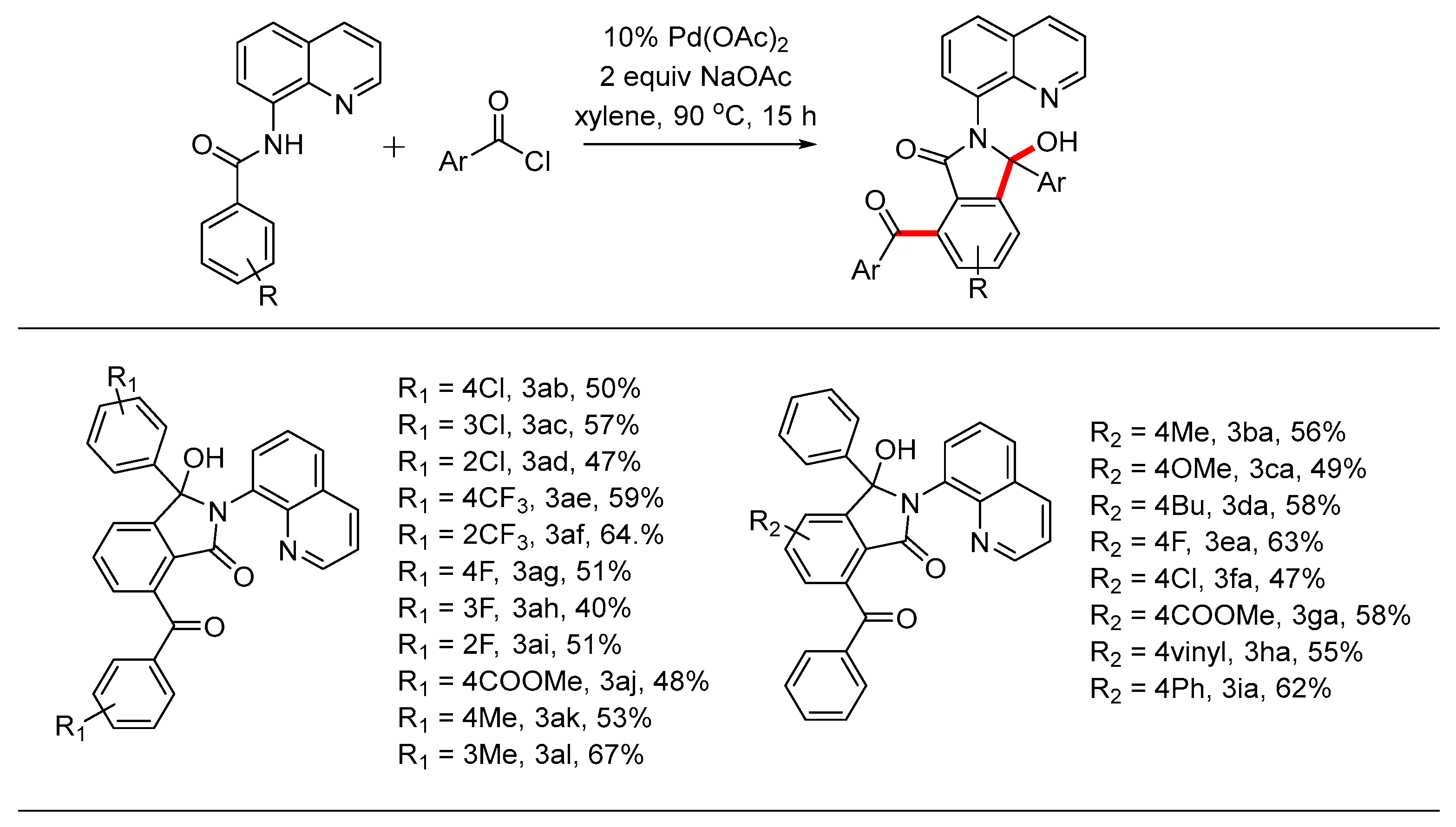
3.3. General Procedure for the Synthesis of Compound 3
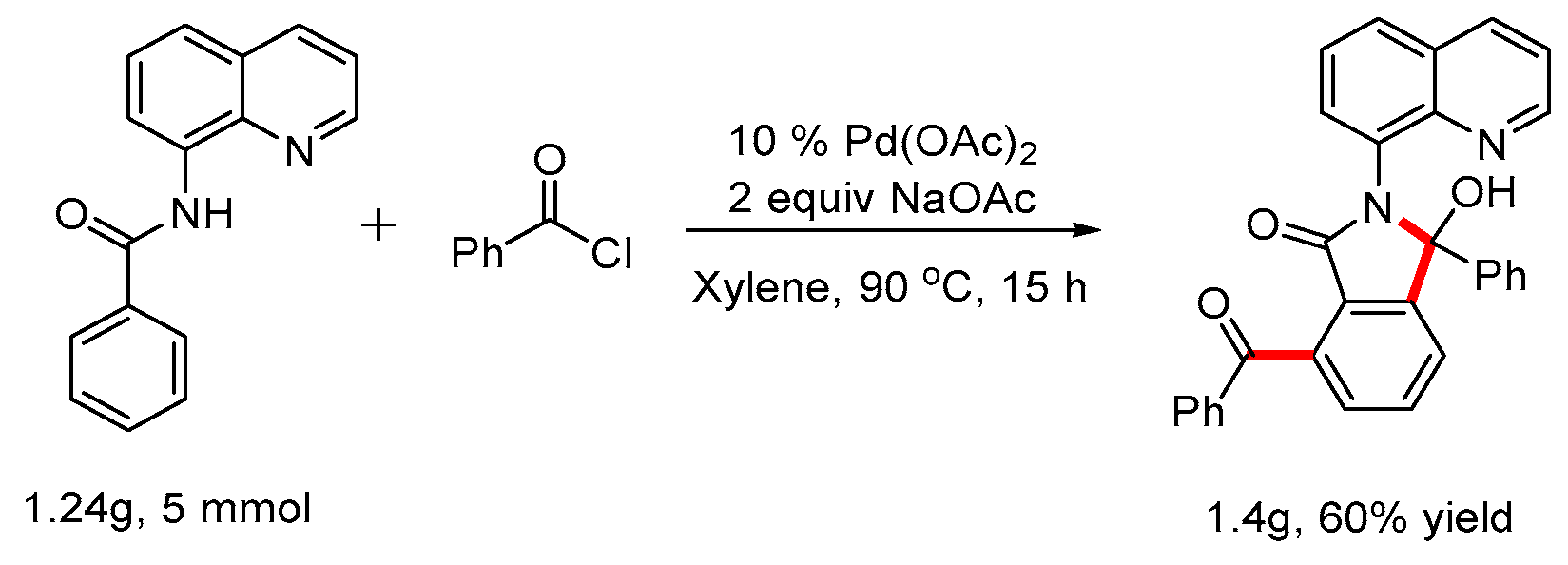
A Schlenk tube was equipped with a magnetic stir bar and charged with substituted N-(quinolin-8-yl)benzamide 1 (0.1 mmol), 2 (0.25 mmol), NaOAc (0.2mmol, 16 mg), Pd(OAc)2 (0.01mmol, 2.3 mg) and xylene (2 mL). Then the flask was sealed under N2 and stirred at 90 oC for 16 h. After the reaction was quenched by addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. Concentration in vacuo followed by silica gel column purification with petroleum ether /ethyl acetate eluent gave the desired product 3.
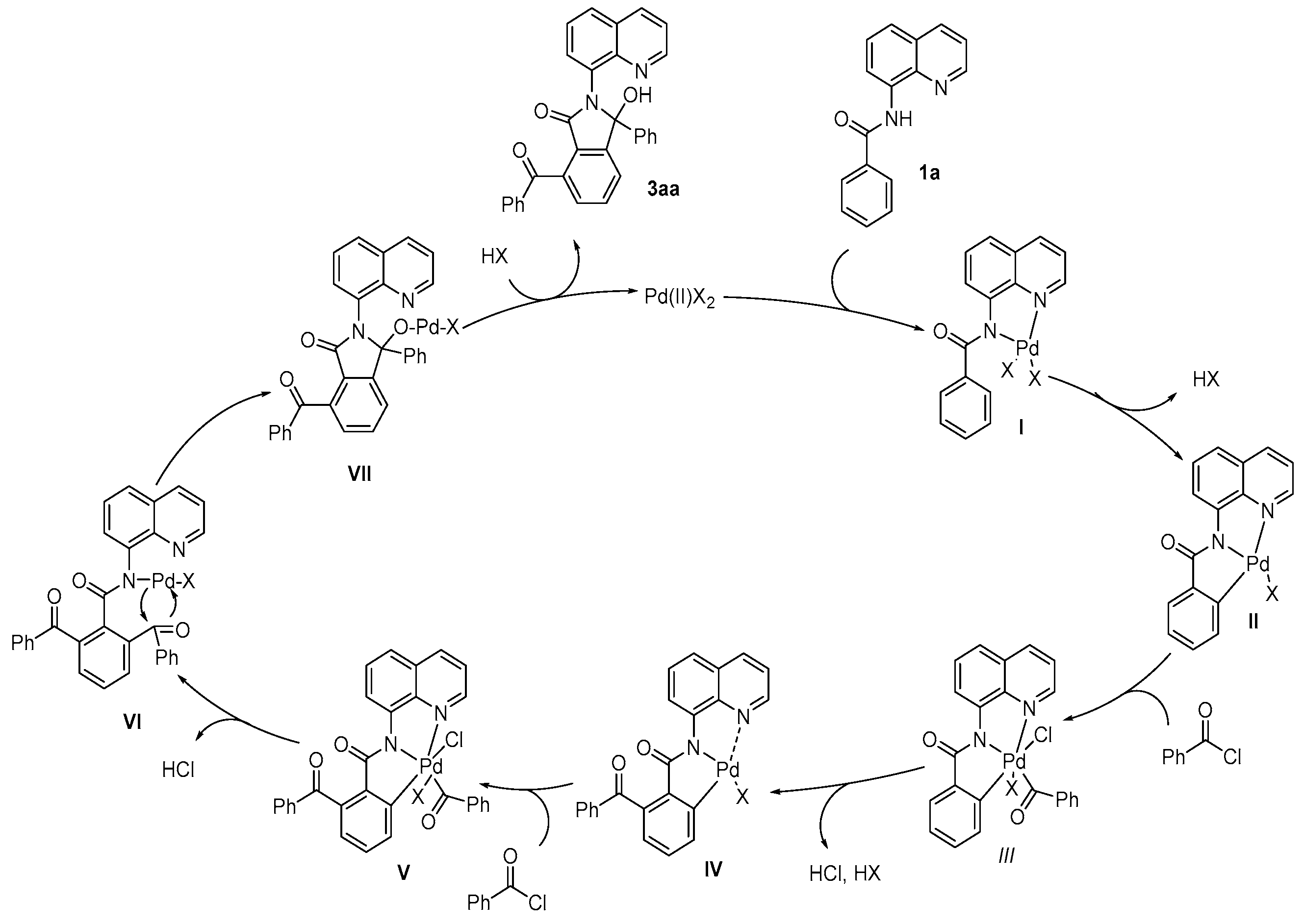
7-Benzoyl-3-hydroxy-3-phenyl-2-(quinolin-8-yl)isoindolin-1-one(3aa, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 83% yield (38 mg), mp 115-116 oC. 1H NMR (600 MHz, CDCl3) δ 10.72 (s, 1H), 8.82 (dd, J = 4.4, 1.7 Hz, 1H), 8.19 (dd, J = 8.3, 1.7 Hz, 1H), 7.93 – 7.89 (m, 2H), 7.70 (t, J = 7.6 Hz, 1H), 7.64 (dd, J = 8.2, 1.4 Hz, 1H), 7.60 (dd, J = 7.6, 1.4 Hz, 1H), 7.55 – 7.48 (m, 3H), 7.47 – 7.38 (m, 6H), 7.18 – 7.11 (m, 3H). 13C NMR (151 MHz, CDCl3) δ 196.6, 167.2, 150.7, 148.4, 143.6, 139.4, 138.1, 137.9, 137.7, 137.7, 137.1, 134.3, 133.6, 133.2, 132.0, 129.8, 129.4, 128.8, 128.2, 127.9, 127.5, 127.3, 127.2, 127.1, 126.9, 126.8, 124.0, 123.7, 121.2, 93.3. HRMS(ESI) m/z [M+H]+ Calcd for C30H20N2O3: 457.5010, found: 457.5086.
7-(4-Chlorobenzoyl)-3-(4-chlorophenyl)-3-hydroxy-2-(quinolin-8-yl)isoindolin-1-one(3ab, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 50% yield (26.25 mg), mp 125-128 oC. 1H NMR (600 MHz, CDCl3) δ 10.87 (s, 1H), 8.82 (dd, J = 4.4, 1.7 Hz, 1H), 8.22 (d, J = 8.2 Hz, 1H), 7.86 – 7.81 (m, 2H), 7.73 (d, J = 7.6 Hz, 1H), 7.71 – 7.67 (m, 1H), 7.59 (d, J = 7.5 Hz, 1H), 7.55 (dd, J = 7.5, 0.9 Hz, 1H), 7.50 – 7.44 (m, 3H), 7.39 – 7.37 (m, 2H), 7.35 – 7.32 (m, 2H), 7.16 – 7.11 (m, 2H). 13C NMR (151 MHz, CDCl3) δ 195.0, 167.0, 150.2, 148.5, 139.9, 138.2, 137.1, 135.5, 134.1, 133.6, 130.9, 129.5, 128.8, 128.4, 128.1, 128.0, 127.6, 124.3, 121.4, 92.9. HRMS(ESI) m/z [M+H]+ Calcd for C30H18Cl2N2O3: 525.0694, found: 525.0770.
7-(3-Chlorobenzoyl)-3-(3-chlorophenyl)-3-hydroxy-2-(quinolin-8-yl)isoindolin-1-one (3ac, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 57% yield (30 mg), mp 120-123 oC. 1H NMR (600 MHz, CDCl3) δ 10.61 (s, 1H), 8.80 (d, J = 4.4 Hz, 1H), 8.16 (d, J = 8.3 Hz, 1H), 7.90 (s, 1H), 7.76 – 7.70 (m, 2H), 7.65 (d, J = 8.2 Hz, 1H), 7.59 – 7.42 (m, 7H), 7.33 (d, J = 8.0 Hz, 1H), 7.25 – 7.21 (m, 1H), 7.11 (d, J = 5.0 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 195.2, 167.3, 150.6, 149.1, 143.9, 142.2, 139.1, 138.5, 137.2, 135.1, 134.6, 134.0, 133.7, 133.4, 132.3, 130.1, 129.9, 129.8, 129.6, 128.8, 128.4, 128.2, 128.1, 127.4, 127.3, 127.2, 125.2, 124.8, 121.8, 93.1. HRMS(ESI) m/z [M+H]+ Calcd for C30H18Cl2N2O3: 525.0694, found: 525.0775.
7-(2-Chlorobenzoyl)-3-(2-chlorophenyl)-3-hydroxy-2-(quinolin-8-yl)isoindolin-1-one (3ad, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 47% yield (25 mg), mp 114-117 oC. 1H NMR (600 MHz, CDCl3) δ 8.83 (dd, J = 4.4, 1.7 Hz, 1H), 8.18 (dd, J = 8.3, 1.7 Hz, 1H), 8.08 (dd, J = 6.9, 2.7 Hz, 1H), 7.84 (dd, J = 7.6, 1.4 Hz, 1H), 7.69 (d, J = 6.7 Hz, 2H), 7.64 (ddd, J = 11.0, 7.9, 1.5 Hz, 2H), 7.48 – 7.44 (m, 2H), 7.41 (dt, J = 6.4, 4.6 Hz, 2H), 7.39 – 7.34 (m, 1H), 7.29 – 7.27 (m, 1H), 7.15 – 7.12 (m, 1H), 7.08 (tt, J = 7.4, 5.3 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 195.1, 167.3, 149.3, 148.2, 143.3, 138.2, 138.1, 137.5, 136.2, 133.4, 133.1, 132.9, 132.5, 132.0, 130.9, 130.9, 130.7, 130.2, 130.0, 129.3, 128.9, 127.2, 127.2, 126.4, 126.2, 124.0, 121.2, 91.5. HRMS(ESI) m/z [M+H]+ Calcd for C30H18Cl2N2O3: 525.0694, found: 525.0774.
3-Hydroxy-2-(quinolin-8-yl)-7-(4-(trifluoromethyl)benzoyl)-3-(4-(trifluoromethyl)phenyl)isoindolin-1-one (3ae, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 59% yield (34.9 mg), mp 95-98 oC. 1H NMR (600 MHz, CDCl3) δ 10.98 (s, 1H), 8.84 (d, J = 4.4 Hz, 1H), 8.23 (d, J = 8.2 Hz, 1H), 8.01 (d, J = 7.9 Hz, 2H), 7.76 (t, J = 7.6 Hz, 1H), 7.68 (d, J = 8.1 Hz, 3H), 7.57 (dd, J = 21.6, 7.9 Hz, 4H), 7.52 – 7.44 (m, 5H) 13C NMR (151 MHz, CDCl3) δ 195.3, 167.0, 150.0, 148.6, 143.6, 139.7, 138.3, 136.8, 133.8, 131.8, 129.7, 129.6, 128.1, 127.8, 127.1, 126.9, 125.7, 125.6, 125.5, 125.4, 125.3, 125.2, 125.1, 125.0, 124.6, 121.5, 92.9. HRMS(ESI) m/z [M+H]+ Calcd for C32H18F6N2O3: 593.1222, found: 593.1296.
3-Hydroxy-2-(quinolin-8-yl)-7-(2-(trifluoromethyl)benzoyl)-3-(2-(trifluoromethyl)phenyl)isoindolin-1-one (3af, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 64% yield (37.9 mg), mp 108-110 oC. 1H NMR (600 MHz, CDCl3) δ 10.15 (s, 1H), 8.69 (dd, J = 4.2, 1.6 Hz, 1H), 8.50 (dd, J = 7.4, 1.6 Hz, 1H), 8.09 (dd, J = 8.3, 1.7 Hz, 1H), 7.68 – 7.63 (m, 4H), 7.59 (d, J = 7.5 Hz, 2H), 7.55 – 7.48 (m, 5H), 7.46 – 7.42 (m, 2H), 7.38 (dd, J = 8.3, 4.2 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 195.2, 165.6, 148.3, 138.6, 137.9, 137.8, 134.6, 134.5, 131.8, 131.2, 130.3, 129.3, 128.9, 128.6, 128.1, 127.5, 127.0, 127.0, 124.6, 122.8, 122.2, 121.8. HRMS(ESI) m/z [M+H]+ Calcd for C32H18F6N2O3: 593.1222, found: 593.1296.
7-(4-Fluorobenzoyl)-3-(4-fluorophenyl)-3-hydroxy-2-(quinolin-8-yl)isoindolin-1-one (3ag, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 51% yield (25 mg), mp 123-126 oC. 1H NMR (600 MHz, CDCl3) δ 10.10 (s, 1H), 8.81 (dd, J = 4.4, 1.8 Hz, 1H), 8.19 (dd, J = 8.3, 1.8 Hz, 1H), 7.95 – 7.90 (m, 2H), 7.72 (t, J = 7.6 Hz, 1H), 7.66 (dd, J = 8.3, 1.4 Hz, 1H), 7.59 – 7.35 (m, 7H), 7.10 – 7.04 (m, 2H), 6.85 (t, J = 8.7 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 194.6, 166.9, 161.5, 150.3, 148.5, 143.4, 138.1, 137.2, 135.4, 135.3, 133.6, 133.5, 133.4, 133.3, 132.2, 132.1, 131.8, 129.4, 128.5, 128.4, 127.8, 127.5, 126.9, 126.8, 124.2, 121.3, 115.6, 115.5, 115.1, 114.9, 92.9. HRMS(ESI) m/z [M+H]+ Calcd for C30H18F2N2O3: 493.1285, found: 493.1358.
7-(3-Fluorobenzoyl)-3-(3-fluorophenyl)-3-hydroxy-2-(quinolin-8-yl)isoindolin-1-one (3ah,new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 40% yield (19.7 mg), mp 108-110 oC. 1H NMR (600 MHz, CDCl3) δ 10.77 (s, 1H), 8.90 – 8.78 (m, 1H), 8.22 (s, 1H), 7.74 (t, J = 7.6 Hz, 1H), 7.69 (d, J = 8.2 Hz, 1H), 7.64 (d, J = 8.5 Hz, 2H), 7.56 (t, J = 8.4 Hz, 2H), 7.52 (d, J = 7.7 Hz, 1H), 7.47 (dt, J = 15.9, 7.2 Hz, 2H), 7.38 (q, J = 7.3 Hz, 1H), 7.24 – 7.19 (m, 2H), 7.15 (q, J = 7.5 Hz, 1H), 7.09 (d, J = 7.9 Hz, 1H), 6.84 (t, J = 8.5 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 195.0, 166.9, 163.4, 161.8, 150.1, 139.1, 136.9, 133.6, 130.1, 130.1, 129.8, 129.7, 129.5, 128.0, 124.4, 122.3, 121.4, 120.5, 120.4, 115.9, 115.7, 115.2, 115.0, 114.0, 113.8, 92.8. HRMS(ESI) m/z [M+H]+ Calcd for C30H18F2N2O3: 493.1285, found: 493.1360.
7-(2-Fluorobenzoyl)-3-(2-fluorophenyl)-3-hydroxy-2-(quinolin-8-yl)isoindolin-1-one (3ai, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 51% yield (25mg), mp 125-127 oC. 1H NMR (600 MHz, CDCl3) δ 8.81 (ddd, J = 12.1, 4.4, 1.7 Hz, 1H), 8.18 (ddd, J = 28.3, 8.4, 1.8 Hz, 1H), 7.90 (td, J = 7.6, 1.9 Hz, 1H), 7.86 – 7.84 (m, 1H), 7.73 – 7.64 (m, 2H), 7.63 – 7.56 (m, 2H), 7.56 – 7.50 (m, 1H), 7.47 – 7.39 (m, 3H), 7.23 – 7.17 (m, 1H), 7.11 (ddt, J = 7.3, 5.0, 2.4 Hz, 1H), 7.05 (dd, J = 10.9, 8.2 Hz, 1H), 6.95 – 6.91 (m, 1H), 6.78 (ddd, J = 11.6, 8.8, 5.4 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 193.2, 167.5, 158.4, 149.3, 148.5, 139.1, 135.0, 134.9, 133.3, 133.2, 131.5, 130.7, 129.4, 127.8, 127.4, 127.3, 124.1, 123.8, 122.0, 121.4, 116.8, 116.6, 115.8, 115.6, 90.8. HRMS(ESI) m/z [M+H]+ Calcd for C30H18F2N2O3: 493.1285, found: 493.1364.
Methyl4-(1-hydroxy-1-(4-(methoxycarbonyl)phenyl)-3-oxo-2-(quinolin-8-yl)isoindoline-4-carbonyl)benzoate (3aj, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 48% yield (27.5 mg), mp 111-113 oC. 1H NMR (600 MHz, CDCl3) δ 10.80 (s, 1H), 8.81 (dd, J = 4.5, 1.8 Hz, 1H), 8.19 – 8.13 (m, 1H), 8.09 – 8.05 (m, 2H), 7.94 (d, J = 8.4 Hz, 2H), 7.86 – 7.78 (m, 2H), 7.73 (t, J = 7.6 Hz, 1H), 7.65 – 7.61 (m, 1H), 7.58 (d, J = 7.5 Hz, 1H), 7.54 (dd, J = 7.6, 1.4 Hz, 1H), 7.52 – 7.45 (m, 3H), 7.44 (dd, J = 8.3, 4.4 Hz, 1H), 7.40 (t, J = 7.9 Hz, 1H), 3.87 (s, 3H), 3.81 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 196.0, 167.3, 166.8, 166.5, 150.4, 149.0, 144.9, 143.7, 140.8, 138.5, 137.3, 134.3, 134.0, 133.5, 132.1, 130.3, 130.0, 129.9, 129.8, 129.7, 128.6, 128.0, 127.4, 127.3, 127.1, 124.9, 121.8, 93.4, 77.6, 77.4, 77.2, 52.7, 52.4. HRMS(ESI) m/z [M+H]+ Calcd for C34H24N2O7: 573.1584, found: 573.1572.
3-Hydroxy-7-(4-methylbenzoyl)-2-(quinolin-8-yl)-3-(p-tolyl)isoindolin-1-one (3ak, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 53% yield (25.6mg), mp 115-118 oC. 1H NMR (600 MHz, CDCl3) δ 10.29 (s, J = 229.3 Hz, 1H), 8.81 (dt, J = 4.4, 2.6 Hz, 1H), 8.17 (d, J = 8.2 Hz, 1H), 7.81 (d, J = 7.8 Hz, 2H), 7.68 (t, J = 7.5 Hz, 1H), 7.61 (dd, J = 15.7, 7.9 Hz, 2H), 7.50 (d, J = 7.4 Hz, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.43 (q, J = 6.9 Hz, 2H), 7.28 (d, J = 7.9 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 6.96 (d, J = 7.9 Hz, 2H), 2.34 (s, 3H), 2.19 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 196.0, 167.2, 150.7, 144.2, 137.7, 136.6, 134.7, 133.2, 129.8, 129.4, 129.1, 128.9, 128.7, 127.6, 127.2, 126.5, 123.9, 121.2, 93.3, 21.6, 20.9. HRMS(ESI) m/z [M+H]+ Calcd for C32H24N2O3: 485.1787, found: 485.1864.
3-Hydroxy-7-(3-methylbenzoyl)-2-(quinolin-8-yl)-3-(m-tolyl)isoindolin-1-one (3al, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 67% yield (32.4mg), mp 122-125 oC. 1H NMR (600 MHz, CDCl3) δ 10.66 (s, 1H), 8.86 – 8.76 (m, 1H), 8.15 (d, J = 8.3 Hz, 1H), 7.80 (s, 1H), 7.70 (dd, J = 17.2, 8.4 Hz, 2H), 7.63 (d, J = 7.9 Hz, 2H), 7.53 (dd, J = 11.6, 7.6 Hz, 2H), 7.43 (q, J = 6.7 Hz, 2H), 7.37 – 7.25 (m, 4H), 7.08 (t, J = 7.6 Hz, 1H), 6.96 (d, J = 7.5 Hz, 1H), 2.37 (s, 3H), 2.22 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 196.6, 167.2, 150.7, 148.4, 143.6, 139.4, 138.1, 137.9, 137.7, 137.6, 137.1, 134.3, 133.6, 133.2, 132.0, 129.8, 129.4, 128.8, 128.2, 127.9, 127.5, 127.3, 127.2, 127.1, 126.9, 124.0, 123.7, 121.2, 93.3, 21.3, 21.2. HRMS(ESI) m/z [M+H]+ Calcd for C32H24N2O3: 485.1787, found: 485.1864.
7-Benzoyl-3-hydroxy-5-methyl-3-phenyl-2-(quinolin-8-yl)isoindolin-1-one (3ba, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 56% yield (26.4 mg), mp 125-127 °C. 1H NMR (600 MHz, CDCl3) δ 10.71 (s, 1H), 8.78 (d, J = 4.5 Hz, 1H), 8.12 (d, J = 8.3 Hz, 1H), 7.92 (d, J = 7.7 Hz, 2H), 7.59 (dd, J = 13.5, 7.8 Hz, 2H), 7.50 (t, J = 7.7 Hz, 1H), 7.45 – 7.37 (m, 6H), 7.31 (d, J = 27.5 Hz, 2H), 7.19 – 7.11 (m, 3H), 2.45 (s, 3H) ; 13C NMR (151 MHz, CDCl3) δ 196.7, 167.4, 151.1, 148.6, 144.7, 143.7, 139.9, 138.2, 137.6, 137.3, 133.9, 133.5, 132.0, 129.8, 129.5, 128.7, 128.5, 128.2, 128.1, 127.3, 127.0, 126.8, 124.7, 124.7, 121.4, 93.3, 22.0. HRMS(ESI) m/z [M+H]+ Calcd for C31H22N2O3: 471.1630, found: 471.1706.
7-Benzoyl-3-hydroxy-5-methoxy-3-phenyl-2-(quinolin-8-yl)isoindolin-1-one (3ca, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 49% yield (23.8 mg), mp 118-120 °C.1H NMR (600 MHz, CDCl3) δ 8.81 (dd, J = 4.4, 1.7 Hz, 1H), 8.15 (dd, J = 8.3, 1.7 Hz, 1H), 8.10 – 8.06 (m, 1H), 7.95 – 7.91 (m, 2H), 7.60 (dd, J = 8.0, 1.4 Hz, 1H), 7.56 (d, J = 7.5 Hz, 1H), 7.53 – 7.49 (m, 1H), 7.46 (t, J = 7.7 Hz, 1H), 7.43 – 7.39 (m, 5H), 7.19 – 7.15 (m, 2H), 7.15 – 7.12 (m, 1H), 7.03 (d, J = 2.2 Hz, 1H), 6.94 (d, J = 2.2 Hz, 1H), 3.84 (s, 3H ); 13C NMR (151 MHz, CDCl3) δ 196.1, 167.1, 164.1, 153.2, 148.5, 139.9, 139.2, 137.0, 133.7, 133.6, 132.1, 130.2, 129.9, 129.5, 128.6, 128.5, 128.2, 128.2, 127.3, 127.1, 126.7, 121.4, 119.7, 114.9, 108.6, 93.1, 56.1. HRMS(ESI) m/z [M+H]+ Calcd for C31H22N2O4: 487.1580, found: 487.1657.
7-Benzoyl-5-(tert-butyl)-3-hydroxy-3-phenyl-2-(quinolin-8-yl)isoindolin-1-one (3da, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 58% yield (29.7 mg), mp 117-120 oC. 1H NMR (600 MHz, CDCl3) δ 10.77 (s, 1H), 8.80 (dd, J = 4.5, 1.8 Hz, 1H), 8.15 (dd, J = 8.2, 1.9 Hz, 1H), 7.95 – 7.91 (m, 2H), 7.62 – 7.58 (m, 1H), 7.57 (d, J = 1.6 Hz, 1H), 7.55 (dd, J = 7.6, 1.3 Hz, 1H), 7.53 – 7.49 (m, 2H), 7.44 – 7.37 (m, 6H), 7.15 (dt, J = 15.0, 6.9 Hz, 3H), 1.34 (s, 9H). 13C NMR (151 MHz, CDCl3) δ 197.08, 167.31, 158.04, 150.82, 148.58, 143.80, 139.96, 138.17, 137.36, 137.21, 133.86, 133.53, 132.09, 129.87, 129.51, 128.55, 128.16, 128.11, 127.37, 127.07, 126.75, 125.51, 124.80, 121.41, 121.04, 93.57, 35.86, 31.34. HRMS(ESI) m/z [M+H]+ Calcd for C34H28N2O3: 513.2100, found: 513.2176.
7-Benzoyl-5-fluoro-3-hydroxy-3-phenyl-2-(quinolin-8-yl)isoindolin-1-one (3ea, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 63% yield (29.9 mg), mp 97-100 oC. 1H NMR (600 MHz, CDCl3) δ 11.27 – 10.51 (s, 1H), 8.81 (dd, J = 4.4, 1.7 Hz, 1H), 8.17 (dd, J = 8.4, 1.8 Hz, 1H), 7.91 (d, J = 7.6 Hz, 2H), 7.62 (dd, J = 15.5, 7.9 Hz, 2H), 7.54 (t, J = 7.4 Hz, 1H), 7.46 – 7.40 (m, 6H), 7.25 – 7.22 (m, 1H), 7.21 – 7.13 (m, 4H). 13C NMR (151 MHz, CDCl3) δ 194.8, 166.4, 148.7, 143.6, 139.2, 138.3, 136.7, 133.9, 133.5, 132.1, 129.8, 129.6, 128.7, 128.5, 128.4, 127.6, 127.1, 126.7, 121.5, 115.9, 115.7, 111.8, 111.6, 93.0. HRMS(ESI) m/z [M+H]+ Calcd for C30H19FN2O3: 475.1380, found: 475.1459.
7-Benzoyl-5-chloro-3-hydroxy-3-phenyl-2-(quinolin-8-yl)isoindolin-1-one (3fa, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 47% yield (23 mg), mp 96-100 oC. 1H NMR (600 MHz, CDCl3) δ 10.77 (s, 1H), 8.81 (dd, J = 4.4, 1.6 Hz, 1H), 8.17 (d, J = 8.3 Hz, 1H), 7.91 (d, J = 7.6 Hz, 2H), 7.64 (d, J = 8.2 Hz, 1H), 7.60 (d, J = 7.5 Hz, 1H), 7.57 – 7.49 (m, 2H), 7.47 – 7.39 (m, 7H), 7.19 (t, J = 7.1 Hz, 2H), 7.16 (dd, J = 8.3, 6.0 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 194.8, 166.4, 152.4, 148.7, 143.6, 139.9, 139.2, 139.1, 138.3, 136.8, 133.9, 133.4, 132.1, 129.9, 129.6, 128.7, 128.5, 128.4, 128.2, 127.7, 127.1, 126.7, 125.5, 124.7, 121.5, 93.1. HRMS(ESI) m/z [M+H]+ Calcd for C30H19ClN2O3: 491.1084, found: 491.1161.
7-Benzoyl-3-hydroxy-1-oxo-3-phenyl-2-(quinolin-8-yl)isoindoline-5-carboxylate (3ga, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 58% yield (29.8mg), mp 113-115 oC. 1H NMR (600 MHz, CDCl3) δ 8.83 (dd, J = 4.3, 2.0 Hz, 1H), 8.20 (d, J = 8.2 Hz, 2H), 8.13 (d, J = 1.6 Hz, 1H), 7.89 (d, J = 7.6 Hz, 2H), 7.67 (dd, J = 8.2, 1.8 Hz, 1H), 7.61 (dd, J = 7.6, 1.5 Hz, 1H), 7.54 (d, J = 7.3 Hz, 1H), 7.49 – 7.46 (m, 1H), 7.43 (ddd, J = 14.5, 8.4, 5.7 Hz, 5H), 7.17 (dt, J = 14.9, 6.9 Hz, 3H), 3.92 (d, J = 1.9 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 195.3, 166.3, 165.4, 150.9, 148.6, 143.4, 138.9, 138.1, 137.7, 136.7, 134.7, 133.7, 133.3, 131.9, 130.5, 129.7, 129.4, 129.1, 128.5, 128.3, 128.2, 127.6, 127.0, 126.6, 125.3, 121.4, 93.3, 52.6. HRMS(ESI) m/z [M+H]+ Calcd for C32H22N2O5: 515.1529, found: 515.1609.
7-Benzoyl-3-hydroxy-3-phenyl-2-(quinolin-8-yl)-5-vinylisoindolin-1-one (3ha, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 55% yield (26.5mg), mp 120-123 oC. 1H NMR (600 MHz, CDCl3) δ 10.61 (s, J = 99.1 Hz, 1H), 8.81 (d, J = 4.6 Hz, 1H), 8.15 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 7.7 Hz, 2H), 7.60 (dd, J = 12.8, 7.8 Hz, 2H), 7.55 (s, 1H), 7.53 – 7.48 (m, 2H), 7.45 – 7.37 (m, 6H), 7.21 – 7.11 (m, 3H), 6.76 (dd, J = 17.6, 10.8 Hz, 1H), 5.87 (d, J = 17.6 Hz, 1H), 5.41 (d, J = 10.9 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 196.2, 166.8, 151.2, 148.4, 142.9, 139.5, 137.8, 137.0, 135.3, 133.4, 129.7, 129.3, 128.4, 128.1, 128.0, 126.9, 126.6, 126.2, 125.8, 121.5, 121.2, 117.7, 93.2. HRMS(ESI) m/z [M+H]+ Calcd for C32H22N2O3: 483.5390, found: 483.1703.
7-Benzoyl-3-hydroxy-3,5-diphenyl-2-(quinolin-8-yl)isoindolin-1-one (3ia, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 62% yield (32.9 mg), mp 118-121 oC. 1H NMR (600 MHz, DMSO-d6) δ 8.83 (dd, J = 4.2, 1.8 Hz, 1H), 8.32 (d, J = 8.3 Hz, 1H), 7.89 (ddd, J = 13.3, 5.6, 3.2 Hz, 4H), 7.75 – 7.72 (m, 2H), 7.69 – 7.65 (m, 2H), 7.57 (ddd, J = 29.5, 14.9, 7.5 Hz, 5H), 7.50 – 7.45 (m, 3H), 7.43 – 7.40 (m, 1H), 7.17 – 7.09 (m, 3H). 13C NMR (151 MHz, DMSO-d6) δ 195.4, 165.0, 151.3, 149.9, 145.0, 143.7, 139.3, 138.2, 137.3, 136.7, 133.7, 133.4, 129.5, 129.3, 128.8, 128.7, 128.6, 128.2, 128.1, 127.8, 127.3, 127.1, 126.7, 126.6, 126.1, 126.0, 122.0, 121.6, 92.7. HRMS(ESI) m/z [M+H]+ Calcd for C36H24N2O3 : 533.1787, found: 533.1861.
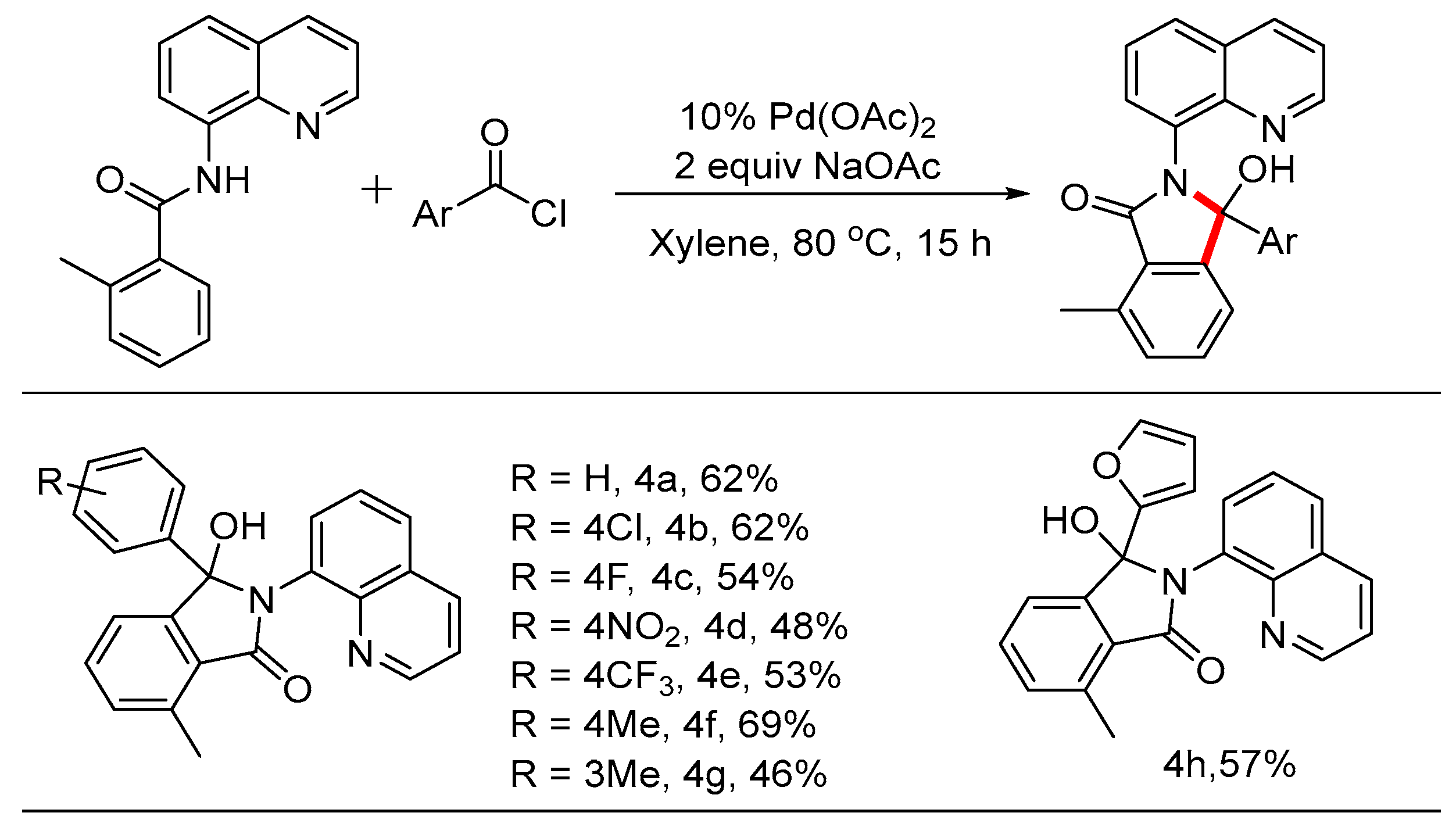
3.4. General Procedure for the Synthesis of Compound 4
A Schlenk tube was equipped with a magnetic stir bar and charged with 2-methyl-N-(quinolin-8-yl)benzamide 1i (0.1 mmol), 2 (0.25 mmol), NaOAc (0.2mmol, 16 mg), Pd(OAc)2 (0.01mmol, 2.3 mg) and xylene (2 mL). Then the flask was sealed under N2 and stirred at 80 °C for 16 h. After the reaction was quenched by addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. Concentration in vacuo followed by silica gel column purification with petroleum ether /ethyl acetate eluent gave the desired product 4.
3-Hydroxy-7-methyl-3-phenyl-2-(quinolin-8-yl)isoindolin-1-one (4a, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 62% yield (22.7 mg), mp 130-132 oC. 1H NMR (600 MHz, CDCl3) δ 9.85 (s, 1H), 8.80 (dd, J = 4.3, 1.7 Hz, 1H), 8.18 (dd, J = 8.3, 1.8 Hz, 1H), 7.69 (dd, J = 8.2, 1.4 Hz, 1H), 7.59 (dd, J = 7.5, 1.4 Hz, 1H), 7.50 – 7.44 (m, 2H), 7.43 – 7.40 (m, 3H), 7.29 (d, J = 7.6 Hz, 1H), 7.20 – 7.10 (m, 4H), 2.81 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 170.2, 151.4, 149.3, 140.6, 138.3, 138.2, 133.2, 132.6, 131.4, 129.8, 128.3, 128.1, 127.9, 127.2, 126.9, 126.8, 121.7, 120.6, 92.7, 17.9. HRMS(ESI) m/z [M+Na]+ Calcd for C24H18N2O2: 389.1368, found: 389.1262.
3-(4-Chlorophenyl)-3-hydroxy-7-methyl-2-(quinolin-8-yl)isoindolin-1-one (4b, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 62% yield (24.8 mg), mp 107-109 oC. 1H NMR (600 MHz, CDCl3) δ 10.08 – 9.88 (m, 1H), 8.79 (d, J = 4.3 Hz, 1H), 8.20 (d, J = 8.3 Hz, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.59 (d, J = 7.4 Hz, 1H), 7.52 – 7.41 (m, 3H), 7.34 (d, J = 8.5 Hz, 2H), 7.29 (d, J = 7.6 Hz, 1H), 7.14 (dd, J = 15.7, 7.9 Hz, 3H), 2.80 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 170.0, 151.0, 149.3, 139.4, 138.4, 138.4, 134.1, 133.3, 132.5, 131.7, 129.9, 128.5, 128.5, 128.1, 127.4, 126.7, 121.8, 120.5, 92.3, 17.9. HRMS(ESI) m/z [M+Na]+ Calcd for C24H17ClN2O2: 423.0979, found: 423.0876.
3-(4-Fluorophenyl)-3-hydroxy-7-methyl-2-(quinolin-8-yl)isoindolin-1-one (4c, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 54% yield (20.7mg), mp 114-116 oC. 1H NMR (600 MHz, CDCl3) δ 9.96 (d, J = 90.1 Hz, 1H), 8.84-8.79 (m, 1H), 8.23 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 8.1 Hz, 1H), 7.56 (dd, J = 7.5, 1.5 Hz, 1H), 7.52 (d, J = 7.9 Hz, 1H), 7.47 (t, J = 7.6 Hz, 2H), 7.40-7.33 (m, 2H), 7.30 (d, J = 7.6 Hz, 1H), 7.17 (d, J = 7.5 Hz, 1H), 6.84 (t, J = 8.7 Hz, 2H), 2.80 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 169.6, 163.0, 161.4, 150.7, 148.9, 138.0, 136.1, 136.1, 132.9, 132.1, 131.2, 129.5, 128.5, 128.4, 127.6, 126.9, 126.3, 121.4, 120.1, 114.9, 114.7, 92.0, 17.5. HRMS(ESI) m/z [M+H]+ Calcd for C24H17FN2O2: 385.1274, found: 385.1346.
3-Hydroxy-7-methyl-3-(4-nitrophenyl)-2-(quinolin-8-yl)isoindolin-1-one (4d, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 48% yield (19.7 mg), mp 118-120 oC. 1H NMR (600 MHz, CDCl3) δ 10.28 (s, 1H), 8.82 (tt, J = 4.5, 1.3 Hz, 1H), 8.25 (ddt, J = 8.5, 4.1, 1.8 Hz, 1H), 8.04 – 7.99 (m, 2H), 7.75 (ddt, J = 8.1, 3.4, 1.6 Hz, 1H), 7.60 (t, J = 8.5 Hz, 3H), 7.55 – 7.45 (m, 3H), 7.35 – 7.30 (m, 1H), 7.13 (d, J = 7.5 Hz, 1H), 2.80 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 169.8, 150.3, 149.4, 148.3, 147.8, 144.3, 138.7, 138.5, 133.7, 133.5, 132.4, 132.0, 129.9, 128.3, 128.1, 127.4, 126.6, 123.7, 121.9, 120.5, 91.9, 17.9. HRMS(ESI) m/z [M+H]+ Calcd for C24H17N3O4: 412.1219, found: 412.1286.
3-Hydroxy-7-methyl-2-(quinolin-8-yl)-3-(4-(trifluoromethyl)phenyl)isoindolin-1-one (4e,new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 53% yield (23.0mg), mp 123-126 oC. 1H NMR (600 MHz, CDCl3) δ 10.11 (s, 1H), 8.82 (d, J = 4.3 Hz, 1H), 8.23 (d, J = 8.3 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.60 (d, J = 7.4 Hz, 1H), 7.54 (t, J = 9.0 Hz, 3H), 7.46 (dt, J = 21.6, 7.9 Hz, 4H), 7.31 (d, J = 7.6 Hz, 1H), 7.14 (d, J = 7.5 Hz, 1H), 2.81 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 170.0, 150.8, 149.4, 145.0, 138.6, 138.4, 133.9, 133.4, 132.6, 131.8, 129.9, 128.2, 127.5, 127.4, 126.6, 125.4, 125.4, 125.4, 125.4, 121.9, 120.6, 92.2, 17.9. HRMS(ESI) m/z [M+H]+ Calcd for C25H17F3N2O2: 457.1242, found: 457.1136.
3-Hydroxy-7-methyl-2-(quinolin-8-yl)-3-(p-tolyl)isoindolin-1-one (4f, new compound):Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 69% yield (26.2mg), mp 116-119 oC. 1H NMR (600 MHz, CDCl3) δ 9.81 (s, 1H), 8.80 (dd, J = 4.4, 1.7 Hz, 1H), 8.20 – 8.16 (m, 1H), 7.70 (dd, J = 8.2, 1.4 Hz, 1H), 7.62 – 7.58 (m, 1H), 7.51 – 7.41 (m, 3H), 7.28 (t, J = 7.9 Hz, 3H), 7.18 (d, J = 7.5 Hz, 1H), 6.97 (d, J = 8.0 Hz, 2H), 2.81 (s, 3H), 2.21 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 169.75, 151.14, 148.84, 137.78, 137.40, 137.19, 132.75, 130.94, 129.39, 128.60, 127.42, 126.84, 126.43, 126.30, 121.24, 120.16, 92.37, 20.91, 17.48. HRMS(ESI) m/z [M+Na]+ Calcd for C25H20N2O2: 403.1525, found: 403.1424.
3-Hydroxy-7-methyl-2-(quinolin-8-yl)-3-(m-tolyl)isoindolin-1-one (4g, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 46% yield (17.5mg), mp 115-117 oC. 1H NMR (600 MHz, CDCl3) δ 9.69 (s, 1H), 8.81 (d, J = 4.2 Hz, 1H), 8.20 (d, J = 8.3 Hz, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.59 (d, J = 7.4 Hz, 1H), 7.52 – 7.43 (m, 3H), 7.28 (d, J = 7.6 Hz, 1H), 7.24 (s, 1H), 7.19 (t, J = 6.7 Hz, 2H), 7.06 (t, J = 7.6 Hz, 1H), 6.95 (d, J = 7.5 Hz, 1H), 2.80 (s, 3H), 2.20 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 169.8, 151.2, 140.1, 137.9, 137.5, 132.8, 131.0, 129.5, 128.5, 127.8, 127.5, 127.1, 123.7, 121.3, 120.2, 92.4, 21.3, 17.5. HRMS(ESI) m/z [M+Na]+ Calcd for C25H20N2O2: 403.1525, found: 403.1424.
3-(Furan-2-yl)-3-hydroxy-7-methyl-2-(quinolin-8-yl)isoindolin-1-one (4h, new compound): Following the general procedure the title compound was isolated by flash chromatography (eluent: petrol ether/ethyl acetate = 2/1~1/1) as a white solid in 57% yield (20.3 mg), mp 122-125 oC. 1H NMR (600 MHz, CDCl3) δ 8.87 (dd, J = 4.4, 1.8 Hz, 1H), 8.31 (dd, J = 8.3, 1.7 Hz, 1H), 7.84 (dd, J = 8.2, 1.3 Hz, 2H), 7.64 (s, 1H), 7.59 (d, J = 7.5 Hz, 1H), 7.53 (dd, J = 8.3, 4.4 Hz, 1H), 7.42 (dd, J = 15.2, 7.5 Hz, 2H), 7.26 (d, J = 1.4 Hz, 1H), 6.50 (d, J = 3.3 Hz, 1H), 6.20 (dd, J = 3.3, 1.8 Hz, 1H), 2.88 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 169.2, 148.8, 147.9, 142.7, 138.0, 132.6, 131.5, 129.3, 127.5, 127.5, 126.9, 126.5, 121.3, 119.9, 109.9, 109.3, 88.9, 17.4. HRMS(ESI) m/z [M+Na]+ Calcd for C22H16N2O3: 379.1161, found: 379.1054.