1. Introduction
Brazil is home to an extraordinary diversity of avian species, with 1,834 species currently identified [
1,
2], many of which are endemic to the Amazon region [
3,
4]. Among these, the genus Aratinga stands out, comprising over 21 species distributed across the Neotropical region [
5]. These birds, some reaching up to 30 cm in length and weighing around 130 grams [
6], are a significant part of the local fauna. One of the most notable species in this genus is
Aratinga jandaya (Jandaya parakeet), a bird distinguished by its vibrant red-orange plumage. Native to northern and central-eastern South America, this species is considered of "Least Concern" in Brazil, although it is listed as endangered in the state of Ceará according to the Brazilian Biodiversity Information System (SiBBr) [
7]. Similar to other psittaciformes, these birds often live in pairs or groups and feed primarily on fruits and seeds, but their diet may also include flowers, nectar, leaves, invertebrates, and minerals from the soil [
5,
8].
However, despite their adaptability,
Jandaya parakeet face significant challenges in their survival. One of the primary threats is illegal wildlife trade, which disrupts their populations [
9]. Additionally, human activities such as mining, urbanization, and port operations pose a serious threat to their health due to the risk of exposure to heavy metals [
10]. This environmental contamination is particularly concerning in urban areas where these birds often come into contact with pollutants, including heavy metals that can be absorbed through their diet as they search for materials for their nests, for reproduction [
9,
10].
Birds possess a unique digestive system that includes a crop, which is present in this species but absent in many other avian orders, followed by two distinct stomach regions: the proventriculus (glandular stomach) and the ventriculus (muscular stomach). While the crop serves as a storage area, the proventriculus is the most common site for food accumulation before digestion progresses to the ventriculus, which is specialized for grinding and mechanical breakdown. [
11]. These are common sites for the accumulation of heavy metals, leading to health issues in the affected birds [
12,
13]. Heavy metals are typically categorized as either essential (such as copper, nickel, chromium, zinc, iron, cobalt, and manganese) or non-essential (such as arsenic, lead, cadmium, mercury, aluminum, titanium, tin, and tungsten) [
13]. While essential metals play a role in metabolic processes, non-essential metals can cause harm at any concentration [
14]. Among these, lead (Pb) and zinc (Zn) are most frequently implicated in cases of heavy metal poisoning in psittaciformes [
15].
Lead poisoning in birds can affect multiple systems, including hematopoietic, nervous, gastrointestinal, renal, cardiovascular, and reproductive systems. Clinical signs of lead toxicity in birds include lethargy, weakness, weight loss, somnolence, paresis, ataxia, convulsions, greenish droppings, diarrhea, crop stasis, regurgitation, and drooping wings. The latter symptom may be related to demyelination of the brachial plexus nerves. Zinc poisoning, on the other hand, primarily affects the gastrointestinal system but can also involve the nervous system, with clinical signs including lethargy, weakness, polydipsia, polyuria, diarrhea, and regurgitation [
16].
The diagnosis of heavy metal poisoning in birds is typically based on laboratory tests, blood metal analysis, and imaging studies [
17]. Both lead and zinc toxicity can impair heme synthesis, shorten erythrocyte lifespan, and cause hemolysis and anemia [
18]. Specific laboratory findings for lead poisoning include hemoglobinuria and elevated biochemical analytes such as glutamate dehydrogenase (GLDH) and aspartate aminotransferase (AST) due to myocyte and hepatocyte damage, as lead can accumulate in hepatocytes. Creatine phosphokinase (CPK) levels may also rise due to muscle damage or hemolysis [
16,
19]. In cases of zinc toxicity, hemolytic anemia and iron deficiency may be observed, as zinc interferes with iron absorption, potentially leading to hypochromia, hypoxia, and, in severe cases, death. Morphological changes in erythrocytes and greenish droppings may also be noted [
18]. Radiography is a valuable tool for detecting radiopaque foreign bodies in the gastrointestinal tract, such as ingested metals [
12].
Once diagnosed, the treatment for heavy metal poisoning typically involves chelation therapy. Calcium disodium ethylenediaminetetraacetic acid (Ca-EDTA) is the chelating agent of choice in avian species [
20] due to its safety profile and minimal adverse effects [
21]. This chelator is distributed primarily in extracellular fluids, undergoes minimal metabolism, and is rapidly excreted by glomerular filtration, with approximately 50% of the administered dose eliminated via urine [
22]. Despite its low gastrointestinal absorption, Ca-EDTA can be administered orally at higher doses than the injectable form, at 8-12 hours intervals [
23]. The recommended dosage is 20-50 mg/kg BID, intramuscularly, for seven days or until blood metal concentrations return to normal [
24].
Another commonly used chelator is meso-2,3-dimercaptosuccinic acid (DMSA), which is administered orally and has good gastrointestinal absorption [
22]. DMSA chelates lead, cadmium, mercury, and arsenic, and is metabolized in the liver and excreted in the urine [
25]. Unlike Ca-EDTA, DMSA does not deplete essential minerals such as calcium, iron, and magnesium [
26]. The recommended dose is 40 mg/kg orally for treating acute and severe metal poisoning in birds. Treatment should continue until clinical signs stabilize, blood metal concentrations decrease, and the metal is fully removed from the gastrointestinal tract [
26]. However, a drawback of DMSA is its strong sulfur odor and taste, which may lead to regurgitation during administration [
26]. It is also important to note that DMSA does not cross cell membranes [
22]. Other treatment options include the use of emollient laxatives, endoscopy, and surgery, although invasive procedures carry additional risks for the patient’s survival [
27,
28].
Given the significant health risks posed by heavy metal poisoning, this study aims to explore two cases of suspected heavy metal toxicity in free-living A. jandaya individuals treated at the Veterinary Hospital of the Federal University of Pará (UFPA) – Wild Animal Sector (HVSAS) in Castanhal, Pará. This study seeks to highlight the effectiveness of the therapeutic protocol used in these birds, emphasizing the importance of early diagnosis and intervention in managing heavy metal exposure in urban environments.
2. Materials and Methods
This study involved two adult Jandaya parakeets (
Aratinga jandaya) admitted to the Wild Animal Sector of the Veterinary Hospital (HVSAS) at the Federal University of Pará (UFPA). The birds (
Figure 1A and B) were voluntarily surrendered by a private individual who found them disoriented in the yard of a residence in the Vila do Apeú district, located in the municipality of Castanhal, Pará, within the Brazilian Amazon Biome (
Figure 2). All procedures followed ethical guidelines for wildlife treatment and handling, and the ethical approval for this study was granted by the UFPA Animal Ethics Committee under the approval code 8888280618 (ID 002193).
2.1. Clinical Evaluation [16,24,29]
Upon arrival at the veterinary hospital, both parakeets underwent a comprehensive clinical examination. The first bird (Bird 1) was active and responsive, weighing 102 grams with a body condition score of 3/5. While its nostrils appeared dry with no nasal discharge, the bird did exhibit signs of diarrhea. The second bird (Bird 2), weighing 98 grams, had a pectoral muscle score of 2/5 and displayed wing weakness as determined by the “wing withdrawal” test. Both body weights were within the expected range for the Aratinga genus. Cloacal temperatures for both birds were recorded at 42°C, with heart rates of 160 beats per minute (bpm) for Bird 1 and 152 bpm for Bird 2. Neither bird showed significant interest in the provided food.
A detailed physical examination was conducted to rule out any potential trauma, particularly in the limbs, with no evidence of wing trauma or fractures found. Transillumination of the trachea revealed no fluid accumulation or presence of parasites. Respiratory auscultation showed no abnormalities. During coelomic palpation, no masses, fluid accumulation, or hepatomegaly were detected. Additionally, there were no signs of external hematomas or bruising on the body, despite the reported fall. From the photographs provided, an inspection revealed both birds had feathers beneath their beaks stained, suggesting possible regurgitation. These findings, in combination with the physical exam, helped guide further diagnostic considerations.
2.2. Radiographic Examination [30]
A comprehensive radiographic examination was performed on both birds to assess for potential traumatic injuries, given that they were found in a disoriented and fallen state. Skull radiographs were taken in rostrocaudal and laterolateral projections to evaluate for cranial trauma, but no signs of bone abnormalities were observed. Additionally, full-body radiographs, including the coelomic cavity, were conducted in ventrodorsal and right laterolateral projections as part of a thorough screening process. These radiographs aimed to rule out traumatic brain injury (TBI), internal organ trauma, and assess the respiratory system and other internal organs. This screening was essential to exclude skeletal or internal damage as the underlying cause of the birds' condition.
2.3. Hematological Analysis and Hemoparasite Screening [14,16]
Blood samples were collected from the jugular vein of each bird after an overnight fast. Chemical restraint was achieved via isoflurane anesthesia, administered through a face mask vaporization system. Hematological evaluations, including a complete blood count (CBC) and a blood smear for hemoparasite (because the animals had diarrhea) screening, were performed. The blood smears were examined microscopically for hemoparasites. However, due to limited laboratory infrastructure, it was not possible to perform further toxicological analyses, such as tissue or blood sample collection for the determination of heavy metal concentrations.
2.4. Therapeutic Intervention [20,21,22]
Based on clinical suspicion of heavy metal poisoning, a therapeutic protocol was initiated. Although confirmatory toxicological testing could not be performed, supportive care and chelation therapy were administered to address potential heavy metal toxicity. The therapeutic plan included fluids, nutritional support, and administration of chelating agents, following established protocols for avian species with suspected metal poisoning. However, details on the specific chelation agents used and their dosages could not be included due to the lack of detailed medical records.
2.5. Limitations of the Study
One of the main limitations of this study was the inability to collect and analyze samples for heavy metal determination due to constraints in physical infrastructure and specialized personnel. This limitation restricted the definitive identification of the type and concentration of heavy metals involved in the suspected poisoning case. Further studies with access to appropriate toxicological testing facilities are necessary to corroborate clinical findings and enhance diagnostic accuracy [
16].
3. Results
On the first day of hospitalization, both Aratinga jandaya birds were subjected to maintenance fluid therapy using lactated Ringer's solution (50 ml/kg), supplemented with B-complex vitamins (3 mg/kg), administered subcutaneously in the left flank. This protocol was maintained for 12 consecutive days, administered once daily (SID). In addition, nebulization therapy with gentamicin sulfate (4 mg/kg) was initiated twice daily (BID) for 14 days.
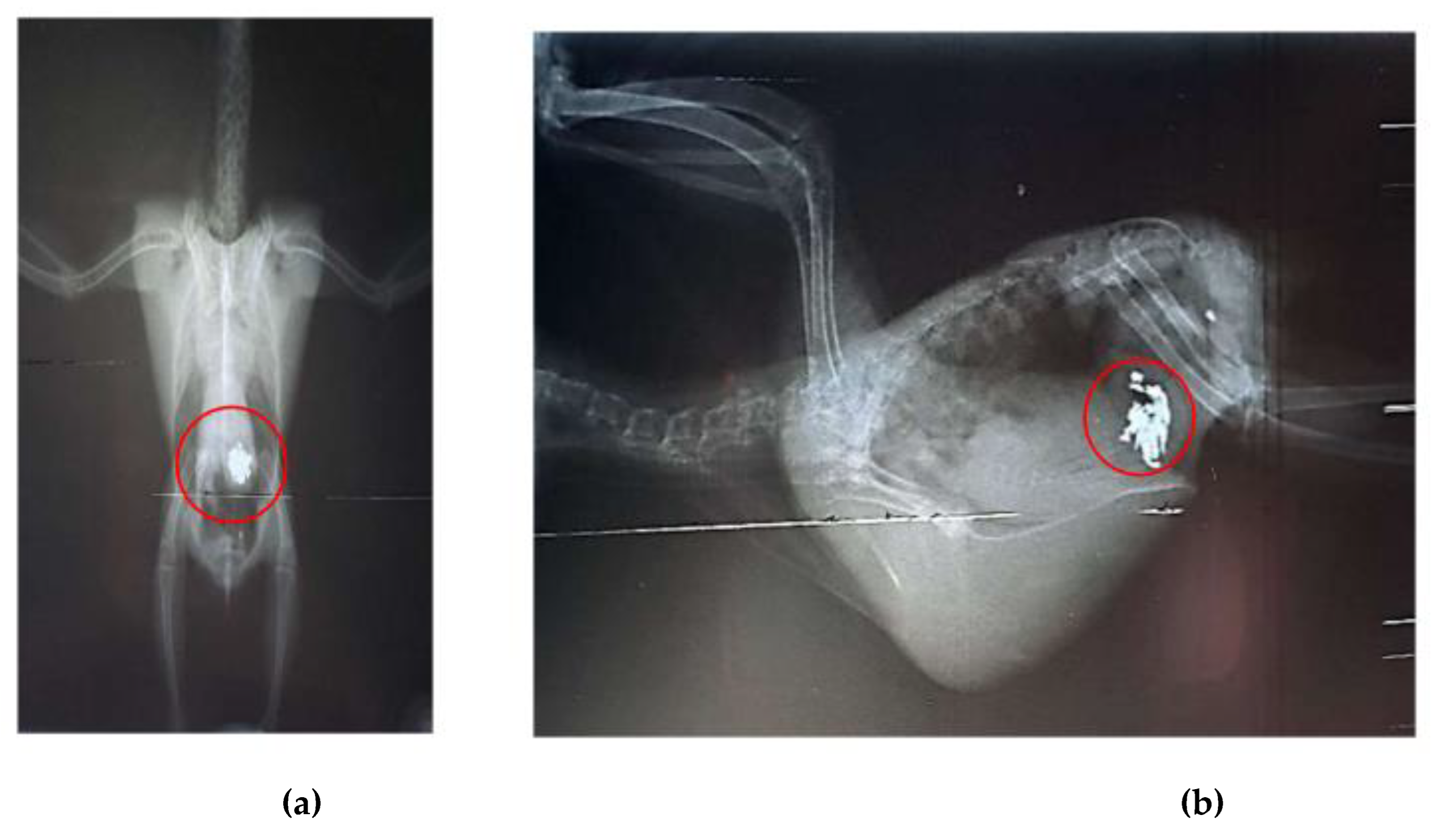
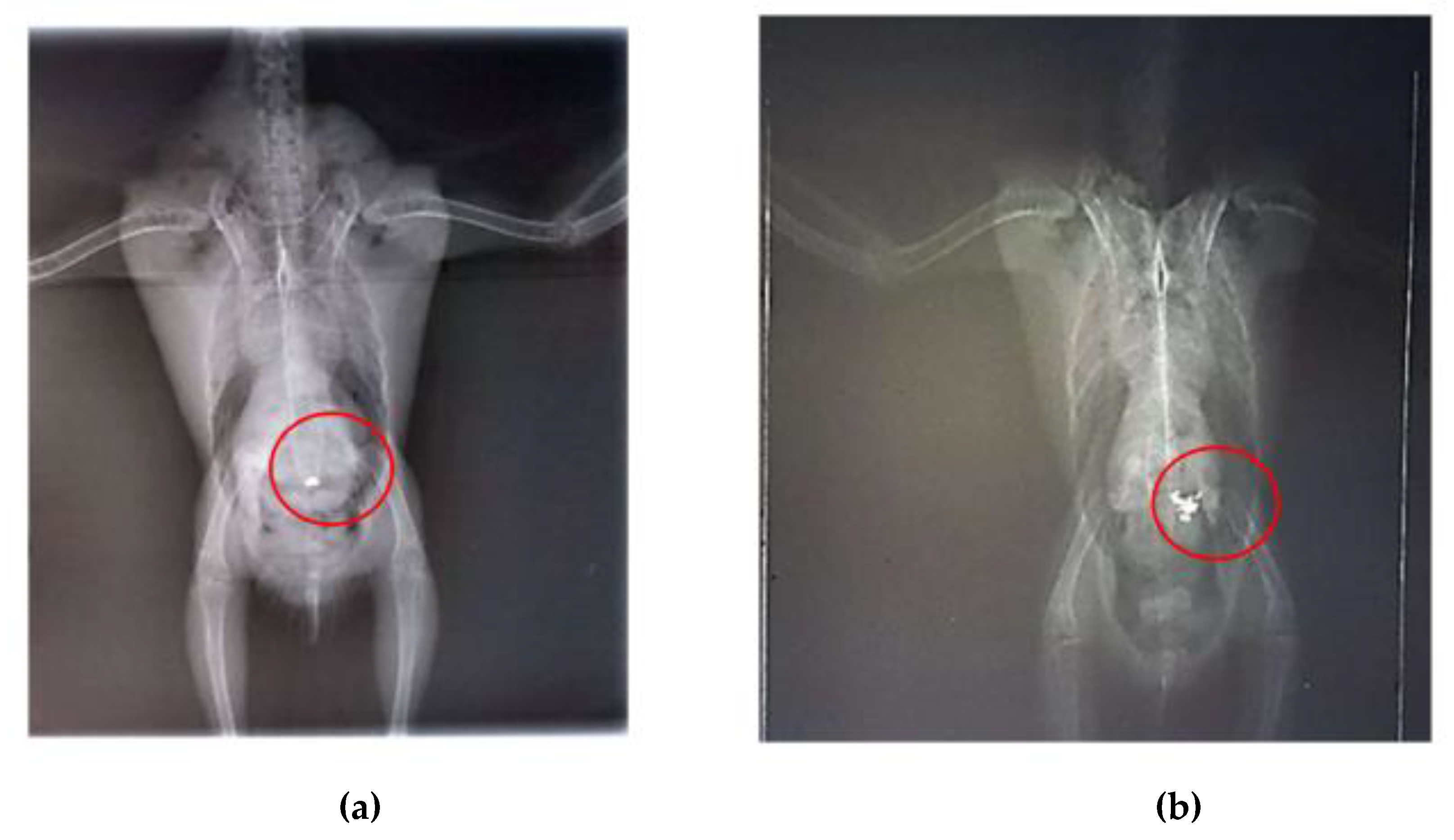
Radiographic examinations revealed a significant accumulation of radiopaque foreign bodies in the ventriculus of both birds, suggestive of metal ingestion (
Figure 3A, B and
Figure 4A, B). The hematological results are presented in
Table 1.
During hospitalization, the two birds were housed together as they appeared to be a bonded pair. By the third day, both patients were active, vocalizing, perching, and feeding well, with the first bird's droppings returning to a normal appearance. On the fifth day, chelation therapy with calcium ethylenediaminetetraacetic acid (calcium EDTA) (70 mg/kg) was initiated as soon as it was delivered by the compounding pharmacy. The therapy was administered orally (PO) twice daily (BID) for seven days.
On the seventh and eighth days of hospitalization, bird 1 exhibited signs of lethargy and puffed-up feathers, while bird 2 also showed puffed-up feathers and had difficulty maintaining its perch. Both birds were placed in the Avian Treatment Unit (ATU), where humidity and temperature were carefully controlled. Once their clinical signs improved, they were returned to the main enclosure.
After seven days of chelation therapy, the birds underwent repeat radiographs of the coelomic cavity under sedation with intranasal midazolam (0.01 ml). In these radiographs, a reduction in the amount of metallic content in the ventriculus of bird 1 was observed, and chelation therapy with calcium EDTA was continued for an additional three days. For bird 2, the radiographs taken after the start of chelation therapy showed no remaining foreign bodies in the ventriculus; however, an area of radiopacity was noted in the pulmonary field (Figures 5A, B). It is important to note that no metallic objects were observed in the feces of either bird.
In addition to the chelation therapy, two supplements were administered to both birds. Glicopan Pet® (0.5 ml/kg) was given orally once daily (SID) for seven days, and Omega 3® (0.22 ml/kg) was also administered orally once daily (SID) for seven days. The doses for both birds were based on a formulary for exotic animals, specifically referencing cockatiels and parrots [
29]. During the hospitalization period, the birds were placed in an outdoor area (solarium) for daily sunbathing, and they were occasionally observed sleeping in close proximity, interacting, and even exhibiting mating behavior.
After 21 days, new radiographs of the coelomic cavity were performed, and no abnormalities were observed. Both birds had normalized appetites, no diarrhea, normal feces, and were alert, active, perching well, and interacting positively. Consequently, after one month and six days of hospitalization and treatment, the A. jandaya pair was medically cleared for discharge and released back into the wild.
4. Discussion
The specimens of
Aratinga jandaya were discovered in a residential backyard in Vila do Apeú, a district or neighborhood of Castanhal in Pará state, Brazil [
31]. This area has undergone extensive landscape transformations due to deforestation, mining, and agriculture [
32], leading to irreversible and persistent habitat changes. These changes negatively impact the local fauna, including species such as
A. jandaya, which seek refuge for shelter and reproduction [
33]. Attracted to shiny metallic objects, birds may inadvertently ingest harmful materials, leading to poisoning [
34]. Common objects like nails, wires, screws, and metal fences, along with materials used in construction like aluminum or galvanized steel roofing, are abundant in residential areas [
35,
36]. Most of these metals, particularly galvanized materials, contain high levels of zinc, which may have been ingested by the birds as used by them as material for building nests, during reproductive periods [
37].
Zinc (Zn) poisoning has been documented in various bird species, particularly those in captivity, as well as in aquatic and zoo birds [
15]. Although zinc is essential for many physiological processes, including protein biosynthesis, immune response, and antioxidant activity, excessive exposure can lead to toxicity, especially in avian species [
38]. Birds, unlike mammals, are more sensitive to elevated zinc levels, with serum or plasma concentrations above 2.0 ppm often indicating overexposure [
39]. Free-ranging birds, like those in this case, may be exposed to zinc through environmental contamination or ingestion of zinc-based rodenticides [
40]. Given the free-ranging nature of the birds in this report, environmental exposure remains a plausible source of intoxication.
Birds tend to suffer more severely from zinc poisoning than mammals due to their accelerated metabolism [
41]. Zinc absorption in birds occurs mainly in the proventriculus and small intestine, with the metal accumulating in vital organs such as the pancreas, liver, kidneys, and gastrointestinal tract [
42,
43]. These organs contain zinc-binding proteins, such as metallothionein, responsible for metal storage and regulation [
44]. Clinical signs of zinc toxicity, including depression, lethargy, anorexia, and weight loss, often reflect the gastrointestinal and systemic damage caused by the metal. In this case, the affected birds exhibited symptoms consistent with zinc poisoning, such as nasal dryness, diarrhea, weight loss, anorexia, apathy, and respiratory distress, confirmed through radiographs [
39].
Behavioral alterations in birds suffering from heavy metal poisoning, such as difficulty standing, posture instability, reduced activity, and loss of appetite, can indicate pain, which should be factored into the therapeutic approach [
45]. Ingestion of foreign metallic objects often causes gastrointestinal obstruction, compounding digestive issues and discomfort [
46]. Despite these symptoms, analgesics were not administered in this case. Instead, environmental management, including housing the birds in a specialized aviary unit, sunbathing, hose baths, and socialization with other birds, appeared sufficient for their recovery.
Regarding diagnosis, imaging techniques like radiography are essential for determining the type and location of ingested metallic objects, as metal particles are highly radiopaque compared to minerals [
30,
47]. Radiographs taken at admission, during treatment, and upon discharge confirmed the presence and subsequent removal of metal objects from the birds' ventriculus. Physical or chemical restraint may be necessary for radiographs, with chemical restraint generally reducing stress and anxiety for avian patients. Midazolam, administered intranasally, was used in this case to sedate the birds safely, as it provides adequate sedation with minimal complications [
48]. This method is less invasive than intramuscular administration and has been shown to maintain cardiovascular stability in birds [
49].
In humans, heavy metal exposure is typically assessed through urine tests, hair mineral analysis for long-term exposure, or blood tests for diet-related exposure [
50]. In birds, feather analysis is a simple, non-invasive method to assess recent exposure to toxic metals, although it may lack sensitivity in low-pollution environments [
51]. Blood metal testing is less common in birds due to the challenges of obtaining sufficient blood volume, specialized equipment, limited laboratory access in Brazil, and high costs. Despite these constraints, chelation therapy is recommended as soon as clinical signs of metal poisoning are evident, even in the absence of specific metal test results [
52]. In this case, radiographs were sufficient to diagnose zinc poisoning, and chelation therapy was promptly initiated.
Chelation therapy with calcium EDTA is commonly used to treat zinc, lead, mercury, and arsenic poisoning [
23]. This chelator works by competing with metals for reactive sites in the body, facilitating their excretion [
26]. Although parenteral administration is preferred for its higher absorption, oral administration was used in this case at a higher dose (70 mg/kg) to compensate for the reduced gastrointestinal absorption [
20,
23]. Despite the delayed start to therapy due to external factors, both birds responded well to the treatment, with no adverse effects and successful metal excretion within a short period. This case represents the first documented use of this chelation protocol for zinc poisoning in
A. jandaya, highlighting its effectiveness even with oral administration.
Supportive therapy is also crucial in managing metal poisoning cases. Lactated Ringer’s solution aids in rehydration, maintaining urinary output, and mitigating renal toxicity associated with both the chelator and the metal [
27,
52]. Additionally, antibiotics may be used prophylactically to prevent secondary bacterial infections, and B-complex vitamins are administered to stimulate appetite and prevent metal accumulation in tissues [
28]. Both birds received Ringer's solution, B-complex vitamins, and oral Glicopan® to support their recovery, alongside gentamicin nebulization for respiratory issues observed on radiographs. This comprehensive approach, including chelation and supportive care, ensured the birds' recovery without further complications.In conclusion, the case of heavy metal poisoning in
A. jandaya demonstrates the environmental risks posed to wildlife in urbanized areas of the Amazon. The successful use of oral calcium EDTA in these birds sets a precedent for future cases and underscores the need for prompt and thorough intervention when managing heavy metal intoxication in avian species.
5. Conclusions
It was concluded that radiographic examinations were sufficient for detecting the presence of metals in Jandaya parakeets (Aratinga jandaya). Despite the lack of more specific tests to identify the exact type of metal involved, the clinical signs observed in the affected birds strongly suggested zinc poisoning. Additionally, the therapeutic protocol employed, using calcium EDTA at a dose of 70 mg/kg, twice daily, administered orally for 7 days in one bird and for 10 days in another of the same species, proved to be effective in improving the clinical condition of the birds and promoting metal excretion, without the need for invasive procedures.
Furthermore, it is crucial that more studies are conducted on bird species of the Aratinga genus, which inhabit the Amazon Biome, to better identify the heavy metals involved in poisoning cases, particularly in relation to the environmental impacts occurring in the region. This would aid in the rapid diagnosis and appropriate selection of therapeutic protocols, thereby improving the chances of survival for the affected animals.
Author Contributions
Conceptualization, K.A.C.S., C.T.d.A.L., S.F.S.D and F.M.S.; methodology, K.A.C.S., K.E.P.G., L.Y.S.C., H.G.d.S.O., C.T.d.A.L., R.M.C.T. and S.F.S.D.; formal analysis, K.A.C.S., K.E.P.G., L.Y.S.C., H.G.d.S.O., C.T.d.A.L., R.M.C.T., S.F.S.D and F.M.S.; investigation, K.A.C.S., K.E.P.G., C.T.d.A.L., S.F.S.D. and F.M.S.; data curation, K.A.C.S., K.E.P.G., L.Y.S.C., R.M.C.T. C.T.d.A.L., S.F.S.D. and F.M.S; writing—original draft preparation, K.A.C.S., H.G.d.S.O., C.T.d.A.L., R.M.C.T., S.F.S.D. and F.M.S.; writing—review and editing, K.A.C.S. and F.M.S.; supervision, F.M.S.; project administration, F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol number CEUA 8888280618 (ID 002193) was approved by the National Council for Control of Animal Experimentation (CONCEA), and was approved by the Ethical Committee on Animal Use of the Federal University of Para (CEUA/UFPA) in the meeting of 03/30/2023. It was also authorized by the Chico Mendes Institute for Biodiversity Conservation (ICMBio) under nº 67300-1.
Informed Consent Statement
All the animals in the current study belong to the Veterinary Hospital (wild animal sector) at UFPA; those are animals that came from rescue by environmental agencies in Brazil, which include animals rescued from highways, traffic, etc.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPESPA (Fundação Amazônia de Amparo a Estudos e Pesquisas do Estado do Pará), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; finance code: 001), Laboratório de Anaeróbios da Universidade Federal de Minas Gerais and PROPESP-UFPA (Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Federal do Pará) for paying the publication fee article via the Programa Institucional de Apoio à Pesquisa (PAPQ/2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carvalho, A.M.; Andrade, M.A.; Linhares, G.F.C.; Jaime, V.S. Pesquisa de Mycoplasma em aves da família Psittacidae mantidas em diferentes cativeiros no Brasil Central. Pesq. Vet. Bras. 2017, 37, 1159–1164. [Google Scholar] [CrossRef]
- Dos Santos, C.B.; Canavessi, L.; Da Silva, A.H.; Telles, P.H.F.; Zat, L.H.S.; Cubas, Z.S. Intoxicação por metal pesado em periquito (Brotogeris Chiriri ): Relato de caso. Braz. J. Dev. 2021, 7, 02570–102580. [Google Scholar]
- De Moraes, K.F.; Santos, M.P.D.; Gonçalves, G.S.R.; De Oliveira, G.L.; Gomes, L.B.; Lima, M.G.M. Climate change and bird extinctions in the Amazon. PLoS ONE 2020, 15, 0236103. [Google Scholar] [CrossRef] [PubMed]
- Instituto Chico Mendes de Conservação da Biodiversidade. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção, 1rd ed.; ICMBio: Brasília, Brasil, 2018; p. 4162. [Google Scholar]
- Silveira, L.F.; De Lima, F.C.T.; Hofling, E. A new species of Aratinga parakeet (psittaciformes: Psittacidae) from Brazil, with taxonomic remarks on the Aratinga solstitialis complex. Ornithology 2005, 122, 292–305. [Google Scholar] [CrossRef]
- Pessoa, C.A.; Machado, C.S.; Loatelli-Dittrich, R.; De Brito, H.F.V. Vídeo-endoscopia para avaliação das gônadas de jandaias-verdadeiras (Aratinga jandaya Gmelin, 1788) mantidas em cativeiro. Sci. Vit. 2013, 1, 28–33. [Google Scholar]
- Sistema da Informação sobre a Biodiversidade Brasileira (SiBBR). Available online: https://ala-bie.sibbr.gov.br (accessed on 07 October 2024).
- Leoni, A.M.; Reis, M.G.; Filho, M.M.D. A food interaction network between psittacines and plants in an urban area in the city of São Carlos – SP, southeastern Brazil. Braz. J. Biol. 2023, 269353. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.R.; Moreira, N. Manejo, reprodução e conservação de psitacídeos brasileiros. Rev. Bras. Reprod. Anim. 2012, 36, 215–219. [Google Scholar]
- Dos Santos, C.C.M.; Nauar, A.R.; Ferreira, J.A.; Montes, C.S.; Adolfo, F.R.; Leal, G.; Reis, G.M.; Lapinsky, J.; Carvalho, L.M.; Amado, L.L. Multiple anthropogenic influences in the Pará River (Amazonia, Brazil): A spatial-temporal ecotoxicological monitoring in abiotic and biotic compartments. Chemosphere 2023, 323, 138090. [Google Scholar] [CrossRef]
- Silva, V.B.C.; Freitas, F.L.C.; Momo, C. Aspectos morfológicos do proventrículo e ventrículo gástrico de Crypturellus parvirostris (wagler, 1827). Cien. Anim. Bras. 2013, 14, 106–112. [Google Scholar] [CrossRef]
- Cotton, R.J.; Divers, S.J. Endoscopic Removal of Gastrointestinal Foreign Bodies in Two African Grey Parrots (Psittacus erithacus) and a Hyacinth Macaw (Anodorhynchus hyacinthinus). J. Avian Med. Surg. 2017, 31, 335–343. [Google Scholar] [CrossRef]
- Costa, A.G.; Borges, A.M.; Soto-Blanco, B. Metais tóxicos e seus efeitos sobre a reprodução dos animais. Revisão. Rev. Bras. Hig. Sanid. Anim. 2020, 14, 108. [Google Scholar] [CrossRef]
- Freitas, K.M.A. Relação entre metais e anormalidades nucleares eritrocitárias em aves de ecossitema estuarino do Rio Grande do Norte. Monografia (Graduação), Universidade Federal Rural do Semi-Árido, Rio Grande do Norte, 2023.
- Osofsky, A.; Jowett, P.L.H.; Hosgood, G.; Tully, T.N. Determination of Normal Blood Concentrations of Lead, Zinc, Copper, and Iron in Hispaniolan Amazon Parrots (Amazona ventralis). J. Avian Med. Surg. 2001, 15, 31–36. [Google Scholar] [CrossRef]
- Guthrie, A,L. ; Jayson, S.l.; Strike, T.B.; Sparrow, S.J.; Flach, E.J. Diagnosis and Treatment of Heavy Metal Toxicosis in Six Waldrapp Ibis (Geronticus eremita). J. Avian Med. Surg. 2020, 34, 371–380. [CrossRef]
- Carneiro, M.A.; Oliveira, P.A.; Brandão, R.; Olga, N.F.; Roser, V.; Lavin, S.; Colaço, B. Lead poisoning Due to Lead-Pellet Ingestion in Griffon Vultures (Gyps fulvus) from the Iberian Peninsula. J. Avian Med. Surg. 2016, 30, 274–279. [Google Scholar] [CrossRef]
- Cristopher, M.M.; Shooshtari, M.P.; Levengood, J.M. Assessment of erythrocyte morphologic abnormalities in mallards with experimentally induced zinc toxicosis. AJVR 2004, 65, 4. [Google Scholar] [CrossRef] [PubMed]
- Luz, D.B. Ingestão de objetos metálicos por calopsita (Nymphicus hollandicus) associado ao uso de enriquecimento ambiental – relato de caso, Monografia (Graduação), Universidade de Brasília, Brasília, 2016.
- Cojean, O.; Larrat, S.; Vergneau-Grosset, C. Clinical Management of Avian Renal Disease. Vet. Clin. Exot. Anim. 2020, 23, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.K.; Doss, G.A.; Mans, C. Suspected peripheral neuropathy secondary to lead intoxication in three psittacine birds. JEPM 2020, 32, 13–17. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pachauri, V. Chelation in Metal Intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Malateaux, I.F.; Muniz, L.M. Intoxicação por chumbo em aves de companhia. Anuário Prod. Acad. Docent. 2013, 7, 89–102. [Google Scholar]
- Jepson, L. Clínica de animais exóticos: Referência rápida, 1rd ed.; Elsevier: Rio de Janeiro, Brasil, 2010; p. 592. [Google Scholar]
- Huali, T.; Honglei, P.; Feng, W.; Shaokang, W.; Ligang, L.; Jianghong, L.; Guiju, S. Effects of combined administration of calcium, iron, zinc, chrysanthemum flavonoids, and DMSA on the treatment of lead intoxication in mice. J. Biochem. Mol. Toxicol. 2020, 34, e22425. [Google Scholar] [CrossRef]
- Denver, M.C.; Tell, L.A.; Galey, F.D.; Trupkiewicz, J.G.; Philip, H.K. Comparison of two heavy metal chelators for treatment of lead toxicosis in cockatiels. Am. J. Vet. Res. 2000, 618, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Puschner, B.; Poppenga, R.H. Lead and zinc intoxication in companion birds. Compend. Contin. Educ. Vet. 2009, 31, E1–E12. [Google Scholar] [PubMed]
- De Francisco, N.; Ruiz Troya, J.D.; Agüera, E.I. Lead and lead toxicity in domestic and free living birds. Avian Pathol. 2003, 32, 3–13. [Google Scholar] [CrossRef]
- Carpenter, J.W.; Harms, C.A. Birds. In Exotic Animal Formulary, 6nd ed.; Elsevier: St. Loius, Missouri; 2023. [Google Scholar]
- Molazem, M.; Soroori, S.; Soflaei, R.; Bahonar, A.; Madani, S.A.; Mokhtari, R.; Hartmann, A. Radiologic features of radiolucent foreign bodies ingestion in common mynah (Acridotheres tristis). Vet. Med. Sci. 2023, 9, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.A.N.; Nogueira, R.J.B. Dispersão urbana e transporte público na região metropolitana de Belém: Uma análise de Castanhal e Santa Izabel do Pará. RIHGP 2021, 8, 54–78. [Google Scholar] [CrossRef]
- Silva, G.N.; Nazaré, M.L. Impactos das ações antrópicas na história ambiental da bacia hidrogáfica do rio Apeú. Rev. Cien. Multid. 2024, 5, 4117. [Google Scholar] [CrossRef]
- Hayes, W.M.; O'Shea, B.J.; Pierre, M.A.; Wilson, A.; Bicknell, J.E. Bird communities across different levels of human settlement: A comparative analysis from two northern Amazonian ecoregions. Sci. Total Environ. 2023, 166535. [Google Scholar] [CrossRef] [PubMed]
- Vetere, A.; Bertocchi, M. , Pelizzone, I.; Moggia, E.; Travaglino, C.; Grotta, M.D.; Casali, S.; Gerosa, S.; Strada, L.; Filia, K.; Casalini, J.; Parmigiani, E.; Di lanni, F. Acute tea tree oil intoxication in a pet cockatiel (Nymphicus hollandicus): A case report. BMC Vet. Res. 2020, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Association of Avian Veterinarians. What is your diagnosis? J. Avian Med. Surg. 2022, 35, 486–493. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Igonin, T.N.; Nenasheva, T.A.; Nikolaeva, L.A.; Ivanenko, A.A. Corrosion Resistance of Zinc and Zinc-Aluminum-Magnesium Coatings in Atmosphere on the Territory of Russia. Materials 2023, 16, 5214. [Google Scholar] [CrossRef] [PubMed]
- Viespoli. , L.M.; Multignani, F.; Reme, H.; Berto, F. Cruciform welded joints: Hot-dip galvanization effect on the fatigue life and local energetic analysis. Proc. Struc.l Integ. 2018, 13, 340–346. [CrossRef]
- Bombik, E.; Bombik, A.; Pietrzkiewicz, K. Analysis of Zinc and Copper Content in Selected Tissues and Organs of Wild Mallard Ducks (Anas platyrhynchos L.) in Poland. Animals 2024, 14, 1176. [Google Scholar] [CrossRef]
- Puschner, B.; Leger, J.S.; Galey, F.D. Normal and toxic zinc concentrations in serum/plasma and liver of psittacines with respect to genus diffferences. J. Vet. Diagn. Invest. 1999, 11, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Aloupi, M.; Karagianni, A.; Kazantzidis, S.; Akriotis, T. Heavy Metals in liver and Brain of waterfowl from the Evros Delta, Greece. Arch. Environ. Contam. Toxicol. 2017, 72, 215–234. [Google Scholar] [CrossRef]
- dos Santos, A.N.D.; Recketenvald, M.C.N.N.; de Carvalho, D.P.; Puerta, E.L.B.; De Sousa-Filho, I.F.; Dórea, J.G.; Bastos, W.R. Mercury in birds (aquatic and scavenger) from the Western Amazon. Envi. Res. 2021, 201, 111574. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.N.S. Metabolismo do zinco na nutrição de frangos de corte e suas respostas no desempenho e no sistema imune. Rev. Elet. Nutrit. 2013, 11, 2287–2799. [Google Scholar]
- Zheng, Y.; Cui, M.; Ni, Le.; Qin, Y.; Li, J.; Pan, Y.; Zhang, X. Heterologous Expression of Human Metallothionein Gene HsMT1L Can Enhance the Tolerance of Tobacco (Nicotiana nudicaulis Watson) to Zinc and Cadmium. Genes 2022, 13, 2413. [Google Scholar] [CrossRef]
- Vignesh, K.S.; Deep Jr, G.S. Metallothioneins: Emerging Modulators in Immunity and Infection. Int. J. Mol. Sci. 2017, 18, 2197. [Google Scholar] [CrossRef]
- Mikoni, N.A.; Guzman, D.S.M.; Fausak, E. , Paul-Murphy, J. Recogintion and Assessment of Pain-Related Behaviors in Avian Species: Na Integrative Review. J. Avian. Med. Surg. 2022, 36, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.O.; Cunha, C.C.C.; Bath, F.V.C.; Sousa, C.A.S.; Abidu-Figueiredo, M. Ingluviotomia para remoção de corpo estranho em calopsita doméstica (Nymphicus hollandicus). Relato de caso. Rev. Med. Vet. 2022, 44, 33–40. [Google Scholar] [CrossRef]
- Lamb, S.K. Obstruction by fibrous foreign object ingestion in two green-cheeked conures (Pyrrhura molinae) and a jenday conure (Aratinga jandaya). J. Exot. Pet. Med. 2019, 31, 127–132. [Google Scholar] [CrossRef]
- Beier, S.L.; Rosa, A.C.; Oleskovicz, N.; Mattoso, C.R.S.; Moraes, A.N. Efeitos anestésicos da administração intranasal ou intramuscular de cetamina S+ e midazolam em pomba-rola (Streptotelia sp.). Pesq. Vet. Bras. 2013, 33, 517–522. [Google Scholar] [CrossRef]
- Bittencourt, E.H.; Padilha, V.S.; Lima, M.P.A.; Beier, S.L.; Moraes, A.N.; Oleskovicz, N. Efeitos sedativos da associação de Cetamina e Midazolam administrados pela via intranasal ou intramuscular em papagaio (Amazona aestiva e Amazona vinacea). Pesq. Vet. Bras. 2013, 33, 1125–1129. [Google Scholar] [CrossRef]
- Langeland, A.L.; Hardin, R.D.; Neitzel, R.L. Mercury levels in human hair and farmed fish near artisanal and small-scale gold mining communities in the Madre de Dios River Basin, Peru. Int. J. Environ. Res. Public Health 2017, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Iemmi, T.; Menozzi, A.; Pérez-López, M.; Basini, G.; Grasselli, F.; Menotta, S.; Serventi, P.; Bertini, S. Heavy Metal Assessment in Feathers of Eurasian Magpies (Pica pica): A Possible Strategy for Monitoring Environmental Contamination? Int. J. Environ. Res. Public Health 2021, 18, 2973. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.C.; Melo, R.C.; Grespan, A.; Peixoto, T.M.B.; Santos, M.H.; Cabral, L.A.R.; Costa, P.P.C. Heavy Metal Poisoning in a Cockatiel (Nymphicus hollandicus). Acta Sci. Vet. 2018, 46, 251. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).