Submitted:
24 October 2024
Posted:
25 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
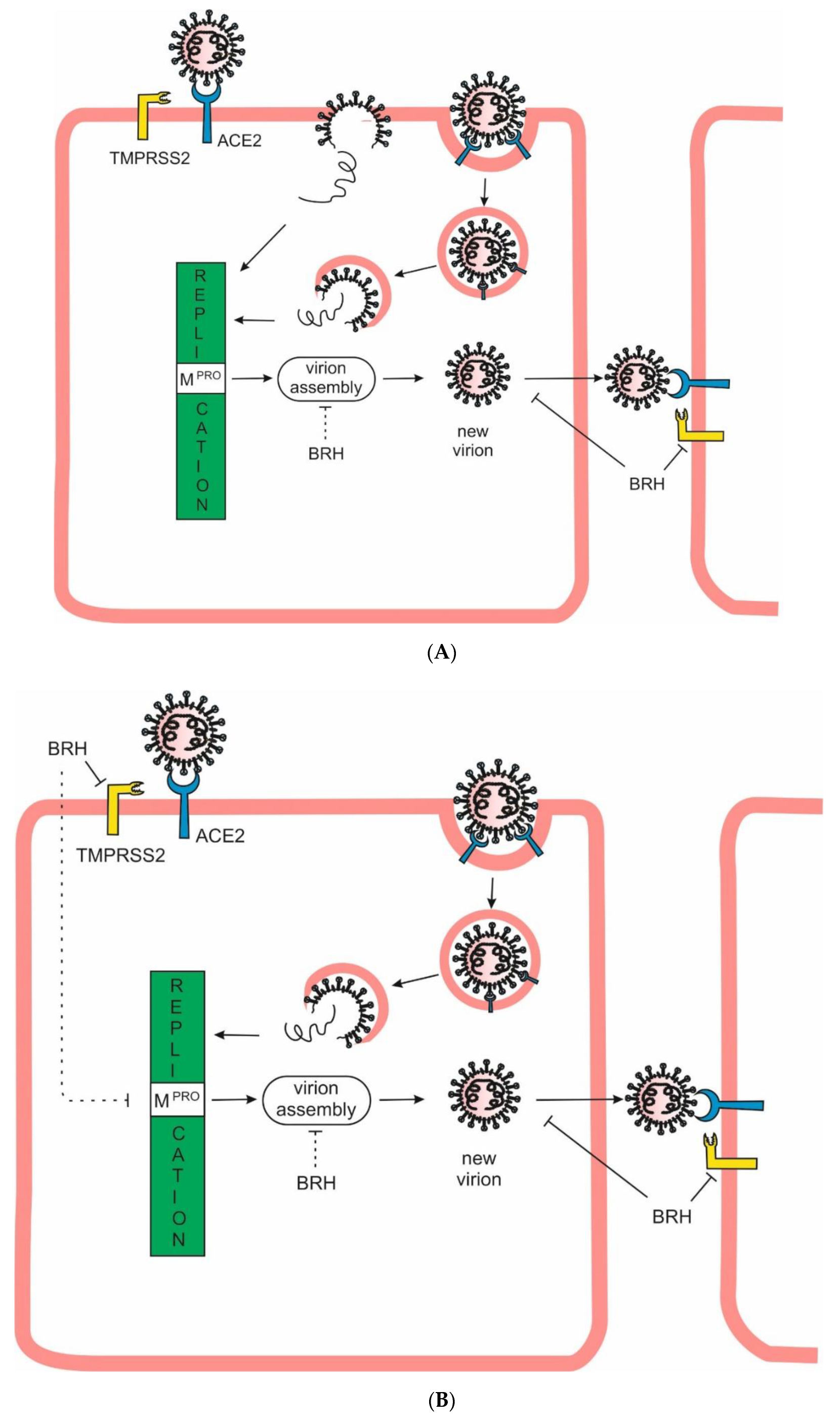
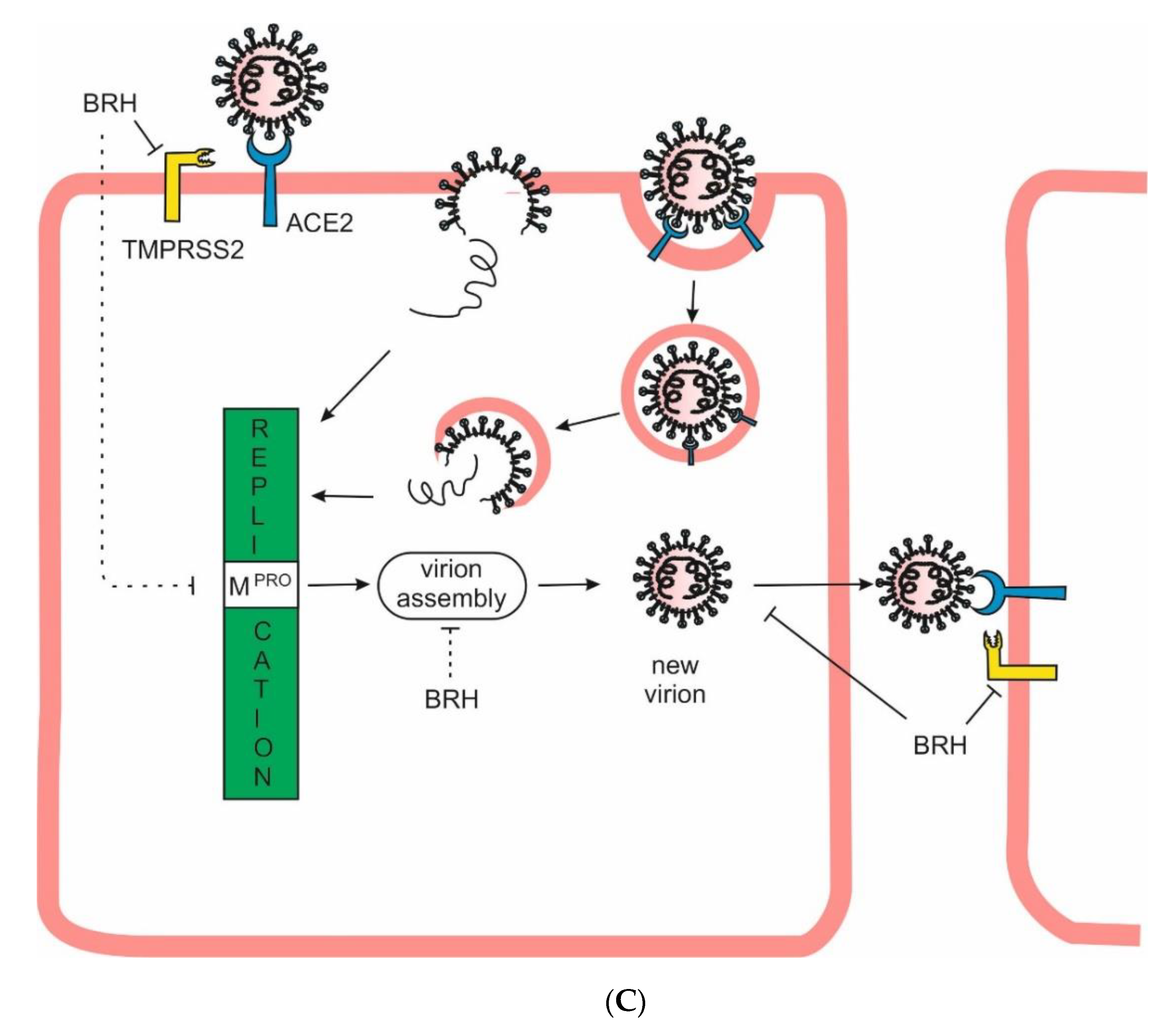
Aim of the Study
Materials and Methods
Recruitment and Procedure
Outcomes
- Fatigue, or up unexplainable tiredness
- Constant fatigue, despite rest
- Finding it “difficult to breathe”
- Chest discomfort (such as tightness, or as if pricked by a needle)
- Coughing fits
- Increased heart-rate without exertion
- Increased body temperature
- Muscle or joint pain
- Headache
- Impaired cognitive reasoning, function, or concentration abilities
- Difficulty sleeping/lower quality of sleep
- Loss of sense of smell.
- Hair-loss
Statistical Analysis
Results
Baseline Responder Characteristics
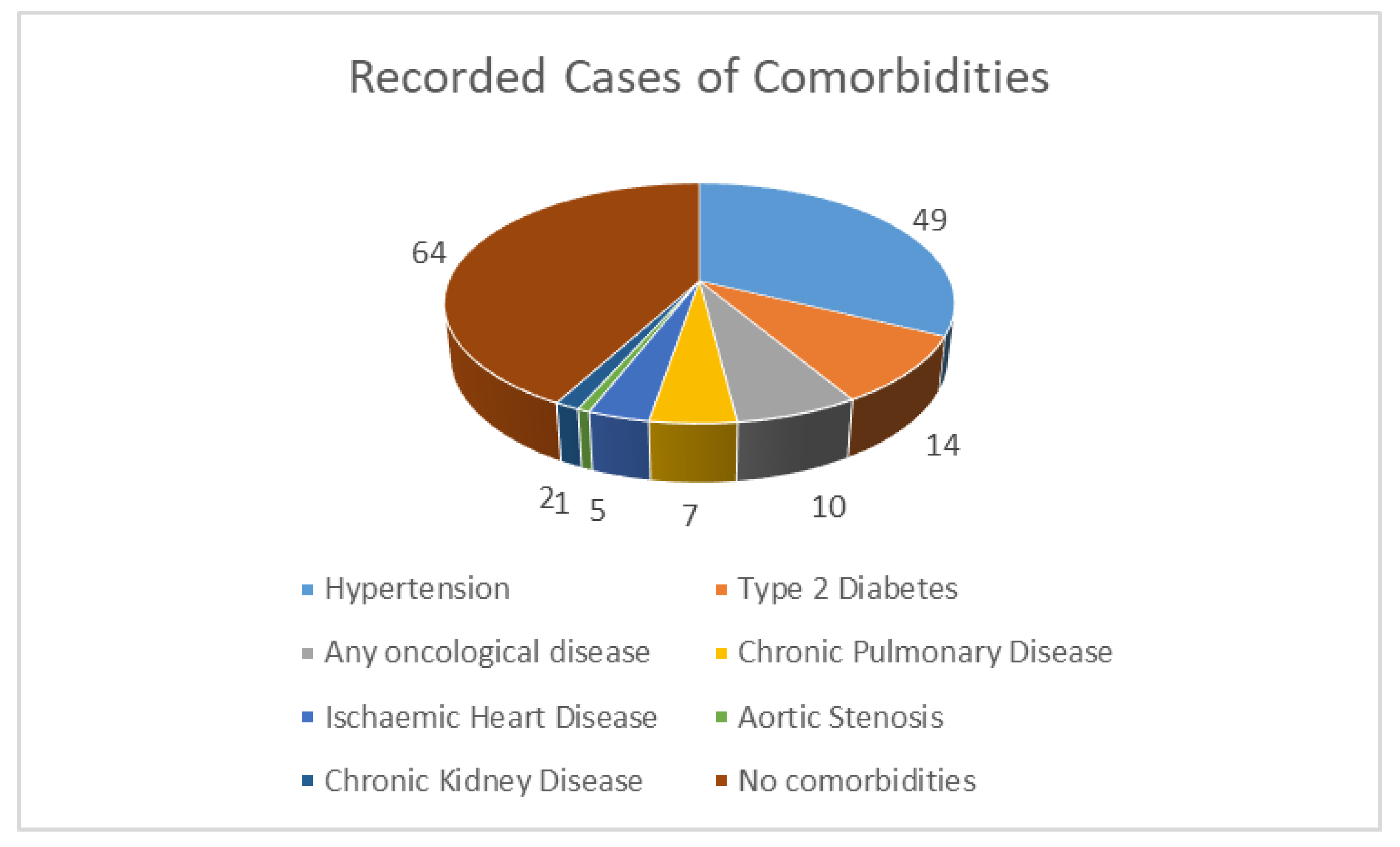
Reported Comorbidities
COVID-19 Status
Duration of Prophylactic Treatment and Outcomes
Analysis of Secondary Outcomes
Discussion
Game of Time
Benefits of Prophylaxis with BRH
Нypothesis
Reasoning
Benefits of Post-Exposure Prophylaxis with BRH
Нypothesis
Reasoning
Benefits of Using BRH as a Remedy
Нypothesis
Results
Conclusions
Supplementary Materials
Acknowledgements
References
- Morgenstern, J. Paxlovid evidence: Still very little reason to prescribe. First10EM 2024. [Google Scholar] [CrossRef]
- Sax, P. The Rise and Fall of Paxlovid—HIV and ID Observations. NEJM Journal Watch. 2024. Available online: https://blogs.jwatch.org (accessed on 3 June 2024).
- Mitev, V. Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib. Pharmacia 2023, 70, 1177–1193. [Google Scholar] [CrossRef]
- Mitev, V. Colchicine—The Divine Medicine against COVID-19. J. Pers. Med. 2024, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol 2020, 11, 1518. [Google Scholar] [CrossRef]
- Mitev, V.; Mondeshki, T.; Marinov, K.; Bilukov, R. ; Colchicine, bromhexine, and hymecromone as part of COVID19 treatment - cold, warm, hot. In Current Overview on Disease and Health Research; Khan , BA, Ed.; BP International: London, UK, 2023; Volume 10, pp. 106–13. [Google Scholar]
- Tiholov, R.; Lilov, A.I.; Georgieva, G.; Palaveev, K.R.; Tashkov, K.; Mitev, V. Effect of increasing doses of colchicine on thetreatment of 333 COVID-19 inpatients. Immun Inflamm Dis. 2024, 12, e1273. [Google Scholar] [CrossRef]
- Ni, Z.; Bin, D.; Li-li, X. ; The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar]
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T. ; Peiyang, Li.; Yiguo, Z.; Yi-Fan, L.; Qibin, D.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. Journal of Infection 2020, 80, 656–665. [Google Scholar] [CrossRef]
- Fung, S.Y.; Yuen, K.S.; Ye, Z.; Chan, C.P.; Jin, D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerging Microbes Infections 2020, 9, 558–570. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersal,l R. S.; Manson, J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Zhang, C,; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Zhao, M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents 2020, 55, 105982. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L.L. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef]
- Mondeshki, T.; Bilyukov, R.; Tomov, T.; Mihaylov, M.; Mitev, V. Complete, rapid resolution of severe bilateral pneumonia and acute respiratory distress syndrome in a COVID-19 patient: role for a unique therapeutic combination of inhalations with bromhexine, higher doses of colchicine, and hymecromone. Cureus 2022, 14, e30269. [Google Scholar] [CrossRef] [PubMed]
- Mondeshki, T.; Bilyukov, R.; Mitev, V. Effect of an Accidental Colchicine Overdose in a COVID-19 Inpatient With Bilateral Pneumonia and Pericardial Effusion. Cureus 2023, 15, e35909. [Google Scholar] [CrossRef] [PubMed]
- Mitev, V. What is the lowest lethal dose of colchicine? Biotechnol. Biotechnol. Equip.. 2023, 37, 2288240. [Google Scholar] [CrossRef]
- Lilov, A.; Palaveev, K.; Mitev, V. High Doses of Colchicine Act As “Silver Bullets” Against Severe COVID-19. Cureus 2024, 16, e54441. [Google Scholar] [CrossRef]
- Mondeshki, T.; Mitev, V. High-Dose Colchicine: Key Factor in the Treatment of Morbidly Obese COVID-19 Patients. Cureus 2024, 16, e58164. [Google Scholar] [CrossRef]
- Bulanov, D.; Yonkov, A.; Arabadzhieva, E.; Mitev, V. Successful Treatment With High-Dose Colchicine of a 101-Year-Old Patient Diagnosed With COVID-19 After an Emergency Cholecystectomy. Cureus 2024, 16, e63201. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angio-tensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–90. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J. ; Raizada, M, K,; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res 2020, 126, 1456–74. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, E.; Matrosovich, T.; Beyerle, M. ; Klenk, H-D.; Garten, W.; Matrosovich, M.. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol 2006, 80, 9896–9898. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ami, Y.; Nakajima, N.; Nakajima, K.; Kitazawa, M.; Anraku, M.; Takayama, I.; Sangsriratanakul, N.; Komura, M.; Sato, Y.; et al. TMPRSS2 independency for haemagglutinin cleavage in vivo differentiates influenza B virus from influenza A virus. Sci Rep 2016, 6, 29430. [Google Scholar] [CrossRef] [PubMed]
- Limburg, H.; Harbig, A.; Bestle, D.; Stein, D. A.; Moulton, H. M.; Jaeger, J.; Janga, H.; Hardes, K.; Koepke, J. Schulte, L. TMPRSS2 is the major activating protease of influenza A virus in primary human airway cells and influenza B virus in human type II pneumocytes. J Virol. 2019, 93, e00649–19. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. W.; Mao, H. J.; Wu, Y. L.; Tanaka, Y.; Zhang, W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie 2017, 142, 1–10. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Vvirol, 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Depfenhart, M.; De Villiers, D.; Lemperle, G.; Meyer, M.; Di Somma, S. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? Intern Emerg Med 2020, 15, 801–812. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Ansarin, K.; Tolouian, R.; Ardalan, M.; Taghizadieh, A.; Varshochi, M.; Teimouri, S.; Vaezi, T.; Valizadeh, H.; Saleh, P.; Safiri, S. Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial. BioImpacts 2020, 10, 209–215. [Google Scholar] [CrossRef]
- Zhao, M M.; Yang, WL.; Yang, F.Y.; Zhang, L.; Huang, W-J,; Hou, W.; Fan, C-F.; Jin, R-H.; Feng, Y-M.; Wang Y-C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct Target Ther 2021, 6, 134. [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L. , Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.W.; Mao, H.J.; Wu, Y.L.; Tanaka, Y.; Zhang, W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie 2017, 142, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maggio, R.; Corsini, G.U. Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacol. Res. 2020, 157, 104837. [Google Scholar] [CrossRef]
- Markus, D.; Gottfried, L.; Markus, M.; Marina, R.; Dario, B.; Danielle de, V. A SARS-CoV-2 prophylactic and treatment; A counter argument against the sole use of chloroquine. Am J Biomed Sci. 2020, 248–351. [Google Scholar]
- Shirato, K.; Kawase, M.; Matsuyama, S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology 2018, 517, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R.; Nunneley, J.W.; Barnard, D.; Pöhlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015, 116, 76–84. [Google Scholar] [CrossRef]
- Atyeo, N.; Perez, P.; Matuck, B.; Byrd, K.M.; Warner, B.M. The Mouth as a Site of SARS-CoV-2 Infection. Curr Oral Health Rep 2024, 11, 167–176. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Ghayour, A.E.; Nazari, S.; Keramat, F.; Shahbazi, F.; Eslami-Ghayour, A. Evaluation of the efficacy of N-acetylcysteine and bromhexine compared with standard care in preventing hospitalization of outpatients with COVID-19: a double blind randomized clinical trial. Rev Clín Esp. (English Edition). 2024, 224, 86–95. [Google Scholar]
- Han, S.; Mallampalli, R. K. The role of surfactant in lung disease and host defense against pulmonary infections. Annals of the American Thoracic Society 2015, 12, 765–774. [Google Scholar] [CrossRef]
- Plomer, M.; de Zeeuw, J. More than expectorant: new scientific data on ambroxol in the context of the treatment of bronchopulmonary diseases. MMW-Fortschritte der Medizin 2017, 159, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Garten, W.; Braden, C.; Arendt, A.; Peitsch, C.; Baron, J.; Lu, Y.; Pawletko, K.; Hardes, K.; Steinmetzer, T.; Böttcher-Friebertshäuser, E. Influenza virus activating host proteases: Identification, localization and inhibitors as potential therapeutics. Eur J Cell Biol. 2015, 94, 375–83. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zheng, X.; Zhou, Y.; Tang, L.; Chen, Z.; Ni, S. Re-recognizing bromhexine hydrochloride: pharmaceutical properties and its possible role in treating pediatric COVID-19. Eur J Clin Pharmacol. 2021, 77, 77,261–263. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.M.; Heinlein, C.; Kim, T.; Hernandez, S.A.; Malik, M.S.; True, L.D.; Morrissey, C.; Corey, E.; Montgomery, B.; Mostaghel, E.; et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microen-vironment and promotes prostate cancer metastasis. Cancer Discovery 2014, 4, 1310–1325. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.A.; Kim, H. J.; Ko, M.; Jee, Y.; Kim, S. TMPRSS2 and RNA-dependent RNA polymerase are effective targets of therapeutic intervention for treatment of COVID-19 caused by SARS-CoV-2 variants (B. 1.1. 7 and B. 1.351). Microbiol Spectr. 2021, 9, e0047221. [Google Scholar] [CrossRef]
- Wettstein, L.; Kirchhoff, F.; Münch, J. The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef]
- Bahadoram, S.; Keikhaei, B.; Bahadoram, M.; Mahmoudian-Sani, R.; Hassanzadeh, S.; Saeedi-Boroujeni, A.; Alikhani, K. Bromhexine is a potential drug for COVID-19; From hypothesis to clinical trials. Vopr Virusol 2022, 67, 126–132. [Google Scholar] [CrossRef]
- Huynh, T.; Wang, H.; Luan, B. In silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2’s main protease. J. Phys. Chem. Lett. 2020, 11, 4413–4420. [Google Scholar] [CrossRef]
- Tolouian ,R.; Moradi, O.; Mulla, Z.D.; Ziaie, S.; Haghighi, M.; Esmaily, H.; Amini, H.; Hassanpour, R.; Poorheidar, E.; Kouchek, M.; et al. Bromhexine for post-exposure COVID-19 prophylaxis: A randomized, double-blind, placebo-controlled trial. Jundishapur J. Microbiol 2022, 15, e130198. [Google Scholar]
- Tolouian, R.; Mulla, Z.D. Controversy with bromhexine in COVID-19; where we stand. Immunopathologia Persa 2021, 7, e12. [Google Scholar] [CrossRef]
- Tolouian, R.; Mulla, Z.D.; Jamaati, H.; Babamahmoodi, A.; Marjani, M.; Eskandari, R.; Dastan, F.J. Effect of bromhexine in hospitalized patients with COVID-19. JIM 2021, 71, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Méndez, M.L.; Antón Sanz, C.; Cárdenas García, A.D.R.; Bravo Malo, A.; Torres Martínez, F.J.; Martín Moros, J.M.; Real Torrijos, M.; Vendrell Covisa, J.F.J.; Guzmán Sierra, O.; Molina Barcena, V.; et al. Efficacy of bromhexine versus standard of care in reducing viral load in patients with mild-to-moderate COVID-19 disease attended in primary care: A randomized open-label trial. J Clin Med. 2022, 12, 142. [Google Scholar] [CrossRef]
- Cuerdo, A.R.M.; Ogbac, M.K.; Tamayo, J.E. Effect of Bromhexine among COVID-19 Patients - A Meta-Anaylsis. ERJ Open Res. 2022, 8, 104. [Google Scholar]
- He, X.; Lau, E. H.; Wu, P.; Deng, X.; Wang, J.; Hao, X. . & Leung, G. M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature medicine 2020, 26, 672–675. [Google Scholar]
- Boechat JL, Chora I, Morais A, Delgado L. The immune response to SARS-CoV-2 and COVID-19 immunopathology - Current perspectives. Pulmonology. 2021, 27, 423–437.
- Mikhaylov, E.N.; Lyubimtseva, T.A.; Vakhrushev, A.D.; Stepanov, D.; Lebedev, D.S.; Vasilieva, E.Y.; . Konradi, A.O.; Shlyakhto, E. V. Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: A randomized open-label study. Interdiscip Perspect Infect Dis. 2022, 4693121. [Google Scholar] [CrossRef]
- Tobback, E.; Degroote, S.; Buysse, S.; Delesie, L.; Van Dooren, L.; Vanherrewege, S.; Barbezange, C.; Hutse, V. ; Romano, M,.;Thomas, I.; et a;. Efficacy and safety of camostat mesylate in early COVID-19 disease in an ambulatory setting: a randomized placebo-controlled phase II trial. Int J Infect Dis 2022, 122, 628–635. [Google Scholar] [CrossRef]
- Khan, U.; Mubariz, M.; Khlidj, Y.; Nasirk M., M.; Ramadan, S.; Saeed, F.; Muhammad, A.; Abuelazm, M. Safety and Efficacy of Camostat Mesylate for Covid-19: a systematic review and Meta-analysis of Randomized controlled trials. BMC Infect Dis 2024, 24, 709. [Google Scholar] [CrossRef]
- Kinoshita, T.; Shinoda, M.; Nishizaki, Y.; Shiraki, K.; Hirai, Y.; Kichikawa, Y.; Tsushima, K.; Shinkai, M.; Komura, N.; Yoshida, K.; et al. A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study). BMC Med 2022, 20, 342. [Google Scholar]
- Chupp, G.; Spichler-Moffarah, A.; Søgaard, O.S.; Esserman, D.; Dziura, J.; Danzig, L.; Chaurasia, R.; Patra, K.P.; Salovey, A.; Nunez, A.; et al. Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste. medRxiv 01.28.22270035. 2022. [Google Scholar]
- Tsampasian V, Elghazaly H, Chattopadhyay R, et al. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
| Mean (95% CI) | Median | Lowest | Highest | |
|---|---|---|---|---|
| Age | 56.46 years (53.23-59.69) | 58 years | 16 years | 98 years |
| BMI | 25.59 (24.53-26.64) | 24.25 | 14.52 | 45.91 |
| Number of Comorbidities | 1.33 (1.15-1.52) | 1 | 1 | 4 |
| Duration of bromehexine intake | 47.51 days (38.04-56.99) | 30 days | 10 days | 365 days |
| Daily bromhexine intake | 41.15 mg (37.82-44.48) | 48 mg | 8 mg | 128 mg. |
| Vaccination rate | 20% | |||
| Age group | Number | Average BMI | Number who reported comorbidities | % in group with comorbidity (% from total) |
|---|---|---|---|---|
| < 40 | 24 people | 21.96 | 5 people | 20.83% (4%) |
| 40 – 44 | 7 people | 21.37 | 1 person | 14.28% (0.8%) |
| 45 – 49 | 9 people | 25.17 | 1 person | 11.11% (0.8%) |
| 50 – 54 | 16 people | 26.46 | 7 people | 43.75% (5.6%) |
| 55 – 59 | 9 people | 27.89 | 7 people | 77.77% (5.6%) |
| 60 – 64 | 11 people | 24.94 | 4 people | 36.36% (3.2%) |
| 65 – 69 | 15 people | 29.39 | 8 people | 53.33% (6.4%) |
| 70 – 74 | 11 people | 26.33 | 8 people | 72.72% (6.4%) |
| 75 + | 23 people | 26.14 | 16 people | 69.56% (12.8%) |
| Total | 125 people | 25.59 | 57 people | 45.6 % |
| Infection % prior to BRH | Infection % after BRH inclusion | |
| Vaccinated (n = 27) | 48.15% (n = 13) | 3.7% (n = 1) |
| Unvaccinated (n = 98) | 66.32% (n = 65) | 13.26% (n = 13) |
| Overall (n = 125) | 62.4% (n = 78) | 11.2% (n =14) |
| Factor | N | Mean reinfection rate | SD | Different (p < 0.05) than factor | |
| 1. | No more than 10 days | 18 | 0.3333 (33.33%) | 0.4851 | 3, 4 |
| 2. | No more than 20 days | 42 | 0.1429 (14.29%) | 0.3542 | - |
| 3. | No more than 30 days | 26 | 0.03846 (3.84%) | 0.1961 | 1 |
| 4. | 30 days or more | 39 | 0.02564 (2.56%) | 0.1601 | 1 |
| F- ratio | 4.927 | ||||
| Significance level | P = 0.003 | ||||
| Levene Statistic | 20.202 | ||||
| Significance | P < 0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





