Submitted:
24 October 2024
Posted:
26 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Saliva Sampling and Analysis for HBD2
2.3. Serum Sampling and Analysis for HBD2
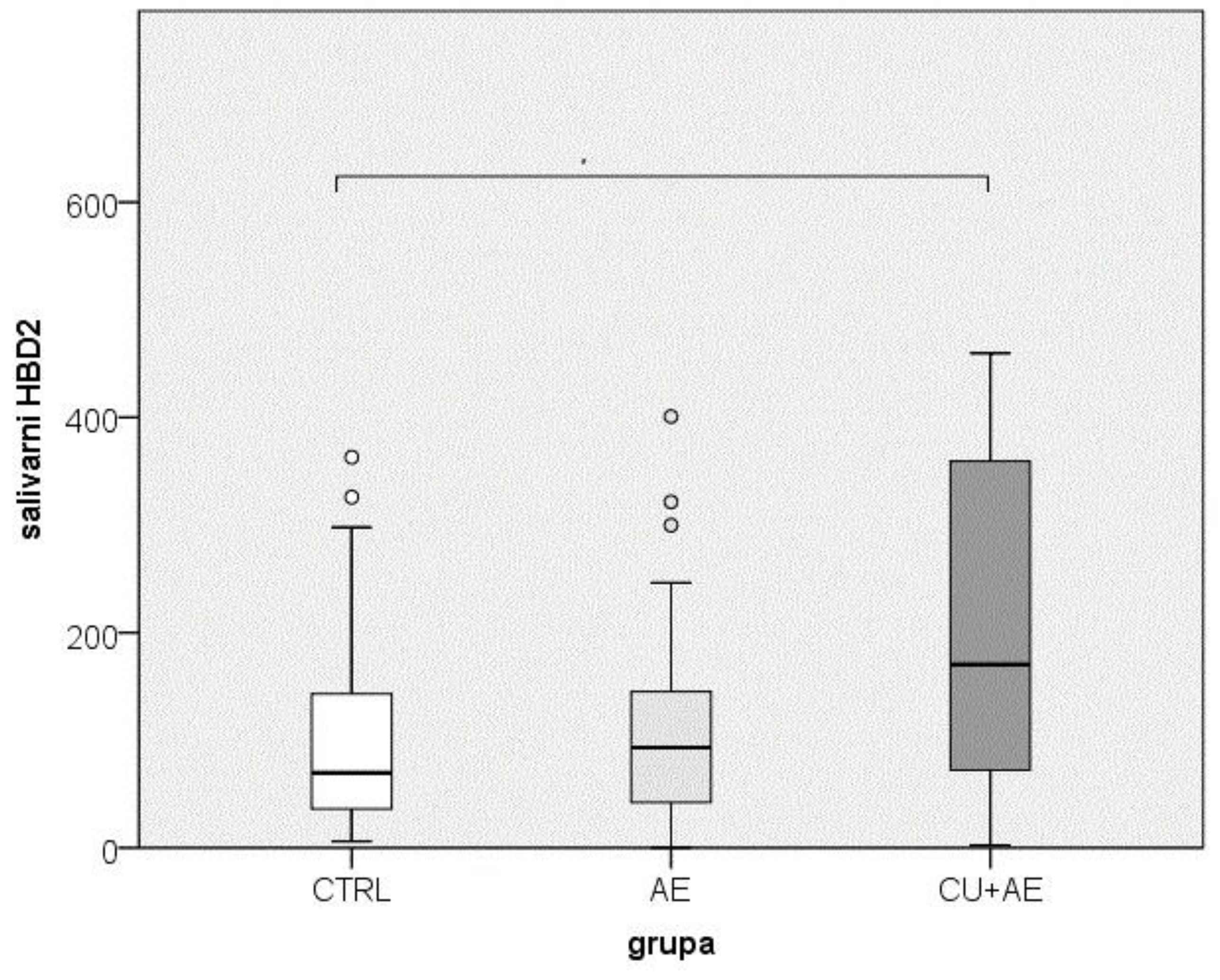
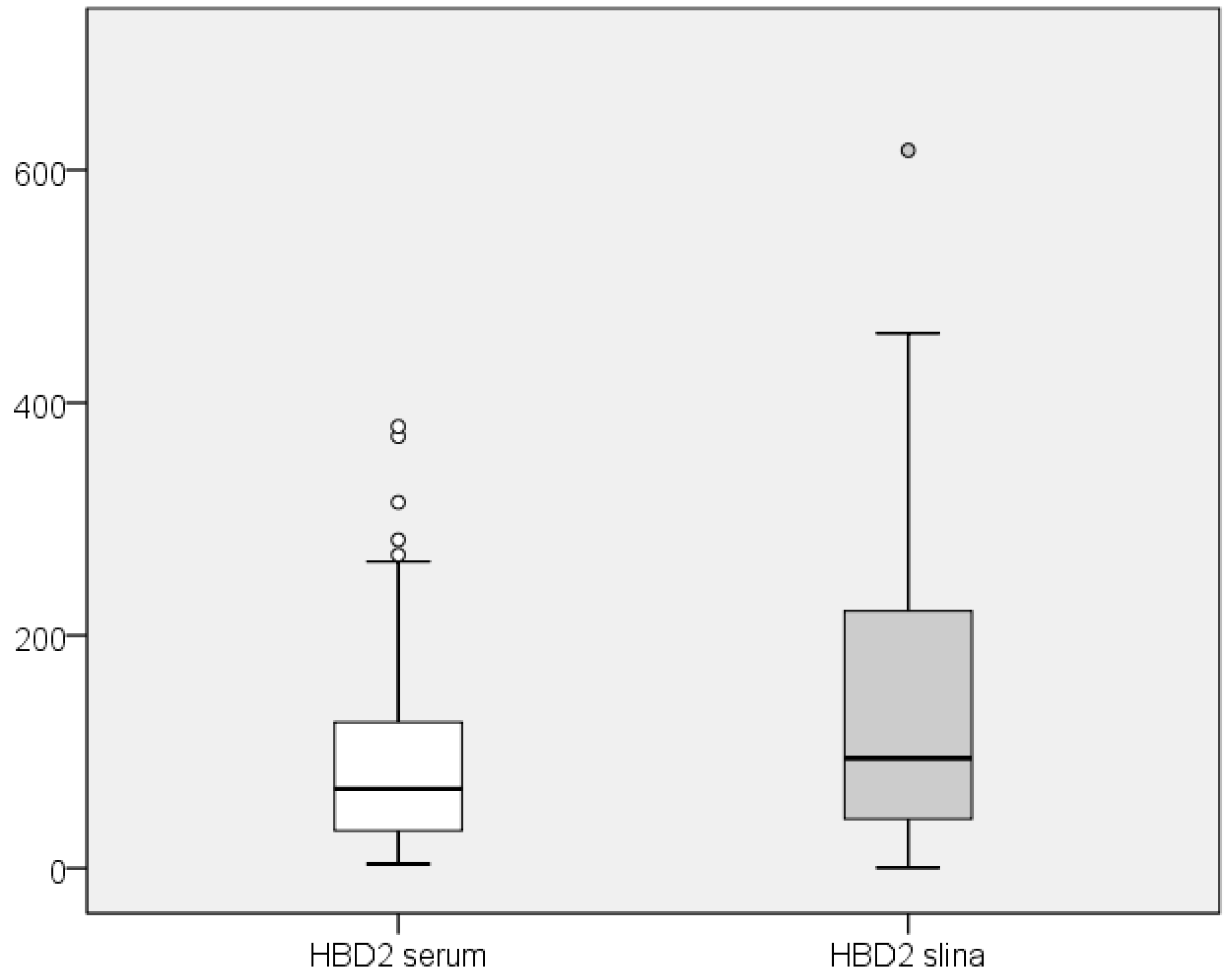
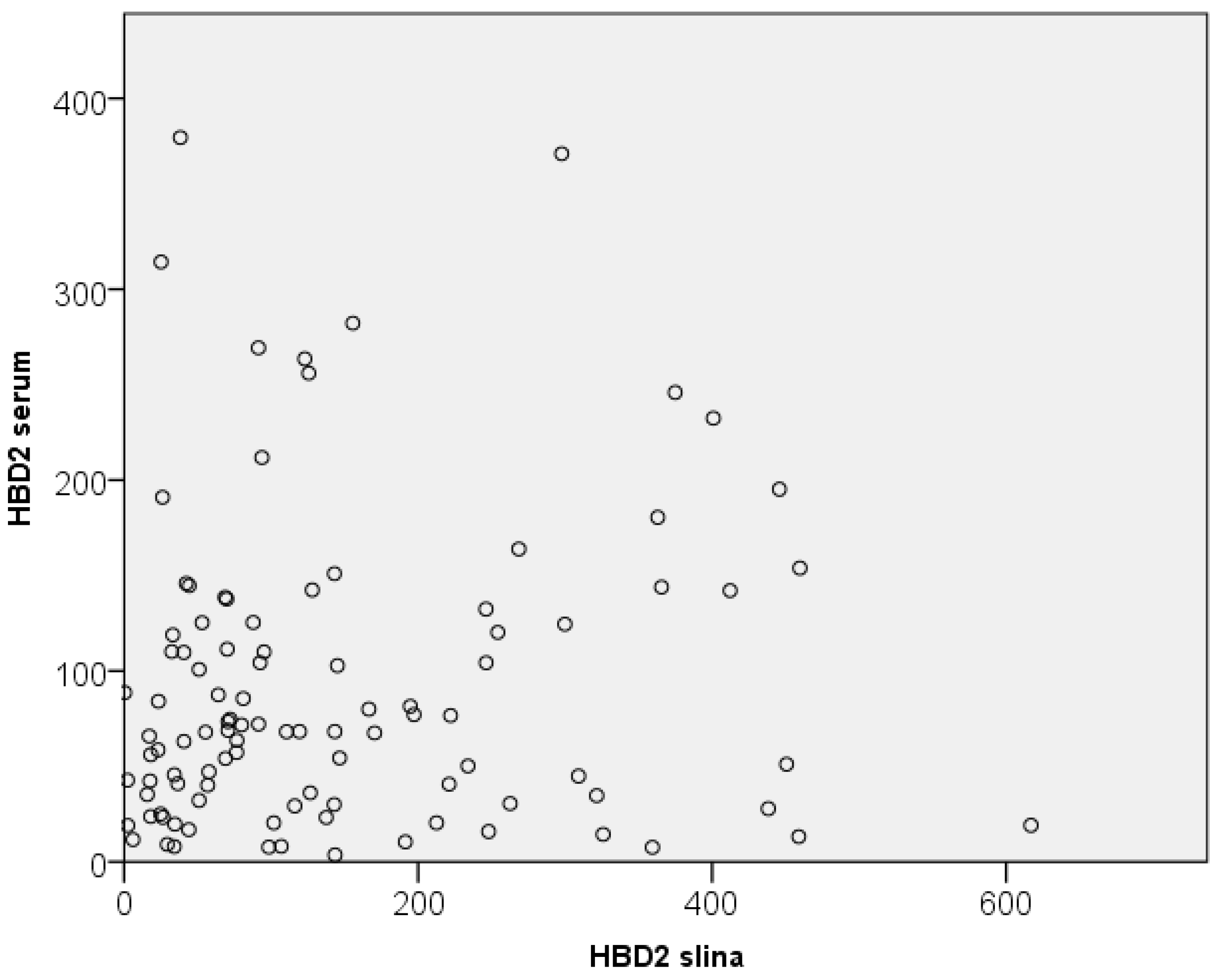
3. Results
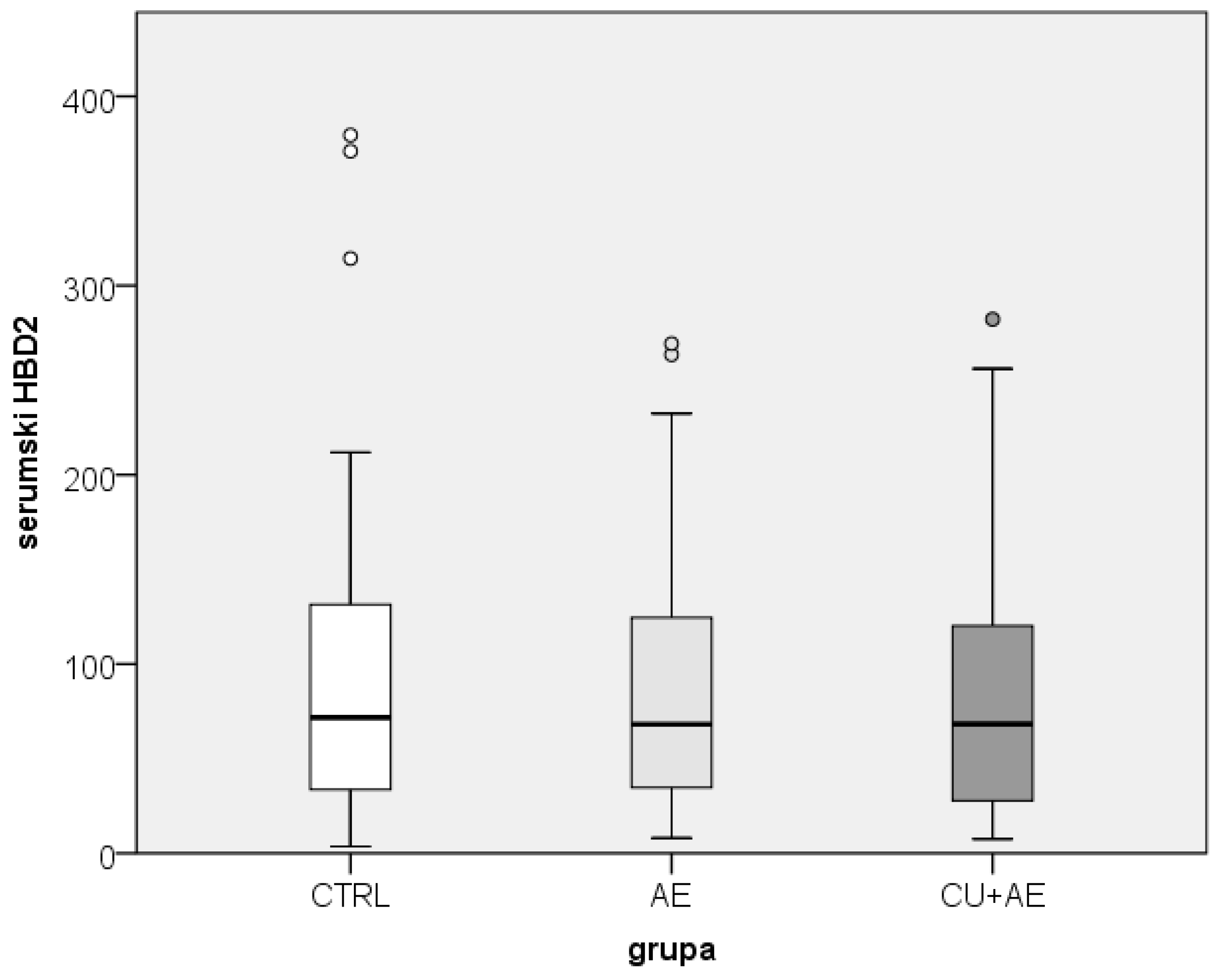
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jin, G.; Kawsar, H.I.; Hirsch, S.A.; Zeng, C.; Jia, X.; Feng, Z.; Ghosh, SK.; Zheng, Q.Y.; Zhou, A.; McIntyre, T.M.; Weinberg, A. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorogenesis. PLos One. 2010, 5, e10993. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Lee, D.; Bayir, A.K.; Ahn, H.; Ali, H. ß-defensins activate human mast cells via mas-related gene X2. J Immunol. 2013, 191, 345–52. [Google Scholar] [CrossRef] [PubMed]
- J., *!!! REPLACE !!!*; Luo, J.; Mack, MR.; Yang, P.; Zhang, F.; Wang, G.; Gong, X.; Cai, T.; Mei, Z.; Kim, BS.; Yin, S.; Hu, H. The antimicrobial peptide human ß-defensin 2 promotes itch trought Toll-like receptor 4 signaling in mice. J Allergy Clin Immunol 2017, 140, 885-e6. [Google Scholar] [CrossRef]
- Zhang, L.; McNeil, B.D. ß-defensins are proinflammatory pruritogens that activate Mrgprs. J Allergy Clin Immunol 2019, 143, 1960–1962-e5. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Watanabe, S. Increased serum human ß-defensin-2 levels in atopic dermatitis: relationship to IL-22 and oncostatin M. Immunobiology. 2012, 2017, 436–45. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Sun, Z.; Chen, X.; Wang, Y.; Li, R.; Ji, S.; Zhao, Y. Serum human ß-defensin-2 is a possible biomarker for monitoring response to JAK inhibitor in psoriasis patient. Dermatology. 2017, 233, 164–169. [Google Scholar] [CrossRef]
- Tra Cao, T.B.; Cha, H.Y.; Yang, E.M.; Choi, B.Y.; Park, H.S.; Ye, Y.M. Serum human ß-defensin 2 is increased in angioedema accompanying chronic spontaneous urticaria. Int Arch Allergy Immunol. 2021, 182, 1066–1071. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Pozdarec, I.; Lugović-Mihić, L.; Artuković, M.; Stipuć-Marković, A.; Kuna, M.; Ferček, I. Chronic inducible urticaria: classification and prominent features of physical and non-physical types. Acta Dermatovenerol Alp Pannonica adria. 2020, 29, 141–148. [Google Scholar] [CrossRef]
- Bulfone-Paus, S.; Nilsson, G.; Draber, P.; Blank, U.; Levi-Schaffer, F. Positive and negative signals in mast cell activation. Trends Immunol. 2017, 38, 657–667. [Google Scholar] [CrossRef]
- Maurer, M.; Eyerich, K.; Eyerich, S.; Ferrer, M.; Gutermuth, J.; Hartmann, K.; Jakob, T.; Kapp, A. , Kolkhir, P.; Larens-Linnemann, D.; et al. Urticaria: Colegium internationale allergologicum (CIA) update 2020. Int Arch Allergy Immunol 2020, 181, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Vena, G.A.; Cassano, N.; Di Leo, E.; Calogiuri, G.F.; Nettis, E. Focus on the role of substance P in chronic urticaria. Clin Mol Allergy. 2018, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gopta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Kasakura, K.; Kawakami, T. Histamine releasimg factor, a new therapeutic target in allergic diseases. Cells. 2019, 8, 1515. [Google Scholar] [CrossRef]
- Ulambayar, B.; Lee, H.; Yang, E.M.; Park, H.S.; Lee, K.; Ye, Y.M. Dimerized, not monomeric, translationally controlled tumor protein induces basophil activation and mast cell degranulation in chronic urticaria. Immune Netw. 2019, 19–e20. [Google Scholar] [CrossRef]
- Sánchez-Borges, M.; Caballero-Fonseca, F.; Capriles-Hulett, A.; González-Aveledo, L.; Maurer, M. Factors linked to disease severity and time to remission in patients with chronic spontaneus urticaria. J Eur Acad Dermatol Venereol. 2017, 31, 964–971. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. Histamine enhances the production of human ß-defensin-2 in human keratinocytes. Am J Physiolαα Cell Physiol 2007, 293, C1916-23. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ogawa, H.; Nagaoka, I. Human ß-defensin-2 functions as a chemotactic agent for tumour necrosis factor-α-treated human neutrophils. Immunology. 2004, 111, 273–281. [Google Scholar] [CrossRef]
- Öztürk, A.; Kurt-Bayrakdar, S.; Avci, B. Comparison of gingival crevicular fluid and serum human beta-defensin-2 levels between periodontal health and disease. Oral Dis. 2021, 27, 993–1000. [Google Scholar] [CrossRef]
- Ribeiro, A.E.R.A.; Lourenco, A.G.; Motta, A.C.F.; Komesu, M.C. Influence of peridontal conditions on levels of human beta defensins 1 and 2 in saliva. J Microbiol Exp. 2018, 6, 00186. [Google Scholar] [CrossRef]
- Güncü, G.N.; Yilmaz, D.; Könönen, E.; Gürsoy, U.K. Salivary antimicrobial peptides in early detection of periodontitis. Front Cell Infect Microbiol. 2015, 5, 99. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Human β-defensin 2 and its postulated role in modulation of the immune response. Cells. 2021, 10, 2991. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Agra, M.; González-Serrano, J.; de Pedro, M:, Virto; Caponio, V.C.A.; Prieto-Ibáñez, E.; Hernández, G.; Pintor-López, M.R. Salivary biomarkers in burning mouth syndrome: A systematic review and meta-analysis. Oral Dis 2023, 229, 2600–2613. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kho, H.S. Blood contamination in salivary diagnostics: Current methods and their limitations. Clin Chem Lab Med. 2019, 57, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Štrajtenberger, M.; Stipić-Marković, A.; Barac, E.; Artuković, M.; Lugović-Mihić, L. Human β -defensin-2 a connection between infections and allergic skin diseases. Acta Dermatovenerol Alp Pannonica Adriat. 2024, 33, 135–139. [Google Scholar] [CrossRef] [PubMed]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995, 5, 521–7. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003, 3, 710–20. [Google Scholar] [CrossRef]
- Hellgren, O.; Sheldon, B.C. Locus-specific protocol for nine different innate immune genes (antimicrobial peptides: β-defensins) across passerine bird species reveals within-species coding variation and a case of trans-species polymorphisms. Mol Ecol Resour. 2011, 11, 686–692. [Google Scholar] [CrossRef]
- van Dijk, A.; Veldhuizen, E.J.; Haagsman, H.P. Avian defensins. Vet Immunol Immunopathol. 2008, 124, 1–18. [Google Scholar] [CrossRef]
- Deptula, J.; Tokarz-Deptula, B.; Deptula, W. Defensins in humans and animals. Postepy hig. Med. Dosw. 2019, 73, 152–158. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.M. A peptide antibiotic from human skin. Nature. 1997, 387, 861. [Google Scholar] [CrossRef]
- Schröder, J.M.; Harder, J. Human beta-defensin-2. Int J Biochem. Cell Biol. 1999, 31, 645–51. [Google Scholar] [CrossRef]
- Akin, C.; Elhosni, M.; Khokar, D.S. Mast cells and mast cell disorders. In: Rich RR, Fleicher TA, Schroeder HW, Weyand CM, Corry DB, Puck J (eds.). Clinical immunology: principles and practice. Elsevier. 2023, p, 563. [CrossRef]
- Kuna, M.; Štefanović, M.; Ladika Davidović, B.; Mandušić, N.; Birkić Belanović, I.; Lugović-Mihić, L. Chronic urticaria biomarkers IL-6, ESR and CRP in correlation with disease severity and patient quality of life-a pilot study. Biomedicines. 2023, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Huston, D.P.; Sabato, V. Decoding the enigma of urticaria and angioedema. J Allergy Clin Immunol Pract. 2018, 6, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Sussman, G.; Abuzakouk, M.; Bérard, F.; Canonica, W.; Oude Elberink, H.; Giménez-Arnau, A.; Grattan, C.; Hollis, K.; Hunteer, S.; Knulst, A.; et al. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact: Analyses from ASSURE-CSU. Allergy. 2018, 73, 1724–1734. [Google Scholar] [CrossRef]
- Puxeddu, I.; Petrelli, F.; Angelotti, F.; Croia, C.; Migliorini, P. Biomarkers in chronic spontaneous urticaria: current targets and clinical implications. J Asthma Allergy. 2019, 12, 285–295. [Google Scholar] [CrossRef]
- Mathews, M.; Jia, H.P.; Guthmiller, J.M.; Losh, G.; Graham, S.; Johnson, G.K.; Tack, B.K.; McCray, P.B.Jr. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect. Immun. 1999, 67, 2740–2745. [Google Scholar] [CrossRef]
- Dale, B.A.; Fredericks, L.P. Antimicrobial peptide sin the oral environment. Expression and function in health and disease. Curr. Issues Mol. Biol. 2005, 7, 119–133. [Google Scholar] [CrossRef]
- Krisanaprakornkit, S.; Kimball, J.R.; Weinberg, A.; Darveau, R.P.; Bainbridge, B.W.; Dale, B.A. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum ino ral epithelial cells: Multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 2000, 68, 2907–2915. [Google Scholar] [CrossRef]
- Jansen, P.A; Rodijk-Olthuis, D.; Hollox, E.J.; Kamsteeg, M.; Tjabringa, G.S.; de Jongh, G.J.; van Vlijmen-Willems, I.M.; Bergboer, J.G.; van Rossum, M.M.; de Jong, E.M.; et al. Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One. 2009, 4–e4725. [Google Scholar] [CrossRef]
- Yu, L.; Li, L. Potential biomarkers of atopic dermatitis. Front Med (Lausanne). 2022, 9, 1028694. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H. Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: anti-allergic, anti-inflammatory, and skin-protective activities. Int J Mol Sci 2020, 24, 4620. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.R.; Gallo, R.L. Antimicrobial peptides, skin infections, and atopic dermatitis. Semin Cutan Med Surg. 2008, 27, 144–150. [Google Scholar] [CrossRef]
- Clausen, M.L.; Jungersted, J.M.; Andersen, P.S.; Slotved, H.C.; Krogfelt, K.A.; Agner, T. Human β-defensin-2 as a marker for disease severity and skin barrier properties in atopic dermatitis. Br J Dermatol. 2013, 169, 587–93. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002, 347, 1151–60. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Mu, Z.L.; Chen, X.; Wen, G.D.; Zhao, Y.; Zhang, J.Z. Atopic dermatitis-like graft-versus-host disease and lichen planus-like graft-versus-host disease: alterations in skin barrier function and related molecules. Chin Med J (Engl). 2017, 130, 1459–1466. [Google Scholar] [CrossRef]
- de Jongh, G.J.; Zeeuwen, P.L.; Kucharekova, M.; Pfundt, R.; van der Valk, P.G.; Blokx, W.; Dogan, A.; Hiemstra, P.S.; van de Kerkhof, P.C.; Schalkwijk, J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005, 125, 1163–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




