigenmann, P.A.; Niggemann, B.; Rancé, F.; Turjanmaa, K.; Worm, M. Eczematous reactions to food in atopic eczema: position paper of the EAACI and GA2LEN. Allergy 2007, 62, 723–728. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hirasawa, N.; Asakawa, S.; Okita, K.; Tokura, Y. Intrinsic atopic dermatitis shows high serum nickel concentration. Allergol Int 2015, 64, 282–284. [Google Scholar] [CrossRef]
- Glocova, I.; Brück, J.; Geisel, J.; Müller-Hermelink, E.; Widmaier, K.; Yazdi, A.S.; Röcken, M.; Ghoreschi, K. Induction of skin-pathogenic Th22 cells by epicutaneous allergen exposure. J Dermatol Sci 2017, 87, 268–277. [Google Scholar] [CrossRef]
- Tsuge, I.; Kondo, Y.; Tokuda, R.; Kakami, M.; Kawamura, M.; Nakajima, Y.; Komatsubara, R.; Yamada, K.; Urisu, A. Allergen-specific helper T cell response in patients with cow's milk allergy: Simultaneous analysis of proliferation and cytokine production by carboxyfluorescein succinimidyl ester dilution assay. Clin Exp Allergy 2006, 36, 1538–1545. [Google Scholar] [CrossRef]
- Noh, J.; Noh, G. Allergen-specific responses of CD19(high) and CD19(low) B Cells in Non-IgE-mediated food allergy of late eczematous reactions in atopic dermatitis: presence of IL-17- and IL-32-producing regulatory B cells (Br17 & Br32). Inflamm Allergy Drug Targets 2012, 11, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Noh, G.; Kim, H.S.; Kim, A.R.; Choi, W.S. Allergen-specific responses of CD19(+)CD5(+)Foxp3(+) regulatory B cells (Bregs) and CD4(+)Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cell Immunol 2012, 274, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sütas, Y.; Kekki, O.M.; Isolauri, E. Late onset reactions to oral food challenge are linked to low serum interleukin-10 concentrations in patients with atopic dermatitis and food allergy. Clin Exp Allergy 2000, 30, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Abernathy-Carver, K.J.; Sampson, H.A.; Picker, L.J.; Leung, D.Y. Milk-induced eczema is associated with the expansion of T cells expressing cutaneous lymphocyte antigen. J Clin Invest 1995, 95, 913–918. [Google Scholar] [CrossRef]
- Nicolàs, L.S.S.; Czarnowicki, T.; Akdis, M.; Pujol, R.M.; Lozano-Ojalvo, D.; Leung, D.Y.M.; Guttman-Yassky, E.; Santamaria-Babí, L.F. CLA+ memory T cells in atopic dermatitis. Allergy 2024, 79, 15–25. [Google Scholar] [CrossRef]
- Glickman, J.W.; Han, J.; Garcet, S.; Krueger, J.G.; Pavel, A.B.; Guttman-Yassky, E. Improving evaluation of drugs in atopic dermatitis by combining clinical and molecular measures. J Allergy Clin Immunol Pract 2020, 8, 3622–3625.e19. [Google Scholar] [CrossRef]
- Homey, B.; Alenius, H.; Müller, A.; Soto, H.; Bowman, E.P.; Yuan, W.; McEvoy, L.; Lauerma, A.I.; Assmann, T.; Bünemann, E.; et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med 2002, 8, 157–165. [Google Scholar] [CrossRef]
- Leonard, A.; Wang, J.; Yu, L.; Liu, H.; Estrada, Y.; Greenlees, L.; McPhee, R.; Ruzin, A.; Guttman-Yassky, E.; Howell, M.D. Atopic Dermatitis Endotypes Based on Allergen Sensitization, Reactivity to Staphylococcus aureus Antigens, and Underlying Systemic Inflammation. J Allergy Clin Immunol Pract 2020, 8, 236–247.e3. [Google Scholar] [CrossRef]
- Acevedo, N.; Benfeitas, R.; Katayama, S.; Bruhn, S.; Andersson, A.; Wikberg, G.; Lundeberg, L.; Lindvall, J.M.; Greco, D.; Kere, J.; et al. Epigenetic alterations in skin homing CD4+CLA+ T cells of atopic dermatitis patients. Sci Rep 2020, 10, 18020. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Malajian, D.; Shemer, A.; Fuentes-Duculan, J.; Gonzalez, J.; Suárez-Fariñas, M.; Krueger, J.G.; Guttman-Yassky, E. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol 2015, 136, 208–211. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Sans-De San Nicolàs, L.; Figueras-Nart, I.; Bonfill-Ortí, M.; De Jesús-Gil, C.; García-Jiménez, I.; Guilabert, A.; Curto-Barredo, L.; Bertolín-Colilla, M.; Ferran, M.; Serra-Baldrich, E.; et al. SEB-induced IL-13 production in CLA+ memory T cells defines Th2 high and Th2 low responders in atopic dermatitis. Allergy 2022, 77, 3448–3451. [Google Scholar] [CrossRef] [PubMed]
- Soul, J.; Carlsson, E.; Hofmann, S.R.; Russ, S.; Hawkes, J.; Schulze, F.; Sergon, M.; Pablik, J.; Abraham, S.; Hedrich, C.M. Tissue gene expression profiles and communication networks inform candidate blood biomarker identification in psoriasis and atopic dermatitis. Clin Immunol 2024, 265, 110283. [Google Scholar] [CrossRef]
- Chang, S.H.; Dong, C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal 2011, 23, 1069–1075. [Google Scholar] [CrossRef]
- Herberth, G.; Daegelmann, C.; Röder, S.; Behrendt, H.; Krämer, U.; Borte, M.; Heinrich, J.; Herbarth, O.; Lehmann, I.; LISAplus study group. IL-17E but not IL-17A is associated with allergic sensitization: results from the LISA study. Pediatr Allergy Immunol 2010, 21, 1086–1090. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Fluhr, J.W.; Ruwwe-Glösenkamp, C.; Stevanovic, K.; Bergmann, K.C.; Zuberbier, T. Role of IL-17 in atopy—A systematic review. Clin Transl Allergy 2021, 11, e12047. [Google Scholar] [CrossRef]
- Żbikowska-Gotz, M.; Pałgan, K.; Gawrońska-Ukleja, E.; Kuźmiński, A.; Przybyszewski, M.; Socha, E.; Bartuzi, Z. Expression of IL-17A concentration and effector functions of peripheral blood neutrophils in food allergy hypersensitivity patients. Int J Immunopathol Pharmacol 2016, 29, 90–98. [Google Scholar] [CrossRef]
- Berin, M.C.; Lozano-Ojalvo, D.; Agashe, C.; Baker, M.G.; Bird, J.A.; Nowak-Wegrzyn, A. Acute FPIES reactions are associated with an IL-17 inflammatory signature. J Allergy Clin Immunol. 2021, 148, 895–901.e6. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Chen, X.; Dunkin, D.; Agashe, C.; Baker, M.G.; Bird, J.A.; Molina, E.; Nowak-Wegrzyn, A.; Berin, M.C. Untargeted serum metabolomic analysis reveals a role for purinergic signaling in FPIES. J Allergy Clin Immunol. 2023, 151, 797–802. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, P.; Anvari, S.; Barrachina, J.; Portillo, A.L.J.; Jimenez, T.; Marco de la Calle, F.M.; Fernandez, J. Egg-induced adult food protein-induced enterocolitis syndrome: Clinical phenotypes, natural history and immunological characteristics. J Allergy Clin Immunol Pract. 2024, 12, 1657–1659. [Google Scholar] [CrossRef]
- Fina, D.; Sarra, M.; Caruso, R.; Del Vecchio Blanco, G.; Pallone, F.; MacDonald, T.T.; Monteleone, G. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 2008, 57, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Omidian, Z.; Ahmed, R.; Giwa, A.; Donner, T.; Hamad, A.R.A. IL-17 and limits of success. Cell Immunol 2019, 339, 33–40. [Google Scholar] [CrossRef]
- Ortega, C.; Fernández, S.; Estévez, O.A.; Aguado, R.; Molina, I.J.; Santamaría, M. IL-17 producing T cells in celiac disease: angels or devils? Int Rev Immunol 2013, 32, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, V.; Sandström, O.; Hedberg, M.; Hammarström, S.; Hernell, O.; Hammarström, M.L. Intestinal T-cell responses in celiac disease - impact of celiac disease associated bacteria. PLoS One 2013, 8, e53414. [Google Scholar] [CrossRef]
- Cicerone, C.; Nenna, R.; Pontone, S. Th17, intestinal microbiota and the abnormal immune response in the pathogenesis of celiac disease. Gastroenterol Hepatol Bed Bench 2015, 8, 117–122. [Google Scholar] [PubMed]
- Lahdenperä, A.I.; Fälth-Magnusson, K.; Högberg, L.; Ludvigsson, J.; Vaarala, O. Expression pattern of T-helper 17 cell signaling pathway and mucosal inflammation in celiac disease. Scand J Gastroenterol 2014, 49, 145–156. [Google Scholar] [CrossRef]
- Madi, M.; Abdelsalam, M.; Elakel, A.; Zakaria, O.; AlGhamdi, M.; Alqahtani, M.; AlMuhaish, L.; Farooqi, F.; Alamri, T.A.; Alhafid, I.A.; Alzahrani, I.M.; Alam, A.H.; Alhashmi, M.T.; Alasseri, I.A.; AlQuorain, A.A. Salivary interleukin-17A and interleukin-18 levels in patients with celiac disease and periodontitis. PeerJ 2024, 12, e17374. [Google Scholar] [CrossRef]
- Caproni, M.; Capone, M.; Rossi, M.C.; Santarlasci, V.; Maggi, L.; Mazzoni, A.; Rossettini, B.; Renzi, D.; Quintarelli, L.; Bianchi, B.; et al. T Cell Response Toward Tissue-and Epidermal-Transglutaminases in Coeliac Disease Patients Developing Dermatitis Herpetiformis. Front Immunol 2021, 12, 645143. [Google Scholar] [CrossRef]
- Collin, P.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Reunala, T. Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Ann Med 2017, 49, 23–31. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; Crespo, L.; Planella, M.; Farrais, S.; Izquierdo, S.; López-Palacios, N.; Roy, G.; Vidal, J.; Núñez, C. Improving the Diagnosis of Dermatitis Herpetiformis Using the Intraepithelial Lymphogram. Nutrients 2024, 16, 232. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Kim, S.J. Dermatitis Herpetiformis: An Update on Diagnosis, Disease Monitoring, and Management. Medicina (Kaunas) 2021, 57, 843. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.; Shahid, M.; Ivanova-Todorova, E.; Drenovska, K.; Tumangelova-Yuzeir, K.; Altankova, I.; Vassileva, S. Celiac-Related Autoantibodies and IL-17A in Bulgarian Patients with Dermatitis Herpetiformis: A Cross-Sectional Study. Medicina 2019, 55, 136. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.S.; Nierkens, S.; Knol, E.F.; Giovannone, B.; Delemarre, E.M.; van der Schaft, J.; van Wijk, F.; de Bruin-Weller, M.S.; Drylewicz, J.; Thijs, J.L. Confirmation of multiple endotypes in atopic dermatitis based on serum biomarkers. J Allergy Clin Immunol 2021, 147, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Sarig, O.; Sun, G.; Samuelov, L.; Ma, C.A.; Zhang, Y.; Dimaggio, T.; Nelson, C.G.; Stone, K.D.; Freeman, A.F.; et al. Loss-of-function mutations in caspase recruitment domain-containing protein 14 (CARD14) are associated with a severe variant of atopic dermatitis. J Allergy Clin Immunol 2019, 143, 173–181.e10. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tsai, T.F. Overlapping Features of Psoriasis and Atopic Dermatitis: From Genetics to Immunopathogenesis to Phenotypes. Int J Mol Sci 2022, 23, 5518. [Google Scholar] [CrossRef]
- Goh, M.S.; Yun, J.S.; Su, J.C. Management of atopic dermatitis: a narrative review. Med J Aust 2022, 216, 587–593. [Google Scholar] [CrossRef]
- Laska, J.; Tota, M.; Łacwik, J.; Sędek, Ł.; Gomułka, K. IL-22 in Atopic Dermatitis. Cells 2024, 13, 1398. [Google Scholar] [CrossRef]
- Sugaya, M. The Role of Th17-Related Cytokines in Atopic Dermatitis. Int J Mol Sci 2020, 21, 1314. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Rodriguez, E.; Stölzl, D.; Wehkamp, U.; Sun, J.; Gerdes, S.; Sarkar, M.K.; Hübenthal, M.; Zeng, C.; Uppala, R.; et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J Allergy Clin Immunol 2020, 145, 1406–1415. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int J Mol Sci 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Honda, T.; Kabashima, K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int Immunol 2018, 30, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Giancotta, C.; Colantoni, N.; Pacillo, L.; Santilli, V.; Amodio, D.; Manno, E.C.; Cotugno, N.; Rotulo, G.A.; Rivalta, B.; Finocchi, A.; Cancrini, C.; Diociaiuti, A.; El Hachem, M.; Zangari, P. Tailored treatments in inborn errors of immunity associated with atopy (IEIs-A) with skin involvement. Front Pediatr 2023, 11, 1129249. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, I.; Knöpfel, N.; Theiler, M.; Bonnet des Claustres, M.; Barbieux, C.; Schwieger-Briel, A.; Brunner, C.; Donghi, D.; Buettcher, M.; Meier-Schiesser, B.; Hovnanian, A.; Weibel, L. Secukinumab Therapy for Netherton Syndrome. JAMA Dermatol 2020, 156, 907–911. [Google Scholar] [CrossRef]
- Pontone, M.; Giovannini, M.; Filippeschi, C.; Oranges, T.; Pedaci, F.A.; Mori, F.; Barni, S.; Barbati, F.; Consonni, F.; Indolfi, G.; Lodi, L.; Azzari, C.; Ricci, S.; Hovnanian, A. Biological treatments for pediatric Netherton syndrome. Front Pediatr 2022, 10, 1074243. [Google Scholar] [CrossRef]
- Christensen, M.J.; Eller, E.; Kjaer, H.F.; Broesby-Olsen, S.; Mortz, C.G.; Bindslev-Jensen, C. Exercise-induced anaphylaxis: causes, consequences, and management recommendations. Expert Rev Clin Immunol 2019, 15, 265–273. [Google Scholar] [CrossRef]
- Morita, E.; Chinuki, Y.; Kohno, K.; Matsuo, H. Cofactors of wheat-dependent exercise-induced anaphylaxis increase gastrointestinal gliadin absorption by an inhibition of prostaglandin production. Clin Exp Allergy 2023, 53, 359–361. [Google Scholar] [CrossRef]
- Shin, M. Food allergies and food-induced anaphylaxis: role of cofactors. Clin Exp Pediatr 2021, 64, 393–399. [Google Scholar] [CrossRef]
- Ferrier, L.; Bérard, F.; Debrauwer, L.; Chabo, C.; Langella, P.; Buéno, L.; Fioramonti, J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol 2006, 168, 1148–1154. [Google Scholar] [CrossRef]
- Feuille, E.; Nowak-Węgrzyn, A. Food Protein-Induced Enterocolitis Syndrome, Allergic Proctocolitis, and Enteropathy. Curr Allergy Asthma Rep 2015, 15, 50. [Google Scholar] [CrossRef]
- Banerjee, A.; Nobleza, K.; Haddad, C.; Eubanks, J.; Rana, R.; Rider, N.L.; Pompeii, L.; Nguyen, D.; Anvari, S. Applying Market Basket Analysis to Determine Complex Coassociations Among Food Allergens in Children With Food Protein-Induced Enterocolitis Syndrome (FPIES). Health Serv Res Manag Epidemiol 2024, 11, 23333928241264020. [Google Scholar] [CrossRef] [PubMed]
- Labrosse, R.; Graham, F.; Caubet, J.C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C.; Lozano-Ojalvo, D.; Agashe, C.; Baker, M.G.; Bird, J.A.; Nowak-Wegrzyn, A. Acute FPIES reactions are associated with an IL-17 inflammatory signature. J Allergy Clin Immunol 2021, 148, 895–901.e6. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Chen, X.; Dunkin, D.; Agashe, C.; Baker, M.G.; Bird, J.A.; Molina, E.; Nowak-Wegrzyn, A.; Berin, M.C. Untargeted serum metabolomic analysis reveals a role for purinergic signaling in FPIES. J Allergy Clin Immunol 2023, 151, 797–802. [Google Scholar] [CrossRef]
- Rizzi, A.; Lo Presti, E.; Chini, R.; Gammeri, L.; Inchingolo, R.; Lohmeyer, F.M.; Nucera, E.; Gangemi, S. Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications. J Clin Med 2023, 12, 2699. [Google Scholar] [CrossRef]
- Wada, T.; Matsuda, Y.; Toma, T.; Koizumi, E.; Okamoto, H.; Yachie, A. Increased CD69 Expression on Peripheral Eosinophils from Patients with Food Protein-Induced Enterocolitis Syndrome. Int Arch Allergy Immunol 2016, 170, 201–205. [Google Scholar] [CrossRef]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Blanco-Pérez, F.; Infante, S.; Zubeldia, J.M.; Pérez-Gordo, M. Non-IgE-Mediated Gastrointestinal Food Protein-Induced Allergic Disorders: Clinical Perspectives and Analytical Approaches. Foods 2021, 10, 2662. [Google Scholar] [CrossRef]
- Koksal, B.T.; Barıs, Z.; Sencelikel, T.; Ozcay, F.; Ozbek, O.Y. Food Protein-Induced Allergic Proctocolitis in Infants Is Associated with Low Serum Levels of Macrophage Inflammatory Protein-3a. J Pediatr Gastroenterol Nutr 2024, 78, 211–216. [Google Scholar] [CrossRef]
- Tsabouri, S.; Nicolaou, N.; Douros, K.; Papadopoulou, A.; Priftis, K.N. Food Protein Induced Proctocolitis: A Benign Condition with an Obscure Immunologic Mechanism. Endocr Metab Immune Disord Drug Targets 2017, 17, 32–37. [Google Scholar] [CrossRef]
- de Boer, J.; Deb, C.; Bornstein, J.; Horvath, K.; Mehta, D.; Smadi, Y. Using Eosinophil Biomarkers from Rectal Epithelial Samples to Diagnose Food Protein-Induced Proctocolitis: A Pilot Study. J Pediatr Gastroenterol Nutr 2020, 71, e109–e112. [Google Scholar] [CrossRef]
- Rycyk, A.; Cudowska, B.; Lebensztejn, D.M. Eosinophil-Derived Neurotoxin, Tumor Necrosis Factor Alpha, and Calprotectin as Non-Invasive Biomarkers of Food Protein-Induced Allergic Proctocolitis in Infants. J Clin Med 2020, 9, 3147. [Google Scholar] [CrossRef] [PubMed]
- Jauregi-Miguel, A. The Tight Junction and the Epithelial Barrier in Coeliac Disease. Int Rev Cell Mol Biol 2021, 358, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Sowińska, A.; Morsy, Y.; Czarnowska, E.; Oralewska, B.; Konopka, E.; Woynarowski, M.; Szymańska, S.; Ejmont, M.; Scharl, M.; Bierła, J.B.; Wawrzyniak, M.; Cukrowska, B. Transcriptional and Ultrastructural Analyses Suggest Novel Insights into Epithelial Barrier Impairment in Celiac Disease. Cells 2020, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Taraz, T.; Mahmoudi-Ghehsareh, M.; Asri, N.; Nazemalhosseini-Mojarad, E.; Rezaei-Tavirani, M.; Jahani-Sherafat, S.; Naseh, A.; Rostami-Nejad, M. Overview of the Compromised Mucosal Integrity in Celiac Disease. J Mol Histol 2024, 55, 15–24. [Google Scholar] [CrossRef]
- Matysiak-Budnik, T.; Moura, I.C.; Arcos-Fajardo, M.; Lebreton, C.; Ménard, S.; Candalh, C.; Ben-Khalifa, K.; Dugave, C.; Tamouza, H.; van Niel, G.; et al. Secretory IgA Mediates Retrotranscytosis of Intact Gliadin Peptides via the Transferrin Receptor in Celiac Disease. J Exp Med 2008, 205, 143–154. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Morselli-Labate, A.M.; Di Sabatino, A.; Giuffrida, P.; Corazza, G.R.; Di Stefano, M.; Caio, G.; Latella, G.; Ciacci, C.; et al. Serum Zonulin and Its Diagnostic Performance in Non-Coeliac Gluten Sensitivity. Gut 2020, 69, 1966–1974. [Google Scholar] [CrossRef]
- Rotondi Aufiero, V.; Fasano, A.; Mazzarella, G. Non-Celiac Gluten Sensitivity: How Its Gut Immune Activation and Potential Dietary Management Differ from Celiac Disease. Mol Nutr Food Res 2018, 62, e1700854. [Google Scholar] [CrossRef]
- Sapone, A.; Lammers, K.M.; Mazzarella, G.; Mikhailenko, I.; Cartenì, M.; Casolaro, V.; Fasano, A. Differential Mucosal IL-17 Expression in Two Gliadin-Induced Disorders: Gluten Sensitivity and the Autoimmune Enteropathy Celiac Disease. Int Arch Allergy Immunol 2010, 152, 75–80. [Google Scholar] [CrossRef]
- Zingone, F.; Bertin, L.; Maniero, D.; Palo, M.; Lorenzon, G.; Barberio, B.; Ciacci, C.; Savarino, E.V. Myths and Facts about Food Intolerance: A Narrative Review. Nutrients 2023, 15, 4969. [Google Scholar] [CrossRef]
- Kleuskens, M.T.A.; Bek, M.K.; Al Halabi, Y.; Blokhuis, B.R.J.; Diks, M.A.P.; Haasnoot, M.L.; Garssen, J.; Bredenoord, A.J.; van Esch, B.C.A.M.; Redegeld, F.A. Mast Cells Disrupt the Function of the Esophageal Epithelial Barrier. Mucosal Immunol 2023, 16, 567–577. [Google Scholar] [CrossRef]
- Pyon, G.C.; Masuda, M.Y.; Putikova, A.; Luo, H.; Gibson, J.B.; Dao, A.D.; Ortiz, D.R.; Heiligenstein, P.L.; Bonellos, J.J.; LeSuer, W.E.; Pai, R.K.; Garg, S.; Rank, M.A.; Nakagawa, H.; Kita, H.; Wright, B.L.; Doyle, A.D. Tissue-Specific Inducible IL-33 Expression Elicits Features of Eosinophilic Esophagitis. J Allergy Clin Immunol 2024, S0091-6749, 00910–00912. [CrossRef]
- Simon, D.; Page, B.; Vogel, M.; Bussmann, C.; Blanchard, C.; Straumann, A.; Simon, H.U. Evidence of an Abnormal Epithelial Barrier in Active, Untreated and Corticosteroid-Treated Eosinophilic Esophagitis. Allergy 2018, 73, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Antolín-Amérigo, D.; Popescu, F.D.; Jutel, M. Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma. Int J Mol Sci 2023, 24, 12725. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.M.; Lenehan, P.J.; Vimalathas, P.; Miller, K.C.; Valencia-Yang, M.; Qiang, L.; Canha, L.A.; Ali, L.R.; Dougan, M.; Garber, J.J.; Dougan, S.K. Tissue Eosinophils Express the IL-33 Receptor ST2 and Type 2 Cytokines in Patients with Eosinophilic Esophagitis. Allergy 2022, 77, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Litosh, V.A.; Rochman, M.; Rymer, J.K.; Porollo, A.; Kottyan, L.C.; Rothenberg, M.E. Calpain-14 and Its Association with Eosinophilic Esophagitis. J Allergy Clin Immunol 2017, 139, 1762–1771.e7. [Google Scholar] [CrossRef]
- McAleer, M.A.; Pohler, E.; Smith, F.J.; Wilson, N.J.; Cole, C.; MacGowan, S.; Koetsier, J.L.; Godsel, L.M.; Harmon, R.M.; Gruber, R.; et al. Severe Dermatitis, Multiple Allergies, and Metabolic Wasting Syndrome Caused by a Novel Mutation in the N-terminal Plakin Domain of Desmoplakin. J Allergy Clin Immunol 2015, 136, 1268–1276. [Google Scholar] [CrossRef]
- Pan, C.; Zhao, A.; Li, M. Atopic Dermatitis-like Genodermatosis: Disease Diagnosis and Management. Diagnostics (Basel) 2022, 12, 2177. [Google Scholar] [CrossRef]
- Ogrodowczyk, A.; Markiewicz, L.; Wróblewska, B. Mutations in the Filaggrin Gene and Food Allergy. Prz Gastroenterol 2014, 9, 200–207. [Google Scholar] [CrossRef]
- Irvine, A.D.; McLean, W.H.; Leung, D.Y. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011, 365, 1315–1327. [Google Scholar] [CrossRef]
- Tham, E.H.; Leung, D.Y. Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. Allergy Asthma Immunol Res 2019, 11, 4–15. [Google Scholar] [CrossRef]
- Leonard, A.; Guttman-Yassky, E. The Unique Molecular Signatures of Contact Dermatitis and Implications for Treatment. Clin Rev Allergy Immunol 2019, 56, 1–8. [Google Scholar] [CrossRef]
- Sashikawa-Kimura, M.; Takada, M.; Hossain, M.R.; Tsuda, H.; Xie, X.; Komine, M.; Ohtsuki, M.; Imokawa, G. Overexpression of the β-Subunit of Acid Ceramidase in the Epidermis of Mice Provokes Atopic Dermatitis-like Skin Symptoms. Int J Mol Sci 2024, 25, 8737. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am J Physiol Cell Physiol 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, O.; Moreno-Corona, N.C.; Cruz-Holguin, V.J.; Garcia-Gonzalez, L.D.; Helguera-Repetto, A.C.; Romero-Valdovinos, M.; Arevalo-Romero, H.; Cedillo-Barron, L.; León-Juárez, M. The Immune Response in Adipocytes and Their Susceptibility to Infection: A Possible Relationship with Infectobesity. Int J Mol Sci 2022, 23, 6154. [Google Scholar] [CrossRef] [PubMed]
- Morąg, B.; Kozubek, P.; Gomułka, K. Obesity and Selected Allergic and Immunological Diseases—Etiopathogenesis, Course and Management. Nutrients 2023, 15, 3813. [Google Scholar] [CrossRef]
- Zhang, P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients 2023, 15, 3683. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Poto, R.; Fusco, W.; Rinninella, E.; Cintoni, M.; Kaitsas, F.; Raoul, P.; Caruso, C.; Mele, M.C.; Varricchi, G.; Gasbarrini, A. The Role of Gut Microbiota and Leaky Gut in the Pathogenesis of Food Allergy. Nutrients 2023, 16, 92. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; Puel, A.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Ichikawa, M.; Lyons, J.J.; Datta, S.; Lamborn, I.T.; Jing, H.; Kim, E.S.; Biancalana, M.; Wolfe, L.A.; et al. Autosomal Recessive Phosphoglucomutase 3 (PGM3) Mutations Link Glycosylation Defects to Atopy, Immune Deficiency, Autoimmunity, and Neurocognitive Impairment. J Allergy Clin Immunol 2014, 133, 1400–1409. [Google Scholar] [CrossRef]
- Stukus, D.R.; Mikhail, I. Pearls and Pitfalls in Diagnosing IgE-Mediated Food Allergy. Curr Allergy Asthma Rep 2016, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P. Food Intolerance and Food Allergy in Adults: An Overview. In: Sicherer, S.H., ed. UpToDate. Wolters Kluwer. Updated May 30, 2024. Available online: www.uptodate.com/contents/food-intolerance-and-food-allergy-in-adults-an-overview (accessed on 8 September 2024).
- Demirbas, D.; Coelho, A.I.; Rubio-Gozalbo, M.E.; Berry, G.T. Hereditary Galactosemia. Metabolism 2018, 83, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Pasta, A.; Formisano, E.; Calabrese, F.; Plaz Torres, M.C.; Bodini, G.; Marabotto, E.; Pisciotta, L.; Giannini, E.G.; Furnari, M. Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients 2024, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.T.; Li, A.; Banerjee, J.; Gao, Z.G.; Kambayashi, T.; Jacobson, K.A.; Civan, M.M. The Role of Activated Adenosine Receptors in Degranulation of Human LAD2 Mast Cells. Purinergic Signal 2014, 10, 465–475. [Google Scholar] [CrossRef]
- Matsukura, S.; Aihara, M.; Sugawara, M.; Kunimi, Y.; Matsuki, M.; Inoue, Y.; Kambara, T.; Ikezawa, Z. Two Cases of Wheat-Dependent Anaphylaxis Induced by Aspirin Administration but Not by Exercise. Clin Exp Dermatol 2010, 35, 233–237. [Google Scholar] [CrossRef]
- Muñoz-Cano, R.; Pascal, M.; Araujo, G.; Goikoetxea, M.J.; Valero, A.L.; Picado, C.; Bartra, J. Mechanisms, Cofactors, and Augmenting Factors Involved in Anaphylaxis. Front Immunol 2017, 8, 1193. [Google Scholar] [CrossRef]
- Pascal, M.; Muñoz-Cano, R.; Milà, J.; Sanz, M.L.; Diaz-Perales, A.; Sánchez-López, J.; García-Moral, A.; Juan, M.; Valero, A.; Yagüe, J.; Picado, C.; Bartra, J. Nonsteroidal Anti-Inflammatory Drugs Enhance IgE-Mediated Activation of Human Basophils in Patients with Food Anaphylaxis Dependent on and Independent of Nonsteroidal Anti-Inflammatory Drugs. Clin Exp Allergy 2016, 46, 1111–1119. [Google Scholar] [CrossRef]
- Süß, H.; Dölle-Bierke, S.; Geier, J.; Kreft, B.; Oppel, E.; Pföhler, C.; Skudlik, C.; Worm, M.; Mahler, V. Contact Urticaria: Frequency, Elicitors and Cofactors in Three Cohorts (Information Network of Departments of Dermatology; Network of Anaphylaxis; and Department of Dermatology, University Hospital Erlangen, Germany). Contact Dermatitis 2019, 81, 341–353. [Google Scholar] [CrossRef]
- Geisslitz, S.; Weegels, P.; Shewry, P.; Zevallos, V.; Masci, S.; Sorrells, M.; Gregorini, A.; Colomba, M.; Jonkers, D.; Huang, X. , et al. Wheat Amylase/Trypsin Inhibitors (ATIs): Occurrence, Function and Health Aspects. Eur J Nutr 2022, 61, 2873–2880. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L. , et al. Wheat Amylase Trypsin Inhibitors Drive Intestinal Inflammation via Activation of Toll-Like Receptor 4. J Exp Med 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Stapel, S.O.; Asero, R.; Ballmer-Weber, B.K.; Knol, E.F.; Strobel, S.; Vieths, S.; Kleine-Tebbe, J.; EAACI Task Force. Testing for IgG4 Against Foods is Not Recommended as a Diagnostic Tool: EAACI Task Force Report. Allergy 2008, 63, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Fuchs, T.; Kleine-Tebbe, J.; Klimek, L.; Lepp, U.; Niggemann, B.; Saloga, J.; Schäfer, C.; et al. German Guideline for the Management of Adverse Reactions to Ingested Histamine: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Association of Allergologists (AeDA), and the Swiss Society for Allergology and Immunology (SGAI). Allergo J Int 2017, 26, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Zuberbier, T.; Bunselmeyer, B.; Erdmann, S.; Henzgen, M.; Fuchs, T.; Jäger, L.; Kleine-Tebbe, J.; Lepp, U.; Niggemann, B.; Raithel, M.; Saloga, J.; Vieths, S.; Werfel, T. Diagnostic Approach for Suspected Pseudoallergic Reaction to Food Ingredients. J Dtsch Dermatol Ges 2009, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Williams, M.; Reeves, L.; Meyer, R.; Venter, C. Sensitivity to Food Additives, Vaso-Active Amines and Salicylates: A Review of the Evidence. Clin Transl Allergy 2015, 5, 34. [Google Scholar] [CrossRef]
- Mohamed, G.G.; El-Hameed, A.K.; El-Din, A.M.; El-Din, L.A. High Performance Liquid Chromatography, Thin Layer Chromatography and Spectrophotometric Studies on the Removal of Biogenic Amines from Some Egyptian Foods Using Organic, Inorganic and Natural Compounds. J Toxicol Sci 2010, 35, 175–187. [Google Scholar] [CrossRef]
- Hare, L.G.; Woodside, J.V.; Young, I.S. Dietary Salicylates. J. Clin. Pathol. 2003, 56, 649–650. [Google Scholar] [CrossRef]
- Paterson, J.R.; Srivastava, R.; Baxter, G.J.; Graham, A.B.; Lawrence, J.R. Salicylic Acid Content of Spices and Its Implications. J. Agric. Food Chem. 2006, 54, 2891–2896. [Google Scholar] [CrossRef]
- Kang, M.G.; Song, W.J.; Park, H.K.; Lim, K.H.; Kim, S.J.; Lee, S.Y.; Kim, S.H.; Cho, S.H.; Min, K.U.; Chang, Y.S. Basophil Activation Test with Food Additives in Chronic Urticaria Patients. Clin. Nutr. Res. 2014, 3, 9–16. [Google Scholar] [CrossRef]
- Ortolani, C.; Bruijnzeel-Koomen, C.; Bengtsson, U.; Bindslev-Jensen, C.; Björkstén, B.; Høst, A.; Ispano, M.; Jarish, R.; Madsen, C.; Nekam, K. , et al. Controversial Aspects of Adverse Reactions to Food. European Academy of Allergology and Clinical Immunology (EAACI) Reactions to Food Subcommittee. Allergy 1999, 54, 27–45. [Google Scholar] [CrossRef]
- Reese, I.; Zuberbier, T.; Bunselmeyer, B.; Erdmann, S.; Henzgen, M.; Fuchs, T.; Jäger, L.; Kleine-Tebbe, J.; Lepp, U.; Niggemann, B. , et al. Diagnostic Approach for Suspected Pseudoallergic Reaction to Food Ingredients. J. Dtsch. Dermatol. Ges. 2009, 7, 70–77. [Google Scholar] [CrossRef]
- Simon, R.A. Allergic and Asthmatic Reactions to Food Additives. In Sicherer, S.H., Ed.; UpToDate. Wolters Kluwer. Updated August 2024. Available online: www.uptodate.com/contents/allergic-and-asthmatic-reactions-to-food-additives (Accessed 8 September 2024).
- Treudler, R.; Simon, J.C. Anaphylaxis to Food Additives. Allergo J. Int. 2022, 31, 141–144. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Georgalas, C.; Jovancevic, L. Gustatory Rhinitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 9–14. [Google Scholar] [CrossRef]
- Liva, G.A.; Karatzanis, A.D.; Prokopakis, E.P. Review of Rhinitis: Classification, Types, Pathophysiology. J. Clin. Med. 2021, 10, 3183. [Google Scholar] [CrossRef]
- Van Gerven, L.; Alpizar, Y.A.; Steelant, B.; Callebaut, I.; Kortekaas Krohn, I.; Wouters, M.; Vermeulen, F.; Boeckxstaens, G.; Talavera, K.; Hellings, P.W. Enhanced Chemosensory Sensitivity in Patients with Idiopathic Rhinitis and Its Reversal by Nasal Capsaicin Treatment. J. Allergy Clin. Immunol. 2017, 140, 437–446.e2. [Google Scholar] [CrossRef]
- Watanabe, T.; Terada, Y. Food Compounds Activating Thermosensitive TRP Channels in Asian Herbal and Medicinal Foods. J. Nutr. Sci. Vitaminol. 2015, 61, S86–88. [Google Scholar] [CrossRef]
- de Bree, R.; van der Waal, I.; Leemans, C.R. Management of Frey Syndrome. Head Neck 2007, 29, 773–778. [Google Scholar] [CrossRef]
- Izikson, L.; English, J.C. III.; Zirwas, M.J. The Flushing Patient: Differential Diagnosis, Workup, and Treatment. J. Am. Acad. Dermatol. 2006, 55, 193–208. [Google Scholar] [CrossRef]
- Young, A.; Okuyemi, O.T. Frey Syndrome. [Updated 2023 Jan 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK562247.
- Diaz, J.H. Amatoxin-Containing Mushroom Poisonings: Species, Toxidromes, Treatments, and Outcomes. Wilderness Environ Med 2018, 29, 111–118. [Google Scholar] [CrossRef]
- Predy, G.; Honish, L.; Hohn, W.; Jones, S. Was it Something She Ate? Case Report and Discussion of Scombroid Poisoning. CMAJ 2003, 168, 587–588. [Google Scholar]
- Smolinska, S.; Jutel, M.; Crameri, R.; O'Mahony, L. Histamine and Gut Mucosal Immune Regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Vilariño, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human Poisoning from Marine Toxins: Unknowns for Optimal Consumer Protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Winiarska, E.; Globinska, A.; Jutel, M. Histamine: A Mediator of Intestinal Disorders—A Review. Metabolites 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
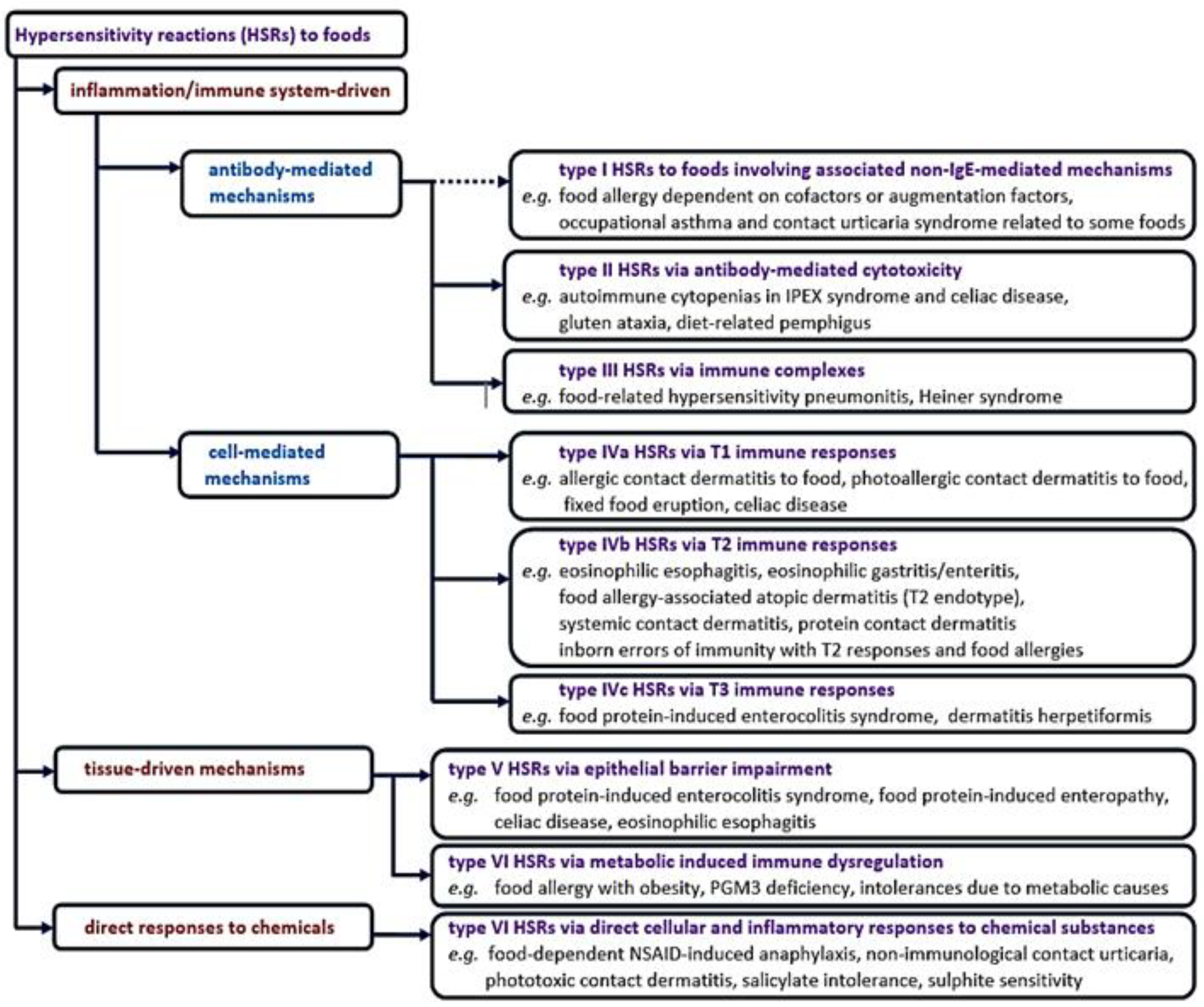
| Subtypes of non-IgE mediated food hypersensitivity reactions | Examples |
| non-IgE mediated food allergies | contact dermatitis, food protein-induced enterocolitis syndrome, food protein-induced allergic proctitis/proctocolitis, food protein-induced enteropathy, Heiner syndrome, celiac disease, dermatitis herpetiformis |
| mixed IgE and non-IgE mediated food allergies | |
| eosinophilic oesophagitis, eosinophilic gastritis/enteritis, food-exacerbation of atopic eczema, food-exacerbation of asthma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





