Submitted:
25 October 2024
Posted:
28 October 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
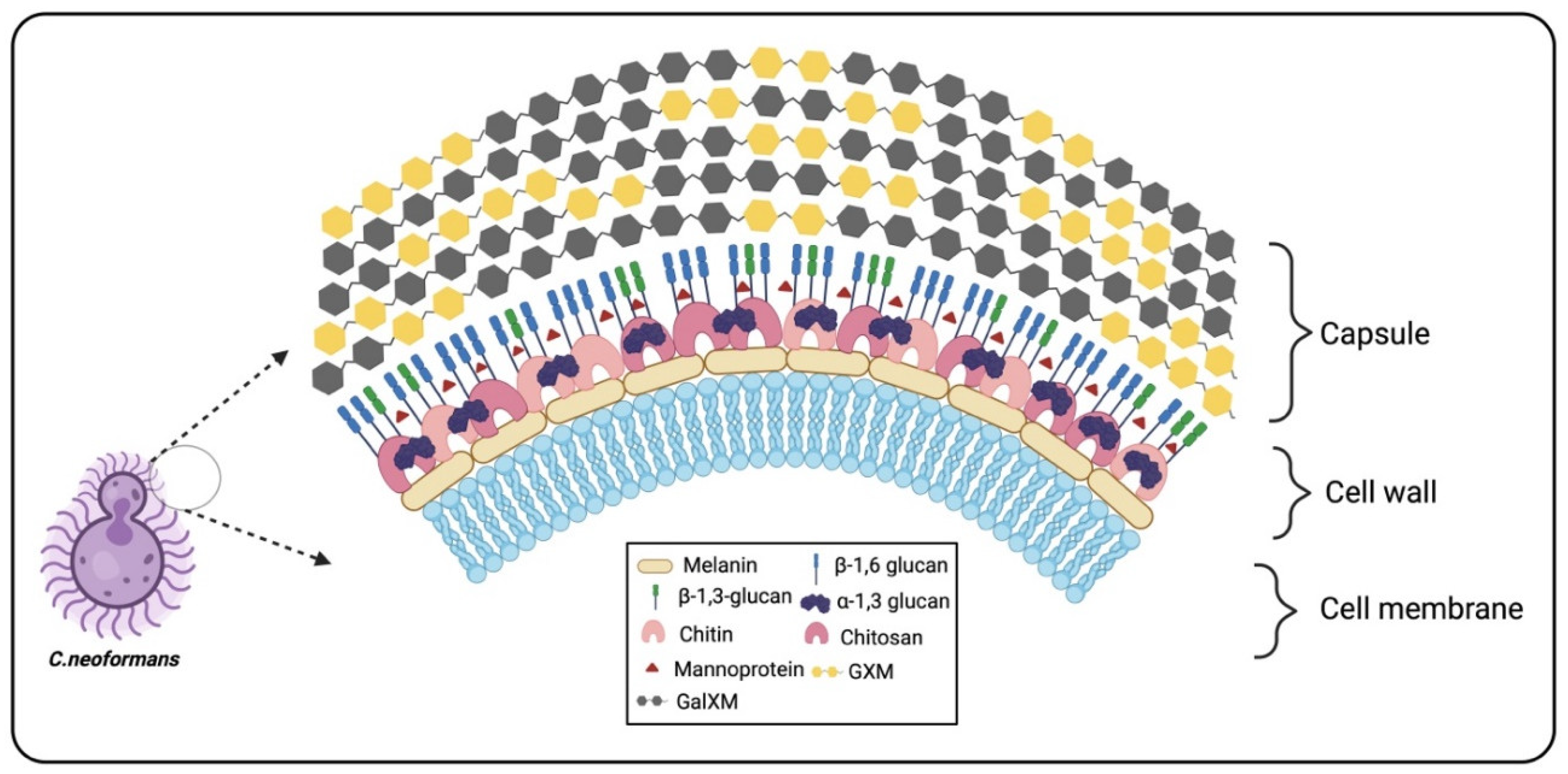
2. Cryptococcal Capsule
3. Cryptococcal Glucans
4. Cryptococcal Chitin and Chitosan
5. Mannoproteins
6. Extracellular Vesicles as Fungal Vaccine Platform
7. Immunological Responses of Current Whole Cell Cryptococcal Vaccine Candidates
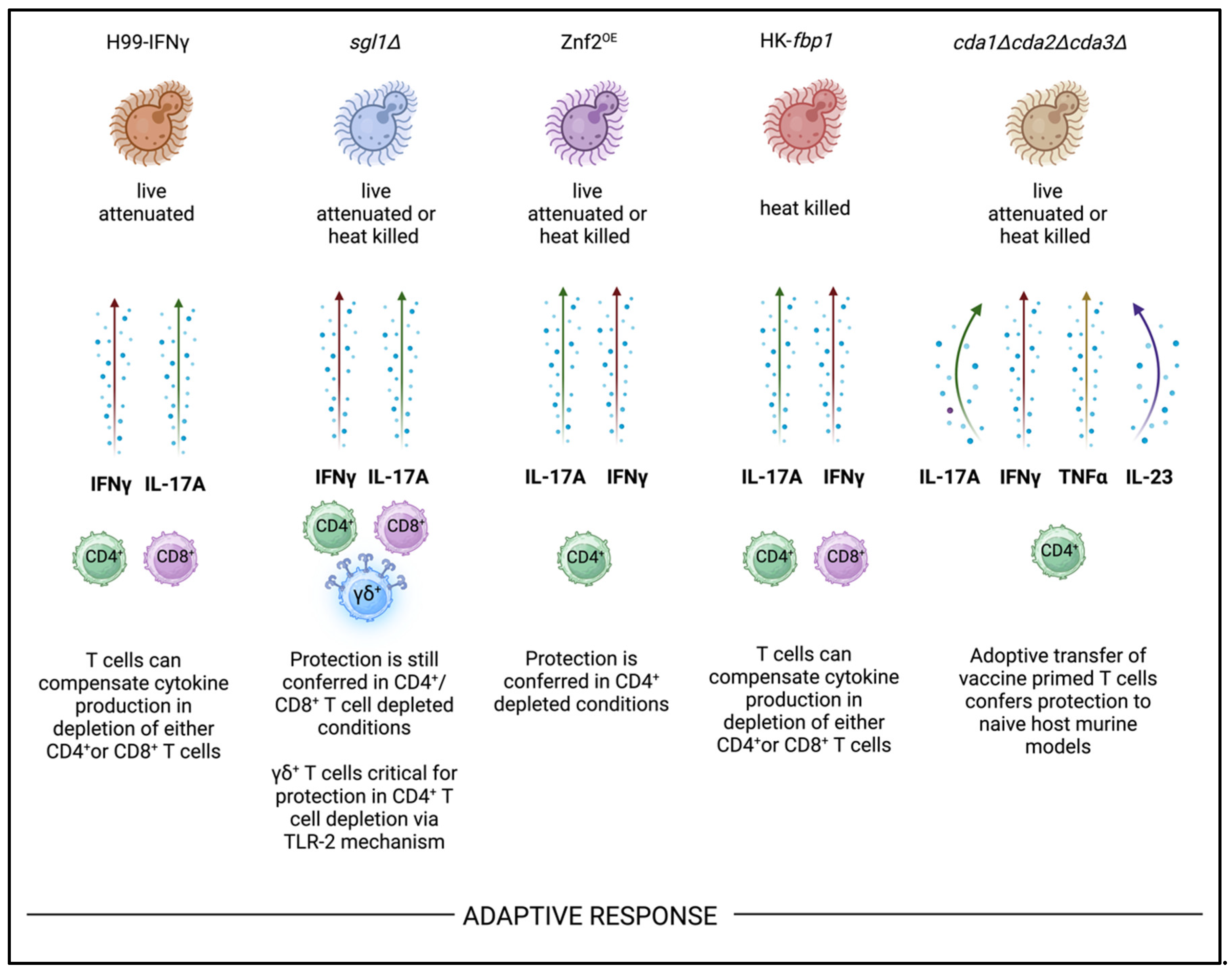
8. Adaptive Immunity
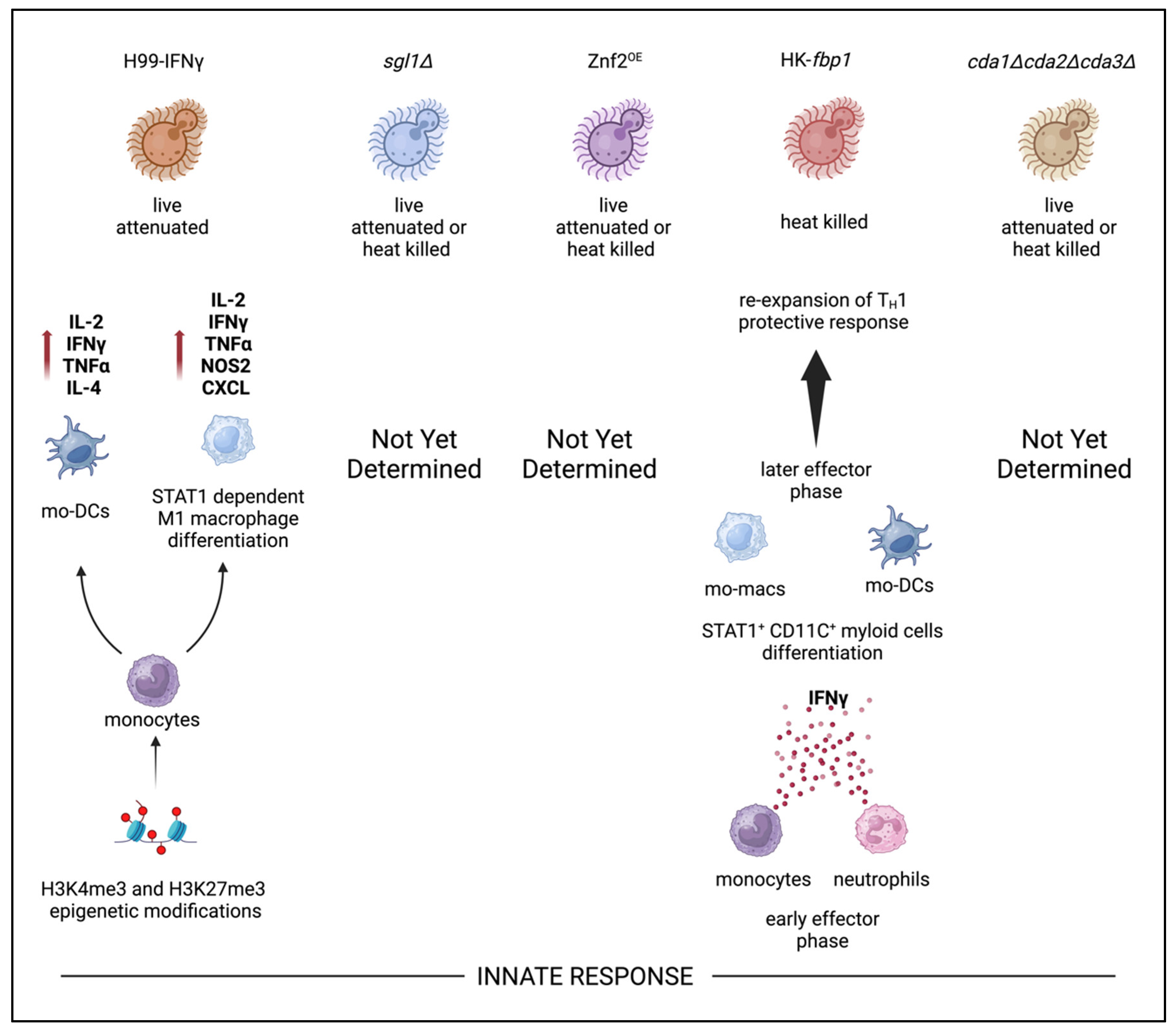
9. Innate Immunity and Trained Innate Immunity Responses
10. Concluding Remarks
Disclosure Statement
Acknowledgments
References
- Dao, A.; Kim, H.Y.; Garnham, K.; et al. Cryptococcosis—a systematic review to inform the world health organization fungal priority pathogens list. Medical Mycology 2024, 62. [Google Scholar] [CrossRef] [PubMed]
- WHO fungal priority pathogens list to guide research, development and public health action.
- Hagen, F.; Khayhan, K.; Theelen, B.; et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal genetics and biology 2015, 78, 16–48. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. The Lancet infectious diseases 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Rivera, A.; Lodge, J.; Xue, C. Harnessing the immune response to fungal pathogens for vaccine development. Annu Rev Microbiol. 2022, 76, 703–726. [Google Scholar] [CrossRef]
- An, H.; Liu, Y.; Qian, C.; et al. Chapter 5 - bacterial capsules. Molecular medical microbiology 2024, 69–96. [Google Scholar]
- Boodwa-Ko, D.; Doering, T.L. A quick reCAP: Discovering Cryptococcus neoformans capsule mutants. Journal of Fungi 2024, 10. [Google Scholar] [CrossRef]
- Cherniak, R.; Reiss, E.; Slodki, M.E.; Plattner, R.D.; Blumer, S.O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol Immunol. 1980, 17, 1025–1032. [Google Scholar] [CrossRef]
- Merrifield, E.H.; Stephen, A.M. Structural investigations of two capsular polysaccharides from Cryptococcus neoformans. Carbohydr Res. 1980, 86, 69–76. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans capsule: A sword and a shield. Clin Microbiol Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef]
- Fonseca, F.L.; Reis, F.C.G.; Sena, B.A.G.; Jozefowicz, L.J.; Kmetzsch, L.; Rodrigues, M.L. The overlooked glycan components of the Cryptococcus capsule. Fungal Physiology and Immunopathogenesis 2019, 31–43. [Google Scholar]
- Bouklas, T.; Jain, N.; Fries, B.C. Modulation of replicative lifespan in Cryptococcus neoformans: Implications for virulence. Frontiers in microbiology 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Tejas, B.; Ximo, P.; Goldman David, L.; Batya, E.; Aviv, B.; Fries Bettina, C. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio 2013, 4, 10.1128/mbio.00455–13. [Google Scholar] [CrossRef]
- Al-Huthaifi, A.M.; Radman, B.A.; Al-Alawi, A.A.; Mahmood, F.; Liu, T. Mechanisms and virulence factors of Cryptococcus neoformans dissemination to the central nervous system. Journal of Fungi 2024, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Carolina, C.; Emma, C.; Antonio, S.; Alexandre, A.; Arturo, C. Intranasal inoculation of Cryptococcus neoformans in mice produces nasal infection with rapid brain dissemination. mSphere 2019, 4, 10.1128/msphere.00483-19. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhao, X.; Lu, W.; Zhong, Y.; Fu, Y.V. Antifungal peptide SP1 damages polysaccharide capsule of Cryptococcus neoformans and enhances phagocytosis of macrophages. Microbiology Spectrum 2023, 11, 4562. [Google Scholar] [CrossRef]
- Zaragoza, O.; Chrisman, C.J.; Castelli, M.V.; et al. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.; Moraes Nicola, A.; Chow, S.; et al. Glucuronoxylomannan, galactoxylomannan, and mannoprotein occupy spatially separate and discrete regions in the capsule of Cryptococcus neoformans. Virulence 2010, 1, 500–508. [Google Scholar] [CrossRef]
- Reuwsaat, J.C.V.; Motta, H.; Garcia, A.W.A.; et al. A predicted mannoprotein participates in Cryptococcus gattii capsular structure. Msphere 2018, 3, 10.1128/msphere.00023-18. [Google Scholar] [CrossRef]
- Chang, Y.C.; Cherniak, R.; Kozel, T.R.; et al. Structure and biological activities of acapsular Cryptococcus neoformans 602 complemented with the CAP64 gene. Infect Immun. 1997, 65, 1584–1592. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J Bacteriol. 1999, 181, 5636–5643. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect Immun. 1998, 66, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun. 1996, 64, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Kwon-Chung, K.J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994, 14, 4912–4919. [Google Scholar] [PubMed]
- Kozel, T.R.; Gotschlich, E.C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. Journal of immunology (Baltimore, Md.: 1950) 1982, 129, 1675–1680. [Google Scholar] [CrossRef]
- Jianfeng, L.; Tuyetnhu, P.; Kenton, H.; Nathan, G.; Yumeng, F.; Xiaorong, L. Immunoprotection against cryptococcosis offered by Znf2 depends on capsule and the hyphal morphology. mBio 2022, 13, 2785. [Google Scholar] [CrossRef]
- Normile, T.G.; Chu, T.H.; Sheridan, B.S.; Del Poeta, M. Vaccine protection by Cryptococcus neoformans Δsgl1 is mediated by γδ T cells via TLR2 signaling. Mucosal Immunology 2022, 15, 1416–1430. [Google Scholar] [CrossRef]
- Datta, K.; Pirofski, L. Towards a vaccine for Cryptococcus neoformans: Principles and caveats. FEMS yeast research 2006, 6, 525–536. [Google Scholar] [CrossRef]
- Maitta Robert, W.; Kausik, D.; Qing, C.; et al. Protective and nonprotective human immunoglobulin M monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan manifest different specificities and gene use profiles. Infect Immun. 2004, 72, 4810–4818. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Cleare, W.; Feldmesser, M.; et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998, 42, 1437–1446. [Google Scholar] [CrossRef]
- Larsen, R.A.; Pappas, P.G.; Perfect, J.; et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother. 2005, 49, 952–958. [Google Scholar] [CrossRef]
- Casadevall, A.; Mukherjee, J.; Devi, S.J.; Schneerson, R.; Robbins, J.B.; Scharff, M.D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992, 165, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.J. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 1996, 14, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Fleuridor, R.; Lees, A.; Pirofski, L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans Infection1. J Immunol. 2001, 166, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Casadevall, A. Evaluation of Cryptococcus neoformans galactoxylomannan–protein conjugate as vaccine candidate against murine cryptococcosis. Vaccine 2011, 29, 1891–1898. [Google Scholar] [CrossRef]
- Crawford, C.J.; Liporagi-Lopes, L.; Coelho, C.; et al. Semisynthetic glycoconjugate vaccine candidates against Cryptococcus neoformans. ACS Infect Dis. 2024, 10, 2089–2100. [Google Scholar] [CrossRef]
- Doering, T.L. How sweet it is! cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol 2009, 63, 223–247. [Google Scholar] [CrossRef]
- Mukaremera, L. The Cryptococcus wall: A different wall for a unique lifestyle. PLOS Pathogens 2023, 19, e1011141. [Google Scholar] [CrossRef]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans melanin and virulence: Mechanism of action. Infect Immun. 1995, 63, 3131–3136. [Google Scholar] [CrossRef]
- James, P.G.; Cherniak, R.; Jones, R.G.; Stortz, C.A.; Reiss, E. Cell-wall glucans of Cryptococcus neoformans cap 67. Carbohydr Res. 1990, 198, 23–38. [Google Scholar] [CrossRef]
- Reese, A.J.; Doering, T.L. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol Microbiol. 2003, 50, 1401–1409. [Google Scholar] [CrossRef]
- Thompson, J.R.; Douglas, C.M.; Li, W.; et al. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J Bacteriol. 1999, 181, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.J.; Yoneda, A.; Breger, J.A.; et al. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol. 2007, 63, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.M.; Donlin, M.J.; Gerik, K.J.; et al. KRE genes are required for β-1, 6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in Cryptococcus neoformans. Mol Microbiol. 2010, 76, 517–534. [Google Scholar] [CrossRef]
- Basso, A.M.M.; De Castro, R.J.A.; de Castro, T.B.; et al. Immunomodulatory activity of β-glucan-containing exopolysaccharides from auricularia auricular in phagocytes and mice infected with Cryptococcus neoformans. Medical Mycology 2019, 58, 227–239. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; et al. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J Immunol. 2012, 189, 312–317. [Google Scholar] [CrossRef]
- Specht, C.A.; Homan, E.J.; Lee, C.K.; et al. Protection of mice against experimental cryptococcosis by synthesized peptides delivered in glucan particles. mBio 2021, 13, e0336721. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 2010, 1. [Google Scholar] [CrossRef]
- Specht, C.A.; Lee, C.K.; Huang, H.; et al. Vaccination with recombinant Cryptococcus proteins in glucan particles protects mice against cryptococcosis in a manner dependent upon mouse strain and cryptococcal species. mBio 2017, 8. [Google Scholar] [CrossRef]
- Wang, R.; Oliveira, L.V.N.; Lourenco, D.; et al. Immunological correlates of protection following vaccination with glucan particles containing Cryptococcus neoformans chitin deacetylases. npj Vaccines 2023, 8, 6. [Google Scholar] [CrossRef]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M.; Lodge, J.K. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2005, 4, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.; Jones, D.; Farmer, V.C.; Webley, D.M. The occurrence of α (1–3) glucan in Cryptococcus, schizosaccharomyces and polyporus species, and its hydrolysis by a streptomyces culture filtrate lysing cell walls of Cryptococcus. Biochimica et Biophysica Acta (BBA)-General Subjects 1968, 158, 313–315. [Google Scholar] [CrossRef]
- Upadhya, R.; Lam, W.C.; Maybruck, B.; Specht, C.A.; Levitz, S.M.; Lodge, J.K. Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of Cryptococcus neoformans. mBio 2016, 7. [Google Scholar] [CrossRef]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot Cell. 2007, 6, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Huang, H.; Ostroff, G.R.; Specht, C.A. Exploiting fungal cell wall components in vaccines. Seminars in Immunopathology 2015, 37, 199–207. [Google Scholar] [CrossRef]
- Baker Lorina, G.; Specht Charles, A.; Lodge Jennifer, K. Cell wall chitosan is necessary for virulence in the opportunistic pathogen Cryptococcus neoformans. Eukaryotic Cell. 2011, 10, 1264–1268. [Google Scholar] [CrossRef]
- Upadhya, R.; Baker, L.G.; Lam, W.C.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Cryptococcus neoformans Cda1 and its chitin deacetylase activity are required for fungal pathogenesis. mBio 2018, 9. [Google Scholar] [CrossRef]
- Lam, W.C.; Upadhya, R.; Specht, C.A.; et al. Chitosan biosynthesis and virulence in the human fungal pathogen Cryptococcus gattii. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Upadhya, R.; Lam, W.C.; Hole, C.R.; Vasselli, J.G.; Lodge, J.K. Cell wall composition in Cryptococcus neoformans is media dependent and alters host response, inducing protective immunity. Frontiers in Fungal Biology 2023, 4, 1183291. [Google Scholar] [CrossRef]
- Hole, C.R.; Lam, W.C.; Upadhya, R.; Lodge, J.K. Cryptococcus neoformans chitin synthase 3 plays a critical role in dampening host inflammatory responses. MBio 2020, 11, 10.1128/mbio. 03373-19. [Google Scholar] [CrossRef]
- Hester, M.M.; Oliveira, L.V.N.; Wang, R.; et al. Cross-reactivity between vaccine antigens from the chitin deacetylase protein family improves survival in a mouse model of cryptococcosis. Front Immunol 2022, 13, 1015586. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Chandrudu, S.; Toth, I. Strategies for intranasal delivery of vaccines. Drug Deliv Transl Res. 2013, 3, 100–109. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Levitz, S.M.; Specht, C.A. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS yeast research 2006, 6, 513–524. [Google Scholar] [CrossRef]
- Specht, C.A.; Nong, S.; Dan, J.M.; Lee, C.K.; Levitz, S.M. Contribution of glycosylation to T cell responses stimulated by recombinant Cryptococcus neoformans mannoprotein. J Infect Dis. 2007, 196, 796–800. [Google Scholar] [CrossRef]
- Levitz, S.M.; Nong, S.; Mansour, M.K.; Huang, C.; Specht, C.A. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proceedings of the National Academy of Sciences 2001, 98, 10422–10427. [Google Scholar] [CrossRef] [PubMed]
- Viudes, A.; Lazzell, A.; Perea, S.; et al. The C-terminal antibody binding domain of Candida albicans mp58 represents a protective epitope during candidiasis. FEMS Microbiol Lett. 2004, 232, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Lau, C.C.; et al. Mp1p is a virulence factor in Talaromyces (penicillium) marneffei. PLoS neglected tropical diseases 2016, 10, e0004907. [Google Scholar] [CrossRef]
- Cao, L.; Chan, C.; Lee, C.; Sai-yin Wong, S.; Yuen, K. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus penicillium marneffei. Infect Immun. 1998, 66, 966–973. [Google Scholar] [CrossRef]
- Pietrella, D.; Corbucci, C.; Perito, S.; Bistoni, G.; Vecchiarelli, A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun. 2005, 73, 820–827. [Google Scholar] [CrossRef]
- Mansour, M.K.; Schlesinger, L.S.; Levitz, S.M. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. The Journal of Immunology 2002, 168, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Messina, L.; Bombaci, M.; et al. Characterization of two novel cryptococcal mannoproteins recognized by immune sera. Infect Immun. 2005, 73, 7348–7355. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Mota, C.; Thak, E.J.; et al. Effects of altered N-glycan structures of Cryptococcus neoformans mannoproteins, MP98 (Cda2) and MP84 (Cda3), on interaction with host cells. Scientific Reports 2023, 13, 1175. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Lam, W.C.; Hole, C.R.; et al. Cryptococcus neoformans Cda1 and Cda2 coordinate deacetylation of chitin during infection to control fungal virulence. The Cell Surface 2021, 7, 1183291. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Miah, M.I.; Rahman, S.R. A comprehensive immunoinformatic analysis of chitin deacetylase’s and MP88 for designing multi-epitope vaccines against Cryptococcus neoformans. Journal of Biomolecular Structure and Dynamics 2023, 1–16. [Google Scholar] [CrossRef]
- Wang, R.; Oliveira, L.V.; Hester, M.M.; et al. Protection against experimental cryptococcosis elicited by cationic adjuvant formulation 01-adjuvanted subunit vaccines. bioRxiv 2024. [CrossRef]
- Biondo, C.; Mancuso, G.; Midiri, A.; et al. Identification of major proteins secreted by Cryptococcus neoformans. FEMS Yeast Res. 2006, 6, 645–651. [Google Scholar] [CrossRef]
- Cadieux, B.; Lian, T.; Hu, G.; et al. The mannoprotein Cig1 supports iron acquisition from heme and virulence in the pathogenic fungus Cryptococcus neoformans. J Infect Dis. 2013, 207, 1339–1347. [Google Scholar] [CrossRef]
- Yu Chen-Hsin Sephton-Clark Poppy Tenor Jennifer, L.; et al. Gene expression of diverse Cryptococcus isolates during infection of the human central nervous system. mBio 2021, 12, 2313. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Norton, D.; Price, M.S.; et al. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS pathogens 2010, 6, e1000776. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Holmer, S.M.; Selvig, K.; Dietrich, F.; Alspaugh, J.A. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. MBio 2013, 4, 10.1128/mbio. 00522-12. [Google Scholar] [CrossRef]
- Geddes, J.M.; Croll, D.; Caza, M.; Stoynov, N.; Foster, L.J.; Kronstad, J.W. Secretome profiling of Cryptococcus neoformans reveals regulation of a subset of virulence-associated proteins and potential biomarkers by protein kinase A. BMC microbiology 2015, 15, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Geddes, J.M.H.; Caza, M.; Croll, D.; Stoynov, N.; Foster, L.J.; Kronstad, J.W. Analysis of the protein kinase A-regulated proteome of Cryptococcus neoformans identifies a role for the ubiquitin-proteasome pathway in capsule formation. mBio 2016, 7, 10.1128/mbio.01862-15. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, K.; Wang, Y.; Liu, T.; Rivera, A.; Xue, C. Ubiquitin proteolysis of a CDK-related kinase regulates titan cell formation and virulence in the fungal pathogen Cryptococcus neoformans. Nature communications 2022, 13, 6397. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nature Reviews Immunology 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of extracellular vesicles in immune response and immunity. Immunity 2024, 57, 1752–1768. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, K.; Hu, H.; Xing, X.; Huang, X.; Gao, H. Extracellular vesicles: Their functions in plant–pathogen interactions. Molecular plant pathology 2022, 23, 760–771. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Castelli, R.F.; Reis, F.C.; Rizzo, J.; Rodrigues, M.L. Pathogenic delivery: The biological roles of cryptococcal extracellular vesicles. Pathogens 2020, 9, 754. [Google Scholar] [CrossRef]
- Rizzo, J.; Wong, S.S.W.; Gazi, A.D.; et al. Cryptococcus extracellular vesicles properties and their use as vaccine platforms. J Extracell Vesicles 2021, 10, e12129. [Google Scholar] [CrossRef]
- Colombo, A.C.; Rella, A.; Normile, T.; et al. Cryptococcus neoformans glucuronoxylomannan and sterylglucoside are required for host protection in an animal vaccination model. MBio 2019, 10, 10.1128/mbio. 02909-18. [Google Scholar] [CrossRef]
- Rella, A.; Mor, V.; Farnoud, A.M.; et al. Role of sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: Potential applications for vaccine development. Frontiers in microbiology 2015, 6, 836. [Google Scholar] [CrossRef]
- Del Poeta, M.; Wormley Floyd, L.; Lin, X., Jr. Host populations, challenges, and commercialization of cryptococcal vaccines. PLOS Pathogens 2023, 19, e1011115. [Google Scholar] [CrossRef]
- Wormley, F.L.J.; Perfect, J.R.; Steele, C.; Cox, G.M. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun. 2007, 75, 1453–1462. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Hole, C.R.; Campuzano, A.; et al. IFN-γ immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against Cryptococcus neoformans. PLoS pathogens 2018, 14, e1007358. [Google Scholar] [CrossRef]
- Van Dyke, M.C.C.; Chaturvedi, A.K.; Hardison, S.E.; et al. Induction of broad-spectrum protective immunity against disparate Cryptococcus serotypes. Frontiers in Immunology 2017, 8, 1359. [Google Scholar] [CrossRef]
- Zhai, B.; Wozniak, K.L.; Masso-Silva, J.; et al. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio 2015, 6, 1433. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Y.; Ferraro, A.R.; Yang, E.; Lewis, Z.A.; Lin, X. Transcription factor Znf2 coordinates with the chromatin remodeling SWI/SNF complex to regulate cryptococcal cellular differentiation. Communications Biology 2019, 2, 412. [Google Scholar] [CrossRef]
- Tuyetnhu, P.; Yeqi, L.; Wendy, W.; Xiaorong, L. Vaccination with a ZNF2oe strain of Cryptococcus provides long-lasting protection against cryptococcosis and is effective in immunocompromised hosts. Infect Immun. 2023, 91, 198. [Google Scholar] [CrossRef]
- Yina, W.; Keyi, W.; Masso-Silva Jorge, A.; Amariliz, R.; Chaoyang, X. A heat-killed Cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio 2019, 10, 10.1128/mbio.02145–19. [Google Scholar] [CrossRef]
- Yina, W.; Keyi, W.; Amariliz, R.; Chaoyang, X. Development of a heat-killed fbp1 mutant strain as a therapeutic agent to treat invasive Cryptococcus infection. Microbiology Spectrum. 2023, 11, 4955. [Google Scholar] [CrossRef]
- Keyi, W.; Vanessa, E.; Yina, W.; et al. Innate cells and STAT1-dependent signals orchestrate vaccine-induced protection against invasive Cryptococcus infection. mBio 2024, 0, 1944. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Masso-Silva, J.; Rivera, A.; Xue, C. A heat-killed Cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.; Espinosa, V.; Liu, T.B.; Wang, Y.; Xue, C.; Rivera, A. The F-box protein Fbp1 shapes the immunogenic potential of Cryptococcus neoformans. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hester, M.M.; Carlson, D.; Lodge, J.K.; Levitz, S.M.; Specht, C.A. Immune evasion by Cryptococcus gattii in vaccinated mice coinfected with C. neoformans. Frontiers in Immunology, 2024; 15. [Google Scholar] [CrossRef]
- Chen, J.; Shao, J.; Dai, M.; Fang, W.; Yang, Y. Adaptive immunology of Cryptococcus neoformans infections—an update. Frontiers in immunology, 2023; 14, 1174967. [Google Scholar]
- Wozniak, K.L.; Young, M.L.; Wormley Jr, F.L. Protective immunity against experimental pulmonary cryptococcosis in T cell-depleted mice. Clinical and Vaccine Immunology 2011, 18, 717–723. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Ravi, S.; Macias, S.; et al. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PloS one 2009, 4, e6854. [Google Scholar] [CrossRef]
- Normile, T.G.; Rella, A.; Del Poeta, M. Cryptococcus neoformans Δ sgl1 vaccination requires either CD4 or CD8 T cells for complete host protection. Frontiers in Cellular and Infection Microbiology 2021, 11, 739027. [Google Scholar]
- Masso-Silva, J.; Espinosa, V.; Liu, T.; Wang, Y.; Xue, C.; Rivera, A. The F-box protein Fbp1 shapes the immunogenic potential of Cryptococcus neoformans. MBio 2018, 9, 10.1128/mbio.01828-17. [Google Scholar] [CrossRef]
- Espinosa, V.; Dutta, O.; Heung, L.J.; et al. Cutting edge: Neutrophils license the maturation of monocytes into effective antifungal effectors. The Journal of Immunology 2022, 209, 1827–1831. [Google Scholar] [CrossRef]
- Espinosa, V.; Dutta, O.; McElrath, C.; et al. Type III interferon is a critical regulator of innate antifungal immunity. Science immunology 2017, 2, eaan5357. [Google Scholar] [CrossRef]
- Espinosa, V.; Jhingran, A.; Dutta, O.; et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS pathogens 2014, 10, e1003940. [Google Scholar] [CrossRef]
- Mukaremera, L.; Nielsen, K. Adaptive immunity to Cryptococcus neoformans infections. Journal of fungi 2017, 3, 64. [Google Scholar] [CrossRef]
- Specht Charles, A.; Ruiying, W.; Oliveira Lorena, V.N.; et al. Immunological correlates of protection mediated by a whole organism, Cryptococcus neoformans, vaccine deficient in chitosan. mBio 2024, 15, 1746. [Google Scholar] [CrossRef]
- Hardison, S.E.; Brown, G.D. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012, 13, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Osorio, F.; Rosas, M.; et al. Dectin-2 is a syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009, 206, 2037–2051. [Google Scholar] [CrossRef]
- Saijo, S.; Ikeda, S.; Yamabe, K.; et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against candida albicans. Immunity 2010, 32, 681–691. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van Het Hof, B.; van Kooyk, Y.; Geijtenbeek, T.B. C-type lectin DC-SIGN modulates toll-like receptor signaling via raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 2007, 26, 605–616. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; Den Dunnen, J.; Litjens, M.; et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through raf-1 and syk. Nat Immunol. 2009, 10, 203–213. [Google Scholar] [CrossRef] [PubMed]
- LeibundGut-Landmann, S.; Groß, O.; Robinson, M.J.; et al. Syk-and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef]
- Cheng, S.; Quintin, J.; Cramer, R.A.; et al. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; et al. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Divangahi, M.; Aaby, P.; Khader, S.A.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Tarang, S.; Kesherwani, V.; LaTendresse, B.; Lindgren, L.; Rocha-Sanchez, S.M.; Weston, M.D. In silico design of a multivalent vaccine against candida albicans. Scientific Reports 2020, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Tarcha Eric, J.; Venkatesha, B.; Hung Chiung-Yu Gardner Malcolm, J.; Cole Garry, T. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect Immun. 2006, 74, 5802–5813. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Q.J.; Ambati, S.; Link, C.D.; Lin, X.; Lewis, Z.A.; Meagher, R.B. Dectin-3-targeted antifungal liposomes efficiently bind and kill diverse fungal pathogens. Mol Microbiol. 2023, 120, 723–739. [Google Scholar] [CrossRef]
- Pham, T.; Shi, R.; Ambati, S.; Meagher, R.; Lin, X. All hands on dec: Treating cryptococcosis with dectin decorated liposomes loaded with antifungals. iScience 2024, 27. [Google Scholar] [CrossRef]
| Vaccine Candidate | Vaccination method | Mutant background | Vaccine Route Administration | Mechanism | Reference |
|---|---|---|---|---|---|
| sgl1D | Whole cell, live attenuated and heat killed | sterylglucosidase deficient strain | intranasal | IFN-g and IL-17A produced by gdT CD4+, and CD8+cells | (Normile et al., 2022) |
| H99g | Whole cell, live attenuated | Mouse IFN-g producing H99 strain | Intranasal | Th-1/proinflammatory cell response | (Wormley et al., 2007) |
| ZNf2OE | Whole cell, live attenuated and heat killed | zinc finger transcription factor 2 overexpressed | Intranasal | Th-1/Th-17 | (Zhai et al., 2015) |
| HK-fbp1D | Whole cell, heat killed | Disruption of SCF E3 ligase complex by deletion of F-box protein 1 | Intranasal | Th-1/Th-17 response | (Masso-Silva et al., 2018) (Wang et al., 2019) |
| cda1D2D3D | Whole cell, live attenuated and heat killed | Deletion of 3 chitin deacetylases | Intranasal | CD4+T cell response; proinflammatory cytokines IL-1b,IL-6, and IL-23 | (Upadhya et al., 2016b) |
| Glucan Particles (GP) | Protein subunit vaccine | Synthesized subunit protein | Intranasal | Antibody and T cell response | (Wang et al., 2023) (Specht et al., 2021) (Huang et al., 2010). |
| b-Glucan antibody | antibody based | monoclonal antibody | Intranasal | Antibody response | (Rachini et al., 2007) |
| Glucosylceramide antibody | antibody based | monoclonal antibody | Intranasal | Reduced pulmonary inflammation | (Rodrigues et al., 2007) |
| P13 (Peptide mimic of Cn GXM) | antibody based | Intranasal | Antibody response | (Fleuridor et al., 2001) | |
| GXM-TT | recombinant | Recombinant protein subunit | Intranasal | Antibody response | (Fleuridor et al., 2001) |
| GalXM-BSA | recombinant | Recombinant protein subunit | Intranasal | Antibody response | (Chow and Casadevall, 2011) |
| GXM antibody 18B7 | antibody based | monoclonal antibody | intranasal | Antibody responseClinical trial phase 1 | (Larsen et al., 2005) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





