1. Introduction
Abnormal placental implantation occurs when trophoblasts invade the superficial uterine endometrium (placenta accreta), the myometrium (placenta increta), or beyond the uterine serosa (placenta percreta). Collectively, these conditions are referred to as placenta accreta spectrum (PAS). The primary cause of PAS is thought to be defective decidualization at the implantation site, leading to the absence of both the decidua basalis and Nitabuch’s layer. This results in the direct attachment of chorionic villi to the myometrium [
1,
2]. The incidence of PAS is estimated to be as high as 1.1% of all births [
3], and this rate is rising globally due to an increase in cesarean deliveries and other uterine surgeries, such as surgical uterine evacuations, myomectomies, and infertility treatments [
4,
5]. Among the types of PAS, placenta accreta is the most common. In a pooled analysis of hysterectomy specimens with confirmed abnormal placentation, the distribution was as follows: placenta accreta (79%), placenta increta (14%), and placenta percreta (7%) [
6].
Several clinical studies have shown that PAS is associated with an increased incidence of respiratory distress syndrome (RDS) and a greater need for neonatal respiratory support, including continuous positive airway pressure [
7] [
8]. RDS occurs due to surfactant deficiency and immature lung development. Although it is well-known that preterm infants (those born before 37 weeks of gestation) are at higher risk for RDS, especially those born before 32 weeks [
9] [
10], and that the risk decreases with increasing gestational age as organ systems mature [
11,
12], earlier analysis at the V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology revealed a more severe course of the early neonatal period and a higher incidence of RDS in preterm infants born to mothers with PAS compared to those born to mothers without PAS [
13]. Despite the increasing number of PAS cases in recent years, its impact on neonatal outcomes and respiratory morbidity has not yet been extensively studied in large multicenter clinical trials. Therefore, it is crucial to understand the underlying mechanisms of neonatal complications in the context of PAS.
Andrew Parsons discussed the concept of a placental-pulmonary connection in his review article [
14], hypothesizing that placental disorders during pregnancy may uniquely affect the developing fetal lungs due to similarities in their structure and function. Both the placenta and lungs undergo parallel branching morphogenesis during gestation, leading to the formation of functional subunits for gas exchange—placental villi in the placenta and the air-epithelial interface in the lung alveoli. Parsons also explored the relationship between bronchopulmonary dysplasia and hypertensive pregnancy disorders, such as preeclampsia, which is associated with the release of the anti-angiogenic soluble fms-like tyrosine kinase 1 (sFLT-1) from the placenta into the maternal circulation. Increased sFLT-1 levels have been detected in fetal cord blood and amniotic fluid. In an antenatal model, intraamniotic exposure to anti-angiogenic sFLT-1 [
15] [
16] led to postnatal lung changes, including simplified alveolar structure, altered vascularization, and flattening of bronchial airway epithelium.
Given that PAS is characterized by a proangiogenic placental phenotype and increased trophoblast invasiveness, which contrasts with the antiangiogenic profile seen in preeclamptic placentas, there may be an antenatal link between placental characteristics in PAS and structural changes in the newborn’s lung tissue. Investigating this potential relationship was the focus of the current study.
Corticosteroids have become the standard of care for women at risk of preterm birth before 32 to 34 weeks of gestation in many countries [
17]. In the fetal lungs, corticosteroids stimulate the production of proteins, promote the biosynthesis of phospholipids, and increase the production of surfactant [
18]. Despite the widespread use of antenatal corticosteroids to prevent RDS in preterm infants, there is still no consensus on the optimal corticosteroid type, dosage, frequency, timing, or administration route [
19]. The reduction in the incidence of RDS with antenatal corticosteroid therapy is effective for up to seven days after treatment [
20]. A Cochrane Review evaluated whether women who remain undelivered and at risk of preterm birth should receive a repeat course of corticosteroids seven days after the initial treatment [
21].
The first large study demonstrating the effectiveness of antenatal corticosteroids for RDS prevention in late preterm infants (34/0–36/6 weeks) was conducted by Gyamfi-Bannerman et al. and published in 2016. It showed a reduction in the need for respiratory and surfactant therapy, as well as supplemental oxygen, in infants whose mothers received antenatal prophylaxis with betamethasone [
22]. The study also found a significant decrease in transient tachypnea of the newborn (TTN) and bronchopulmonary dysplasia (BPD). However, there was a noted increase in neonatal hypoglycemia. A systematic review with meta-analysis, published in 2016 and including Gyamfi-Bannerman's study, showed that neonates whose mothers received corticosteroids after 34 weeks had a significantly lower risk of developing RDS, TTN, and required less surfactant and mechanical ventilation. These infants also had shorter NICU stays, higher Apgar scores, and required lower peak oxygen concentrations [
23].
The effectiveness of antenatal corticosteroids for preventing RDS in late preterm infants (34/0 – 36/6 weeks) born to mothers with placenta accreta was specifically assessed at the Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology [
24]. The study found that when corticosteroids were administered no later than seven days before delivery, there was a reduction in the severity of respiratory disorders and a decrease in the need for invasive respiratory therapy, including high-frequency oscillatory ventilation (HFOVL). RDS prevention earlier in pregnancy was not always determinative, and the positive effects did not depend on the frequency of corticosteroid administration.
Despite these findings, no study has yet demonstrated the effectiveness of antenatal corticosteroid therapy in newborns of mothers with PAS at the molecular level. In earlier research, we identified microRNA markers of PAS in the blood of women during the first trimester of pregnancy [
25] and near the time of delivery [
26]. MicroRNAs (miRNAs) are small non-coding RNAs that regulate protein-coding mRNAs post-transcriptionally [
27]. They are expressed in various cell types and act as biological regulators. In reproductive biology, miRNAs are involved in processes such as spermatogenesis, folliculogenesis, endometrial functions, embryogenesis, maternal recognition of pregnancy, embryo implantation, and placental development [
28] [
29] [
30] [
31]. Aberrant miRNA expression has been linked to numerous pathological conditions, including pregnancy complications [
32,
33,
34]. Their ability to be secreted into biological fluids, combined with their measurability, sensitivity, and stability (average half-life of 119 hours), makes them promising markers for identifying pathological conditions [
35] [
36].
In this study, we aimed to investigate whether there are changes in plasma miRNA levels in premature infants born to mothers with PAS compared to infants of similar gestational age born to mothers without PAS. Additionally, we explored whether these changes are associated with the morphological type of PAS, the severity of respiratory and cardiovascular disorders in the newborn, and the timing of antenatal RDS prophylaxis.
2. Materials and Methods
2.1. Patients
All patients included in the study were admitted to the National Medical Research Center for Obstetrics, Gynecology, and Perinatology, named after Academician V.I. Kulakov of the Ministry of Healthcare of the Russian Federation, for pregnancy and delivery management. They signed informed consent to participate, and the study was approved by the Ethics Committee of the Center.
In the main group (n=69), all women underwent operative delivery via cesarean section due to PAS. In 66 cases, delivery was planned, while 3 cases required emergency cesarean section due to bleeding.
In the control group (n=11), all women also underwent cesarean sections. In 2 cases, the procedure was planned, with indications being preeclampsia in one case and threatened preterm labor in the other. Nine women required emergency cesarean sections, for reasons including bleeding (1 case), onset of labor (2 cases), fetal condition deterioration (3 cases), preeclampsia (1 case), suspected uterine scar failure (1 case), and maternal somatic pathology (1 case).
Antenatal prophylaxis for RDS was conducted following current clinical guidelines for preterm labor management. The drug "Dexamethasone" (manufacturer "Ellara," Russia) was administered intramuscularly at a dose of 8 mg three times, with an 8-hour interval between doses (total dose: 24 mg).
2.2. Isolation of RNA from Peripheral Blood Plasma Samples
Peripheral blood samples were collected into VACUETTE® EDTA tubes, centrifuged for 20 minutes at 300g at 4°C, plasma was collected and centrifuged again for 10 minutes at 16,000g. RNA was isolated from 200 μl of plasma using the miRNeasy Serum/Plasma kit (Qiagen).
2.3. Deep Sequencing of miRNA
cDNA libraries were synthesized using 6 μl of total RNA eluate from neonatal plasma samples with the NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (Set2, New England Biolab®, Germany), following the manufacturer’s protocol. The cDNA libraries were amplified and purified using 6% polyacrylamide gel, with the 140–160 base pair fraction extracted. The quantity and quality of the cDNA libraries were assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, USA) using the High Sensitivity DNA reagents kit (Agilent Technologies, USA). Sequencing of the cDNA libraries was performed on the NextSeq 500 platform (Illumina, USA), following the manufacturer’s instructions. For sequence annotation, the GRCh38.p15 and miRBase v21 databases were utilized, with the STAR RNAseq aligner program. The DESeq2 software package was used to normalize the cDNA read counts in each sample.
2.4. Reverse Transcription and Quantitative Real-Time PCR
Five microliters of the 14 µL eluate obtained from the miRNeasy Serum/Plasma Kit column (Qiagen, Hilden, Germany), which contained plasma RNA, were used for cDNA synthesis following the manufacturer's protocol with the miRCURY LNA RT Kit (Qiagen, Hilden, Germany). Quantitative real-time PCR was then carried out using the miRCURY LNA SYBR Green PCR Kit (Qiagen, Hilden, Germany) and miRNA-specific primers (miRCURY LNA miRNA PCR Assay, Qiagen, Hilden, Germany) according to the manufacturer's instructions, using a StepOnePlus™ thermal cycler (Applied Biosystems). Relative miRNA expression in plasma was calculated using the ∆Ct method, with UniSp6 serving as the reference RNA.
2.5. Statistical Data Processing
Scripts written in R [
37] and the RStudio software [
38] were used for statistical analysis. The Shapiro-Wilk test was applied to assess the normality of the data. For non-normally distributed data, paired comparisons were made using the Mann-Whitney test. Variables that did not follow a normal distribution were described as the median (Me) and quartiles Q1 and Q3 in the format Me (Q1; Q3). A significance threshold of p = 0.05 was set, and if the p-value was less than 0.001, it was indicated as p < 0.001.
Logistic regression models were developed in RStudio through stepwise inclusion and exclusion of miRNA marker molecules based on their contribution to the model. The predictive performance of the model was evaluated using ROC (Receiver Operating Characteristic) analysis, assessing the AUC (Area Under the Curve), statistical significance, specificity, and sensitivity.
4. Discussion
While maternal outcomes following pregnancies complicated by PAS are well documented, reports on neonatal outcomes in these cases are limited. Previous retrospective studies consistently indicated high rates of admissions to neonatal intensive care units (NICUs) and a significant need for mechanical ventilation in pregnancies affected by PAS [
45]. The primary perinatal complications observed in premature infants born to mothers with PAS in this study included transient tachypnea of the newborn (44%), RDS (12%), congenital pneumonia (41%), congenital anemia (20%), and intraventricular hemorrhage (8%). RDS, which results from a primary deficiency of surfactant and the immaturity of lung tissue due to prematurity, along with congenital pneumonia, can lead to the development of acute respiratory distress syndrome (ARDS) [
46]. The mortality rate associated with ARDS remains high, accounting for 30% of all fatalities in intensive care units. [
47] [
48] [
49]. Morphologically, RDS and ARDS exhibit similar characteristics, including immaturity and antenatal damage to the structures of the air-blood barrier, as well as pneumonia and pulmonary ischemia with the formation of hyaline membranes [
46].
Recently, numerous studies have been published on the molecular mechanisms involved in the pathogenesis and pathophysiology of ARDS, many of which are detailed in a review article by Huang Q. et al.[
50]. The author summarizes that lung barrier dysfunction during ARDS results from the death of alveolar epithelial and pulmonary endothelial cells, which can be triggered by apoptosis pathways such as FasL, TNF-α/TNFR1, and TNF-related apoptosis-inducing ligand (TRAIL) signaling events. Additionally, the article discusses various signals that regulate inflammatory processes during ARDS, particularly those known to activate the RhoA/ROCK pathway, including IL-1, TGF-β, thrombin, sphingosine-1 phosphate (S1P), and endothelin-1. It also highlights factors that alter the activity of the PI3K/AKT pathway through the mammalian target of rapamycin (mTOR) or NF-κB, leading to NLRP3 inflammasome activation or increased levels of inflammatory cytokines. Furthermore, the epithelial–mesenchymal transition (EMT) is identified as a major factor contributing to epithelial barrier dysfunction and worsening pulmonary edema through the modulation of Wnt signaling in the alveolar epithelium. This process results in the loss of epithelial morphology and the acquisition of mesenchymal characteristics, along with the expression of profibrotic proteins that contribute to pulmonary fibrosis. In this study, we found that these signaling pathways are potentially regulated by two microRNAs, miR-382-5p and miR-199a-3p, which were significantly elevated in the blood plasma of day-old neonates and/or their mothers with PAS.
In recent times, there has been an increasing emphasis on the role of miRNAs in RDS, particularly through their ability to target specific genes to regulate signaling pathways [
51] [
52]. Certain miRNAs play significant roles in the inflammatory response associated with ARDS. For instance, miR-199a-3p has been linked to inflammatory lung diseases, including sepsis-induced ARDS [
53]. Notably, this miRNA regulates the synthesis and release of various inflammatory mediators by macrophages [
54], which account for nearly half of the immune cells in the lungs[
55] [
56]. Emerging evidence highlights the critical role of extracellular vesicles from alveolar macrophages in the inflammatory processes of ARDS, particularly secretory autophagosomes (SAPs) [
57]. One of the regulators of SAP secretion is miR-199a-3p, which influences the expression of the target gene PAK4 [
54], a serine/threonine kinase identified as a key regulator of TNF-induced microparticle release [
58]. Studies have shown that SAPs derived from alveolar macrophages contribute to ARDS through excessive secretion of IL-1β, which exacerbates inflammation and pathological injury in lung tissue [
57]. Overexpression of miR-199a-3p has been observed in the lungs of mice with ARDS, where the miR-199a-3p antagomir significantly inhibited SAP release, while the miR-199a-3p mimetic promoted SAP release in bronchoalveolar lavage fluid (BALF), resulting in alleviation or intensification of LPS-stimulated ARDS, respectively [
54]. These results are consistent with findings from this study that noted an increase in hsa-miR-199a-3p levels in the blood plasma of newborns from mothers with PAS. This increase manifests as severe respiratory distress in the early neonatal period, necessitating invasive ventilation or high-frequency ventilation (HFVL).
Another possible pathogenetic mechanism for respiratory disorders in premature infants born to mothers with PAS, particularly concerning the elevated levels of hsa-miR-199a-3p circulating in maternal and fetal blood, is its negative impact on the differentiation of alveolar type II cells, consequently affecting surfactant protein production [
59]. The major protein component of pulmonary surfactant, SP-A (a product of the SFTPA gene), is developmentally regulated in fetal lung. It serves as a marker of alveolar type II cell differentiation Additionally, SP-A plays a vital role in innate immunity by enhancing the uptake and destruction of various pathogens by alveolar macrophages [
60] [
61]. Moreover, it is secreted into the amniotic fluid from the fetal lung, acting as a signaling molecule for the initiation of labor [
62] [
63] [
64].
During a normal pregnancy, there is a developmental decline in the expression of the miR-199a/-214 cluster in the fetal lung, which leads to increased expression of key gene targets responsible for alveolar type II cell differentiation and enhanced SP-A expression by term [
59]. This dependence of miR-199a/-214 cluster expression on gestational age can be explained by increased TGF-β signaling during early to mid-gestation, when the fetal lung is relatively hypoxic. This signaling enhances the expression of ZEB1, a transcription factor that stimulates miR-199a/miR-214 cluster expression. As vascularization of the fetal lung increases during the third trimester and near term, heightened oxygen tension leads to decreased TGF-β signaling and repression of ZEB1, resulting in reduced expression of miR-199a/miR-214.Overexpression of miR-199a-3p, -5p, and miR-214 in human fetal lung epithelial cells has been shown to inhibit SP-A expression as well as the expression of transcription factors CREB1 and C/EBPβ, which are crucial for fetal lung development [
65] [
66]. Interestingly, ZEB1 is an EMT (epithelial-mesenchymal transition) factor that downregulates epithelial genes while activating mesenchymal genes, promoting a highly invasive cell phenotype [
67] [
68]. This is typical for extravillous trophoblast cells of the placenta in the case of PAS, which exhibit abnormally aggressive EMT that does not cease at the end of the first trimester but continues throughout pregnancy [
69] [
70].
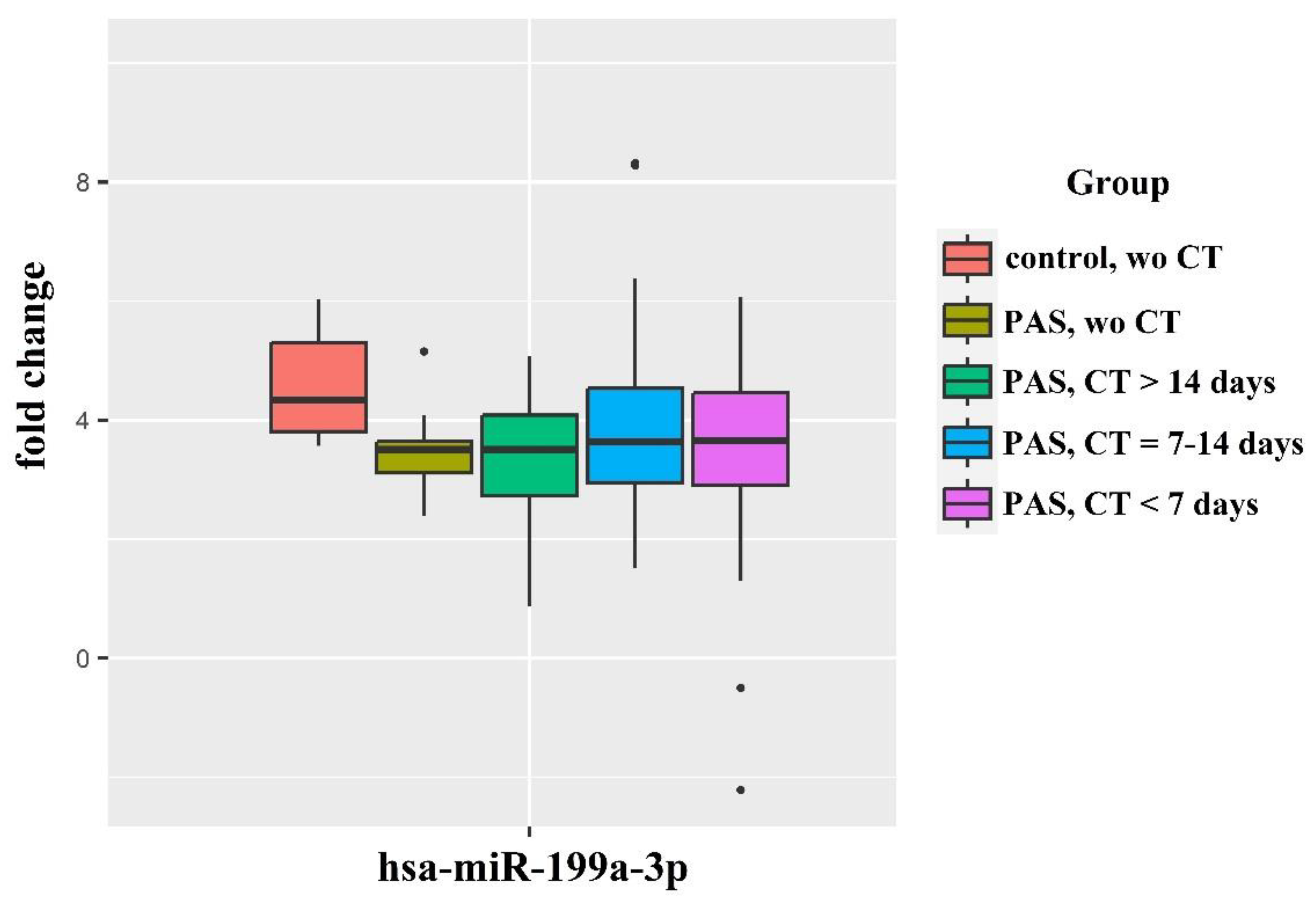
Thus, the following mechanism of pathogenesis of respiratory disorders in neonates from mothers with PAS. The elevated level of miR-199a-3p in maternal blood plasma in cases of PAS may reflect excessive EMT of extracellular trophoblasts under chronic inflammatory conditions in the uterine decidua due to endometritis, antecedent curettage, or incompetent uterine scars following cesarean sections. According to Kalluri R. [
71], macrophages and activated resident fibroblasts secrete growth factors such as TGF-β, chemokines, and matrix metalloproteinases (MMP-2, -3, -9) in these circumstances. The presence of chorionic villi in the layers of the myometrium results in abnormal gas exchange in the maternal-fetal system, creating hypoxic conditions for the fetus, including the lung tissue. Under these conditions, TGF-β signaling in lung tissue increases, raising the expression of ZEB1 and, consequently, hsa-miR-199a-3p, leading to immature lung structures and reduced surfactant synthesis.Additionally, we observe elevated levels of hsa-miR-199a-3p in the blood plasma of neonates. As indicated in
Figure 3, in cases of PAS, the level of hsa-miR-199a-3p in maternal blood plasma is higher than that in neonatal blood plasma compared to pregnancies without PAS. This represents an additional negative factor influencing the damage to fetal lung tissue due to circulating maternal hsa-miR-199a-3p. Moreover, this study revealed significant negative correlations between the levels of hsa-miR-199a-3p in maternal and fetal blood plasma and the severity of PAS; specifically, lower levels of hsa-miR-199a-3p in the maternal and fetal bloodstream are associated with deeper placental invasion into the myometrial layers. The elevated level of hsa-miR-382-5p detected in the blood plasma of newborns from mothers with placenta accreta may represent an additional pathogenetic link in the occurrence of neonatal complications. Furthermore, levels of hsa-miR-199a-3p and hsa-miR-382-5p in the blood plasma of newborns were found to correlate significantly and positively with each other. This correlation may be explained by the presence of a common experimentally validated target gene, PTEN (according to miRTargetLink), which is involved in cell functions including proliferation, migration, and metabolism [
72]. Dysregulated PTEN expression was found in blastocyst implantation [
73], preeclampsia [
74] [
75], pulmonary diseases [
76], and PAS [
77]. Localized primarily in the syncytiotrophoblast (STB), endothelial cells surrounding fetal blood vessels, and to a lesser extent in the stroma of normal placenta [
77], increased expression of PTEN impairs human trophoblast cell invasion and is associated with the development of preeclampsia [
78]. In contrast, PTEN mRNA and protein levels are reduced in placenta tissue affected by PAS compared to normal placenta [
77], suggesting its critical role during pregnancy.
It is known that miR-382-5p is a member of the chromosome 14 miRNA cluster (C14MC), which is one of the largest clusters of pregnancy-related miRNAs, comprising 52 miRNAs [
28]. This cluster is involved in embryonic development, endothelial cell migration, and angiogenesis during placental development [
79]. miR-382-5p, as an ortholog of the C14MC found in equines, has been shown to be enriched in the blood serum of pregnant mares compared to non-pregnant mares [
80]. Additionally, aberrant expression of miR-382-5p in rat lung tissues has been reported as a potential cause of bronchopulmonary dysplasia (BRD) through the suppression of M1 macrophage polarization[
81,
82].
Regarding the regulation of macrophage function, miR-382-5p may play a significant role in the pathogenesis of ARDS, as macrophages are a crucial component of pulmonary innate immunity, comprising nearly half of the immune cells in the lungs, and the balance between M1 and M2 macrophage phenotypes influences the various stages of ARDS [
83] [
84] [
85] [
86] [
87]. In the acute exudative phase of ARDS, macrophages are predominantly M1-polarized, releasing pro-inflammatory factors that induce a severe inflammatory response. In the later stages of ARDS, macrophages mainly adopt an M2-polarized phenotype, which can lead to pathological fibroplasia and pulmonary fibrosis.
Mechanisms regulating macrophage function involving miR-382-5p have been demonstrated using microglial cells, which are resident macrophages in the central nervous system and perform immune surveillance in the brain and spinal cord [
88]. Through the upregulation of Circ_0006640, which can directly sequester miR-382-5p, and the elevation of IGF1, a target of miR-382-5p, microglial cells showed protection from LPS-induced apoptotic, inflammatory, and oxidative injuries. IGF-1 is a major growth hormone critical for prenatal lung growth and organogenesis [
89]. Local synthesis of IGF-1 in lung tissue occurs in type II pneumocytes, alveolar macrophages, and mesenchymal cells. In animal models, mutations in the IGF-1 gene disrupt the architecture of lung tissue, leading to atelectatic lungs, respiratory failure, and high postnatal mortality.
In our study, the level of hsa-miR-382-5p in the blood plasma of premature infants born to mothers with PAS was significantly higher in cases where antenatal prophylaxis for RDS was absent or implemented more than 14 days before delivery, compared to premature infants born to mothers without PAS and without antenatal prophylaxis for RDS. The level of hsa-miR-382-5p in the blood plasma of newborns from mothers with PAS tended to normalize after antenatal prophylaxis for RDS 2-14 days before delivery and did not significantly differ from levels in the blood plasma of newborns from mothers without PAS. A markedly increased level of hsa-miR-382-5p in the blood plasma of premature infants from mothers with PAS, particularly in the absence of antenatal prophylaxis for RDS or when implemented more than 14 days before delivery, likely causes a decrease in IGF-1 across various organs and tissues of the newborn, including the lungs. This decrease helps explain the presence of respiratory disorders in this group of patients, as well as the statistically significant correlations between the level of hsa-miR-382-5p in the blood plasma of the newborn and factors such as weight (r = -0.39; p = 0.0027), required oxygen fraction in the NICU (r = 0.41; p = 0.0016), length of stay in the NICU (r = 0.31; p = 0.019), and severity of the newborn's condition according to the NEOMOD scale (r = 0.36; p = 0.0051).
In addition to respiratory support, newborns from mothers with PAS require cardiotonic therapy due to cardiovascular dysfunction. It was found that miRNAs derived from the precursor miR-199a play a key role in maintaining cardiac homeostasis, particularly through the regulation of endothelial nitric oxide synthase (eNOS) in the endothelium [
90] [
91] [
92]. A common mechanism underlying many cardiovascular diseases is endothelial dysfunction, which is characterized by reduced availability of nitric oxide (NO) [
93]. It has been demonstrated that inhibition of miR-199a-3p enhances eNOS activity and decreases the degradation of NO, thereby increasing its bioavailability and modulating vascular contractility [
92].
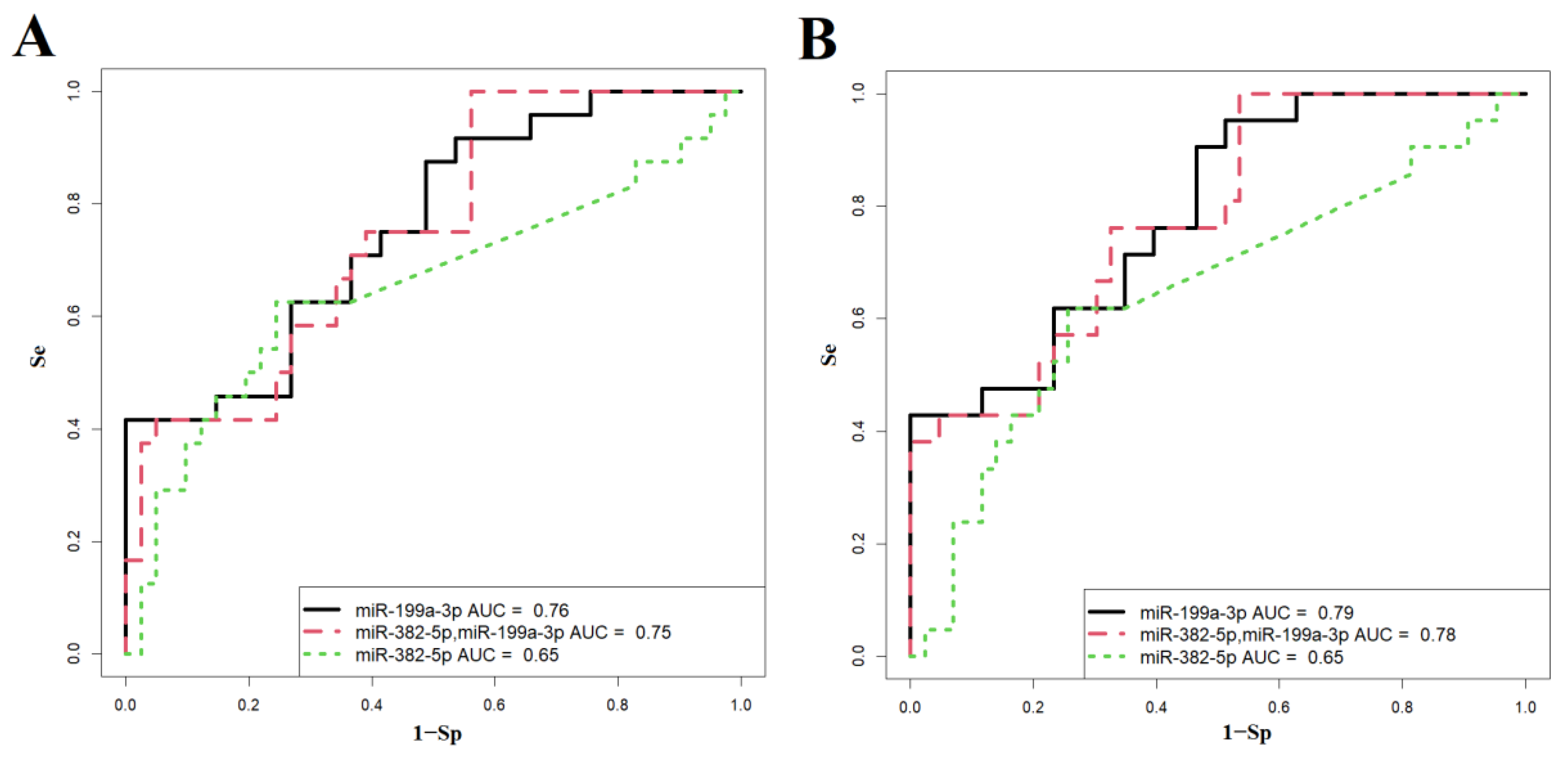
Given the relationships identified in this study between the levels of hsa-miR-199a-3p and hsa-miR-382-5p in the blood plasma of pregnant women and their newborns, as well as the severity of respiratory and cardiac disorders during the neonatal period, we constructed logistic regression models to predict these disorders. These models take into account the established roles of these miRNAs in surfactant synthesis by alveolar cells, fetal organogenesis, the formation of proper lung tissue architecture, and the regulation of the cardiovascular system as reported in the literature. The models developed in this study allow for the prediction of the need for cardiotonic therapy and invasive mechanical ventilation (IMV) or high-frequency oscillatory ventilation (HFOV) for newborns in the early neonatal period, with a sensitivity of 95-100%. However, the implementation of these models in clinical practice will require large-scale studies using independent test samples.
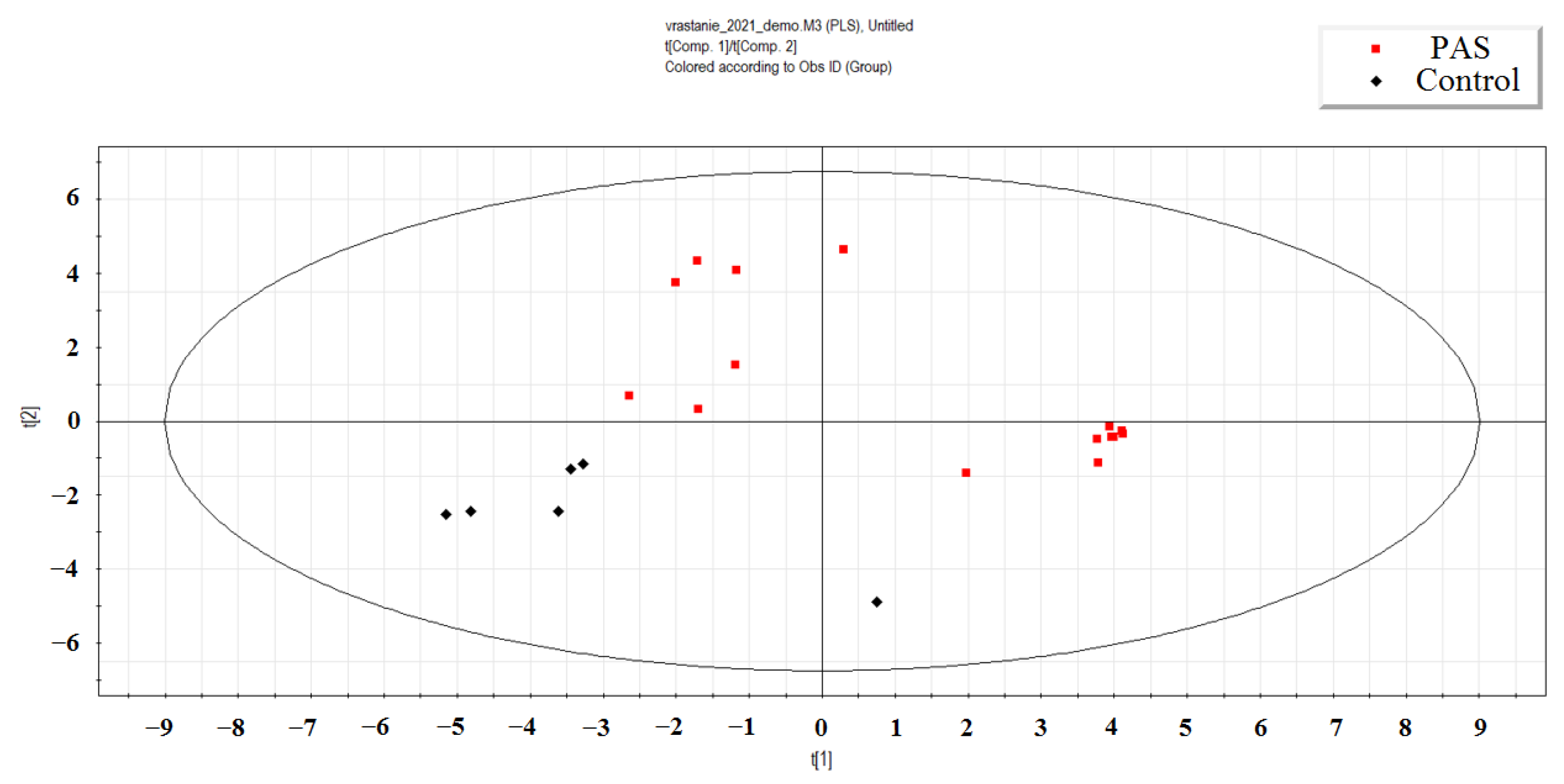
Figure 1.
PLS-A analysis of deep sequencing data of miRNA in the peripheral blood plasma of day-old newborns from mothers with PAS and without PAS (control).
Figure 1.
PLS-A analysis of deep sequencing data of miRNA in the peripheral blood plasma of day-old newborns from mothers with PAS and without PAS (control).
Figure 2.
The dependence of hsa-miR-382-5p and hsa-miR-199a-3p content in the blood plasma of newborns and their mothers on the severity of placenta accreta spectrum (PAS) and the timing of corticosteroid therapy (CT).
Figure 2.
The dependence of hsa-miR-382-5p and hsa-miR-199a-3p content in the blood plasma of newborns and their mothers on the severity of placenta accreta spectrum (PAS) and the timing of corticosteroid therapy (CT).
Figure 3.
Dynamics of changes in hsa-miR-199a-3p levels in the blood plasma of newborns compared to their mothers' blood plasma, with and without PAS, depending on the timing of antenatal corticosteroid therapy (CT).
Figure 3.
Dynamics of changes in hsa-miR-199a-3p levels in the blood plasma of newborns compared to their mothers' blood plasma, with and without PAS, depending on the timing of antenatal corticosteroid therapy (CT).
Figure 4.
Levels of hsa-miR-199a-3p and hsa-miR-382-5p in the blood plasma of newborns with PAS, categorized by their severity score according to the Neomod scale.
Figure 4.
Levels of hsa-miR-199a-3p and hsa-miR-382-5p in the blood plasma of newborns with PAS, categorized by their severity score according to the Neomod scale.
Figure 5.
Levels of miR-181a-5p, miR-199a-3p and miR-382-5p in blood plasma of pregnant women with/without PAS and with/without antenatal corticosteroid therapy.
Figure 5.
Levels of miR-181a-5p, miR-199a-3p and miR-382-5p in blood plasma of pregnant women with/without PAS and with/without antenatal corticosteroid therapy.
Figure 6.
Logistic regression models for predicting neonatal complications by plasma miR-199a-3p and/or miR-382-5p levels in pregnant women with PAS using miR-181a-5p as a reference endogenous RNA. (A) Respiratory complications probability models. (B) Cardiovascular complications probability models.
Figure 6.
Logistic regression models for predicting neonatal complications by plasma miR-199a-3p and/or miR-382-5p levels in pregnant women with PAS using miR-181a-5p as a reference endogenous RNA. (A) Respiratory complications probability models. (B) Cardiovascular complications probability models.
Figure 7.
Enrichment analysis of gene-targets of hsa-miR-382-5p and hsa-miR-199a-3p using FunRich software tool.
Figure 7.
Enrichment analysis of gene-targets of hsa-miR-382-5p and hsa-miR-199a-3p using FunRich software tool.
Table 1.
Statistically significant changes in the level of miRNA in the blood plasma of newborns from mothers with PAS and RDS prophylaxis 7-14 days before delivery compared to newborns from mothers without PAS and without RDS prophylaxis.
Table 1.
Statistically significant changes in the level of miRNA in the blood plasma of newborns from mothers with PAS and RDS prophylaxis 7-14 days before delivery compared to newborns from mothers without PAS and without RDS prophylaxis.
| |
miRNA |
baseMean |
log2FoldChange |
lfcSE |
p-value |
| 1 |
hsa-miR-215-5p |
98.71519 |
5.819687 |
1.265354 |
4.24E-06 |
| 2 |
hsa-miR-516b-5p |
215.1577 |
5.248942 |
1.166537 |
6.81E-06 |
| 3 |
hsa-miR-182-5p |
55.26162 |
4.71655 |
1.105862 |
2.00E-05 |
| 4 |
hsa-miR-183-5p |
143.454 |
4.126976 |
1.034286 |
6.60E-05 |
| 5 |
hsa-miR-192-5p |
503.9136 |
1.635789 |
0.46376 |
0.00042 |
| 6 |
hsa-miR-1323 |
30.67836 |
3.847168 |
1.192338 |
0.001253 |
| 7 |
hsa-miR-760 |
15.02699 |
-3.21625 |
1.009398 |
0.001441 |
| 8 |
hsa-let-7f-5p |
992.7216 |
2.217035 |
0.745282 |
0.002932 |
| 9 |
hsa-miR-26a-5p |
1195.92 |
1.756481 |
0.610171 |
0.003994 |
| 10 |
hsa-miR-199a-3p |
320.7235 |
-1.81477 |
0.635226 |
0.004278 |
| 11 |
hsa-miR-200c-3p |
121.4978 |
-4.12153 |
1.450851 |
0.004501 |
| 12 |
hsa-miR-199b-3p |
160.3617 |
-1.7853 |
0.631627 |
0.004706 |
| 13 |
hsa-let-7g-5p |
1207.634 |
1.872991 |
0.679862 |
0.00587 |
| 14 |
hsa-miR-10a-5p |
1121.615 |
2.724361 |
1.00493 |
0.006708 |
| 15 |
hsa-miR-146b-5p |
130.2105 |
1.470428 |
0.550239 |
0.007532 |
| 16 |
hsa-miR-99b-3p |
8.964436 |
-3.41312 |
1.28876 |
0.008088 |
| 17 |
hsa-miR-218-5p |
9.343058 |
-4.06315 |
1.600639 |
0.011134 |
| 18 |
hsa-miR-150-5p |
24.77196 |
1.48045 |
0.631319 |
0.019026 |
| 19 |
hsa-miR-29a-3p |
35.61049 |
1.99514 |
0.86954 |
0.021763 |
| 20 |
hsa-miR-181b-5p |
124.9393 |
-2.39485 |
1.093218 |
0.028478 |
| 21 |
hsa-miR-378c |
8.895485 |
1.825745 |
0.838232 |
0.029399 |
| 22 |
hsa-miR-26b-5p |
102.9558 |
1.269355 |
0.58317 |
0.029507 |
| 23 |
hsa-miR-30e-3p |
45.69012 |
1.591149 |
0.740817 |
0.031727 |
| 24 |
hsa-miR-483-3p |
37.44106 |
2.100013 |
0.979484 |
0.032033 |
| 25 |
hsa-miR-194-5p |
209.4698 |
1.657005 |
0.780456 |
0.033743 |
| 26 |
hsa-miR-99a-5p |
1362.079 |
-1.48541 |
0.714392 |
0.037592 |
| 27 |
hsa-miR-2110 |
38.68886 |
-1.90428 |
0.917955 |
0.038034 |
| 28 |
hsa-let-7d-3p |
244.3945 |
1.280001 |
0.628354 |
0.041643 |
| 29 |
hsa-miR-382-5p |
125.1091 |
-2.21816 |
1.205711 |
0.04581 |
Table 2.
Clinical characteristics of premature infants in mothers with and without antenatal corticosteroid therapy, along with comparisons of the corresponding groups using the Mann-Whitney U test.
Table 2.
Clinical characteristics of premature infants in mothers with and without antenatal corticosteroid therapy, along with comparisons of the corresponding groups using the Mann-Whitney U test.
| Clinical parameters |
Control, without CT (n=11) |
PAS, Without CT (n=10) |
“Control without CT” vs “PAS without CT” |
PAS, CT more than 14 days before delivery (n=13) |
“Control, without CT” vs “PAS, CT more than 14 days before delivery” |
PAS, CT during 7-14 days before delivery (n=25) |
“Control, without CT” vs PAS from 7 to 14 |
PAS, CT during 7 days before delivery (n=21) |
“Control, without CT” vs “PAS, CT during 7 days before delivery” |
| Me(Q1;Q3) |
Me(Q1;Q3) |
p-value |
Me(Q1;Q3) |
p-value |
Me(Q1;Q3) |
p-value |
Me(Q1;Q3) |
p-value |
| Mother’sbloodless during delivery |
750(750;825) |
1350(812.5;2675) |
0.033 |
800(700;1200) |
0.841 |
800(750;1000) |
0.279 |
800(750;1000) |
0.419 |
| Weight of newborn, g |
2250(1965;2437.5) |
2795.5(2542;3042.25) |
0.001 |
2520(2390;2652) |
0.089 |
2863(2780;3030) |
<0.001 |
2850(2730;2960) |
0.001 |
| Apgar score, 1 min |
8(7.5;8) |
7(7;8) |
0.205 |
8(7;8) |
0.702 |
8(7;8) |
0.606 |
8(7;8) |
0.973 |
| Apgar score, 5 min |
8(8;9) |
8(8;8) |
0.084 |
8(8;8) |
0.067 |
8(8;9) |
0.425 |
8(8;9) |
0.447 |
| WBC |
11.42(9.75;12.68) |
12.25(9.94;18.02) |
0.417 |
10.46(9.39;13.35) |
0.757 |
14.11(9.5;16.98) |
0.207 |
13.28(10.64;16.5) |
0.189 |
| ACHN |
4225(3806.5;4561) |
4776.5(3236.25;8941) |
0.475 |
3872(3448;5440) |
0.937 |
5664(4323;7874) |
0.148 |
6190(4131;7722) |
0.155 |
| Ni |
0.07(0.04;0.08) |
0.05(0.02;0.11) |
0.659 |
0.06(0.03;0.09) |
0.781 |
0.07(0.03;0.11) |
0.714 |
0.06(0.05;0.09) |
0.979 |
| RBС |
4.51(4.36;4.84) |
4.78(4.11;4.9) |
1 |
4.76(4.42;4.89) |
0.938 |
4.46(4.06;4.83) |
0.48 |
4.66(4.45;4.84) |
0.75 |
| RDW-CV |
16(15.35;17.2) |
15.75(15.27;16.28) |
0.769 |
15.8(15.4;16.6) |
0.721 |
15.8(15.4;16.1) |
0.437 |
15.8(15.3;16.5) |
0.652 |
| RDW-SD |
63.1(61.9;67.95) |
57.45(51.85;59.35) |
0.007 |
58.8(55.9;60.4) |
0.047 |
58.9(56.7;59.7) |
0.009 |
60.1(57.7;62.9) |
0.08 |
| MCV |
105.8(105;108.3) |
98(95.38;102.12) |
0.001 |
101.4(99.4;103.2) |
0.008 |
102.2(98.5;103.3) |
0.002 |
101.9(100.4;105.6) |
0.027 |
| HGB, g/L |
163(155.5;180.5) |
161(145.5;167.75) |
0.806 |
168(158;179) |
0.936 |
158(146;173) |
0.583 |
168(161;171) |
0.121 |
| MCH |
36.6(35.8;38.2) |
35.05(34;35.4) |
0.01 |
36.2(35.2;36.7) |
0.427 |
35.5(35.1;36.5) |
0.068 |
35.9(35.1;36.6) |
0.185 |
| MCHC |
34.6(34.55;34.95) |
35.45(35.05;36.22) |
0.05 |
35.7(35.2;36.1) |
0.039 |
35.4(35;35.7) |
0.079 |
35.1(34.6;35.6) |
0.287 |
| HTC |
47.3(45.1;52.15) |
42.75(40.07;49.5) |
0.13 |
47.2(45.1;49.8) |
0.606 |
44.8(41.2;50.6) |
0.171 |
47.7(46.4;48.9) |
0.958 |
| Platelets |
324(288;356) |
323(280.25;399) |
0.696 |
281(224;335) |
0.428 |
354(317;402) |
0.092 |
339(296;413) |
0.533 |
| MPV |
9.7(9.05;9.95) |
9.45(9.2;9.67) |
0.302 |
9.8(9.4;10) |
0.72 |
9.5(8.9;10) |
1 |
9.6(9;10.1) |
0.811 |
| PTC |
0.31(0.26;0.38) |
0.3(0.27;0.37) |
0.883 |
0.28(0.22;0.32) |
0.341 |
0.35(0.3;0.38) |
0.283 |
0.34(0.28;0.37) |
0.594 |
| PDW |
10.4(9.55;10.55) |
9.7(8.98;10.88) |
0.807 |
10.2(9.5;10.7) |
0.873 |
9.1(8.6;10) |
0.273 |
9.8(9;10.1) |
0.381 |
| PLCR |
22.3(17.6;24) |
19.95(18.5;23.18) |
0.66 |
22.8(19.2;24.5) |
0.751 |
19.7(15.9;24.2) |
0.789 |
21(17.8;25.1) |
1 |
| DHR |
2(1;4) |
4.5(3;6) |
0.115 |
5(2;6) |
0.118 |
2(2;4) |
0.591 |
2(2;3) |
0.978 |
| HD |
13(9;14.5) |
10(8;14) |
0.305 |
11(11;13) |
0.937 |
10(7;15) |
0.315 |
9(7;11) |
0.77 |
Table 3.
Quantitative RT-PCR data assessing hsa-miR-382-5p and hsa-miR-199a-3p levels in the blood plasma of newborns from mothers without PAS in the absence of antenatal corticosteroid therapy, as well as from mothers with PAS without CT or with CT administered during different time periods.
Table 3.
Quantitative RT-PCR data assessing hsa-miR-382-5p and hsa-miR-199a-3p levels in the blood plasma of newborns from mothers without PAS in the absence of antenatal corticosteroid therapy, as well as from mothers with PAS without CT or with CT administered during different time periods.
| |
RT-PCR data, -ΔCt |
p-value, Mann-Whitney U test |
| Group |
Me |
Q1 |
Q3 |
Without CT |
Without CT |
CT more than 14 days before delivery |
CT during 7-14 days before delivery |
CT during 7 days before delivery |
| miR-382-5p |
| |
control |
placenta accreta |
| Control, without CT |
-13.28 |
-13.34 |
-12.81 |
1 |
0.0471 |
0.2 |
0.486 |
0.415 |
| placenta accreta, without CT |
-10.28 |
-10.74 |
-10.05 |
0.0471 |
1 |
0.1 |
0.049 |
0.05 |
| placenta accreta, CT more than 14 days before delivery |
-11.66 |
-11.67 |
-11.47 |
0.2 |
0.1 |
1 |
0.8 |
0.25 |
| placenta accreta, CT during 7-14 days before delivery |
-11.68 |
-12.36 |
-11.32 |
0.486 |
0.049 |
0.8 |
1 |
0.9 |
| placenta accreta, CT during 7 days before delivery |
-12.02 |
-12.89 |
-11.72 |
0.415 |
0.05 |
0.25 |
0.9 |
1 |
| |
control |
placenta increta |
| Control, without CT |
-13.28 |
-13.34 |
-12.81 |
1 |
0.0491 |
0.016 |
0.079 |
0.19 |
| placenta increta, without CT |
-10.38 |
-11.06 |
-10.07 |
0.0491 |
1 |
0.7 |
0.1 |
0.1 |
| placenta increta, CT more than 14 days before delivery |
-10.51 |
-10.98 |
-9.78 |
0.016 |
0.7 |
1 |
0.008 |
0.03 |
| placenta increta, CT during 7-14 days before delivery |
-11.31 |
-12.01 |
-11.11 |
0.079 |
0.1 |
0.008 |
1 |
0.5 |
| placenta increta, CT during 7 days before delivery |
-12.13 |
-12.15 |
-11.65 |
0.19 |
0.1 |
0.03 |
0.5 |
1 |
| |
control |
Placenta percreta |
| Control, without CT |
-13.28 |
-13.34 |
-12.81 |
1 |
0.2 |
0.171 |
0.28 |
0.226 |
| placenta percreta, without CT |
-11.89 |
-12.48 |
-11.35 |
0.2 |
1 |
0.7 |
0.55 |
0.785 |
| placenta percreta, CT more than 14 days before delivery |
-11.33 |
-12.11 |
-10.89 |
0.171 |
0.7 |
1 |
0.122 |
0.462 |
| placenta percreta, CT during 7-14 days before delivery |
-12.5 |
-13 |
-11.98 |
0.28 |
0.55 |
0.122 |
1 |
0.101 |
| placenta percreta, CT during 7 days before delivery |
-11.89 |
-11.99 |
-11.72 |
0.226 |
0.785 |
0.462 |
0.101 |
1 |
| miR-199a-3p |
| |
control |
placenta accreta |
| Control, without CT |
-11.44 |
-11.87 |
-10.91 |
1 |
0.05 |
0.05 |
0.1 |
0.28 |
| placenta accreta, without CT |
-9.72 |
-9.86 |
-9.72 |
0.05 |
1 |
0.7 |
0.2 |
0.9 |
| placenta accreta, CT more than 14 days before delivery |
-9.91 |
-9.96 |
-9.87 |
0.05 |
0.7 |
1 |
0.6 |
0.3 |
| placenta accreta, CT during 7-14 days before delivery |
-10.13 |
-10.38 |
-9.72 |
0.1 |
0.2 |
0.6 |
1 |
0.9 |
| placenta accreta, CT during 7 days before delivery |
-10.07 |
-10.41 |
-9.99 |
0.28 |
0.9 |
0.3 |
0.9 |
1 |
| |
control |
placenta increta |
| Control, without CT |
-11.44 |
-11.87 |
-10.91 |
1 |
0.05 |
0.05 |
0.1 |
0.06 |
| placenta accreta, without CT |
-9.72 |
-10.05 |
-9.38 |
0.05 |
1 |
0.7 |
0.07 |
0.45 |
| placenta accreta, CT more than 14 days before delivery |
-9.51 |
-10.08 |
-8.39 |
0.05 |
0.7 |
1 |
0.04 |
0.3 |
| placenta accreta, CT during 7-14 days before delivery |
-10.43 |
-10.54 |
-9.87 |
0.1 |
0.07 |
0.04 |
1 |
0.1 |
| placenta accreta, CT during 7 days before delivery |
-9.82 |
-10.14 |
-9.63 |
0.06 |
0.45 |
0.3 |
0.1 |
1 |
| |
|
|
|
control |
placenta percreta |
| Control, without CT |
-11.44 |
-11.87 |
-10.91 |
1 |
0.6 |
0.35 |
0.4 |
0.6 |
| placenta accreta, without CT |
-10.51 |
-10.96 |
-10.44 |
0.6 |
1 |
0.9 |
0.6 |
0.9 |
| placenta accreta, CT more than 14 days before delivery |
-11.09 |
-11.49 |
-10.12 |
0.35 |
0.9 |
1 |
0.8 |
0.8 |
| placenta accreta, CT during 7-14 days before delivery |
-10.32 |
-11.46 |
-9.89 |
0.4 |
0.6 |
0.8 |
1 |
0.5 |
| placenta accreta, CT during 7 days before delivery |
-10.59 |
-11.94 |
-10.24 |
0.6 |
0.9 |
0.8 |
0.5 |
1 |
Table 4.
The quantitative RT-PCR data for evaluating hsa-miR-382-5p and hsa-miR-199a-3p levels in the blood plasma of pregnant women without PAS in the absence of antenatal CT, in mothers with PAS without CT, or with CT at various time points.
Table 4.
The quantitative RT-PCR data for evaluating hsa-miR-382-5p and hsa-miR-199a-3p levels in the blood plasma of pregnant women without PAS in the absence of antenatal CT, in mothers with PAS without CT, or with CT at various time points.
| |
RT-PCR data, -ΔCt |
p-value, Mann-Whitney U test |
| Group |
Me |
Q1 |
Q3 |
Without CT |
Without CT |
CT more than 14 days before delivery |
CT during 7-14 days before delivery |
CT during 7 days before delivery |
| hsa-miR-382-5p |
| |
control |
placenta accreta |
| Control, without CT |
-19.23 |
-19.35 |
-19.06 |
1 |
0.0167 |
0.9 |
0.23 |
0.106 |
| placenta accreta, without CT |
-17.05 |
-17.06 |
-15.68 |
0.0167 |
1 |
0.1 |
0.161 |
0.39 |
| placenta accreta, CT more than 14 days before delivery |
-19.16 |
-19.27 |
-19.11 |
0.9 |
0.1 |
1 |
0.22 |
0.25 |
| placenta accreta, CT during 7-14 days before delivery |
-18.67 |
-19.1 |
-18.24 |
0.23 |
0.161 |
0.22 |
1 |
0.9 |
| placenta accreta. CT during 7 days before delivery |
-18.92 |
-19.13 |
-18.2 |
0.106 |
0.39 |
0.25 |
0.9 |
1 |
| |
control |
placenta increta |
| Control, without CT |
-19.23 |
-19.35 |
-19.06 |
1 |
0.41 |
0.1 |
0.07 |
0.0177 |
| placenta accreta, without CT |
-19.13 |
-19.22 |
-19.01 |
0.41 |
1 |
0.413 |
0.304 |
0.111 |
| placenta accreta, CT more than 14 days before delivery |
-18.81 |
-19.2 |
-18.11 |
0.1 |
0.413 |
1 |
0.9 |
0.548 |
| placenta accreta, CT during 7-14 days before delivery |
-18.9 |
-19.08 |
-16.86 |
0.07 |
0.304 |
0.9 |
1 |
0.513 |
| placenta accreta, CT during 7 days before delivery |
-18.64 |
-18.99 |
-16.07 |
0.0177 |
0.111 |
0.548 |
0.513 |
1 |
| |
control |
placenta percreta |
| Control, without CT |
-19.23 |
-19.35 |
-19.06 |
1 |
0.383 |
0.731 |
0.0853 |
0.0268 |
| placenta accreta, without CT |
-19.14 |
-19.17 |
-19.06 |
0.383 |
1 |
0.905 |
0.368 |
0.291 |
| placenta accreta, CT more than 14 days before delivery |
-19.18 |
-19.32 |
-16.11 |
0.731 |
0.905 |
1 |
0.808 |
0.216 |
| placenta accreta, CT during 7-14 days before delivery |
-18.98 |
-19.1 |
-18.36 |
0.0853 |
0.368 |
0.808 |
1 |
0.606 |
| placenta accreta, CT during 7 days before delivery |
-18.9 |
-19.07 |
-15.96 |
0.0268 |
0.291 |
0.216 |
0.606 |
1 |
| hsa-miR-199a-3p |
| |
control |
placenta accreta |
| Control, without CT |
-15.52 |
-15.88 |
-15.3 |
1 |
0.015 |
0.0167 |
0.006 |
0.002 |
| placenta accreta, without CT |
-12.88 |
-13.53 |
-12.67 |
0.015 |
1 |
0.7 |
0.161 |
0.786 |
| placenta accreta, CT more than 14 days before delivery |
-13.69 |
-13.77 |
-13.33 |
0.0167 |
0.7 |
1 |
0.629 |
0.786 |
| placenta accreta, CT during 7-14 days before delivery |
-12.96 |
-13.33 |
-12.67 |
0.006 |
0.161 |
0.629 |
1 |
0.19 |
| placenta accreta, CT during 7 days before delivery |
-13.41 |
-13.85 |
-13.39 |
0.002 |
0.786 |
0.786 |
0.19 |
1 |
| |
control |
placenta increta |
| Control, without CT |
-15.52 |
-15.88 |
-15.3 |
1 |
0.00606 |
0.04 |
0.0185 |
0.00253 |
| placenta accreta, without CT |
-13.54 |
-14.11 |
-12.84 |
0.00606 |
1 |
0.9 |
0.188 |
0.905 |
| placenta accreta, CT more than 14 days before delivery |
-13.09 |
-13.46 |
-12.39 |
0.04 |
0.9 |
1 |
0.206 |
0.841 |
| placenta accreta, CT during 7-14 days before delivery |
-14.37 |
-15.01 |
-13.32 |
0.0185 |
0.188 |
0.206 |
1 |
0.31 |
| placenta accreta, CT during 7 days before delivery |
-13.84 |
-14.09 |
-13.04 |
0.00253 |
0.905 |
0.841 |
0.31 |
1 |
| |
control |
placenta percreta |
| Control, without CT |
-15.52 |
-15.88 |
-15.3 |
1 |
0.0167 |
0.05 |
0.0346 |
0.0154 |
| placenta accreta, without CT |
-14.72 |
-14.78 |
-14.59 |
0.0167 |
1 |
0.7 |
0.885 |
0.291 |
| placenta accreta, CT more than 14 days before delivery |
-14.87 |
-15.17 |
-13.85 |
0.05 |
0.7 |
1 |
0.961 |
0.462 |
| placenta accreta, CT during 7-14 days before delivery |
-14.56 |
-15.27 |
-14.08 |
0.0346 |
0.885 |
0.961 |
1 |
0.3 |
| placenta accreta, CT during 7 days before delivery |
-14.13 |
-14.78 |
-13.59 |
0.0154 |
0.291 |
0.462 |
0.3 |
1 |
Table 5.
Relative content of hsa-miR-199a-3p in the blood plasma of newborns from pregnant women without PAS in the absence of corticosteroid therapy, as well as in the blood plasma of newborns from pregnant women with PAS with or without CT administered during different time periods.
Table 5.
Relative content of hsa-miR-199a-3p in the blood plasma of newborns from pregnant women without PAS in the absence of corticosteroid therapy, as well as in the blood plasma of newborns from pregnant women with PAS with or without CT administered during different time periods.
| |
RT-PCR data, -ΔCt |
p-value, Mann-Whitney U test |
| group |
Ме |
Q1 |
Q3 |
Control, without CT |
PAS, without CT |
PAS, CT more than 14 days before delivery |
PAS, CT during 7-14 days before delivery |
PAS, CT during 7 days before delivery |
| Control, without CT |
4.34 |
3.81 |
5.3 |
1 |
0.005 |
0.037 |
0.2 |
0.09 |
| PAS, without CT |
3.51 |
3.12 |
3.64 |
0.005 |
1 |
0.93 |
0.68 |
0.66 |
| PAS, CT more than 14 days before delivery |
3.51 |
2.73 |
4.09 |
0.037 |
0.93 |
1 |
0.55 |
0.8 |
| PAS, CT during 7-14 days before delivery |
3.64 |
2.95 |
4.53 |
0.2 |
0.68 |
0.55 |
1 |
0.58 |
| PAS, CT during 7 days before delivery |
3.66 |
2.91 |
4.46 |
0.09 |
0.66 |
0.8 |
0.58 |
1 |
Table 6.
Comparison of newborns groups from mothers with PAS based on the levels of hsa-miR-199a-3p and hsa-miR-382-5p relative to their scores on the Neomod scale.
Table 6.
Comparison of newborns groups from mothers with PAS based on the levels of hsa-miR-199a-3p and hsa-miR-382-5p relative to their scores on the Neomod scale.
| |
miR-382-5p |
miR-199a-3p |
| |
RT-PCR data, -ΔCt |
p-value, Mann-Whitney U test |
ОТ-ПЦР данные, -ΔCt |
p-value, Mann-Whitney U test |
| Groups according to the Neomod scale |
Me |
Q1 |
Q3 |
Neomod, 0 |
Me |
Q1 |
Q3 |
Neomod, 0 |
| Neomod, 0 |
-12.15 |
-12.81 |
-11.89 |
1 |
-10.36 |
-11.08 |
-10.13 |
1 |
| Neomod, 1 |
-11.75 |
-12.81 |
-11.08 |
0.251 |
-10.3 |
-11.12 |
-9.68 |
0.672 |
| Neomod, 2 |
-11.25 |
-11.59 |
-10.13 |
0.073 |
-9.72 |
-10.17 |
-9.38 |
0.1807 |
| Neomod, 4 |
-11.21 |
-11.64 |
-10.82 |
0.0134 |
-10.25 |
-10.58 |
-9.82 |
0.8868 |
| Neomod, 5 |
-11.43 |
-11.68 |
-10.88 |
0.0503 |
-10.15 |
-10.98 |
-9.45 |
0.927 |
| Neomod, > 4 |
-11.23 |
-11.65 |
-10.82 |
0.0096 |
-10.24 |
-10.58 |
-9.81 |
0.855 |
Table 7.
Comparison of groups of pregnant women by the level of hsa-miR-382-5p, hsa-miR-199a-3p and hsa-miR-181a-5p depending on the presence of PAS and the time of corticosteroid therapy.
Table 7.
Comparison of groups of pregnant women by the level of hsa-miR-382-5p, hsa-miR-199a-3p and hsa-miR-181a-5p depending on the presence of PAS and the time of corticosteroid therapy.
| Group |
miR-382-5p |
miR-199a-3p |
miR-181a-5p |
| RT-PCR data, -ΔCt |
p-value, Mann-Whitney U test |
RT-PCR data, -ΔCt |
p-value, Mann-Whitney U test |
RT-PCR data, -ΔCt |
p-value, Mann-Whitney U test |
| Me |
Q1 |
Q3 |
Control, without CT |
Me |
Q1 |
Q3 |
Control, without CT |
Me |
Q1 |
Q3 |
Control, without CT |
| Control, without CT |
-19.23 |
-19.35 |
-19.06 |
1 |
-15.52 |
-15.88 |
-15.3 |
1 |
-16.12 |
-17.05 |
-15.99 |
1 |
| PAS, without CT |
-18.93 |
-19.12 |
-17.35 |
0.02 |
-14.13 |
-14.39 |
-12.91 |
<0.001 |
-15.72 |
-19.03 |
-14.48 |
0.66 |
| PAS, CT more than 14 days before delivery |
-19.13 |
-19.3 |
-18.29 |
0.3 |
-13.61 |
-14.89 |
-13.18 |
0.004 |
-15.63 |
-17.87 |
-14.45 |
0.255 |
| PAS, CT during 7-14 days before delivery |
-18.98 |
-19.1 |
-18.31 |
0.03 |
-14.25 |
-15.01 |
-13.22 |
0.003 |
-17.03 |
-18.79 |
-15.03 |
0.89 |
| PAS, CT during 7 days before delivery |
-18.9 |
-19.09 |
-16.07 |
0.007 |
-13.9 |
-14.43 |
-13.39 |
<0.001 |
-18.49 |
-19.13 |
-15.2 |
0.53 |
Table 8.
Parameters of the logistic regression models presented in Figure 6A and Figure 6B.
Table 8.
Parameters of the logistic regression models presented in Figure 6A and Figure 6B.
|
Figure 6A |
Wald |
p_value |
coefficients |
threshold |
sensitivity |
specificity |
| 1 model |
|
|
|
0.642 |
0.4167 |
1 |
| (Intercept) |
1.879 |
0.060 |
0.974 |
|
|
|
| miR-199a-3p |
-3.281 |
0.001 |
-0.548 |
|
|
|
| 2 model |
|
|
|
0.2028 |
1 |
0.439 |
| (Intercept) |
1.706 |
0.088 |
1.540 |
|
|
|
| miR-382-5p |
0.796 |
0.426 |
0.119 |
|
|
|
| miR-199a-3p |
-2.662 |
0.008 |
-0.699 |
|
|
|
| 3 model |
|
|
|
0.4223 |
0.625 |
0.7561 |
| (Intercept) |
-2.616 |
0.009 |
-0.804 |
|
|
|
| miR-382-5p |
-2.049 |
0.040 |
-0.206 |
|
|
|
| |
|
|
|
|
|
|
| Рисунoк 6В |
Wald |
p_value |
coefficients |
threshold |
sensitivity |
specificity |
| 1 model |
|
|
|
0.16 |
0.95 |
0.49 |
| (Intercept) |
1.887 |
0.050 |
1.046 |
|
|
|
| miR-199a-3p |
-3.473 |
0.001 |
-0.635 |
|
|
|
| 2 model |
|
|
|
0.15 |
1 |
0.47 |
| (Intercept) |
2.005 |
0.045 |
2.127 |
|
|
|
| miR-382-5p |
1.282 |
0.200 |
0.217 |
|
|
|
| miR-199a-3p |
-2.940 |
0.003 |
-0.924 |
|
|
|
| 3 model |
|
|
|
0.38 |
0.62 |
0.74 |
| (Intercept) |
-3.092 |
0.002 |
-1.002 |
|
|
|
| miR-382-5p |
-2.031 |
0.042 |
-0.217 |
|
|
|