Submitted:
24 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Search Strategy
Eligibility Criteria
Data Extraction
Risk of Bias Assessment and Meta-Evidence
Statistical Analysis
Results
Literature Search and Study Characteristics
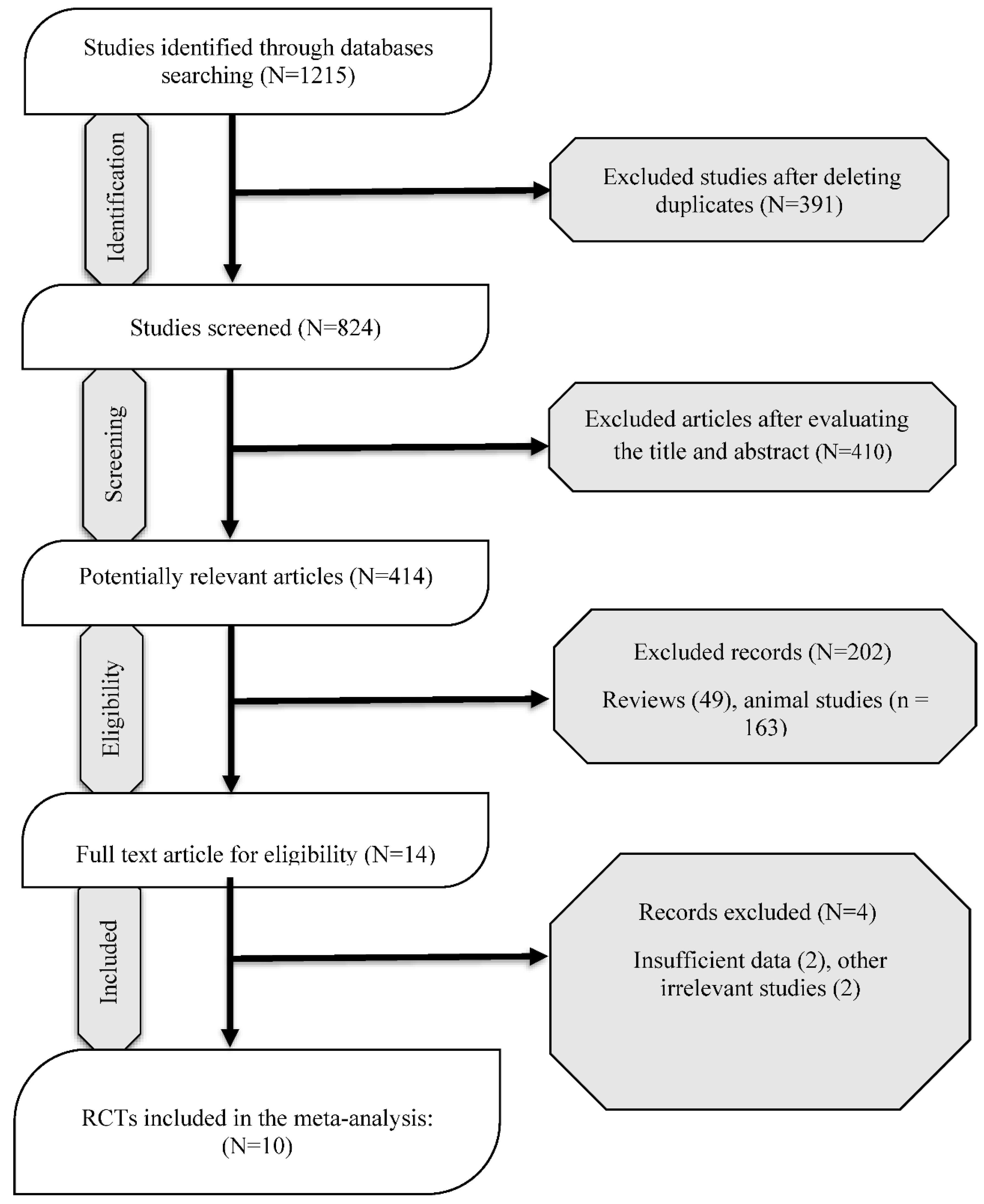
Quality Assessment and Grade Approach
| Included study | Arjmandi,1998. | Lucas, , 2002 | Demark-Wahnefried, USA, 2008 | Patade, USA, 2008 | Simbalista, Brazil, 2009 | Vargas, USA, 2011 | Colli, Brazil, 2012 | Mirmasoumi, Iran, 2017 | Chang, Canada, 2018 | Haidari, Iran, 2020 |
| Year | 2020 | 2020 | 2013 | 2008 | 2019 | 2021 | 2021 | 2017 | 2019 | 2018 |
| Location**Duration (week) | USA 6 | USA 12 | USA5 | USA12 | Brazil 12 | USA6 | Brazil 24 | Iran12 | Canada7 | Iran12 |
| Mean dosage of flaxseed /Intervention | 38g/day Whole Flaxseed | 40 g/day Ground Whole Flaxseed | 30 g/day Flaxseed-Supplemented Die | 30 g/day Flaxseed | 25 g/day Ground Flaxseed | 3.5 g/day Flaxseed Oil | 1g/d Flaxseed Extract | 1 g/day Flaxseed Oil | 15 g/day Ground Flaxseed | 30 g/day Brown Milled Flaxseed Powder + Lifestyle Modification |
| Target population | Postmenopausal Women | Postmenopausal Women | Prostate Cancer | Postmenopausal Women | Postmenopausal Women | PCOS | Menopausal | PCOS | Postmenopausal Women | PCOS |
| Participants’ age (year) | 56 | 54 | 60 | 47-63 | 52 | 29 | 54 | 28 | 60 | 27 |
| Participants’ sex | Women | Women | Men | Women | Women | Women | Women | Women | Women | Women |
| Control group | Sunflower Seed | Wheat | Usual Diet | Control | Wheat Bran | Soybean Oil | Collagen | Liquid Paraffin | Usual Diet | Lifestyle Modification |
| Study | Random Sequence Generation | Allocation concealment | Reporting bias | Other sources of bias | Performance bias | Detection bias | Attrition bias |
| Arjmandi, US, 1998 | L | U | L | L | L | L | H |
| Lucas, USA, 2002 | L | U | L | H | L | L | L |
| Wahnefried, USA, 2008 | L | U | L | H | L | H | L |
| Patade, USA, 2008 | L | U | L | H | L | H | L |
| Simbalista, Brazil, 2009 | L | L | L | H | L | L | L |
| Vargas, USA, 2011 | L | L | L | H | L | L | L |
| Colli, Brazil, 2012 | L | U | H | H | U | U | L |
| Mirmasoumi, Iran, 2017 | L | L | L | L | L | L | L |
| Chang, Canada, 2018 | L | L | L | L | L | H | L |
| Haidari, Iran, 2020 | L | L | L | L | U | U | L |
Meta-Analysis Results
Flaxseed on Sex Hormones Profile
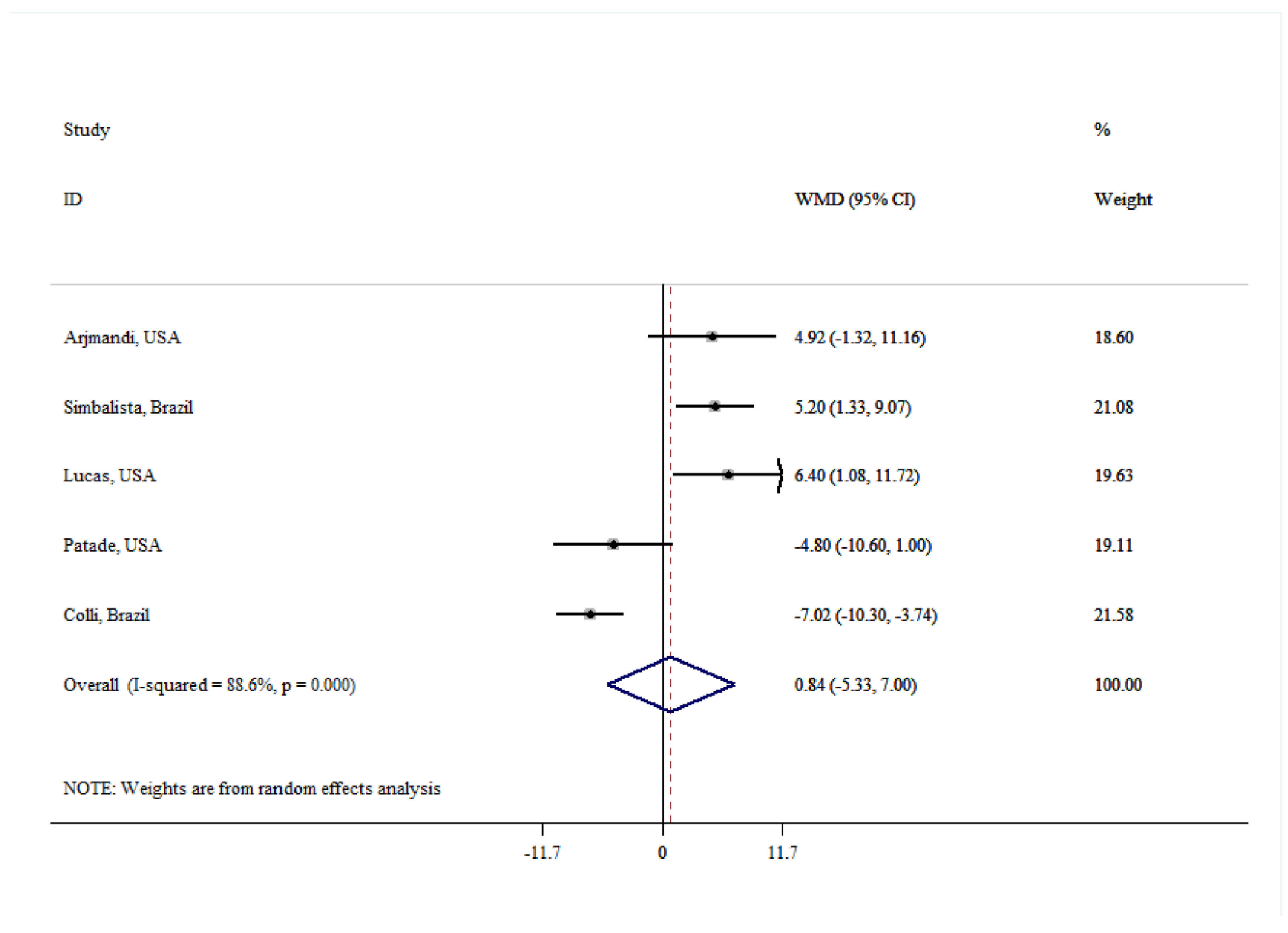
| Number | WMD (95% CI) | P-within | I2 (%) | P-heterogeneity | |
| FSH | |||||
| Overall | 5 | 0.84 (-5.53, 7.00) | 0.791 | 88.6 | <0.001 |
| Age(year) | |||||
| ≤50 | 3 | 1.39 (-7.70, 10.48) | 0.764 | 93.3 | <0.001 |
| >50 | 2 | -0.01 (-9.54, 9.51) | 0.998 | 80.0 | <0.001 |
| Dose (g/day) | |||||
| <30 | 2 | -0.96 (-12.93, 11.02) | 0.876 | 95.0 | <0.001 |
| ≥30 | 3 | 2.19 (-4.77, 9.16) | 0.537 | 77.0 | 0.013 |
| Quality | |||||
| Low | 2 | -6.48 (-9.34, -3.63) | <0.001 | 0.0 | 0.513 |
| High | 3 | 5.48 (2.68, 8.27) | <0.001 | 0.0 | 0.920 |
| Duration (week) | |||||
| <8 | 1 | 4.92 (-1.32, 11.16) | 0.123 | - | - |
| ≥8 | 4 | -0.09 (-7.26, 7.07) | 0.981 | 90.6 | <0.001 |
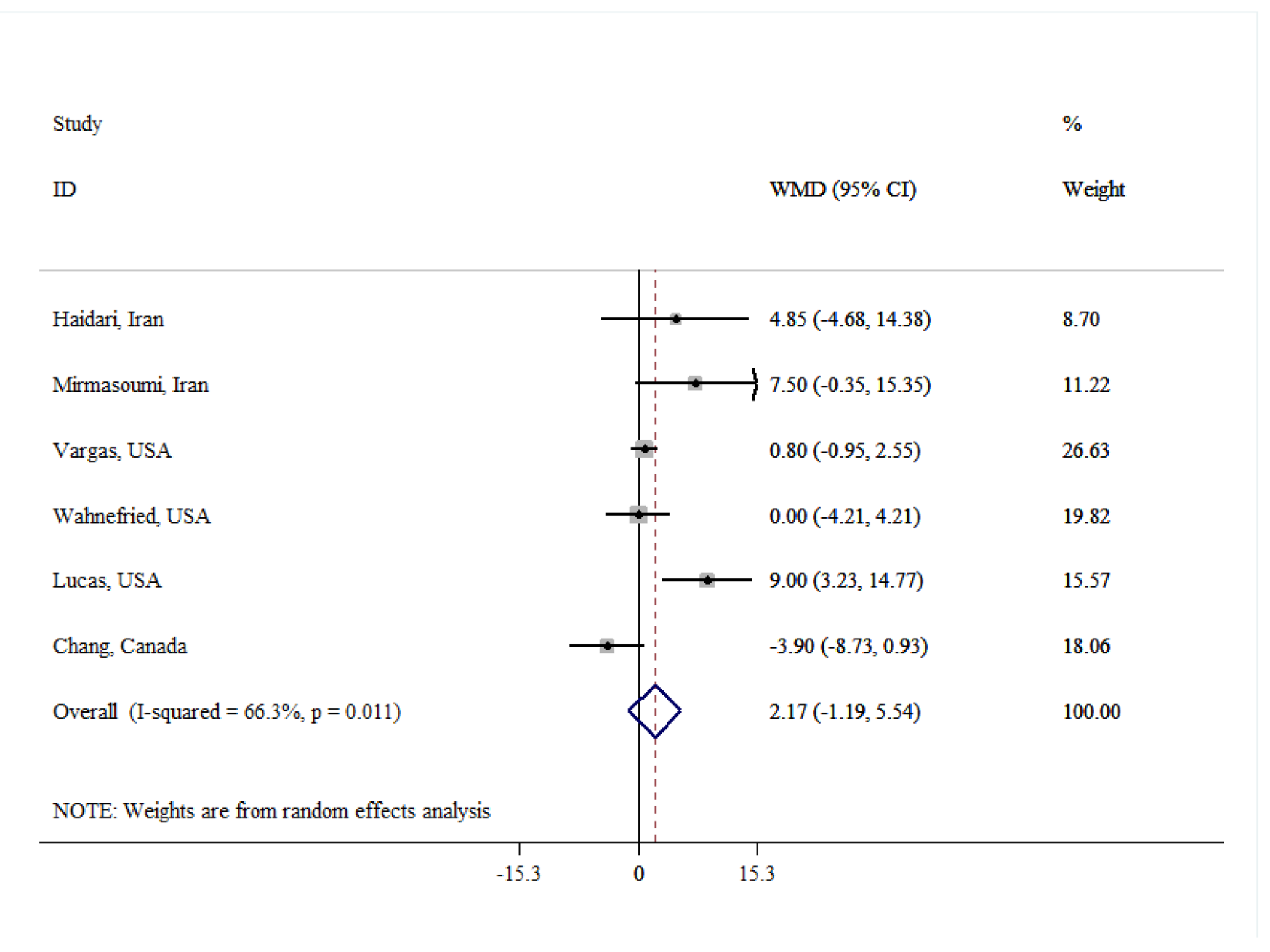
| SHBG | |||||
| Overall | 6 | 2.17 (-1.19, 5.54) | 0.206 | 66.3 | 0.011 |
| Age(year) | |||||
| ≤50 | 4 | 4.94 (-0.10, 9.99) | 0.055 | 68.9 | 0.022 |
| >50 | 2 | -1.76 (-5.57, 2.04) | 0.634 | 29.8 | 0.233 |
| Dose (g/day) | |||||
| <30 | 3 | 0.43 (-4.96, 5.81) | 0.887 | 66.4 | 0.051 |
| ≥30 | 3 | 4.36 (-1.59, 10.31) | 0.114 | 73.5 | 0.023 |
| Quality | |||||
| Low | 2 | 0.79 (-3.06, 4.64) | 0.687 | 0.0 | 0.361 |
| High | 4 | 2.73 (-2.23, 7.69) | 0.280 | 78.5 | 0.003 |
| Duration (week) | |||||
| <8 | 3 | -0.31 (-2.76, 2.14) | 0.806 | 38.1 | 0.199 |
| ≥8 | 3 | 7.78 (3.60, 11.95) | <0.001 | 0.0 | 0.763 |
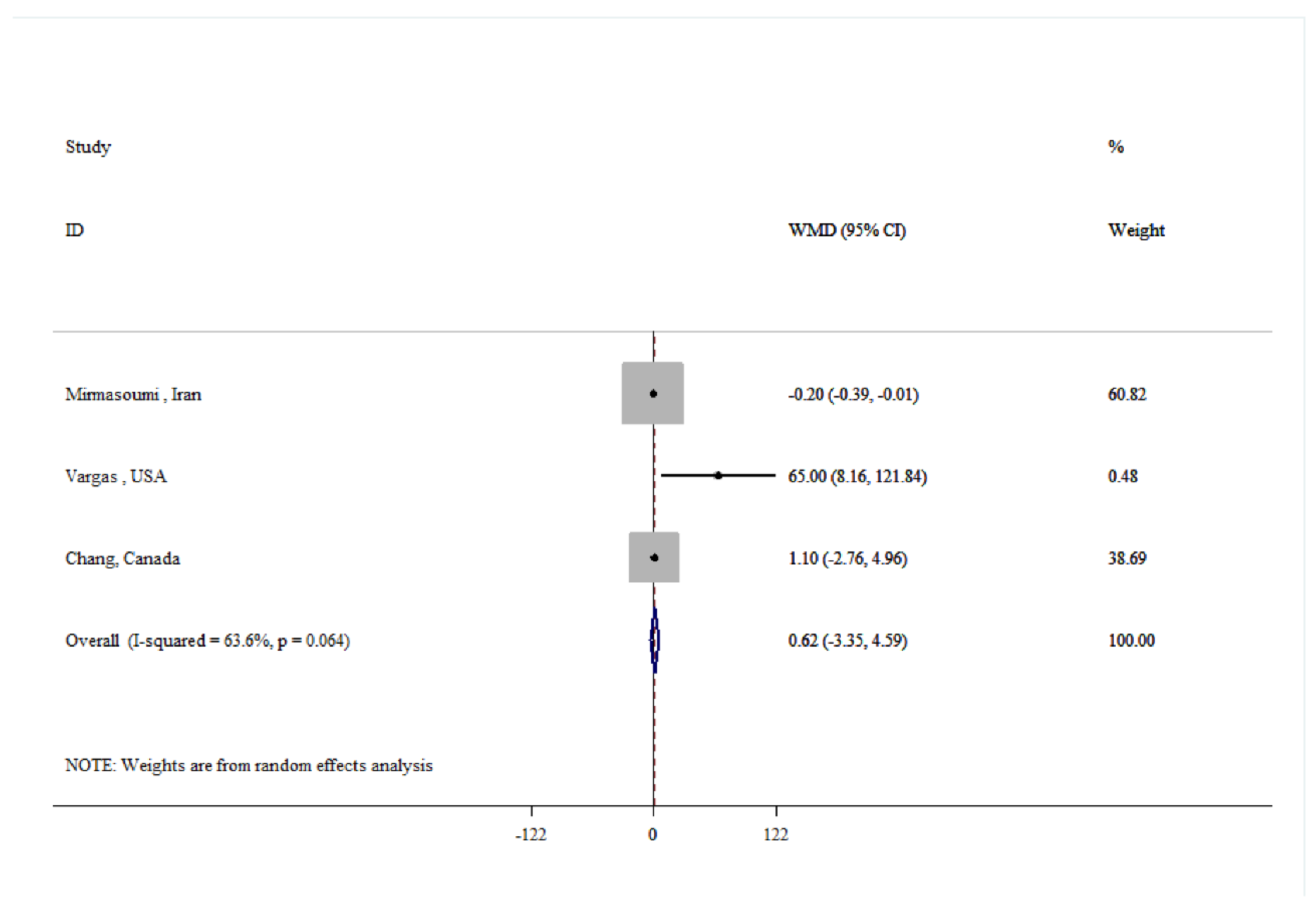
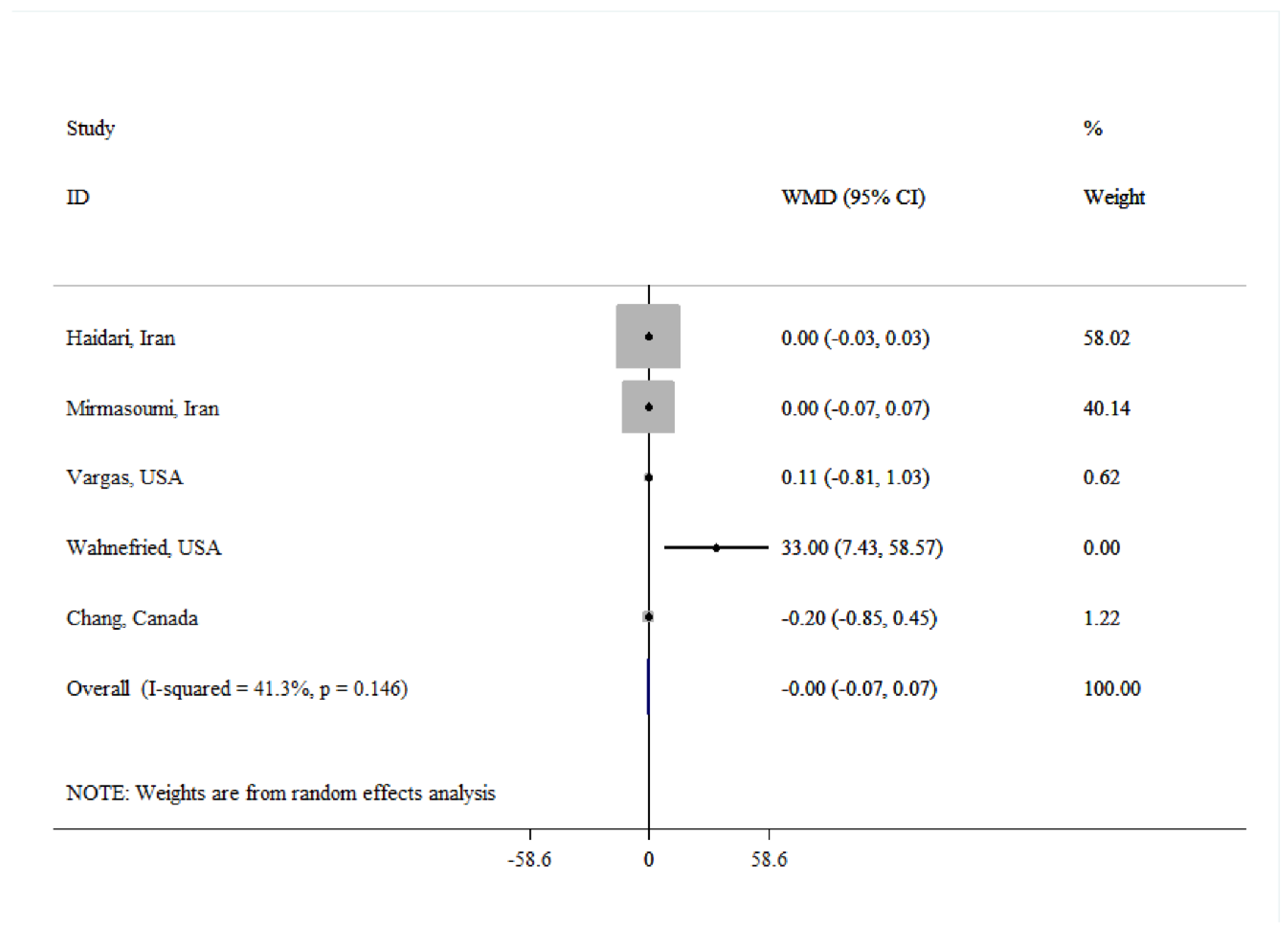
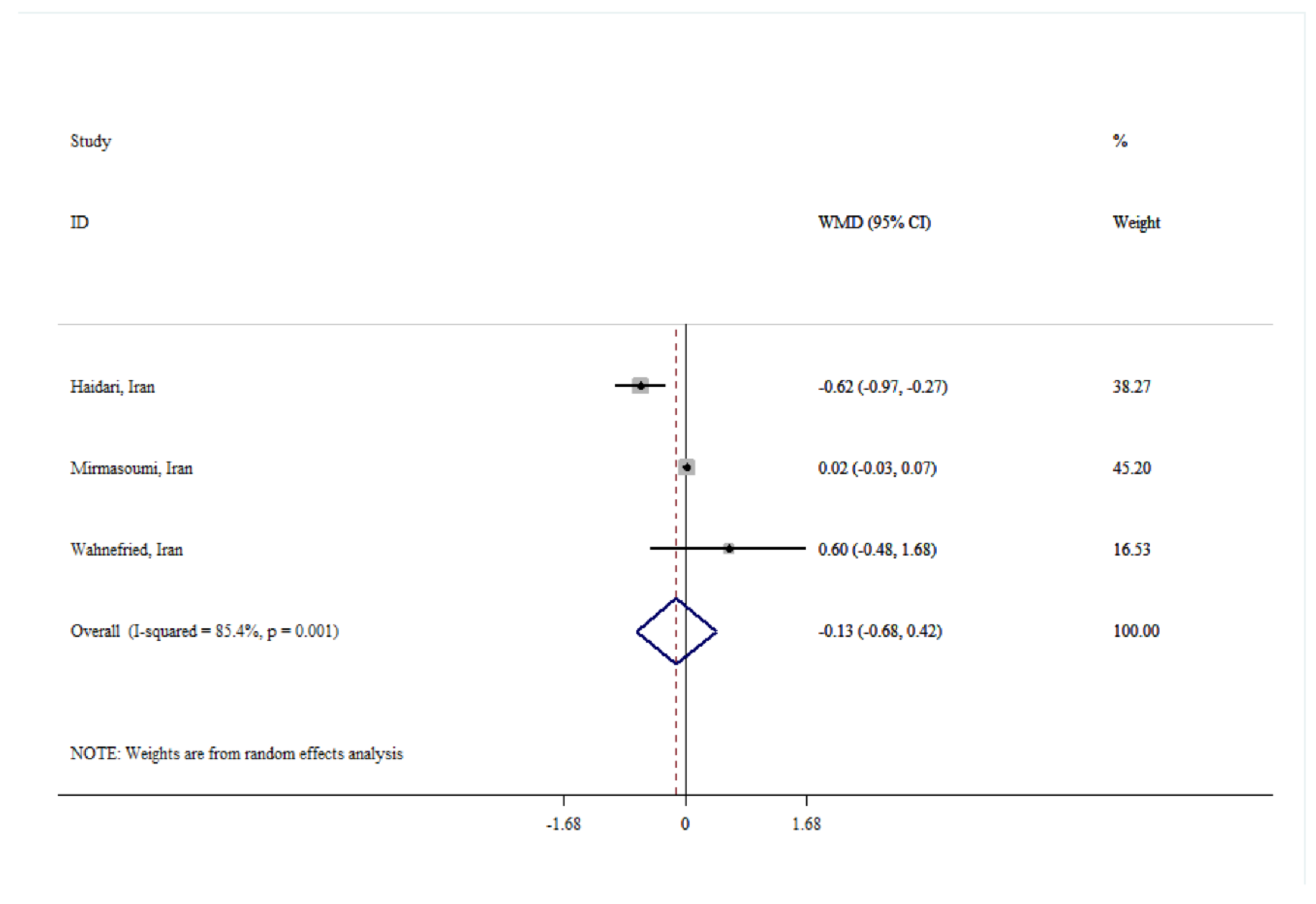
| Total testosterone | |||||
| Overall | 5 | -0.00 (-0.07, 0.07) | 0.968 | 41.3 | 0.146 |
| Age(year) | |||||
| ≤50 | 3 | 0.00 (-0.03, 0.03) | 0.994 | 0.0 | 0.980 |
| >50 | 2 | 13.84 (-18.31, 45.98) | 0.399 | 84.5 | 0.012 |
| Dose (g/day) | |||||
| <30 | 2 | -0.00 (-0.07, 0.07) | 0.949 | 0.0 | 0.554 |
| ≥30 | 3 | 0.10 (-0.95, 1.15) | 0.851 | 69.0 | 0.040 |
| Quality | |||||
| Low | 2 | 13.90 (-17.91, 45.72) | 0.957 | 84.4 | 0.011 |
| High | 3 | -0.00 (-0.07, 0.07) | 0.999 | 0.0 | 0.845 |
| Duration (week) | |||||
| <8 | 3 | 0.04 (-1.31, 1.38) | 0.392 | 70.2 | 0.035 |
| ≥8 | 2 | 0.00 (-0.03, 0.03) | 0.963 | 0.0 | 0.999 |
Discussion
Limitations
Conclusions
Author Contributions
Data Availability
Acknowledgments
Conflicts of Interest
References
- Aubead, N. M. in Reproductive Hormones (IntechOpen, 2021).
- Tan, I. J., Peeva, E. & Zandman-Goddard, G. Hormonal modulation of the immune system—a spotlight on the role of progestogens. Autoimmunity reviews 14, 536-542 (2015). [CrossRef]
- Khmil, M., Khmil, S. & Marushchak, M. Hormone imbalance in women with infertility caused by polycystic ovary syndrome: is there a connection with body mass index? Open Access Macedonian Journal of Medical Sciences 8, 731-737 (2020).
- Chandel, S., Das, S., Ojha, S. & Pandey, M. in Women's Health: A Comprehensive Guide to Common Health Issues in Women 101-128 (Bentham Science Publishers, 2024).
- Peixoto, C. et al. Relationship between sexual hormones, quality of life and postmenopausal sexual function. Trends in psychiatry and psychotherapy 41, 136-143 (2019). [CrossRef]
- Rohr, U. D. The impact of testosterone imbalance on depression and women's health. Maturitas 41, 25-46 (2002).
- Allen, N. E. & Key, T. J. The effects of diet on circulating sex hormone levels in men. Nutrition research reviews 13, 159-184 (2000). [CrossRef]
- Frische, E. J., Hutchins, A. M., Martini, M. C., Thomas, W. & Slavin, J. L. Effect of flaxseed and wheat bran on serum hormones and lignan excretion in premenopausal women. Journal of the American College of Nutrition 22, 550-554 (2003). [CrossRef]
- Hutchins, A. M., Martini, M. C., Olson, B. A., Thomas, W. & Slavin, J. L. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutrition and cancer 39, 58-65 (2001). [CrossRef]
- Webb, A. L. & McCullough, M. L. Dietary lignans: potential role in cancer prevention. Nutrition and cancer 51, 117-131 (2005). [CrossRef]
- Dobrowolska, K. & Regulska-Ilow, B. The legitimacy of using dietary supplement diglycoside secoisolariciresinol (SDG) from flaxseed in cancer. Roczniki Państwowego Zakładu Higieny 72 (2021). [CrossRef]
- Zhang, J. et al. Secoisolariciresinol diglycoside (SDG) lignan content of oil flax: genotypic and environmental variations and association with other traits. Oil Crop Science 7, 1-8 (2022). [CrossRef]
- Raole, V. M. & Raole, V. V. Flaxseed and seed oil: Functional food and dietary support for health. EAS Journal of Nutrition and Food Sciences 4, 68-77 (2022).
- Landete, J. M. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Research International 46, 410-424 (2012). [CrossRef]
- Mason, J. K. & Thompson, L. U. Flaxseed and its lignan and oil components: can they play a role in reducing the risk of and improving the treatment of breast cancer? Applied Physiology, Nutrition, and Metabolism 39, 663-678 (2014).
- McCann, S. E. et al. Dietary lignan intakes in relation to survival among women with breast cancer: the Western New York Exposures and Breast Cancer (WEB) Study. Breast cancer research and treatment 122, 229-235 (2010). [CrossRef]
- Adlercreutz, H. et al. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG). Journal of steroid biochemistry 27, 1135-1144 (1987). [CrossRef]
- Adlercreutz, H. Lignans and human health. Critical reviews in clinical laboratory sciences 44, 483-525 (2007).
- Chang, V. C. et al. Effect of dietary flaxseed intake on circulating sex hormone levels among postmenopausal women: a randomized controlled intervention trial. Nutrition and cancer 71, 385-398 (2019). [CrossRef]
- Markakis, J. The horn of conflict. Review of African political economy 30, 359-362 (2003).
- Haidari, F., Banaei-Jahromi, N., Zakerkish, M. & Ahmadi, K. The effects of flaxseed supplementation on metabolic status in women with polycystic ovary syndrome: a randomized open-labeled controlled clinical trial. Nutrition journal 19, 1-11 (2020). [CrossRef]
- Shamseer, L. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical research ed.) 350, g7647, doi:10.1136/bmj.g7647 (2015). [CrossRef]
- Sterne, J. A. et al. RoB 2. BMJ: British Medical Journal 366, 1-8 (2019).
- Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336, 924-926 (2008). [CrossRef]
- Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557-560 (2003). [CrossRef]
- Egger, M., Higgins, J. P. & Smith, G. D. Systematic Reviews in Health Research: Meta-Analysis in Context. (John Wiley & Sons, 2022).
- Higgins, J. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane; 2021. Updated February 2021. Reference Source (2021).
- Arjmandi, B. H. et al. Whole flaxseed consumption lowers serum LDL-cholesterol and lipoprotein (a) concentrations in postmenopausal women. Nutrition Research 18, 1203-1214 (1998). [CrossRef]
- Lucas, E. A. et al. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. The journal of clinical endocrinology & metabolism 87, 1527-1532 (2002).
- Demark-Wahnefried, W. et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiology Biomarkers & Prevention 17, 3577-3587 (2008). [CrossRef]
- Patade, A. et al. Flaxseed reduces total and LDL cholesterol concentrations in Native American postmenopausal women. Journal of women's health 17, 355-366 (2008). [CrossRef]
- Simbalista, R. L., Sauerbronn, A. V., Aldrighi, J. M. & Arêas, J. A. Consumption of a flaxseed-rich food is not more effective than a placebo in alleviating the climacteric symptoms of postmenopausal women. The Journal of nutrition 140, 293-297 (2010). [CrossRef]
- Vargas, M. L., Almario, R. U., Buchan, W., Kim, K. & Karakas, S. E. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism 60, 1711-1718 (2011). [CrossRef]
- Colli, M. C. et al. Evaluation of the efficacy of flaxseed meal and flaxseed extract in reducing menopausal symptoms. Journal of medicinal food 15, 840-845 (2012). [CrossRef]
- Mirmasoumi, G. et al. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Experimental and Clinical Endocrinology & Diabetes 126, 222-228 (2018).
- Sturgeon, S. R. et al. Effect of dietary flaxseed on serum levels of estrogens and androgens in postmenopausal women. Nutrition and cancer 60, 612-618 (2008). [CrossRef]
- Granata, O. M. et al. Dietary enterolactone affects androgen and estrogen levels in healthy postmenopausal women. Annals of the New York Academy of Sciences 1155, 232-236 (2009). [CrossRef]
- Low, Y.-L. et al. Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants. Cancer Epidemiology Biomarkers & Prevention 16, 1009-1016 (2007). [CrossRef]
- Zeleniuch-Jacquotte, A. et al. Circulating enterolactone and risk of breast cancer: a prospective study in New York. British journal of cancer 91, 99-105 (2004). [CrossRef]
- Zeleniuch-Jacquotte, A. et al. Circulating enterolactone and risk of endometrial cancer. International journal of cancer 119, 2376-2381 (2006). [CrossRef]
- Evans, B. A. J., Griffiths, K. & Morton, M. Inhibition of 5α-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. Journal of Endocrinology 147, 295-302 (1995). [CrossRef]
- Adlercreutz, H. et al. Dietary phytoestrogens and cancer: in vitro and in vivo studies. The Journal of steroid biochemistry and molecular biology 41, 331-337 (1992). [CrossRef]
- Martin, M. E., Haourigui, M., Pelissero, C., Benassayag, C. & Nunez, E. A. Interactions between phytoestrogens and human sex steroid binding protein. Life sciences 58, 429-436 (1995). [CrossRef]
- Haggans, C. J. et al. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutrition and cancer 33, 188-195 (1999). [CrossRef]
- McCann, S. E. et al. Changes in 2-hydroxyestrone and 16α-hydroxyestrone metabolism with flaxseed consumption: modification by COMT and CYP1B1 genotype. Cancer Epidemiology Biomarkers & Prevention 16, 256-262 (2007). [CrossRef]
- Brooks, J. D. et al. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. The American journal of clinical nutrition 79, 318-325 (2004). [CrossRef]
- Lord, R. S., Bongiovanni, B. & Bralley, J. A. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Alternative Medicine Review 7, 112-129 (2002).
- Fuhrman, B. J. et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute 104, 326-339 (2012). [CrossRef]
- Ziegler, R. G., Fuhrman, B. J., Moore, S. C. & Matthews, C. E. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids 99, 67-75 (2015). [CrossRef]
- JD, B. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol 94, 461-467 (2005).
- Adlercreutz, H. et al. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. The Journal of steroid biochemistry and molecular biology 44, 147-153 (1993). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




