1. Introduction
Dimethyl fumarate (DMF) and diroximel fumarate (DRF) are two treatments used in multiple sclerosis (MS), an autoimmune disease of the central nervous system that affects millions of people worldwide [
1,
2]. Both compounds have been shown to be effective in reducing inflammatory activity and progression of flare-dependent disability in MS patients [
3,
4].
DMF, marketed under Tecfidera [
5], was approved by the European Medicines Agency (EMA) in 2013 and reached the Spanish market that same year. It functions as an immune system modulator, although its precise mechanism of action in MS is not yet fully understood. It is believed to act by activating antioxidant and anti-inflammatory pathways, which reduces the autoimmune response that characterizes the disease [
6,
7].
On the other hand, DRF, marketed as Vumerity [
8], is a delayed-acting form of fumarate developed to reduce the GI side effects associated with the use of dimethyl fumarate [
9]. It was approved by the FDA in the United States in 2019 and subsequently by the EMA in 2020, reaching the Spanish market that same year. Like DMF, DRF is believed to exert its action primarily through modulation of the immune system, albeit with an enhanced side effect profile.
Both drugs cause, as a consequence of their mechanism of action, a decline in lymphocytes, which is not always constant but is of essential concern when monitoring since it can lead to a higher probability of developing opportunistic infections and progressive multifocal leukopathy.
Few studies compare these drugs, particularly the levels of lymphopenia produced, which are well known with DMF but little explored with DRF. Considering the importance of medical research and evidence-based conclusions, we conducted this study.
2. Materials and Methods
2.1. Ethical Aspects
The study followed the ethical principles agreed upon in the Declaration of Helsinki [
10] and received a favorable certificate from the corresponding Ethics Committee. The protocol code is DMF001, and the internal validation code is 63/2024.
2.2. Patients' Selections
This is a retrospective descriptive study on patients diagnosed with Relapsing-Remitting Multiple Sclerosis (RRMS) according to the 2017 McDonald criteria, treated with DMF at the Hospital Universitario Torrecardenas. Thirty patients undergoing treatment with DRF were selected. An equal number of cases (N=30) were chosen from patients who transitioned to DMF after previously receiving a different treatment. Specifically, these patients began DMF treatment between 2019 and 2022 and are still on the drug. Data were extracted from the medical records collected in the HUT Multiple Sclerosis database.
2.3. Data Collection
Variables from the regular follow-up of the patients were collected at the demographic, clinical, and analytical levels. Regarding demographic variables, we collected data on sex, age, and time of disease duration. Regarding clinical variables, we collected the Expanded Disability Status Scale (EDSS) score and the appearance of clinical or radiological flares during follow-up. We also wanted to study the safety of the drug, and for this purpose, we reviewed the use of antibiotherapy required by each patient, recorded as “infections”. As for the analytical variables, we focused mainly on lymphopenia. We measured lymphocyte levels, taken from a regular blood test, using quantitative variables (x 10^3/µL) and categorically, in which mild are < 1000 lymphocytes, moderate between 500 - 1000 and severe < 500 lymphocytes.
2.4. Statistical Analysis
Initially, a descriptive analysis of the patients’ sociodemographic and clinical was carried out. Quantitative variables were expressed as the mean and standard deviation or the median, accompanied by the interquartile range. Qualitative variables were represented by frequency and percentage. The normality of quantitative variables was evaluated with the Kolmogorov-Smirnov or Shapiro-Wilk test.
A bivariate analysis was conducted to assess whether significant differences existed in sociodemographic and clinical variables between the treatment groups. A Student’s t-test was used for quantitative variables when the data followed a normal distribution, and the Mann-Whitney U test was applied for non-normally distributed data.
Subsequently, the lymphocytes levels were compared, distinguishing groups at different times using dependent comparisons and the Student´s t-test for dependent samples or Wilcoxon's Sign-Rank based on the previous results of normality.
The results are presented, including the respective 95% confidence intervals. The calculations were conducted with R Statistical Software (v4.1.2; R Core Team 2021) and SPSS version 29 (IBM Inc., Armonk, NY, USA).
3. Results
3.1. Demographic Data
A total of 60 patients were analyzed, 30 treated with DRF and 30 on treatment with DMF. Their basic demographic data were examined, including age, sex, and disability measured by the EDSS. The average age was 40, with a predominance of the female sex (65%) and an average EDSS of 1.50. The data specified by groups are described in
Table 1.
3.2. Higher Incidence of Side Effects in DMF Treated Patients
Of the 30 patients treated with DRF, 20 patients (66.7%) had previously been on DMF, however, they needed to change it due to intolerance. Nineteen of them suffered relevant side effects that did not improve with symptomatic treatment. Only 2 patients maintained these side effects when changing to DRF.
Table 1 shows the side effects that patients experienced with each treatment. We observed 38% of patients with GI symptoms, being significantly higher in patients treated with DMF (p<0.001). 37% of patients presented flushings being also higher in patients treated with DMF, but it was not significant.
Regarding efficacy, four patients (6.7%) had flares that led to a change in treatment; one was treated with DRF, and the other three with DMF. No patients showed progression independent of the detected flares.
3.3. Lymphopenia Detected in Patients Treated with DRF
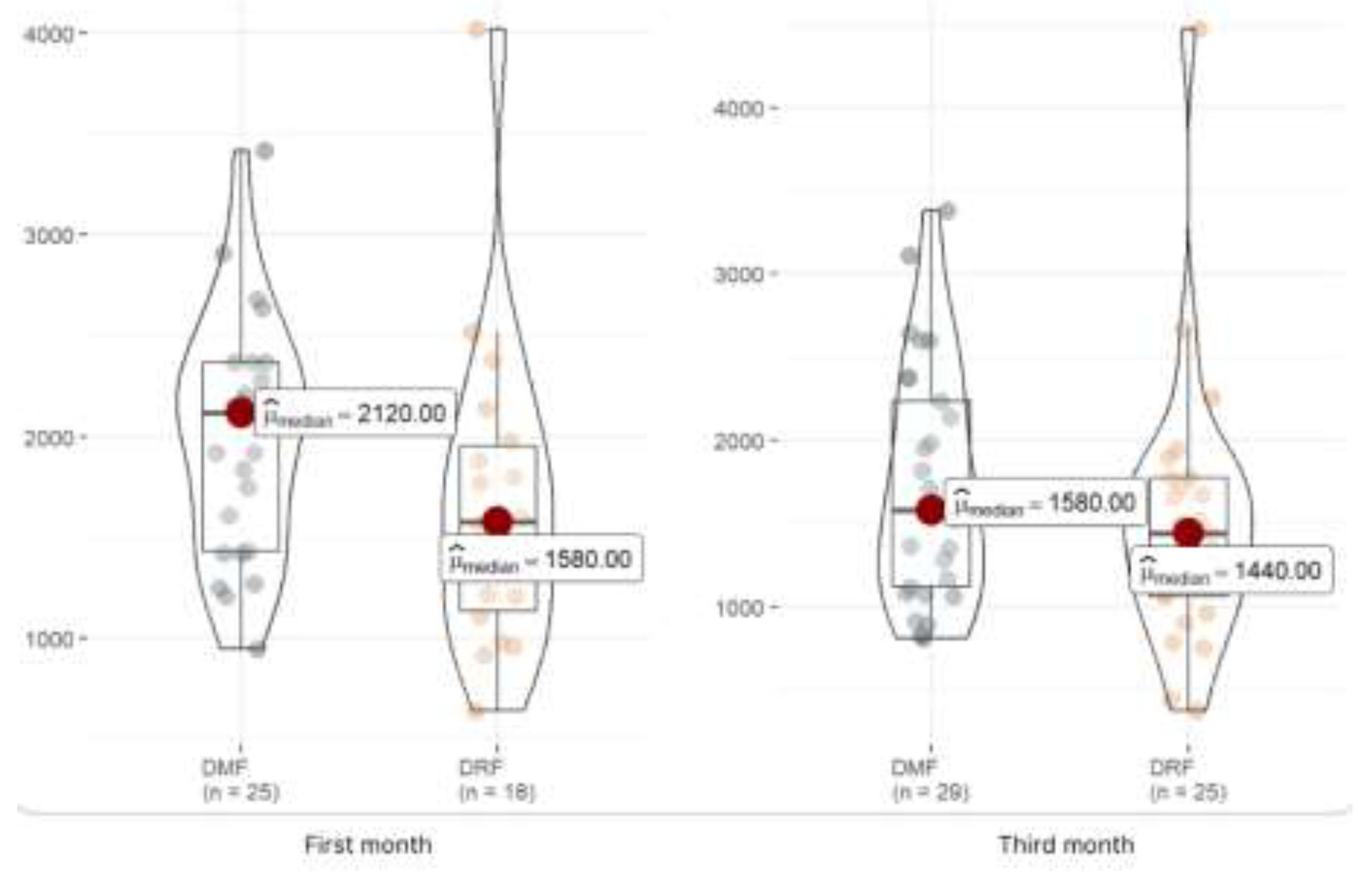
We have also evaluated the immunosuppression caused by both drugs, analyzing lymphocytes one month and three months after starting treatment and finding lower mean levels in those with DRF at the first month of treatment, as shown in
Table 2. However, when analysing the lymphopenias categorically, we can observe how 2 patients in the DRF group have suffered moderate lymphopenias and another two severe ones, nevertheless, we cannot identify these results in the DMF group.
Figure 1 shows that the lymphocyte levels are lower in patients treated with DRF in the first and third months, but this difference is significant only in the first month.
Since two-thirds of the patients treated with DRF had previously been on DMF and were exposed to longer immunosuppression, we wanted to investigate whether the depletion of lymphocytes could be related to this. To do so, we analysed lymphocyte levels in each patient who had been on DMF before switching to DRF, comparing their levels before and after the switch. As shown in the figure 2, there is indeed a decrease in lymphocyte levels after the change from DMF to DRF. DRF showed a greater decrease in levels compared to DMF, with lower mean lymphocyte levels in this drug and the appearance of moderate and severe lymphopenia rates, which did not occur with DMF.
Figure 2.
Graphical distribution of lymphocyte levels detected before the treatment changed from DMF to DRF and after one month of treatment, paired by cases.
Figure 2.
Graphical distribution of lymphocyte levels detected before the treatment changed from DMF to DRF and after one month of treatment, paired by cases.
On the other hand, we also analysed the drugs with which patients had been treated before DMF and DRF, to assess whether a more prolonged effect of immunosuppression could justify the drop in lymphocytes. In our sample, six patients in the DMF group were previously treated with other disease-modifying drugs (two with glatiramer acetate and four with interferons), while 17 patients in the DRF group received prior treatment (nine with glatiramer acetate and eight with interferons).
3.4. Higher Incidence of Infections in DMF Treated Patients
We also aimed to analyse the infections patients experienced with both drugs. To do this, we reviewed clinical records to identify those who had undergone antibiotic treatment since starting the medication. As shown in table 1, 43% of patients (n=26) had been treated with antibiotics, but patients treated with DMF (n=17) required more antibiotics than those on DRF (n=9).
4. Discussion
DMF is a widely known drug in MS clinics, as it has been presented for years as an effective drug for the control of relapsing-remitting diseases with a mild-to-moderate course. Nonetheless, one of the major problems with DMF has always been the side effects, especially GI features and flushings. In the aforementioned real-life study [
11], 44% of patients had flushings and 35.7% had GI effects, of which about half remained after 3 months of treatment. Therefore, up to 44% of patients discontinued treatment because of the several side effects.
This appears to be a solved obstacle in DRF treatment. A phase III comparative study [
9] concluded that DRF showed a significantly lower incidence of GI side effects than DMF. In addition, Liseno et al. [
13] conducted a study on 160 US patients, describing only 11% of GI side effects compared to the 44% previously reported in other real-life studies of DMF. These data are consistent with those observed in our sample, in which up to 63% presented GI symptoms with DMF, 90% of which resolved after switching to DRF.
It is important to note that the side effects of DMF began to manifest more consistently and intolerably after the introduction of generic DMF. However, due to challenges in tracking these patients, we cannot determine how many who switched from DMF to DRF had been using Tecfidera or a generic version.
Regarding efficacy, outbreaks were recorded in 3 patients on DMF, which needed switching to a more highly effective drug, while only one patient on DRF had a relapse. However, to properly evaluate this difference, it is important to note that the DRF-treated patients started their treatment no more than two years ago, whereas the DMF group includes patients who began treatment as early as 2020, giving them a longer treatment duration and, consequently, a higher likelihood of experiencing an outbreak.
In the published studies [
9,
10], no increased risk of infection has been described with either drug. Although in our sample, there is a higher use of antibiotics by patients treated with FMD, one possible explanation is the longer treatment duration for DMF patients. However, this is an important factor to consider in future studies.
Several studies have reported an increased risk of severe lymphopenia in those patients that present over 21% decrease during the first three months of DMF treatment [
13,
14,
15]. In a real-life study in Spain [
11], 30% of patients presented lymphopenia (<1000 lymphocytes). Although the effects of DMF have been thoroughly evaluated over its decade-long use, no comparable studies have been conducted for DRF. The results of the EVOLVE-MS-1 [
16], an open-label, phase 3 study to evaluate the long-term safety and tolerability of DRF, have recently been published, analysing lymphocyte levels in different groups of patients (those with de novo treatment, those with previously treated with DMF and those previously treated with DRF). Their results show moderate levels of maintained lymphopenia, being 13.7% in the first group, 12.4% in the DMF group and 16.4% in the DRF group.
In our sample, we have observed a higher number of patients with lymphopenia and more severe lymphopenia in those treated with DRF compared to those treated with DMF. Two studies [
17,
18] have recently been published with similar results, in which they show that patients who were stable with DMF experience greater lymphopenia when they switch to DRF. We have also been able to observe this in our patients, where a decrease in lymphocytes was observed after the change from DMF to DRF, as shown in
Figure 2, although it is not a significant difference.
As for the immunosuppressive treatments with which patients have been previously treated, it is true that they are higher in the DRF group than in the DMF group. Further research is needed to find out whether this may have an effect on the higher lymphopenia found in the DRF group.
We acknowledge the limitations of our study, primarily due to the small sample size. However, the findings from our analysis are intriguing and pave the way for future research into the potential variability in efficacy and safety between these two drugs, particularly regarding lymphopenia.
5. Conclusions
Our real-life analysis of multiple sclerosis patients treated with DMF or DRF supports the findings in studies of decreased gastrointestinal side effects with DRF versus DMF, without decreasing efficacy. However, our data show greater reduction in lymphocytes in patients with DRF compared to DMF, so more studies are necessary to verify these results.
Funding
This research received no external funding
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data on the variables collected are publicly available at the following DOI: 10.6084/m9.figshare.26207342
Acknowledgments
Fibao and AAM, for their collaboration in the statistical analysis of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol. 2019 Jan;26(1):27-40. [CrossRef] [PubMed]
- Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Curr Opin Neurol. 2018 Dec;31(6):752-759. [CrossRef] [PubMed]
- Popova EV, Boyko AN, Orlova EV. Dimetilfumarat v terapii remittiruyushchego rasseyannogo skleroza [Dimethylfumarate in the treatment of relapsing-remitting multiple sclerosis]. Zh Nevrol Psikhiatr Im S S Korsakova. 2016;116(10 Pt 2):68-72. Russian. [CrossRef] [PubMed]
- Wang Y, Bhargava P. Diroximel fumarate to treat multiple sclerosis. Drugs Today (Barc). 2020 Jul;56(7):431-437. [CrossRef] [PubMed]
- Biogen. Tecfdera® (dimethyl fumarate) prescribing information and patient information. 2023. https://www.tecfdera.com/conte nt/dam/commercial/multiple-sclerosis/tecfdera/pat/en_us/pdf/ full-prescribing-info.pdf. Revised Dec 2023.
- Tastan B, Arioz BI, Tufekci KU, Tarakcioglu E, Gonul CP, Genc K, Genc S. Dimethyl Fumarate Alleviates NLRP3 Inflammasome Activation in Microglia and Sickness Behavior in LPS-Challenged Mice. Front Immunol. 2021 Nov 10;12:737065. [CrossRef] [PubMed]
- Kornberg MD, Bhargava P, Kim PM, Putluri V, Snowman AM, Putluri N, Calabresi PA, Snyder SH. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. 2018 Apr 27;360(6387):449-453. [CrossRef] [PubMed]
- Biogen. Vumerity® (diroximel fumarate) prescribing information and patient information. 2023. https://www.vumerity.com/conte nt/dam/commercial/vumerity/pat/en_us/pdf/vumerity-prescribin g-information.pdf. Revised Dec 2023.
- R. T. Naismith et al., “Diroximel Fumarate Demonstrates an Improved Gastrointestinal Tolerability Profile Compared with Dimethyl Fumarate in Patients with Relapsing–Remitting Multiple Sclerosis: Results from the Randomized, Double-Blind, Phase III EVOLVE-MS-2 Study,” CNS Drugs, vol. 34, no. 2, pp. 185–196, Feb. 2020. [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27; 310(20): 2191-4. [CrossRef] [PubMed]
- J. Sabin et al., “Tolerability and safety of dimethyl fumarate in relapsing multiple sclerosis: a prospective observational multicenter study in a real-life Spanish population,” J Neurol, vol. 267, no. 8, pp. 2362–2371, Aug. 2020. [CrossRef]
- J. Liseno, B. Lager, C. Miller, S. L. Shankar, J. P. Mendoza, and J. B. Lewin, “Multiple Sclerosis Patients Treated With Diroximel Fumarate in the Real-World Setting Have High Rates of Persistence and Adherence,” Neurol Ther, vol. 10, no. 1, pp. 349–360, Jun. 2021. [CrossRef]
- S. Sainz de la Maza et al., “Early predictive risk factors for dimethyl fumarate-associated lymphopenia in patients with multiple sclerosis,” Mult Scler Relat Disord, vol. 59, Mar. 2022. [CrossRef]
- Jaboob A, Asmi AA, Islam MM, Rezvi S, Redha I, Al-Khabouri J, Al-Zakwani I, Al-Qassabi A, Al-Abri H, Gujjar AR. Frequency of Dimethyl Fumarate-Induced Lymphopenia among Omani Patients with Multiple Sclerosis. Sultan Qaboos Univ Med J. 2024 Feb;24(1):44-51. [CrossRef] [PubMed]
- Ravn J, Jensen HB, Kant M, Andersen PB, Góra MK, Sejbaek T. Risk factors for development of lymphopenia in dimethyl fumarate-treated patients with multiple sclerosis. Mult Scler Relat Disord. 2022 Nov;67:104081. [CrossRef] [PubMed]
- Singer BA, Wray S, Gudesblatt M, Bumstead B, Ziemssen T, Bonnell A, Scaramozza M, Levin S, Shanmugasundaram M, Chen H, Mendoza JP, Lewin JB, Shankar SL. Lymphopenia is Not the Primary Therapeutic Mechanism of Diroximel Fumarate in Relapsing-Remitting Multiple Sclerosis: Subgroup Analyses of the EVOLVE-MS-1 Study. Neurol Ther. 2024 Jun 27. [CrossRef] [PubMed]
- Dempsey JP, Wu L, Balshi A, Jun C, Baber U, Sloane JA. Worsening of lymphopenia in patients with multiple sclerosis when switched from dimethyl fumarate to diroximel fumarate. Mult Scler Relat Disord. 2024 Sep;89:105737. [CrossRef] [PubMed]
- Schneider M, Kramer J, Banks A, Moses H. New onset lymphopenia in patients with relapsing multiple sclerosis switching from long-standing dimethyl fumarate treatment to diroximel fumarate: A case series. Mult Scler. 2024 Sep;30(10):1379-1382. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).